Abstract
Although intervention trials have demonstrated significant improvement in mammography adherence for African American women, many of the current measurement tools used in these interventions have not been assessed for validity and reliability in ethnic minorities. This study assessed the validity and reliability of Health Belief Model (HBM) variables that are often the target of mammography interventions. Scale validity and reliability was assessed for HBM scales in a sample of 344 low-income African American women. Validity was supported through exploratory factor analysis and theoretical prediction of relationships. Internal consistency reliability was above .73 for all scales.
Keywords: African American, health beliefs, mammography, measurement
Introduction
Breast cancer ranks as the most common cancer in African American women and is the second leading cause of cancer deaths within low-income populations (Ward et al., 2004). Although the occurrence of breast cancer in African American women is less than in Caucasians, the five-year survival is only 75 percent as compared to 89 percent for Caucasians (Jemal et al., 2005). It has been estimated that as much as one-half of the disparity in breast cancer mortality for African American women, as compared to Caucasian women, can be explained by less frequent breast cancer screening that would have led to treatment at earlier stages of disease when the survival rates are much more favorable (American Cancer Society, 1999, 2000, 2004; Burns et al., 1996; Eley et al., 1994; Jazieh & Buncher, 2002; Ries et al., 2003).
There are many factors that may influence mammography screening behavior. A recent report identified patient, provider, and institutional factors that were causally linked to differences in health care treatment among racial and ethnic groups (Panel on Racial and Ethnic Disparities in Medical Care, 2003). Cultural beliefs about health and medical care were also cited as important contributors to disparities in medical care.
Attempts to increase mammography screening have successfully used the Health Belief Model to tailor interventions to women’s health beliefs for both African American and Caucasian women, and the variables in the Health Belief Model have been found to predict mammography screening in both African American and Caucasian populations (Champion & Springston, 1999; Mayne & Earp, 2003). Furthermore, an intervention using a tailored in-person approach with the variables of perceived risk, benefits, and barriers increased the number of African American women who received mammograms as compared to usual care (Champion, Ray, Heilman, & Springston, 2000). Similarly, a large study of Caucasian and African American women using an intervention which incorporated components to address beliefs of perceived risk, benefits, barriers, and self-efficacy to mammography significantly increased the rate of mammography adherence compared to usual care (Champion et al., 2003).
Rationale for scale development
Although intervention trials have found evidence of significant improvement in mammography adherence, many of the current measurement tools have not been assessed for validity in ethnic minorities. Identifying valid and reliable instruments specific to race and ethnicity is essential in light of the increasing national goals to eliminate health disparities (US Census Bureau, 2000). Using instruments that include culturally biased items can obviously produce invalid conclusions about the effectiveness of interventions (US Department of Health and Human Services, 2000). Furthermore, the subsequent implementation of interventions and initiatives, based on these biased findings may, in fact, be ineffective for specific racial or ethnic groups. Examination of the validity and reliability of standardized measures of women’s health beliefs related to mammography has been attempted by only a few researchers (Mikhail & Petro-Nustas, 2001; Rakowski et al., 1997; Secginli & Nahcivan, 2004; Wu & Yu, 2003). More research is needed to understand how to best measure health beliefs in culturally diverse populations.
The purpose of this study was to assess the validity and reliability of previously developed measures of Health Belief Model variables and other beliefs that may impact mammography utilization among African American women.
Theoretical framework
The Extended Parallel Process Model (EPPM), the Health Belief Model (HBM), and the Transtheoretical Model (TTM) were employed to identify constructs relevant to low-income African American women. The constructs of perceived risk, perceived benefits, perceived self-efficacy and perceived barriers were identified in the HBM. Most studies have found significant correlations between these perceived benefits and barriers, respectively, and mammography use (Champion, 1991; Fulton et al., 1991; Rimer, Davis, Engstrom, Myers, & Rosan, 1988; Schechter, Vanchieri, & Crofton, 1990; Taplin & Montano, 1993; Vernon, Laville, & Jackson, 1990). Unique concepts identified in the HBM include perceived risk of contracting the health problem and self-efficacy or confidence in the ability to take an action (Becker, 1974; Janz & Becker, 1984; Rosenstock, Strecher, & Becker, 1988). Several studies have found perceived risk to be positively related to mammography use (Champion, 1991; Schechter et al., 1990; Vernon et al., 1990).
The construct of fear was identified by the EPPM which addresses characteristics of the person and situation (perceived threat and efficacy) that are linked to health screening behavior and incorporates the anxiety/fear response resulting from cancer fatalism relevant to low-income African American women (Witte, 1992). The EPPM is an extension of Levanthal’s prediction that one of two processes will occur in response to threat. Individuals can employ cognitive strategies to select behaviors that avert danger (danger control processes) or emotion-based strategies to control fear that lead to defensive avoidance or denial (fear control processes). Phillips, Cohen and Moses (1999) discussed the significant impact fear of breast cancer has on African American women and encouraged future research in this area.
Finally, the TTM was initially developed by Prochaska and DiClemente to describe the smoking cessation process, but it has been applied to a wide range of health-related behaviors, including mammography (Prochaska & DiClemente, 1983; Prochaska, Velicer, DiClemente, & Fava, 1988). The TTM considers health behavior change as a continuum wherein the individual moves from not considering an action or health behavior to maintaining adoption of the behavior. Included in the staging algorithms are the history of the targeted behavior and intention regarding future behavior. Changing or initiating a health action involves a gradual change in decisional balance between the pros and cons. In our proposed intervention, decisional balance will be addressed through communication directed toward the specific screening benefits and barriers perceived by the individual intervention recipient.
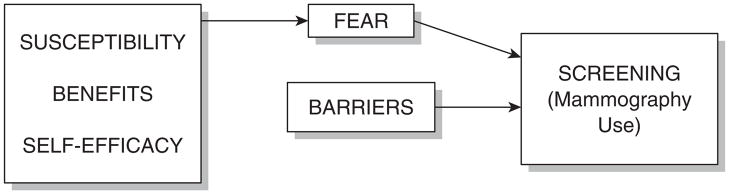
Figure 1 identifies the relationship of variables. An increase in perceived breast cancer risk should theoretically increase fear, whereas perceived benefits of mammography and self-efficacy should decrease fear. It is hypothesized that greater fear will be positively related to mammography screening and that barriers will have an additive effect on screening.
Figure 1.
Proposed integrated model.
Methods
This study was reviewed and approved by the Institutional Review Board of Indiana University.
Revision of scale measures
The perceived susceptibility scale, initially developed in 1997, included five items with an alpha of 0.83 and a test–retest of 0.68 (Champion & Scott, 1997). The first three susceptibility items involved the likelihood of getting breast cancer at five years, 10 years and during a lifetime. The fourth item stated that getting breast cancer was a possibility. The fifth item addressed concerns regarding getting breast cancer. The fourth and firth items were replaced with an item that reflected the risk of getting breast cancer compared to other women.
The perceived benefits component is defined as the perceived effectiveness of the action or behavior to decrease the threat—in this case, death from breast cancer. The benefits of mammography scale included four items with an internal reliability alpha of 0.65 and test–retest value of 0.40. Item wording was changed slightly based on feedback from an African American focus group.
The 1997 barrier scale had 13 items with an internal reliability alpha of 0.85 and test–retest alpha of 0.66. The current barriers scale had 19 items. Several changes were made following the focus group feedback. The 1997 item about being afraid that something was wrong was altered to reflect being afraid of finding a breast lump and not wanting to know if breast cancer were discovered. The 1997 items about difficulty with transportation and child care were changed to one item about inconvenience. A 1997 item about understanding the mammography process was deleted because it was felt that this item reflected self-efficacy. The six items that were added included: (1) forgetting to get a mammogram; (2) considering the treatment worse than the cure; (3) Fear of a mammogram causing breast cancer; (4) feeling too old to get a mammogram; (5) no need because the doctor already examines my breast; and (6) the feeling that getting a mammogram would cause breast cancer.
The self-efficacy scale, consisting of 10 items, had an internal reliability coefficient of 0.91 (Champion, Skinner, & Menon, 2005). A one-dimensional factor structure indicated all items in this scale loaded at 0.60 or above. This scale also discriminated between adherent and non-adherent women (p < .003).
The fear scale included eight items that measured emotional reaction to thinking about breast cancer. The items were found to be valid and reliable in another study that included Caucasian and African American women (Champion, Menon, Rawl, & Skinner, 2004). The internal consistency reliability alpha was found to be 0.91. Construct validity of this scale has been documented using factor analysis and testing of theoretical relationship (Champion et al., 2004).
Mammography stage of readiness
Three items were used to create algorithms that identified participants as being in the pre-contemplation, contemplation, or action stage of readiness to obtain a mammogram. Items obtained the participants’ past mammography history, their intent to be screened in the next six months and the date of their most recent mammogram. Definitions were as follows:
Pre-contemplation—Never had mammogram or had one > 18 months ago and does not plan to have one within six months.
Contemplation—Never had mammogram or had one > 18 months ago and plans to have another within six months.
Action—Had one mammogram since intervention, intends to have another mammogram one year following their last one, or plans to have one as recommended by their health care provider.
Sample
A total of 344 women agreed to participate out of 492 who were eligible resulting in a response rate of 69.9 percent. Reasons for not participating included no interest or time. Women were accrued into the study in three ways, which differed only by location of the initial contact. First, direct accrual occurred at multi-servicecenters located in a Midwestern city, at an African American convention, and at a general medicine clinic serving low-income clients. In all locations, research assistants approached women and asked if they met the project’s eligibility criteria, which included not having a mammogram within the last 18 months, being 41 to 75 years of age, and being at the 175 percent of poverty level or lower. Second, a low-income health center participated in referring women for the study. After screening for initial eligibility, the health center forwarded the names of women who met study criteria to the project manager. Letters written on the health center’s stationery were sent to these women. Research assistants then called the women to reconfirm eligibility and explain the study. If a woman verbally agreed to participate, an appointment was made for her to meet the research assistant at the health center during which time the informed consent was signed, a baseline interview was completed, and the intervention delivered. Third, women were recruited through churches and public housing. Staff at the church or housing tenant council identified women, and if they were willing, arranged times when research assistants were available to meet with them. Eligibility was established, the consent formed was signed, the baseline interview was completed, and intervention was delivered. Data collected included demographic variables, beliefs, knowledge, and information about mammography experiences. All women were surveyed at baseline (Time 1) and at one month following the intervention (Time 2) in order to detect intervention effects on cognitive stage and belief change. Stage and belief change as well as mammography adherence were also measured at six months post-intervention (Time 3).
Results
Analysis
First, exploratory factor analysis was conducted on all scale items to provide evidence of construct validity. The principal component method of factor extraction was used. Varimax rotation was performed. A Scree plot was used to identify the number of factors. Second, scales that were refined through factor analysis were individually assessed for reliability using Cronbach’s alpha.
Third, item discrimination was estimated with item-total scale correlation coefficients (after excluding each item from the total). Fourth, construct validity was assessed by determining how well the data fit the theoretical relationships. Two regression models were computed based on the theoretical model. A linear regression model was computed with fear as the dependent variable and perceived susceptibility, benefits and self-efficacy as the predictors. A binary logistic regression was then computed to predict mammography utilization using the total fear scale and the barriers scale. Finally, construct validity was assessed by determining how well the scales measured at follow-up discriminated the stage of readiness to obtain a mammography (pre-contemplation vs contemplation vs action). If the scales were sensitive to the behavior change, there should be significant differences between women in each stage of behavior for all the scales.
Assessment of scale validity and reliability
Scale validity and reliability were assessed using several criteria. Construct validity was demonstrated by showing that items factor on their respective scales (as shown in Fig. 1) with a value of 0.4 or greater. Construct validity was demonstrated by showing that the scales discriminate between pre-contemplators, contemplators and women in the action stage after intervention. Convergent validity was demonstrated by item and total correlations of not less than 0.20. In addition, internal consistency (reliability) was found to be 0.7 or higher.
The age of the participants ranged from 41 to 79 with a mean of 50.6 (8.8, SD). For education, the mean highest grade achieved was 12.3 (2.2, SD). All of the study subjects were African American females at or below an income of 150 percent of poverty.
Factor analysis results
A five-factor solution produced the results shown in Table 1, labeled as susceptibility, benefits, barriers, self-efficacy, and fear. All items loaded higher on their a priori factor than on any other factor. Only three items (one each from benefits, barriers, and self-efficacy) loaded less than 0.40 (but greater than 0.30). However, even for those three items, the loading was much greater on the item’s a priori factor than on any other factor. The Eigenvalue for each factor after Varimax rotation was (with proportion of common variance accounted for in parenthesis): barriers—6.4 (24.3), fear—5.7 (21.6), self-efficacy—4.7 (17.9), susceptibility—2.2 (8.5), benefits—2.0 (7.7). The five factors accounted for 80 percent of the variance common to all items and 47 percent of total variance.
Table 1.
Factor analysis of scales
| Item/factor loading | Item/total correlation | ||
|---|---|---|---|
| Susceptibility | Factor 4 | ||
| 1. | How likely is it that ‘I will get breast cancer in five years’? | .74 | .65 |
| 2. | How likely is it that ‘I will get breast cancer in the next 10 years’? | .79 | .72 |
| 3. | How likely is it that ‘I will get breast cancer during my lifetime’? | .70 | .65 |
| 4. | ‘Compared to other women my age, would you say your chances of getting breast cancer are higher or lower?’ | .47 | .42 |
| Benefits | Factor 5 | ||
| 1. | ‘If breast cancer was found early, how likely is it that the cancer could be successfully treated?’ | .30 | .41 |
| 2. | How likely is it that ‘Having a mammogram would help me find breast cancer when it is just getting started’? | .64 | .66 |
| 3. | How likely is it that ‘Having a mammogram could help me find a breast lump before it is big enough to feel’? | .64 | .61 |
| 4. | How likely is it that ‘Having a mammogram will decrease my chances of dying from breast cancer’? | .44 | .48 |
| Barriers | Factor 1 | ||
| 1. | How likely is it that ‘Getting a mammogram would be inconvenient for me’? | .45 | .43 |
| 2. | How likely is it that ‘Getting a mammogram could cause breast cancer’? | .38 | .40 |
| 3. | How likely is it that ‘The treatment I would get for breast cancer would be worse than the cancer itself’? | .44 | .42 |
| 4. | How likely is it that ‘Being treated for breast cancer would cause me a lot of problems’? | .41 | .41 |
| 5. | How likely is it that ‘Other health problems would keep me from having a mammogram’? | .57 | .55 |
| 6. | How likely is it that ‘My age would keep me from having a mammogram’? | .67 | .62 |
| 7. | How likely is it that ‘I would not get a mammogram because my doctor already examines my breasts’? | .51 | .49 |
| 8. | How likely is it that ‘Being afraid of finding a breast lump would keep me from having a mammogram’? | .60 | .58 |
| 9. | How likely is it that ‘The trouble of having a mammogram would keep me from getting one’? | .75 | .69 |
| 10. | How likely is it that ‘Concern about pain with having a mammogram would keep me from having one’? | .66 | .60 |
| 11. | How likely is it that ‘Being embarrassed about my body would keep me from having a mammogram’? | .60 | .58 |
| 12. | How likely is it that ‘I will not have time to have a mammogram’? | .54 | .50 |
| 13. | How likely is it that ‘Not being able to afford a mammogram would keep me from having one’? | .47 | .46 |
| 14. | How likely is it that ‘Worrying about breast cancer would keep me from having a mammogram’? | .66 | .63 |
| 15. | How likely is it that ‘Concerns about being exposed to the x-ray would keep me from having a mammogram’? | .57 | .56 |
| 16. | How likely is it that ‘I find it difficult to remember to make an appointment for a mammogram’? | .50 | .45 |
| 17. | How likely is it that ‘Forgetting my appointment would keep me from getting a mammogram’? | .57 | .53 |
| 18. | How likely is it that ‘Being treated rudely at the mammogram centers would keep me from having a mammogram’? | .48 | .47 |
| 19. | How likely is it that ‘Not wanting to know would keep me from having a mammogram’? | .61 | .59 |
| Self-efficacy | |||
| 1. | How likely is it that ‘I can get a mammogram even if my doctor does not tell me to get one’? | .58 | .45 |
| 2. | How likely is it that ‘I can get transportation to have a mammogram’? | .60 | .64 |
| 3. | How likely is it that ‘I can arrange other things in my day to have a mammogram’? | .65 | .66 |
| 4. | How likely is it that ‘I can talk to people at the mammogram center if I have a problem’? | .70 | .65 |
| 5. | How likely is it that ‘I will get a mammogram even if I am worried’? | .73 | .69 |
| 6. | How likely is it that ‘I will get a mammogram even if I don’t know what to expect’? | .76 | .69 |
| 7. | How likely is it that ‘I can find a way to pay for a mammogram’? | .37 | .47 |
| 8. | How likely is it that ‘I can make an appointment for a mammogram’? | .68 | .64 |
| 9. | How likely is it that ‘I can find a place to get a mammogram (or know where to go to get a mammogram)’? | .59 | .64 |
| 10. | How likely is it that ‘I can get a mammogram’? | .62 | .63 |
| Fear (Strongly agree to Strongly disagree) | |||
| 1. | ‘When I think about breast cancer, I get scared.’ | .75 | .71 |
| 2. | ‘When I think about breast cancer, I feel nervous.’ | .82 | .78 |
| 3. | ‘When I think about breast cancer, I get upset.’ | .82 | .80 |
| 4. | ‘When I think about breast cancer, I get depressed.’ | .81 | .79 |
| 5. | ‘When I think about breast cancer, I get edgy.’ | .86 | .85 |
| 6. | ‘When I think about breast cancer, my heart beats faster.’ | .80 | .78 |
| 7. | ‘When I think about breast cancer, I feel uneasy.’ | .86 | .82 |
| 8. | ‘When I think about breast cancer, I feel anxious.’ | .78 | .77 |
Reliability analysis
Reliability analysis was conducted following exploratory factor analysis. Cronbach’s alpha was computed for each scale. Item means and variances along with the alpha coefficient are listed in Table 2. All coefficients were above 0.70 even though susceptibility and benefits had only four items each. Along with the reliability analysis, item/total correlations were computed. All item/total correlations were at 0.40 or above as shown in Table 1.
Table 2.
Reliability
| Scale | # items | Mean of item | Mean of item SDs | Alpha |
|---|---|---|---|---|
| Susceptibility | 4 | 2.86 | 1.70 | .79 |
| Benefits | 4 | 6.02 | 1.50 | .73 |
| Barriers | 19 | 2.01 | 1.64 | .89 |
| Self-efficacy | 10 | 5.98 | 1.70 | .88 |
| Fear | 8 | 3.01 | 1.36 | .94 |
Theoretical prediction
The relationship of perceived susceptibility, benefits and self-efficacy and fear was examined using linear multiple regression. Simultaneous entry of the independent variables yielded an F value of 3.78 (d.f. = 3 & 295), p =.01. Self-efficacy and susceptibility were significant predictors of fear, while benefits was not significant, as shown in Table 3. The model estimated that the correlation between susceptibility and fear was 0.14 after adjusting for the variance that susceptibility shared with benefits and self-efficacy. Influence diagnostics indicated no significant collinearity between the independent variables. Since benefits was moderately correlated with self-efficacy (r = 0.41, p < .0001), and weakly correlated with susceptibility (r = −0.04, p = .51), it was speculated whether benefits alone, unadjusted for self-efficacy, might significantly predict fear. However, benefits was an even weaker predictor when entered alone (p = .945).
Table 3.
Prediction of fear by susceptibility, benefits and self-efficacy
| Variable | Standardized beta | Partial t-test t value | Partial t-test p value | Semi-partial correlation |
|---|---|---|---|---|
| Susceptibility | .139 | 2.41 | .017 | .14 |
| Benefits | .055 | 0.87 | .382 | .05 |
| Self-efficacy | −.131 | −2.08 | .038 | −.12 |
As a second step, binary logistic regression was used to determine the significance of fear and barriers (at Time 1) in predicting whether women obtained a mammogram by the Time 3 survey. A separate univariate logistic regression, performed to determine if demographic variables should be used as covariates in the analysis, found that age did not predict mammography adherence (p = .31), but that education was a marginal predictor (p = .07). Further, education was negatively correlated with fear (r = −.12, p = .035); thus, education was included as a covariate. The model using fear and barriers as independent variables along with education as a covariate, significantly predicted which women would have obtained a mammogram within the six months post-intervention (likelihood ratio chi-square = 23.19, d.f. = 3, p < .0001). Both barriers (p < .0001) and fear (p = .018) were significant predictors of women obtaining a mammography after adjusting for each other and for education (Table 4). The model estimated that the odds of obtaining a mammogram by Time 3 decreased by a factor 0.84 for every five-unit increase in barriers score, and increased by a factor of 1.19 for every five-unit increase in fear score, adjusted for education.
Table 4.
Prediction of mammography adherence by fear and barriers
| Scale | Estimated coefficient | Estimated standard error | Chi-square (d.f. = 1) | p-value | Adjusted odds ratio | 95% Wald confidence interval |
|---|---|---|---|---|---|---|
| Education | .108 | .062 | 3.14 | .077 | 1.72 | .93, 3.16 |
| Barriers | −.035 | .009 | 18.09 | <.0001 | .84 | .77, .92 |
| Fear | .035 | .015 | 5.61 | .018 | 1.19 | 1.03, 1.37 |
Notes: Model chi-square = 23.19, d.f. = 3, p < 0001. Chi-square for model and predictors are likelihood ratio. Odds ratios are for a five-unit increase in predictors
Sensitivity to differences in mammography stage of readiness
Data collected one month post-intervention (Time 2) was used to determine if scales were sensitive to differences in stage. Table 5 indicates that there were significant differences in benefits, barriers and self-efficacy by stage, but not susceptibility or fear. All group means are in the anticipated direction. For instance, the mean benefit scale is lowest for those in the pre-contemplation stage, significantly higher for those considering mammography (contemplators) and significantly higher for those who already received a mammogram (action). For barriers, as would be expected, the mean scale decreased as women progressed towards action; those in the action stage had a significantly lower mean barriers scale than those in the pre-contemplation stage. For self-efficacy, the mean scale value increased significantly as women progressed towards action.
Table 5.
Scale differences by stage
| Scale | Precontemplation mean (n = 38) | Contemplation mean (n = 250) | Action mean (n = 32) | F value (d.f. = 2&317) | Significant group differencesa |
|---|---|---|---|---|---|
| Susceptibility | 10.97 | 12.54 | 11.28 | 1.91 | NS |
| Benefits | 22.87 | 24.90 | 25.16 | 5.45** | 1 vs 2 1 vs 3 |
| Barriers | 46.45 | 38.89 | 35.80 | 3.20* | 1 vs 3 |
| Self-efficacy | 56.92 | 62.22 | 63.66 | 6.15** | 1 vs 2 1 vs 3 |
| Fear | 26.37 | 23.88 | 24.47 | 1.25 | NS |
Notes:
p < 05;
p < 01;
Post hoc tests for each pair of two groups performed with Student-Newman-Keuls test at alpha of 0.05
Discussion
The factor analysis supports the validity of the scale items loading appropriately into the five distinct dimensions (susceptibility, benefits, barriers, self-efficacy, and fear) for African American women. Future research should include conducting factor analyses on the present set of 45 items using a larger sample size. However, the moderate-to-high magnitudes of the factor loading values of each item on its a priori factor are suggestive of strong validity of items in each scale.
Testing of theoretical relationships demonstrated adequate construct validity. Self-efficacy and benefits were related to fear. Women with increased perceived self-efficacy and benefits had decreased fear. This finding is consistent with previous research (Champion et al., 2004). No relationship existed between perceived benefits and fear. One possible explanation is that with the increasing media attention and community and church education programs about breast cancer, more African American women may know about breast cancer benefits, regardless of whether they are or are not fearful of this disease. This assumption is further substantiated in that no significant differences existed between susceptibility and stages of mammography screening adoption. Thus, level of perceived susceptibility is fairly stable for women who have not been screened (pre-contemplators and contemplators) and those who have received screening (actors).
Increased fear and decreased barriers were related to adherence to mammography screening behavior. These findings support the theoretical relationships depicted in Fig. 1 and are congruent with past research (Champion et al., 2004).
Conclusion
The utility of using the health belief model in the development and refinement of scales for predicting mammography screening behavior in African American women is evident. Not withstanding acknowledgment of the contributions of other determinants of screening, including socio-cultural, environment, health system, and public policy factors (Meissner et al., 2004), health beliefs as conceptualized by the health belief model do add to the prediction of mammography screening behavior. Our challenge was to refine and add to current health belief scales that are reflective of African American women. For this study we tested the psychometric properties of health belief scales in low-income African American women living in a Midwestern urban area and found the scales to have acceptable validity and reliability. However, since cultural norms can vary among subpopulations within racial and ethnic groups, it is important to validate these scales with other subgroups of African American women for conceptual, scale, and norm equivalence (Rubio & Williams, 2004). Thus, we recommend continued psychometric assessment of these scales with African American women across acculturation levels, socioeconomic strata, educational background, geographic location, and national origin.
Acknowledgments
This research was funded by the National Cancer Institute, R01 CA77736.
Biographies
VICTORIA CHAMPION is a Distinguished Professor and Associate Dean for Research at Indiana University, USA. Her NIH funded research has spanned over 25 years with a primary focus on increasing adherence to cancer screening. She leads the Cancer Prevention Program at Indiana University Cancer Center.
PATRICK O. MONAHAN [to follow]
JEFFERY K. SPRINGSTON is a Professor and Associate Dean for Research and Graduate Studies in the College of Journalism and Mass Communication, University of Georgia.
KATHLEEN M. RUSSELL is an Associate Professor at Indiana University School of Nursing. Her research focuses on investigating behavioral and social determinants of cancer screening in medically underserved African Americans and developing access enhancing lay health advisor interventions.
TERRELL W. ZOLLINGER received his DrPH in Epidemiology and MSPH in Biostatistics from Loma Linda University. He is a Professor in the Department of Family Medicine and the Department of Obstetrics and Gynecology, Indiana University School of Medicine and serves as the Associate Director of the I. U. Bowen Research Center.
ROBERT M. SAYWELL, JR, received his PhD in Economics from Colorado State University and his MPH from the Johns Hopkins University. He is a Professor Emeritus in the Department of Family Medicine, Indiana University School of Medicine and serves as a Senior Researcher in the I. U. Bowen Research Center.
MALTIE MARAJ [to follow]
Footnotes
COMPETING INTERESTS: None declared.
Contributor Information
VICTORIA L. CHAMPION, Indiana University, USA
PATRICK O. MONAHAN, Indiana University, USA
JEFFERY K. SPRINGSTON, University of Georgia, USA
KATHLEEN RUSSELL, Indiana University, USA.
TERRELL W. ZOLLINGER, Indiana University, USA
ROBERT M. SAYWELL, JR, Indiana University, USA.
MALTIE MARAJ, Indiana University, USA.
References
- American Cancer Society. Cancer facts and figures. Atlanta, GA: American Cancer Society; 1999. [Google Scholar]
- American Cancer Society. Cancer facts & figures. Atlanta, GA: American Cancer Society; 2000. [Google Scholar]
- American Cancer Society. Cancer facts & figures 2004. Atlanta, GA: American Cancer Society; 2004. [Google Scholar]
- Becker MH. The Health Belief Model and personal health behavior. San Francisco, CA: Society for Public Health Education; 1974. [Google Scholar]
- Burns RB, McCarthy EP, Freund KM, Marwill SL, Shwartz M, Ash A, et al. Black women receive less mammography even with similar use of primary care. Annals of Internal Medicine. 1996;125(3):173–182. doi: 10.7326/0003-4819-125-3-199608010-00002. [DOI] [PubMed] [Google Scholar]
- Champion VL. The relationship of selected variables to breast cancer detection behaviors in women 35 and older. Oncology Nursing Forum. 1991;18(4):733–739. [PubMed] [Google Scholar]
- Champion V, Maraj M, Hui S, Perkins AJ, Tierney WM, Menon U, et al. Comparison of tailored interventions to increase mammography screening in nonadherent older women. Preventive Medicine. 2003;36(2):150–158. doi: 10.1016/s0091-7435(02)00038-5. [DOI] [PubMed] [Google Scholar]
- Champion VL, Menon U, Rawl S, Skinner CS. A breast cancer fear scale: Psychometric development. Journal of Health Psychology. 2004;9(6):769–778. doi: 10.1177/1359105304045383. [DOI] [PubMed] [Google Scholar]
- Champion VL, Ray DW, Heilman DK, Springston JK. A tailored intervention for mammography among low-income African-American women. Journal of Psychosocial Oncology. 2000;18(4):1–13. [Google Scholar]
- Champion VL, Scott CR. Reliability and validity of breast cancer screening belief scales in African American women. Nursing Research. 1997;46(6):331–337. doi: 10.1097/00006199-199711000-00006. [DOI] [PubMed] [Google Scholar]
- Champion V, Skinner CS, Menon U. Development of a self-efficacy scale for mammography. Research in Nursing and Health. 2005;28(4):329–336. doi: 10.1002/nur.20088. [DOI] [PubMed] [Google Scholar]
- Champion VL, Springston J. Mammography adherence and beliefs in a sample of low-income African American women. International Journal of Behavioral Medicine. 1999;6(3):228–240. doi: 10.1207/s15327558ijbm0603_2. [DOI] [PubMed] [Google Scholar]
- Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, et al. Racial differences in survival from breast cancer: Results of the National Cancer Institute Black/White Cancer Survival Study. Journal of the American Medical Association. 1994;272(12):947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- Fulton JP, Buechner JS, Scott HD, DeBuono BA, Feldman JP, Smith RA, et al. A study guided by the Health Belief Model of the predictors of breast cancer screening of women ages 40 and older. Public Health Reports. 1991;106(4):410–420. [PMC free article] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: A decade later. Health Education Quarterly. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Jazieh AR, Buncher CR. Racial and age-related disparities in obtaining screening mammography: Results of a statewide database. Southern Medical Journal. 2002;95(10):1145–1148. [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA: A Cancer Journal for Clinicians. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Mayne L, Earp J. Initial and repeat mammography screening: Different behaviors/different predictors. Journal of Rural Health. 2003;19(1):63–71. doi: 10.1111/j.1748-0361.2003.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Meissner HI, Vernon SW, Rimer BK, Wilson KM, Rakowski W, Briss PA, et al. The future of research that promotes cancer screening. Cancer. 2004;101(5 Suppl):1251–1259. doi: 10.1002/cncr.20510. [DOI] [PubMed] [Google Scholar]
- Mikhail BI, Petro-Nustas WI. Transcultural adaptation of Champion’s Health Belief Model Scales. Journal of Nursing Scholarship. 2001;33(2):159–165. doi: 10.1111/j.1547-5069.2001.00159.x. [DOI] [PubMed] [Google Scholar]
- Panel on Racial and Ethnic Disparities in Medical Care. The right to equal treatment: An action plan to end racial and ethnic disparities in clinical diagnosis and treatment in the United States. [accessed 31 March 2004];2003 http://www.phr.usa.org/research/domestic/race/race_report/report.html.
- Phillips JM, Cohen MZ, Moses G. Breast cancer screening and African American women: Fear, fatalism, and silence. Oncology Nursing Forum. 1999;26(3):561–571. [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF, DiClemente CC, Fava J. Measuring processes of change: Applications to the cessation of smoking. Journal of Consulting and Clinical Psychology. 1988;56(4):520–528. doi: 10.1037//0022-006x.56.4.520. [DOI] [PubMed] [Google Scholar]
- Rakowski W, Stoddard AM, Rimer BK, Anderson MR, Urban N, Lane DS, et al. Confirmatory analysis of opinions regarding the pros and cons of mammography. Health Psychology. 1997;16(5):433–441. doi: 10.1037//0278-6133.16.5.433. [DOI] [PubMed] [Google Scholar]
- Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, et al. [accessed 31 March 2004];SEER cancer statistics review, 1975–2000. 2003 http://seer.cancer.gov/csr/1975_2000.
- Rimer BK, Davis SW, Engstrom PF, Myers RE, Rosan JR. Some reasons for compliance and noncompliance in a health maintenance organization breast cancer screening program. Journal of Compliance in Health Care. 1988;3(2):103–114. [PubMed] [Google Scholar]
- Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Education Quarterly. 1988;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- Rubio M, Williams DR. The social dimension of race. In: Beech BM, Goodman M, editors. Race & research: Perspectives on minority participation in research. Washington, DC: American Public Health Association; 2004. pp. 1–25. [Google Scholar]
- Schechter C, Vanchieri CF, Crofton C. Evaluating women’s attitudes and perceptions in developing mammography promotion messages. Public Health Reports. 1990;105(3):253–257. [PMC free article] [PubMed] [Google Scholar]
- Secginli S, Nahcivan NO. Reliability and validity of the breast cancer screening belief scale among Turkish women. Cancer Nursing. 2004;27(4):287–294. doi: 10.1097/00002820-200407000-00005. [DOI] [PubMed] [Google Scholar]
- Taplin SH, Montano DE. Attitudes, age, and participation in mammographic screening: A prospective analysis. Journal of the American Board of Family Practice. 1993;6:13–23. [PubMed] [Google Scholar]
- US Census Bureau. US interim projections by age, sex, race, and Hispanic origin. 2000 http://www.census.gov/ipc/www/usinterimproj/
- US Department of Health and Human Services. Healthy people 2010: Understanding and improving health. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- Vernon SW, Laville EA, Jackson GL. Participation in breast screening programs: A review. Social Science & Medicine. 1990;30(10):1107–1118. doi: 10.1016/0277-9536(90)90297-6. [DOI] [PubMed] [Google Scholar]
- Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA: A Cancer Journal for Clinicians. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- Witte K. Putting the fear back into fear appeals: The extended parallel process model. Communication Monographs. 1992;59(4):329–349. [Google Scholar]
- Wu TY, Yu MY. Reliability and validity of the mammography screening beliefs questionnaire among Chinese American women. Cancer Nursing. 2003;26(2):131–142. doi: 10.1097/00002820-200304000-00007. [DOI] [PubMed] [Google Scholar]