Abstract
Patterned, spontaneous activity occurs in many developing neural circuits, including the retina, the cochlea, the spinal cord, the cerebellum and the hippocampus, where it provides signals that are important for the development of neurons and their connections. Despite differences in adult architecture and output across these various circuits, the patterns of spontaneous network activity and the mechanisms that generate it are remarkably similar and can include a depolarizing action of GABA, transient synaptic connections, extrasynaptic transmission, gap junction coupling and the presence of pacemaker-like neurons. Interestingly, spontaneous activity is robust; if one element of a circuit is disrupted another will generate similar activity. This research suggests that developing neural circuits exhibit transient and tunable features that maintain a source of correlated activity during critical stages of development.
Introduction
One way to understand the complexity of neural circuits is to understand how their connectivity emerges during development. The traditional model of brain development includes two phases: an early phase during which a coarse wiring of the nervous system is laid out, followed by a later phase during which the coarse connections are refined. In this model, the developmental events that underlie the coarse wiring are the result of predetermined genetic programs and occur independent of neural activity, whereas the refinement is a result of interactions between the nervous system and the outside world. For example, the traditional view of visual system development is that a genetic program specifies the organization of projections from the retina to the brain and among visual areas within the brain, whereas once vision matures, neural activity driven by visual experience refines the coarse neuronal circuits into their adult pattern of connectivity.
This traditional model is slowly being modified to accommodate an overwhelming number of observations that neural activity and genetic programs interact to specify the composition and organization of neural circuits during all stages of development. Even at extremely early stages, well before synapses form, neurons and neuronal precursors exhibit spontaneous electrical and chemical activity. These early forms of activity, which often occur on a cell-by-cell basis and are not typically correlated across cells, influence developmental events such as neuronal differentiation, establishment of neurotransmitter phenotype, and neuronal migration (for reviews, see1,2).
As neurons start to form synaptic connections and functional circuits begin to emerge, spontaneous activity becomes correlated across large groups of neighboring cells. This spontaneous network activity has been observed in many parts of the developing nervous system, and it serves a variety of purposes. In developing sensory epithelia, in particular the retina3, 4 and cochlea5, spontaneous network activity correlates action potential firing among projection neurons during a period of development when these projections are forming sensory maps6–8. Spontaneous activity is also observed in the developing spinal cord9, where it contributes to motor neuron path-finding10, maturation of synapses11, and development of pattern-generating circuits within the cord12, 13. In forebrain structures such as the hippocampus14, 15 and the neocortex16, 17, as well as in the hindbrain18, the midbrain19, and the cerebellum20, it has been postulated that spontaneous activity contributes to the development of local circuits21, 22. Each brain area comprises a unique circuit, but there are striking similarities in some aspects of the mechanisms used to generate spontaneous activity.
This Review describes the cellular mechanisms that underlie the generation of correlated firing patterns in immature neural circuits soon after the onset of synapse formation. We do not attempt to review all of the mechanisms that underlie spontaneous activity in multiple brain areas. Rather, our goal is to highlight the remarkable parallels found in the mechanisms used by different circuits. In general, in these developing circuits, transient excitatory networks correlate spontaneous activity in neurons. These networks are formed by different combinations of various mechanisms, such as a depolarizing action of GABA, transient synaptic connections, extrasynaptic transmission, and gap junction coupling. The recurrent excitatory connections in these networks amplify interactions between spontaneously active cells, initiating correlated network activity. In addition, these networks can be resistant to perturbation in the sense that pharmacological or genetic disruptions of critical network components lead to an expression of alternative circuit mechanisms that generate activity similar to the endogenous pattern, suggesting that redundancy is built into neural circuits to ensure that the spontaneous activity is maintained.
Features of spontaneous network activity
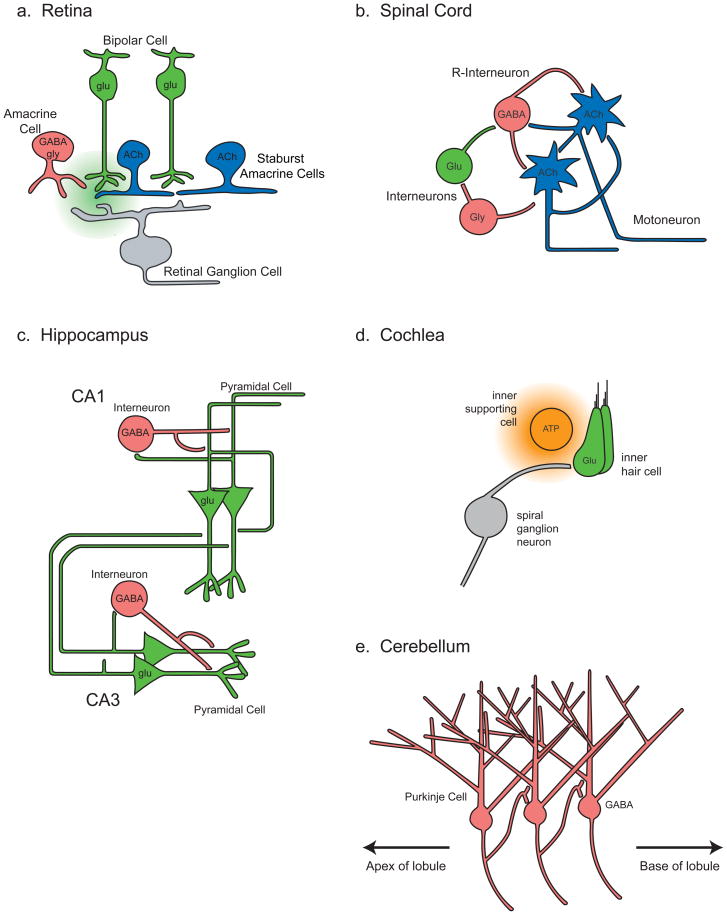
Spontaneous network activation has been observed in multiple developing circuits, but has been best characterized in the retina, the spinal cord and the hippocampus. (A wide range of spontaneous activity patterns analogous to those observed in the hippocampus has also been described in the neocortex; for a review, see23.) The activity patterns in these diverse structures are grossly similar during development. In all three cases, spontaneous network events are comprised of large, slow depolarizations crested by bursts of action potentials (Table 1). Another common feature is that excitatory interneurons have a role in the generation of spontaneous activity. Recently, spontaneous network activity has also been described in developing cochlea5 and cerebellum20. Although the details are not yet fully understood, the strategies used by the cochlea and cerebellum are comparable to those previously described in the retina, spinal cord, and hippocampus. Schematics of the functional circuits that mediate spontaneous network depolarizations in each of these brain structures are provided in Box 1.
Table 1.
Summary of important features of spontaneous network activity recorded in rodents.
| Retina | Spinal Cord | Hippocampus | Cochlea | Cerebellum | |||||
|---|---|---|---|---|---|---|---|---|---|
| Stage | 1. E17-P1 | 2. P1–P10 | 3. P10–P14 | 1. E12–E15 | 2. E15–E18 | 1. E18 – P5 (SPAs) |
2. P3–P10 (GDPs) |
P7–P10 | P4–P6 |
| Description of projection neuron firing patterns | Bursts that propagate over a limited region of the GCL | Bursts that propagate over a large region of the GCL | Clusters of bursts that propagate over a large region of the GCL | Bursts of oscillatory activity that propagate within and between segments | Bursts of oscillatory activity that propagate within and between segments | Ca2+ spikes correlated over few pyramidal cells | Bursts correlated across CA3 and CA1 subfields | Bursts of action potentials; correlation pattern unknown | Travelling waves of action potentials that propagate from apex to base of cerebellar lobules |
| Inter-event interval | 30 s | 1 – 2 min | 1 min | 2–3 min | 1 min | 8 s | 3–10 s | 5–60 s | 100 ms |
| Mechanisms of Initiation | Unknown | Spontaneous Ca2+ spikes in starburst amacrine cells | Unknown | Network interactions | Network interactions | Spontaneous Ca2+ spikes in pyramidal cells | Intrinsic bursts in CA3 interneurons | Unknown | Spontaneous firing in Purkinje neurons |
| Primary source of depolarization | Gap junctions | nACh receptors | iGlu receptors | nACh, GABAA, and glycine receptors | iGlu, nACh, glycine, and GABAA receptors | L-type Ca2+ channels and gap junctions | GABAA and NMDA receptors | ATP release from supporting cells in Kölliker’s organ | GABAA receptors |
| State of network at end | Maturation of cholinergic circuit | Maturation of glutamatergic circuits | Onset of vision | Loss of requisite role for nAChR signaling | GABA signaling becomes inhibitory | Maturation of GDP circuits | GABA signaling becomes inhibitory | Kölliker’s organ disappears | GABA signaling becomes inhibitory |
| Recorded in vivo | No | Yes3 | Yes26, 27 | Yes (chick29) | Yes (chick29) | No | Yes125 | Yes41 | No |
E, embryonic; P, postnatal; GCL, ganglion cell layer; SPA, synchronous plateau assembly; GDP, giant depolarizing potential; iGlu receptor, ionotropic glutamate receptor; nACh receptor, nicotinic acetyl choline receptor; GABAA receptor, γ-Aminobutyric acid receptor A
Box 1. Circuits mediating spontaneous network activity during development.
In the retina (see the figure, part a) three distinct circuits mediate retinal waves at different stages of development (for review, see6); the circuit that mediates waves perinatally is not shown. The cholinergic circuit that mediates waves during the first postnatal week consists of cholinergic interneurons (starburst amacrine cells, blue) forming excitatory synaptic connections with other starburst amacrine cells and projection neurons (retinal ganglion cells, grey) (for details, see Figure 2). It is postulated that wave propagation is mediated by excitatory connections among starburst amacrine cells, which in turn release acetylcholine that depolarizes the ganglion cells. Waves are initiated by spontaneous depolarization in starburst amacrine cells that are amplified by recurrent excitatory connections, and the interval between waves is set by a slow after-hyperpolarization in these cells. The circuit that mediates glutamatergic waves, which occur between postnatal day 10 and 14, consists of glutamatergic interneurons (bipolar cells, green), inhibitory interneurons (amacrine cells, pink), and retinal ganglion cells. One hypothesis is that bipolar cells are coupled by high levels of extrasynaptic glutamate (green cloud), which spills out of the synaptic cleft.
In each segment of the developing spinal cord the same circuit mediates spontaneous network activity (see the figure, part b)66, 80. The circuit consists of glutamatergic (green) and GABAergic and glycinergic interneurons (represented here as a single cell in red), and cholinergic projection neurons (motor neurons, blue), which transiently make nAChR-mediated connections with local interneurons. It is postulated that spontaneous network events initiate in motor neurons, which depolarize a population of GABAergic interneurons, Renshaw cells (R-interneurons). Later in development the propagation of spontaneous network activity in the spinal cord becomes more dependent on GABAergic and glutamatergic transmission. Event initiation is due to a slow buildup of synaptic activity via recurrent excitatory connections until an event threshold is reached. During an event, strong activation of GABAA receptors lowers the intracellular chloride concentration, which diminishes the depolarizing force of GABA. The inter-event interval is set by the time it takes to restore chloride concentrations such that the depolarizing action of GABA is restored71. Schematic modified from66. The circuits that mediate event propagation along the length of the cord are not described here66.
In the hippocampus, the circuit that mediates giant depolarizing potentials (GDPs) consists of pyramidal cells (green) and local GABAergic interneurons (pink) in both the CA3 region and CA1 regions of the hippocampus (see the figure, part c; for review, see47, 48, 124). GDPs are most likely to initiate in the CA3 region, where intrinsic bursting activity in CA3 pyramidal cells is coupled with network interactions mediated by depolarizing GABA and recurrent excitatory connections. The inter-event interval is set by an after-hyperpolarization in CA3 neurons49, 50.
The cochlear circuit (see the figure, part d) consists of glutamatergic inner hair cells (green), a transient population of inner support cells (orange) located in a developmentally transient structure called Kölliker’s organ, and the projection neurons5 (spiral ganglion cells, gray). Spontaneous network activity in the cochlea is initiated by a diffuse release of ATP (orange cloud) from inner support cells, which drives depolarization in nearby inner hair cells by activating both metabotropic and ionotropic ATP receptors. Inner hair cells in turn release glutamate, which depolarizes spiral ganglion cells via activation of ionotropic glutamate receptors. The mechanisms determining the inter-event interval are not known.
The circuit mediating spontaneous network activity in the cerebellum (see the figure, part e) consists solely of projection neurons20, which are GABAergic Purkinje cells. Purkinje cells are transiently connected via local axon collaterals, which entrain the spontaneous firing of nearby Purkinje cells via depolarizing GABA signaling. The direction of propagation is dictated by the asymmetric wiring of local collaterals, with Purkinje cells located toward the base of a lobule receiving more connections than those located toward the apex.
The projection neurons of the retina — retinal ganglion cells — exhibit spontaneous bursts of action potentials that are separated by extended periods of silence during development3. These bursts of action potentials spread as waves of depolarization across the retina4, 24, which earned them the name retinal waves (see Supplemental Movie). Retinal waves propagate through the developing visual system, inducing similar burst patterns in the dorsal lateral geniculate nucleus of the thalamus25, 26 and in visual cortex27. Such spontaneous network activation appears very early in development — after retinal ganglion cells have extended axons to their primary targets in the brain — and lasts until the eyes open, which occurs on postnatal day 13–14 in mice. During this time, as the circuits that mediate retinal waves change (Table 1, Box 1), so too do the details of the resulting firing patterns (Table 1). In the last stage, retinal waves briefly co-exist with visual responses, presumably using parallel circuitry.
In spinal cord, motor neurons exhibit episodes of large rhythmic depolarizations that are separated by extended periods of silence, a firing pattern that drives embryonic limb movements28, 29. This spontaneous network activity has been observed over an extended period of development, from before motor neurons innervate muscle fibers30 until central pattern generator circuits are functional, which occurs in late embryonic development31–33. As in the retina, the circuits that mediate spontaneous activity in the spinal cord and the resulting pattern of activity change during development34 (Table 1, Box 1).
In the developing hippocampus, pyramidal cells exhibit two distinct patterns of spontaneous correlated firing35. Synchronous plateau assemblies (SPAs), which in mice span the period from a few days before to a few days after birth, are characterized by bursts of plateau potentials and are correlated across 3–7 neurons. Later, hippocampal neurons exhibit giant depolarizing potentials (GDPs), which occur for a week, overlapping briefly with the end of the SPAs (Table 1, Box 1). GDPs are characterized by slow depolarizations that are correlated across many neurons15, 36, 37.
Prior to the onset of hearing, spontaneous bursts of action potentials have been recorded in the auditory nerve. These bursts follow a pattern similar to those in the retina: short active periods are followed by quiet periods that range from seconds to minutes, depending on the species38–41. A recent study revealed that this activity originates in the developing cochlea5 (Table 1, Box 1). This correlated spontaneous activity dissipates at the onset of hearing39, 41.
Recently, spontaneous network activation has been characterized in the developing cerebellum20. Here, the cerebellar projection neurons, known as Purkinje cells, fire bursts of action potentials that propagate from the apex toward the base of cerebellar lobules. Intervals between bursts are much shorter here compared to the other circuits described above. The spontaneous rhythmic activity in the cerebellum is found in the first postnatal week of development, preceding the formation of the primary inputs to Purkinje cells (Table 1, Box 1).
Pacemaker-like neurons trigger activity
In the absence of external stimuli, what triggers the large correlated depolarizations that characterize spontaneous activity in developing circuits? In the adult nervous system, spontaneous firing in various networks, such as motor circuits42, is driven by pacemaker neurons. Pacemaker neurons exhibit unstable membrane potentials, caused by a cyclical interplay of depolarizing and hyperpolarizing conductances. Pacemakers in the adult nervous system are typically depolarized by either a hyperpolarization-activated cation conductance43 or a persistently active sodium conductance (e.g. in respiratory system44). Depolarization activates a calcium-activated potassium conductance, generating an afterhyperpolarization (AHP)45. The AHP prevents further depolarization, and the duration of the AHP therefore sets the period of depolarizing events. Such a complement of conductances in adult pacemaker neurons typically leads to membrane potential oscillations with a period of tens of milliseconds to seconds. However, spontaneous network depolarizations during development typically have longer intervals between events. To initiate network activity, developing circuits use varying combinations of pacemaker-like intrinsic membrane properties and network interactions.
Perhaps the simplest example of the interaction between pacemaker-like conductances and network properties is found in the developing cerebellum. Purkinje cells spontaneously fire in the absence of synaptic input46, and therefore serve as the pacemaker-like neurons. During development, network interactions in the form of depolarizing GABAergic synapses (see below) entrain nearby Purkinje cells to fire such that waves of depolarization propagate down a chain of Purkinje cells20 (Box 1). Consistent with computational models20, this leads to an inter-event interval of about 100 msec.
Giant depolarizing potentials (GDPs) in the hippocampus are triggered by an interaction between CA3 pyramidal cells and GABAergic interneurons (Box 1). GABA-induced depolarization causes CA3 pyramidal cells to fire periodic bursts of action potentials (reviewed in47, 48). The pacemaker-like bursts of CA3 pyramidal cells, both during development and in the adult, are driven by a persistent sodium current and are terminated by a slow AHP, which lasts 3–4 seconds and is mediated by a calcium-activated potassium conductance49, 50. Blockade of the AHP decreases the inter-event interval from 3 seconds to less than 2 seconds, suggesting that the frequency of GDPs is set by the kinetics of these conductances. Similar to cerebellum, network interactions mediated by recurrent excitatory connections between CA3 pyramidal cells and excitatory connections with GABAergic interneurons (see below) entrain depolarizations among neighboring cells, thereby prolonging the AHP and setting the frequency of GDPs. A similar organization is observed in neocortex51 and midbrain19, where clusters of pacemaker-like neurons are the sites of repeated event initiation.
The periodicity of spontaneous activity in the developing retina is not fixed by the membrane conductances of the network’s pacemaker-like neurons, as it is in cerebellum and hippocampus. Instead, it emerges from an interplay between the connectivity of the network and the properties of the developing retina’s pacemaker-like neurons, as has been explored both computationally52, 53 and experimentally54. Early retinal waves are initiated by a class of cholinergic interneurons called starburst amacrine cells55 (Box 1). In the absence of synaptic input individual starburst amacrine cells spontaneously depolarize approximately every 15 s 54. It has been postulated that starburst cells, which are densely interconnected through excitatory, cholinergic synapses, depolarize each other, thus generating a retinal wave56. During such waves, starburst amacrine cells undergo a large depolarization, which causes a large calcium influx. The calcium triggers a calcium-dependent, slow AHP, which follows the wave-associated depolarization and lasts 15–30 seconds54, which is roughly the minimum interval between wave initiations. These extremely slow AHPs, which are similar to slow AHPs in the hippocampus, the thalamus, and the peripheral nervous system57–61, are thought to be regulated by the cAMP/PKA second messenger pathway62, 63. Consistent with this hypothesis, elevating cAMP significantly reduces the duration of slow AHPs in starburst amacrine cells54 and increases the frequency of retinal waves64. The AHP makes starburst cells refractory to further depolarization and therefore sets the minimum inter-wave interval. As more starburst cells recover from this refractory period the likelihood of another network depolarization increases 52. Hence, the minute-long interval between retinal waves is due to pacemaker conductances that are activated by network interactions65.
In contrast to the retina, hippocampus and cerebellum, no pacemaker-like neuron has been conclusively identified in the developing spinal cord. Some evidence suggests that motor neurons, which are cholinergic, could be responsible for triggering episodes of spontaneous activity: nicotinic acetylcholine receptor antagonists block spontaneous activity early in development 30, 66 and motor neurons are the first population of neurons to be active in each episode 67, 68. Although motor neurons might trigger episodes of spontaneous activity, recurrent excitatory interactions in the network are thought to set the periodicity of activity. The spinal cord contains cholinergic, glutamatergic, GABAergic, and glycinergic neurons, and all of the connections in the developing spinal cord are excitatory (see below). Immature spinal neurons continuously release neurotransmitters onto one another, but the efficacy of synaptic connections changes as a function of activity69–73: immediately after an episode of spontaneous activity the network is the most depressed, so the ongoing synaptic excitation within the network is not powerful enough to trigger another event. As the network recovers from the previous event the ongoing synaptic excitation increases in efficacy, until eventually the neurons reciprocally excite one another enough to trigger another network-encompassing event. An important component of the network in the spinal cord is a population of GABAergic interneurons that form strong synapses onto motor neurons67. During an episode of network activity, which can last as long as 60 seconds, sustained activation of GABAA receptors on motor neurons leads to a massive efflux of chloride69. As an episode progresses the intracellular concentration of chloride is reduced to such an extent that the reversal potential for chloride becomes more negative than before the episode, causing GABA and glycine to be less excitatory. In this scenario, the long interval between events is due to the relatively slow re-accumulation of chloride in motor neuron dendrites via chloride transporters69, 71, 73. Evidence for a reduction in the excitatory drive is provided by the reduction of the size of GABAA-mediated postsynaptic currents following a network event. In addition, blockade of the chloride-accumulating transporter NKCC1 (in the presence of ionotropic glutamate receptor antagonists, so that excitatory glutamate transmission was also absent) blocks spontaneous network activity during development71, indicating that lowering levels of intracellular chloride reduces the excitability of the network.
Recent research has provided a model for the generation of spontaneous bursts of action potentials in the auditory nerve. In the developing rat cochlea, periodic release of ATP from a developmentally transient population of inner supporting cells depolarizes inner hair cells, which then release glutamate onto the afferent dendrites of spiral ganglion neurons and initiate bursts of action potentials5. Although ATP-mediated currents occur in hair cells at a rate of about three to four per minute, action potential bursts appear in spiral ganglion neurons only once per minute5, possibly because only a subset of ATP-mediated currents are large enough to depolarize hair cells sufficiently to trigger glutamate release. At present, little is known about the mechanisms that regulate the timing of ATP release from supporting cells and thus the timing of action potential bursts in the auditory nerve5.
Transient network features
The patterns of spontaneous network activity observed during development differ in many ways from the activity patterns of the adult nervous system. A dramatic example is found in the retina: here, adult circuits are organized along a “vertical” axis, which limits the lateral spread of excitatory signals in order to preserve a high-acuity representation of visual space. By contrast, during development spontaneous network activity in the form of retinal waves propagates laterally across large areas of tissue that represent several degrees of the visual field. This lateral spread of activity is a result of several connectivity features that are present only during a finite period of development, and which are described below.
Depolarizing GABA
A prominent feature of several developing circuits that is crucial for activity propagation is the excitatory action of GABA and glycine, which in the adult brain act as inhibitory neurotransmitters. This depolarizing action of canonically inhibitory transmitters is primarily due to high intracellular concentrations of chloride at early ages: when a GABAA receptor is activated, chloride diffuses out of the cell, which causes depolarization. As neurons mature, they change their complement of chloride transporters, which leads to a decrease in intracellular chloride74. In the spinal cord, hippocampus, neocortex and cerebellum, the cells that will become inhibitory interneurons in adulthood are a primary source of depolarization during development47, 75. In the developing retina, activation of GABAA receptors on retinal ganglion cells is depolarizing 76, 77, but it is not clear whether GABA signaling is required for cholinergic retinal wave generation. GABAA receptor antagonists block retinal waves in turtle78, but not in ferret or mouse (though they do modulate wave properties64, 79). There is no evidence for GABA signaling during spontaneous activity in the developing cochlea5.
Depolarizing GABA is crucial for the generation of GDPs in the developing hippocampus15, 36, 37. GDPs are blocked by ionotropic glutamate and GABAA receptor antagonists and the age at which activation of GABAA receptors is no longer depolarizing is the age at which GDPs disappear37. This is in contrast to the earlier form of spontaneous network activity in the hippocampus — SPAs — which are not dependent on GABAA signaling, but rather on L-type calcium channel activation and gap junction coupling35 (Table 1 and below).
Spontaneous activity in the developing spinal cord is also strongly influenced by depolarizing GABA and glycine. In the spinal cord, the frequency of network activation is reduced by GABAA receptor antagonists80. Furthermore, episodes of bursting activity and the underlying waves of depolarization are likely to be triggered at least in part by massive GABA release, and then terminated by a switch in the chloride gradient such that GABA temporarily becomes less excitatory71, 73. Also similar to the hippocampus, spontaneous network activity in the spinal cord disappears around the time when activation of GABAA receptors ceases to be excitatory31, 33.
Depolarizing GABA is the sole source of coupling involved in generating spontaneous network activity in the developing cerebellum20. GABAergic Purkinje cells, which are the primary projection neurons of the cerebellum, make local synaptic connections with neighboring Purkinje cells. These local axon collaterals are not distributed uniformly within the cerebellar network. Instead, the density of Purkinje-Purkinje connections is higher for cells located closer to the base of each cerebellar lobule. Purkinje cells spontaneously spike at all ages, but the existence of depolarizing GABAergic connections between nearby Purkinje cells during the first postnatal week entrains the firing of neighboring Purkinje cells, generating a propagating wave that travels preferentially in the direction of higher-density local connections, i.e. towards the base of the cerebellar lobules. A computational model predicts that when GABAA signaling becomes inhibitory in the second postnatal week, Purkinje cells would still be entrained, but that the direction of propagation would switch20, with waves starting from the base of a lobule and propagating toward the apex. Local axon collaterals among Purkinje cells persist until adulthood but form many fewer synaptic connections. Hence, as the cerebellum matures and the functional connections between nearby Purkinje cells are reduced, the substrate for propagation disappears.
Transient connections
A second feature common to many networks that generate spontaneous activity is that they transiently express unique circuit components. These transient components, such as the local axon collaterals of cerebellar Purkinje cells described above and the expression of neurotransmitter receptors, provide a substrate for correlating activity across populations of cells that are not directly connected in adulthood.
The retina provides an example of developmentally transient components that form a substrate for wave propagation. During the first postnatal week in mice, retinal waves propagate via a network of starburst amacrine cells55. Immature starburst amacrine cells undergo spontaneous depolarizations and express nicotinic acetylcholine receptors54, 56 (nAChRs). Starburst cells form a dense, recurrent excitatory network via cholinergic and GABAergic synapses56 (Box 1). Hence, it has been proposed that cholinergic waves are initiated by spontaneous depolarizations in starburst amacrine cells and propagate via connections with other starburst amacrine cells. However, nAChRs are only expressed at starburst-starburst synapses during development 56. At the age when starburst amacrine cells stop expressing nAChRs and are therefore no longer connected via excitatory synapses, the cholinergic waves disappear55 and are replaced by glutamatergic waves, as discussed below.
Similar to the retina, spontaneous network activity in the spinal cord might also depend on connections that exist early in development but that become functionally insignificant in the adult. During development, motor neurons form local excitatory connections with other motor neurons81 and with local GABAergic interneurons called Renshaw cells81, 82 (Box 1). Renshaw cells also receive glutamatergic inputs from sensory neurons81, 83. Although motor neuron inputs to Renshaw cells persist into adulthood, motor neuron—motor neuron synapses and sensory neuron—Renshaw cell synapses do not remain functional83.
The developing cochlea uses a similar strategy to sustain spontaneous correlated activity early in development. Prior to the onset of hearing, hair cells are periodically depolarized through activation of purinergic receptors by ATP released from neighboring supporting cells5. The supporting cells comprise a transient structure, Kölliker’s organ, which is present only during a short period of development7. Furthermore, preliminary studies in rats indicate that hair cells express purinergic receptors only for a transient period from a few days after birth to around the time of hearing onset [N. X. Tritsch and D. E. Bergles, “Developmental Regulation of Spontaneous Cochlear Activity”, Association for Research in Otolaryngology, Annual Meeting, 2009]. Hence, the transient source of ATP-secreting cells and the transient expression of receptors probably dictate the period of development during which spontaneous activity in the cochlea is present.
Extrasynaptic glutamate
There is growing evidence that extrasynaptic transmission plays a part in propagating the waves of depolarization in developing networks before synaptic structures achieve their mature state84. In addition to mediating direct synaptic communication, neurotransmitters released from a presynaptic cell can “spill” out of the synaptic cleft and activate extrasynaptic receptors: on the postsynaptic cell, on the presynaptic terminal and on other neighboring neurons and glia. Extrasynaptic glutamate has been implicated in regulating the early differentiation of neurons in the ventricular zone85 and might modulate neuronal migration86. It is thought that at later developmental stages, retinal waves and hippocampal GDPs are mediated, at least in part, by extrasynaptic glutamate.
In the retina, during the period just prior to eye-opening, spontaneous correlated activity is no longer dependent on acetylcholine release from starburst amacrine cells, but rather on glutamate release from bipolar cells (for review see6). In contrast to the starburst amacrine cells, whose processes form a dense lateral network, neighboring bipolar cells are not synaptically connected. Each bipolar cell has a very small axonal process, forming glutamatergic synapses on a small part of the total dendritic tree of its target ganglion cell. Recently we demonstrated that retinal waves are accompanied by large transient increases in extrasynaptic glutamate87. This extrasynaptic glutamate provides a possible source of depolarization that is not limited to cells that are directly postsynaptic to bipolar cell release sites.
Does extrasynaptic glutamate mediate wave propagation? Interestingly, elevating extrasynaptic glutamate by pharmacologically blocking glutamate transporters, which tightly regulate glutamate levels outside the synaptic cleft, significantly reduces variability in wave speed, making slow waves faster and fast waves slower87. This observation indicates that extrasynaptic glutamate positively and negatively regulates wave propagation. Extrasynaptic glutamate is known to be both excitatory and inhibitory in the adult retina88–90. Furthermore, low concentrations of glutamate receptor antagonists have been shown to reduce wave propagation speed in the developing turtle78 and chick91 retina, although complete blockade of either AMPA/Kainate or NMDA receptors does not affect wave speed in the developing mouse retina87. However, as there is no reliable way to block extrasynaptic glutamate signaling independently of synaptic glutamate signaling, it is not known whether extrasynaptic glutamate transmission is required for wave propagation.
A role for extrasynaptic glutamate has also been demonstrated in the developing cortex92, 93, hippocampus94 and brain stem95, where increasing extracellular glutamate profoundly alters the patterns of spontaneous network activation. In the hippocampus, episodic elevations of extrasynaptic glutamate levels depolarize interneurons via activation of NMDA receptors, causing an increase in the frequency of events compared to endogenous GDPs94. Whether extrasynaptic glutamate has a role in the endogenous activity patterns remains to be determined.
Gap junctions
Several studies have implicated gap junctions as potential substrates for propagating neural activity during development. There are three lines of evidence that support these claims. First, there are several examples of spontaneous network events that persist in the presence of a broad spectrum of neurotransmitter receptor antagonists and are thus non-synaptic. Such non-synaptic waves are detected perinatally in hippocampus35 and embryonically in retina96, 97, and they can be induced in cases when the synaptic pathways for mediating waves are disrupted98 (see next section). Second, spontaneous network activity patterns can be suppressed by pharmacological blockade of gap junctions. Indeed, in the spinal cord, the cochlea and the retina, spontaneous network activity is blocked by gap junction inhibitors5, 30, 66, 97, 98, at least at some stages of development. Unfortunately, gap junction blockers have several non-specific effects that could underlie the overall reduction of activity, including blockade of voltage-gated calcium channels that mediate synaptic transmission99, 100, activation of large-conductance calcium-activated potassium channels100–102, and inhibition of synaptic release103, which makes these experiments difficult to interpret. Third, transgenic mice lacking specific gap junction proteins (connexins) have altered spontaneous firing patterns. For example, in the spinal cord the expression of a number of connexin proteins in motor neurons changes with development104, and in mice lacking connexin 40 (Cx40), spontaneous activity is uncorrelated between motor neurons105. In mice lacking Cx36, spontaneous network activity in the retina is altered such that retinal ganglion cells fire many more spikes between waves than is observed in wild-type animals106, 107, suggesting that Cx36-containing retinal gap junctions have a role in mediating the silences between waves.
Homeostatic regulation
One of the striking features of spontaneous network activity during development is its robustness. Throughout their development, circuits use a multitude of strategies to spontaneously generate activity and, although the details of the temporal and spatial correlations change, the overall pattern of activity remains the same – large depolarizations generated by excitatory synaptic inputs are followed by extended periods of silence.
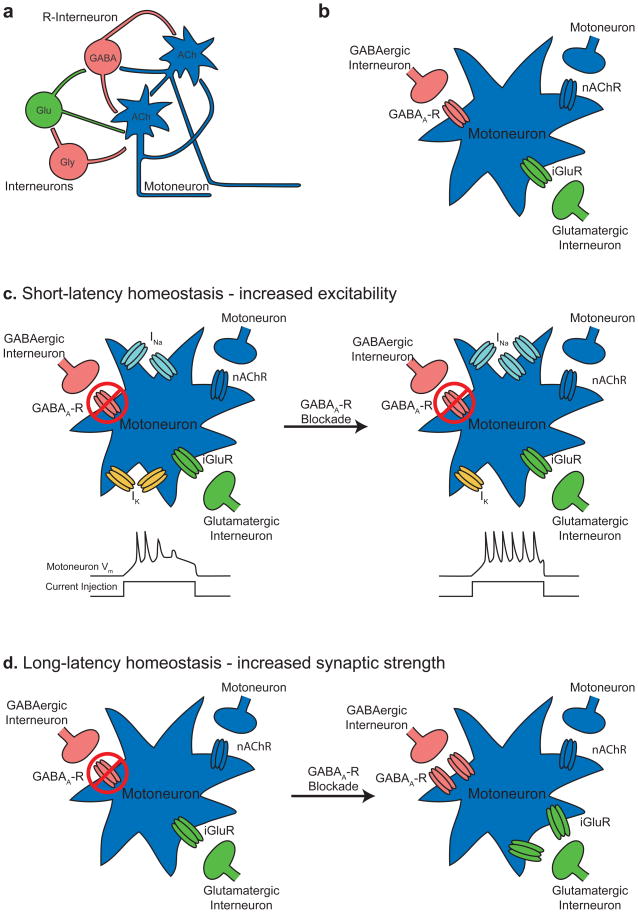
The removal of a crucial component of a circuit showing spontaneous depolarizations often leads to compensation by the remaining components, providing further evidence of the robustness of the network activity108. We refer to this compensation as homeostatic regulation under the assumption that the network is adjusting its inputs to achieve a baseline level of activity. This phenomenon was first described in the developing spinal cord, where extended blockade of receptors for a primary excitatory transmitter (acetylcholine during the early stage of development12, 30 and glutamate or GABA during a later stage109) led to an initial block followed by a restoration of spontaneous network activity. Homeostatic compensation has also been observed in ovo, where recovery from blockade of glutamate or GABA-A receptors takes significantly longer than that observed in vitro (12 hours vs 30–60 minutes). A recent dissection of mechanisms that underlie a homeostatic phenomenon in ovo revealed that changes in synaptic strength11, 110 and changes in the expression of ion channels that control cellular excitability in motor neurons111 compensate for the loss of excitatory transmitter (Figure 1).
Figure 1. Homeostatic regulation of spontaneous network activity in the chick spinal cord.
When a part of the spinal cord network is blocked, activity becomes temporarily less frequent, but recovers to pre-block levels. Here we provide schematics of the changes that take place after activity blockade.
a. Schematic of the circuits that mediate activity in the developing spinal cord. Neurons are color-coded by the transmitter they release. ACh, acetylcholine, blue; Glu, glutamate, green; Gly, glycine, pink; GABA, pink.
b. Motor neurons provide a crucial drive in the generation of activity. They receive input from other motor neurons and from interneurons. nAChR, nicotinic acetylcholine receptor; GABAA-R, GABAA receptor; iGluR, ionotropic glutamate receptor.
c. When GABAA receptors are blocked in ovo (left), activity becomes temporarily less frequent but recovers 11. After 12 hours of GABAA-R blockade, motor neurons become more excitable, an effect that is mediated by an increase in the density of sodium current and a decrease in the density of potassium current111 (right). Bottom: schematics illustrating an increase in motor neuron excitability, with the bottom curve showing current injection into a motor neuron, and the top curve showing membrane potential. A more excitable motor neuron fires more action potentials in response to the same stimulus (right). INa, sodium current, represented by sodium channels; IK, potassium current, represented by potassium channels.
d. When GABAA receptors are blocked for long periods (24–48 hours) glutamatergic and GABAergic postsynaptic currents in motor neurons increase in size110. The exact mechanisms underlying this increase in postsynaptic current are not fully understood, but are schematized here as increases in the number of glutamate and GABAA receptors.
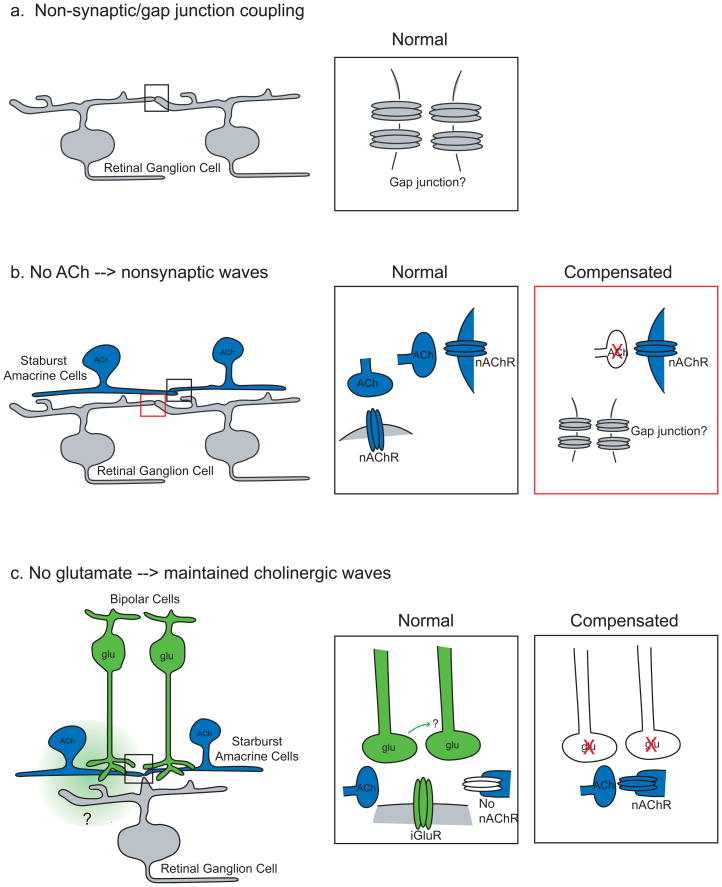
Homeostatic compensation has also been observed in the circuits that mediate retinal waves (Figure 2). Transgenic mice lacking choline acetyltransferase (ChAT), an enzyme crucial for acetylcholine production, do not exhibit cholinergic waves. Instead, they exhibit compensatory waves, which are not blocked by any fast neurotransmitter receptor antagonists112, indicating that the compensatory mechanism here is different from the one observed in the spinal cord and the hippocampus. The compensatory waves are, however, blocked by gap junction antagonists112, suggesting that they are an extension of an earlier, nonsynaptic wave-generating mechanism that has been observed in embryonic mice96 and rabbits97. One of the interesting features of the compensatory retinal waves is that they require a few days to appear, suggesting that significant circuit rearrangements need to take place. A more complex form of compensation occurs in mice lacking the β2 subunit of nicotinic acetylcholine receptors. Under some experimental conditions, no wave activity is detected in these β2-nAChR-KO mice96, 113, whereas in other recording conditions114 -- characterized, for example, by increased temperature115 -- compensatory waves are observed. As these waves are not blocked by fast-neurotransmitter receptor antagonists114, they may be the same gap-junction mediated waves as those in transgenic mice lacking ChAT112. Although the circuit mediating these compensatory waves is not yet understood, one probable homeostatic mechanism is based on an increased excitability of retinal neurons, because bath application of voltage-gated calcium channel agonists leads to the generation of similar non-synaptic waves in both wild-type and β2-nAChR-KO retinas98, 116. Additionally, in β2-nAChR-KO mice retinal waves that are dependent on glutamatergic signaling appear 3–4 days earlier than in wild-type mice 96, indicating that the absence of endogenous signaling induces an early maturation of the next stage of network activity.
Figure 2. Homeostatic regulation of spontaneous network activity in the mammalian retina.
In the absence of a requisite circuit component, the retina regresses to the previous wave-generating mechanism. Here we provide schematics of the circuits that mediate retinal waves at different ages, including the changes that are thought to take place when one form of activity is disrupted.
a. Perinatally in mice, waves are mediated by a non-synaptic circuit, thought to be mediated by gap junction coupling (inset). Here the coupling is shown to be between retinal ganglion cells, although the location of the relevant coupling is not known.
b. During the first postnatal week, starburst amacrine cells (blue) form synaptic connections with other starburst amacrine cells and retinal ganglion cells (gray). Retinas from mice lacking acetyl choline (Ach; bottom inset) exhibit non-synaptic waves112, potentially through a reactivation of non-synaptic connections that mediate network activity in the perinatal period (see panel a). Furthermore, blocking nAChRs soon after the onset of cholinergic waves leads to the reappearance of non-synaptic waves97.
c: In the few days before eye opening in mice, when glutamatergic interneurons begin to form synapses with their postsynaptic targets, waves are mediated by glutamatergic circuits. Inset: Glutamatergic bipolar cells (green), which make glutamatergic synapses onto amacrine and ganglion cells and have no direct connections with each other, release glutamate that is detected both synaptically and extrasynaptically87. After the first postnatal week, starburst cells no longer express nAChRs56. Retinas from mice in which bipolar cells do not release glutamate (bottom inset) exhibit waves that are mediated by the cholinergic network87.
The observation that transgenic mice with disrupted cholinergic circuitry exhibit a reappearance of non-synaptic waves suggests that normally, activity in the cholinergic circuit suppresses non-synaptic waves. Similarly, the disappearance of cholinergic waves depends on the maturation of glutamatergic circuits, suggesting that glutamatergic activity suppresses cholinergic circuit activity. Transgenic mice lacking the vesicular glutamate transporter VGLUT1 in bipolar cells continue to exhibit cholinergic waves at the age when cholinergic circuits disappear in wild-type mice87. A similar switch from cholinergic to glutamatergic transmission has been observed in the developing hindbrain; however, it is not known whether this transition is influenced by the absence of network activity as in the developing retina and spinal cord117.
Homeostatic regulation of spontaneous network activity has also been observed in the developing hippocampus. Activity is maintained during acute blockade of GDPs by a strengthening of SPAs35. Furthermore, although CA3 pyramidal neurons trigger endogenous GDPs, they are not required for GDP generation—other hippocampal areas, such as CA1, are capable of generating GDPs when they are surgically isolated from CA3, albeit at a lower frequency than endogenous GDPs 47. This suggests that CA3 pyramidal neurons generate activity at a higher frequency than other hippocampal areas, but that latent circuits present in other areas generate activity in the absence of CA3.
Another instance of homeostatic regulation has recently been observed in the hippocampus. In a knockout mouse lacking the chloride-accumulating transporter NKCC1, activation of GABAA receptors is never depolarizing, and therefore the major depolarizing drive for GDPs is absent. Nonetheless, GDPs are detectable118, although fewer hippocampal neurons participate in the events119. In NKCC1 knockout mice, the compensatory activity was partially mediated by an increase in the intrinsic excitability of CA3 pyramidal cells rather than by a change in network properties as seen in the developing spinal cord and retina118. To our knowledge, homeostatic regulation of spontaneous network activity has not been observed in the developing cerebellum and cochlea.
The observation that many circuits compensate for the disruption of one form of activity by generating another form leads to the question what aspect of the activity is being homeostatically regulated. In addition, it is not known whether homeostatically generated activity can serve the same function as normal activity. In the case of the retina, the pattern of endogenous and homeostatically generated activity differs. For example, β2-nAChR-KO mice exhibit waves that are larger and faster than waves in wild-type retinas114, 115, and retinal projections to the brain in β2-nAChR-KO mice are abnormal6. This indicates that the feature of activity that is regulated in β2-nAChR-KO mice is not the feature that is required for circuit refinement. Determining what aspects of cellular and/or network function are being homeostatically regulated and what aspects drive circuit maturation will require specific manipulations of endogenous activity patterns.
Conclusions and future directions
A fundamental feature of developing neural circuits is the presence of spontaneous network activity, often taking the form of propagating waves. The circuits that mediate this activity, while differing in the particulars, rely on similar cell-intrinsic and synaptic properties that are observed for only a brief time during development. Robust compensatory mechanisms seem to be in place to ensure that spontaneous network activity is actively maintained throughout this crucial period of development.
Although tremendous insights have been gained into the mechanisms generating spontaneous activity, a remaining question concerns the purpose that this activity serves during development. In developing sensory epithelia, such as the retina and the cochlea, propagating neural activity contains topographic information that may be useful in the establishment of early sensory maps in the brain. Propagating activity in the spinal cord may be used to define circuits within the cord that will serve as central pattern generators in adulthood, or to target motor neurons in neighboring spinal segments to neighboring muscles. Spontaneous activity in the developing cortex and hippocampus may serve to strengthen the networks that mediate oscillatory activity in adulthood. Insights into how spontaneous correlated activity influences the development of neural circuits will require manipulations that alter the pattern of activity rather than block it entirely. Understanding the mechanisms that underlie the generation of correlated patterns will allow us to design such manipulations.
Continued insights into the mechanisms underlying early network activity, as well as an increased awareness of its crucial role in brain development, could have profound implications for clinical treatments of pregnant women. Alcohol, for example, is known to affect network activity patterns, and many regions of the brain are particularly sensitive to fetal alcohol exposure. Alcohol disrupts normal firing patterns in the developing hippocampus120, and extended fetal exposure prevents the normal development of primary sensory systems121, 122. Another potential clinical implication relates to a decrease in spontaneous activity during birth --a transient, neuroprotective effect that is triggered by a large increase in oxytocin, a hormone that modulates the depolarizing action of GABA transmission123. A deeper understanding of the mechanisms that mediate spontaneous activity during development will help to prevent neuropathologies associated with fetal exposure to neuroactive pharmacological agents.
Glossary
- Central pattern generator
A neural circuit that produces self-sustaining patterns of behaviour independently of sensory input
- Gap Junctions
Intercellular channels composed of connexin proteins that are the basis of electrical synapses between neurons
- Pacemaker-like neuron
In the adult nervous system, pacemaker neurons possess a set of voltage-gated ion channels that lead to regular patterns of depolarization and hyperpolarization. In developing circuits, pacemaker-like neurons are neurons with unstable membrane potentials, but whose pacemaker properties also depend upon network interactions
- Cell autonomous
Describes activity that is generated by a cell independent of any input from other cells, as in pacemaker neurons
- Amacrine cell
Retinal interneurons located in the inner nuclear layer of the retina that provide local inhibition in the adult retina
- Bipolar cell
An interneuron of the retina that provides excitatory glutamatergic input to retinal ganglion cells. In the adult retina, bipolar cells receive input from photoreceptors
- Retinal ganglion cells
The projection neurons of the retina, the axons of which form the optic nerve
- Renshaw cells
GABAergic interneurons that receive excitatory input from motor neurons
References
- 1.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 2.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 3.Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242:90–1. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- 4.Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–43. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 5.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–5. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 6.Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–35. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Forsythe ID. Hearing: a fantasia on Kolliker’s organ. Nature. 2007;450:43–44. doi: 10.1038/450043a. [DOI] [PubMed] [Google Scholar]
- 8.Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landmesser LT, O’Donovan MJ. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol. 1984;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson MG, Milner LD, Landmesser LT. Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions. Brain Res Rev. 2008;57:77–85. doi: 10.1016/j.brainresrev.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Islas C, Wenner P. Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron. 2006;49:563–75. doi: 10.1016/j.neuron.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Myers CP, et al. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron. 2005;46:37–49. doi: 10.1016/j.neuron.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Marder E, Rehm KJ. Development of central pattern generating circuits. Curr Opin Neurobiol. 2005;15:86–93. doi: 10.1016/j.conb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol (Lond) 1998;507:219–36. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–9. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- 17.Corlew R, Bosma MM, Moody WJ. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J Physiol. 2004;560:377–90. doi: 10.1113/jphysiol.2004.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gust J, Wright JJ, Pratt EB, Bosma MM. Development of synchronized activity of cranial motor neurons in the segmented embryonic mouse hindbrain. J Physiol. 2003;550:123–133. doi: 10.1113/jphysiol.2002.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockhill W, Kirkman JL, Bosma MM. Spontaneous activity in the developing mouse midbrain driven by an external pacemaker. Dev Neurobiol. 2009 doi: 10.1002/dneu.20725. [DOI] [PubMed] [Google Scholar]
- 20.Watt AJ, et al. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci. 2009;12:463–73. doi: 10.1038/nn.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev. 2005;85:883–941. doi: 10.1152/physrev.00017.2004. [DOI] [PubMed] [Google Scholar]
- 22.Mohajerani MH, Cherubini E. Role of giant depolarizing potentials in shaping synaptic currents in the developing hippocampus. Crit Rev Neurobiol. 2006;18:13–23. doi: 10.1615/critrevneurobiol.v18.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 23.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–38. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- 25.Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 26.Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- 27.Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26:6728–36. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamburger V. Some Aspects of the Embryology of Behavior. Q Rev Biol. 1963;38:342–365. doi: 10.1086/403941. [DOI] [PubMed] [Google Scholar]
- 29.Provine RR. Ontogeny of bioelectric activity in the spinal cord of the chick embryo and its behavioral implications. Brain Res. 1972;41:365–378. doi: 10.1016/0006-8993(72)90508-2. [DOI] [PubMed] [Google Scholar]
- 30.Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci. 1999;19:3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvert B, Branchereau P, Meyrand P. Multiple spontaneous rhythmic activity patterns generated by the embryonic mouse spinal cord occur within a specific developmental time window. J Neurophysiol. 2004;91:2101–2109. doi: 10.1152/jn.01095.2003. [DOI] [PubMed] [Google Scholar]
- 32.Ren J, Greer JJ. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. J Neurophysiol. 2003;89:1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Whelan PJ, Wenner P. Development of an inhibitory interneuronal circuit in the embryonic spinal cord. J Neurophysiol. 2005;93:2922–2933. doi: 10.1152/jn.01091.2004. [DOI] [PubMed] [Google Scholar]
- 34.Bekoff A. Ontogeny of leg motor output in the chick embryo: a neural analysis. Brain Res. 1976;106:271–291. doi: 10.1016/0006-8993(76)91025-8. [DOI] [PubMed] [Google Scholar]
- 35.Crepel V, et al. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron. 2007;54:105–20. doi: 10.1016/j.neuron.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Allene C, et al. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 2008;28:12851–63. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–55. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- 38.Gummer AW, Mark RF. Patterned neural activity in brain stem auditory areas of a prehearing mammal, the tammar wallaby (Macropus eugenii) Neuroreport. 1994;5:685–688. doi: 10.1097/00001756-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Jones TA, Jones SM, Paggett KC. Primordial rhythmic bursting in embryonic cochlear ganglion cells. J Neurosci. 2001;21:8129–8135. doi: 10.1523/JNEUROSCI.21-20-08129.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones TA, Leake PA, Snyder RL, Stakhovskaya O, Bonham B. Spontaneous discharge patterns in cochlear spiral ganglion cells before the onset of hearing in cats. J Neurophysiol. 2007;98:1898–1908. doi: 10.1152/jn.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris-Warrick RM. Voltage-sensitive ion channels in rhythmic motor systems. Curr Opin Neurobiol. 2002;12:646–651. doi: 10.1016/s0959-4388(02)00377-x. [DOI] [PubMed] [Google Scholar]
- 43.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 44.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 48.Sipila ST, Kaila K. GABAergic control of CA3-driven network events in the developing hippocampus. Results Probl Cell Differ. 2008;44:99–121. doi: 10.1007/400_2007_033. [DOI] [PubMed] [Google Scholar]
- 49.Sipila ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+-activated K+ current. Eur J Neurosci. 2006;23:2330–8. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- 50.Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25:5280–5289. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lischalk JW, Easton CR, Moody WJ. Bilaterally propagating waves of spontaneous activity arising from discrete pacemakers in the neonatal mouse cerebral cortex. Dev Neurobiol. 2009;69:407–414. doi: 10.1002/dneu.20708. [DOI] [PubMed] [Google Scholar]
- 52.Butts DA, Feller MB, Shatz CJ, Rokhsar DS. Retinal waves are governed by collective network properties. J Neurosci. 1999;19:3580–3593. doi: 10.1523/JNEUROSCI.19-09-03580.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godfrey KB, Swindale NV. Retinal wave behavior through activity-dependent refractory periods. PLoS Comput Biol. 2007;3:e245. doi: 10.1371/journal.pcbi.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–71. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- 55.Zhou ZJ. The function of the cholinergic system in the developing mammalian retina. Prog Brain Res. 2001;131:599–613. doi: 10.1016/s0079-6123(01)31047-6. [DOI] [PubMed] [Google Scholar]
- 56.Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 57.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sah P, Isaacson JS. Channels underlying the slow afterhyperpolarization in hippocampal pyramidal neurons: neurotransmitters modulate the open probability. Neuron. 1995;15:435–441. doi: 10.1016/0896-6273(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 59.Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 60.Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 61.Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci. 2003;18:3155–3166. doi: 10.1111/j.1460-9568.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- 62.Goaillard JM, Vincent P. Serotonin suppresses the slow afterhyperpolarization in rat intralaminar and midline thalamic neurones by activating 5-HT(7) receptors. J Physiol. 2002;541:453–465. doi: 10.1113/jphysiol.2001.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neylon CB, Fowler CJ, Furness JB. Regulation of the slow afterhyperpolarization in enteric neurons by protein kinase A. Auton Neurosci. 2006;126–127:258–263. doi: 10.1016/j.autneu.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 64.Stellwagen D, Shatz CJ, Feller MB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- 65.Hennig MH, Adams C, Willshaw D, Sernagor E. Early-stage waves in the retinal network emerge close to a critical state transition between local and global functional connectivity. J Neurosci. 2009;29:1077–1086. doi: 10.1523/JNEUROSCI.4880-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wenner P, O’Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol. 2001;86:1481–98. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- 68.Arai Y, Mentis GZ, Wu JY, O’Donovan MJ. Ventrolateral origin of each cycle of rhythmic activity generated by the spinal cord of the chick embryo. PLoS One. 2007;2:e417. doi: 10.1371/journal.pone.0000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chub N, O’Donovan MJ. Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J Neurophysiol. 2001;85:2166–2176. doi: 10.1152/jn.2001.85.5.2166. [DOI] [PubMed] [Google Scholar]
- 70.Fedirchuk B, et al. Spontaneous network activity transiently depresses synaptic transmission in the embryonic chick spinal cord. J Neurosci. 1999;19:2102–2112. doi: 10.1523/JNEUROSCI.19-06-02102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marchetti C, Tabak J, Chub N, O’Donovan MJ, Rinzel J. Modeling spontaneous activity in the developing spinal cord using activity-dependent variations of intracellular chloride. J Neurosci. 2005;25:3601–3612. doi: 10.1523/JNEUROSCI.4290-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabak J, Senn W, O’Donovan MJ, Rinzel J. Modeling of spontaneous activity in developing spinal cord using activity-dependent depression in an excitatory network. J Neurosci. 2000;20:3041–3056. doi: 10.1523/JNEUROSCI.20-08-03041.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chub N, Mentis GZ, O’donovan MJ. Chloride-sensitive MEQ fluorescence in chick embryo motoneurons following manipulations of chloride and during spontaneous network activity. J Neurophysiol. 2006;95:323–330. doi: 10.1152/jn.00162.2005. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 75.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Zhang LL, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol. 2006;95:2404–2416. doi: 10.1152/jn.00578.2005. [DOI] [PubMed] [Google Scholar]
- 77.Leitch E, Coaker J, Young C, Mehta V, Sernagor E. GABA type-A activity controls its own developmental polarity switch in the maturing retina. J Neurosci. 2005;25:4801–4805. doi: 10.1523/JNEUROSCI.0172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. J Neurosci. 2003;23:7621–7629. doi: 10.1523/JNEUROSCI.23-20-07621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang CT, et al. GABA(A) receptor-mediated signaling alters the structure of spontaneous activity in the developing retina. J Neurosci. 2007;27:9130–9140. doi: 10.1523/JNEUROSCI.1293-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Donovan MJ, Chub N, Wenner P. Mechanisms of spontaneous activity in developing spinal networks. J Neurobiol. 1998;37:131–145. doi: 10.1002/(sici)1097-4695(199810)37:1<131::aid-neu10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 81.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mentis GZ, et al. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mentis GZ, Siembab VC, Zerda R, O’Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demarque M, et al. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- 85.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 86.Manent JB, et al. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 2005;25:4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blankenship AG, et al. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62:230–41. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–73. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–48. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 90.Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci. 2006;9:1388–96. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- 91.Sernagor E, Eglen SJ, O’Donovan MJ. Differential effects of acetylcholine and glutamate blockade on the spatiotemporal dynamics of retinal waves. J Neurosci. 2000;20:RC56. doi: 10.1523/JNEUROSCI.20-02-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Demarque M, et al. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J Neurosci. 2004;24:3289–94. doi: 10.1523/JNEUROSCI.5338-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milh M, Becq H, Villeneuve N, Ben-Ari Y, Aniksztejn L. Inhibition of glutamate transporters results in a “suppression-burst” pattern and partial seizures in the newborn rat. Epilepsia. 2007;48:169–74. doi: 10.1111/j.1528-1167.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 94.Cattani AA, Bonfardin VD, Represa A, Ben-Ari Y, Aniksztejn L. Generation of slow network oscillations in the developing rat hippocampus after blockade of glutamate uptake. J Neurophysiol. 2007;98:2324–36. doi: 10.1152/jn.00378.2007. [DOI] [PubMed] [Google Scholar]
- 95.Sharifullina E, Nistri A. Glutamate uptake block triggers deadly rhythmic bursting of neonatal rat hypoglossal motoneurons. J Physiol. 2006;572:407–23. doi: 10.1113/jphysiol.2005.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bansal A, et al. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560:533–49. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singer JH, Mirotznik RR, Feller MB. Potentiation of L-type calcium channels reveals nonsynaptic mechanisms that correlate spontaneous activity in the developing mammalian retina. J Neurosci. 2001;21:8514–22. doi: 10.1523/JNEUROSCI.21-21-08514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vessey JP, et al. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–6. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- 100.Takeda Y, Ward SM, Sanders KM, Koh SD. Effects of the gap junction blocker glycyrrhetinic acid on gastrointestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G832–41. doi: 10.1152/ajpgi.00389.2004. [DOI] [PubMed] [Google Scholar]
- 101.Sheu SJ, Bee YS, Chen CH. Resveratrol and large-conductance calcium-activated potassium channels in the protection of human retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2008;24:551–555. doi: 10.1089/jop.2008.0013. [DOI] [PubMed] [Google Scholar]
- 102.Wu SN, Jan CR, Chiang HT. Fenamates stimulate BKCa channel osteoblast-like MG-63 cells activity in the human. J Investig Med. 2001;49:522–533. doi: 10.2310/6650.2001.33629. [DOI] [PubMed] [Google Scholar]
- 103.Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J Neurophysiol. 2009;102:974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. J Neurosci. 1999;19:10813–28. doi: 10.1523/JNEUROSCI.19-24-10813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Personius KE, Chang Q, Mentis GZ, O’Donovan MJ, Balice-Gordon RJ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci U S A. 2007;104:11808–11813. doi: 10.1073/pnas.0703357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torborg CL, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat Neurosci. 2005;8:72–8. doi: 10.1038/nn1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansen KA, Torborg CL, Elstrott J, Feller MB. Expression and function of the neuronal gap junction protein connexin 36 in developing mammalian retina. J Comp Neurol. 2005;493:309–20. doi: 10.1002/cne.20759. [DOI] [PubMed] [Google Scholar]
- 108.Turrigiano G. Maintaining your youthful spontaneity: microcircuit homeostasis in the embryonic spinal cord. Neuron. 2006;49:481–483. doi: 10.1016/j.neuron.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 109.Chub N, O’Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J Neurosci. 1998;18:294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilhelm JC, Wenner P. GABA(A) transmission is a critical step in the process of triggering homeostatic increases in quantal amplitude. Proc Natl Acad Sci U S A. 2008;105:11412–11417. doi: 10.1073/pnas.0806037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilhelm JC, Rich MM, Wenner P. Compensatory changes in cellular excitability, not synaptic scaling, contribute to homeostatic recovery of embryonic network activity. Proc Natl Acad Sci U S A. 2009;106:6760–6765. doi: 10.1073/pnas.0813058106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stacy RC, Demas J, Burgess RW, Sanes JR, Wong RO. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci. 2005;25:9347–9357. doi: 10.1523/JNEUROSCI.1800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- 114.Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 2008;105:13638–13643. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stafford BK, Sher A, Litke AM, Feldheim DA. Spatial-Temporal Patterns of Retinal Waves Underlying Activity-Dependent Refinement of Retinofugal Projections. Neuron. 2009;64:200–12. doi: 10.1016/j.neuron.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Torborg C, Wang CT, Muir-Robinson G, Feller MB. L-type calcium channel agonist induces correlated depolarizations in mice lacking the beta2 subunit nAChRs. Vision Res. 2004;44:3347–3355. doi: 10.1016/j.visres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 117.Mochida H, Sato K, Momose-Sato Y. Switching of the transmitters that mediate hindbrain correlated activity in the chick embryo. Eur J Neurosci. 2009;29:14–30. doi: 10.1111/j.1460-9568.2008.06569.x. [DOI] [PubMed] [Google Scholar]
- 118.Sipila ST, et al. Compensatory enhancement of intrinsic spiking upon NKCC1 disruption in neonatal hippocampus. J Neurosci. 2009;29:6982–6988. doi: 10.1523/JNEUROSCI.0443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pfeffer CK, et al. NKCC1-dependent GABAergic excitation drives synaptic network maturation during early hippocampal development. J Neurosci. 2009;29:3419–3430. doi: 10.1523/JNEUROSCI.1377-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galindo R, Valenzuela CF. Immature hippocampal neuronal networks do not develop tolerance to the excitatory actions of ethanol. Alcohol. 2006;40:111–118. doi: 10.1016/j.alcohol.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stromland K. Visual impairment and ocular abnormalities in children with fetal alcohol syndrome. Addict Biol. 2004;9:153–7. doi: 10.1080/13556210410001717024. discussion 159–60. [DOI] [PubMed] [Google Scholar]
- 122.Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol. 2005;93:1317–1325. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- 123.Tyzio R, et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 124.Leinekugel X. Developmental patterns and plasticities: the hippocampal model. J Physiol Paris. 2003;97:27–37. doi: 10.1016/j.jphysparis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 125.Leinekugel X, et al. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–52. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]