Abstract
Given the failure of conventional treatments for glioblastoma, gene therapy has gained interest considerable in recent years. Gliomas are associated with a state of immunosuppression, which appears to be partially mediated by an increase in secretion of transforming growth factor-β (TGF-β) from glioma cells. Decorin, a small proteoglycan which can bind to and inactivate TGF-β, has been successfully used as an antitumor strategy on stably transfected tumor cells and has been shown to cause growth suppression in neoplastic cells of various histological origins. In this paper, we investigated the use of gene therapy to deliver the decorin transgene in a site-specific manner in an experimental model of intracranial gliomas. Our aim was to inhibit the glioma-associated immunosuppressive state, and prolong the survival of tumor-bearing rats.
We studied the effects of decorin gene transfer in the rat CNS-1 glioma model. To assess the effect of ectopic expression of decorin on glioma progression in vivo, stably transfected CNS-1 cells expressing decorin were implanted into the brain parenchyma of syngeneic Lewis rats. The rats implanted with CNS-1 cells expressing decorin survived significantly longer than those in the control groups which received CNS-1 cells that did not express decorin (P<.0001). We then investigated whether the survival observed with decorin expressing cells could be mimicked in vivo, using recombinant adenoviruses (RAds) expressing the decorin gene under the control of two different promoters: the human immediate-early cytomegalovirus (h-IE-CMV) and the glial fibrillary acidic protein (GFAP). In vivo results showed that administration of RAd expressing the human decorin under the control of h-IE-CMV promoter has a small, but significant effect in prolonging the survival of experimental tumor bearing rats (P<.0001). Our data indicate that ectopic decorin expression has the potential to slow glioma progression in vivo. Our results also indicate that expression of decorin has to be present in all cells which constitute the intracranial tumor mass for the inhibition of tumor growth and prolongation of the life expectancy of tumor-bearing rats to be effective.
Keywords: glioma, recombinant adenovirus, transfected glioma cells, CNS-1 cells
Decorin plays different roles in regulating matrix organization, growth factor activity, angiogenesis, and cell proliferation and differentiation.1–3 Overexpression of decorin inhibits TGF-β-induced proliferation and slows growth of a Chinese hamster ovary cell line.4 In gene transfer studies of human colon cancer, cells stably transfected with decorin exhibited reduced growth rates in vitro and failed to generate tumors in severe combined immunodeficiency (SCID) mice.5 Decorin has been shown to arrest cells in the G1 phase of the cell cycle, and growth suppression could be restored by treatment with decorin antisense nucleotides.6–8 The mechanism of action of decorin-induced growth suppression appears to be mediated by interaction with the epidermal growth factor receptor (EGFR),8–10 which leads to an upregulation of p21, a potent inhibitor of cyclin-dependent kinases.6,7 In agreement with this, it has been shown that adenovirus-mediated gene transfer of decorin attenuates EGFR activity and suppresses in vivo tumorigenesis.11 The function of decorin to inactivate the oncogenic ErbB2 protein in breast carcinoma cells has also been reported.12 Additionally, it has been indicated that decorin could adversely affect in vivo tumor growth by suppressing the endogenous tumor cell production of angiogenic stimulus.3 Finally, it has been shown that ectopic expression of decorin in C6 rat glioma cells resulted in the inhibition of tumor formation in vivo.13
These unique features make decorin a very good candidate gene for many experimental cancer gene therapy applications. Malignant gliomas frequently secrete TGF-β which creates a state of immunosuppression in the glioma micro-environment, and due to the invasive nature of glioma, throughout the surrounding brain parenchyma.14–16 Decorin, by inhibiting TGF-β or EGFR activity could display antiglioma activity. We therefore proposed to test the hypothesis of using decorin as a therapeutic gene to inhibit intracranial glioma growth in a syngeneic rat model.
The development of an appropriate gene therapy approach requires not only a suitable therapeutic gene, but an effective gene delivery system. Malignant brain tumors are attractive targets for local gene therapy because of their exclusive location in the CNS. Adenoviral vectors are highly efficient at in vivo transduction and can be concentrated to high titers, thus it is possible to stereotactically inject high titers of viruses directly into the area of brain infiltrated by tumor cells.17–19 In addition to the high efficiency of transduction, adenoviruses mediate high-level transgene expression and pose little risk of insertional mutagenesis.
Unlike transient transgene expression observed following systemic administration of adenoviral vectors, in the absence of prior systemic immune priming to adenovirus, first-generation adenoviral vectors injected into the brain parenchyma can sustain prolonged transgene expression for at least 6–18 months.17,20–23 We have assessed this for up to 3 months after CNS-1 tumor implantation in the survivors after gene therapy using RAd/HSV1-TK plus ganciclovir. The reasons for this are two-fold. Firstly, although the tumors are fast growing, due to the fact that they are encased within the cranium, they have a limited space to grow. Second, due to the fact that the treated animals survive long term, either the tumor is completely eliminated or the growth of the tumor is severely impaired. If the tumor is completely eliminated, transgene expression is observed in the surrounding normal brain.17,18 Therefore, RAds are very good gene transfer vectors to test experimental therapies in animal models of brain pathologies, such as brain tumors and neurodegenerative diseases.
Finally, a relevant animal model is critical for most gene therapy approaches, particularly in cancer gene therapy. We chose the rat CNS-1 glioma17,24 model to conduct our studies since it is accepted as a highly reproducible syngeneic model with immunological and invasive characteristics similar to those of human gliomas.25 This constitutes one of the most stringent animal models to study the effectiveness of novel therapies for glioblastoma multiforme.
Results
Generation of a CNS-1 glioma clonal cell subline engineered to stably express human decorin
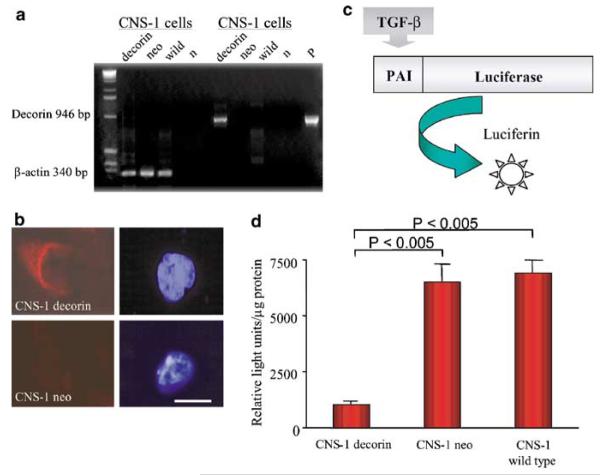
Human decorin cDNA was cloned into the BCMGS neo-expression plasmid26 and transfected into CNS-1 glioma cells by electroporation. The introduction of transgene into CNS-1 cell line was confirmed by PCR amplification of the human decorin gene. A 946 bp human decorin-specific PCR product was detected in decorin-transfected cells, but not in untransfected or neo-transfected cells (Fig 1a,b). The capability of decorin-transfected CNS-1 cells to express decorin was assessed by immunocytochemical analysis (Fig 1b). The ability of secreted decorin to suppress TGF-β bioactivity was evaluated using a mink lung epithelial cell line stably transfected with a plasminogen activator inhibitor-1 promoter-Luciferase construct (MLE/PAI/L cells). Exposure of MLE/PAI/L cells to TGF-β induces a dose-dependent increase in Luciferase activity27 (Fig 1c). The conditioned medium containing secreted decorin was collected from decorin CNS-1 cell cultures and incubated with MLE/PAI/L cells. Wild-type and neo-transfected CNS-1 cells were used as controls. Lysates from MLE/PAI/L cells incubated with CNS-1 cell supernatant were tested for Luciferase activity. Following incubation with supernatant from decorin-transfected cells a statistically significant (P<.005) reduction in Luciferase activity was seen (Fig 1d).
Figure 1.
Evaluation of bioactivity of CNS-1 cells expressing decorin. (a) Decorin expression in rat CNS-1 glioma cells. A volume of 20 μl of the PCR reaction were loaded on a 2% agarose gel containing 0.5 μg/ml ethidium bromide, and the DNA fragments fractionated by electrophoresis. The gel indicates that the decorin gene is amplified in transfected CNS-1 cells (946 bp band) compared to the negative band in control lanes; β-actin serves as an internal control; “n” is a negative control lacking genomic DNA in the PCR reaction, and “p” shows amplification from the plasmid (pAL119/decorin), used here as positive control. (b) Immunocytochemical detection of decorin in CNS-1 cells. Cells were paraformaldehyde fixed, permeabilized with Triton X-100, labeled with a sheep anti-human decorin polyclonal antibody followed by an anti-sheep FITC-conjugated secondary antibody and counterstained with 4′-6-diamidino-2 phenylindole (DAPI). The positive signal (in CNS-1 decorin cells) was not detected in neo-transfected cell samples (CNS-1 neo cells). Corresponding DAPI staining of the same cells show cell nuclei. Scale bar represents 10 μm. (c) Schematic representation of the Luciferase assay experiment. (d) Exposure of MLE/PAI/L cells to supernatant from decorin-transfected cells results in a statistically significant reduction in Luciferase activity. Results are shown as means±SEM (n=3).
Assessment of the growth rate of decorin-transfected CNS-1 cells in vitro
The growth rate of decorin-transfected CNS-1 cells was assessed and compared with the growth rate of control (neo-) transfected CNS-1 cells and wild-type CNS-1 cells. Cells were seeded into 12-well plates at a density of 2500 cells/well and incubated at 37°C in 5% CO2 with constant humidity. Cells were counted every 24 hours during 12 consecutive days. Decorin-transfected cells were the slowest growing cell population (Fig 2). In comparison with the neo-transfected cells or wild-type CNS-1 cells, the growth rate was inhibited by 25 and 40%, respectively (P<.0001) as analyzed using the Student’s t-test. The experiment was repeated two times, giving identical results.
Figure 2.
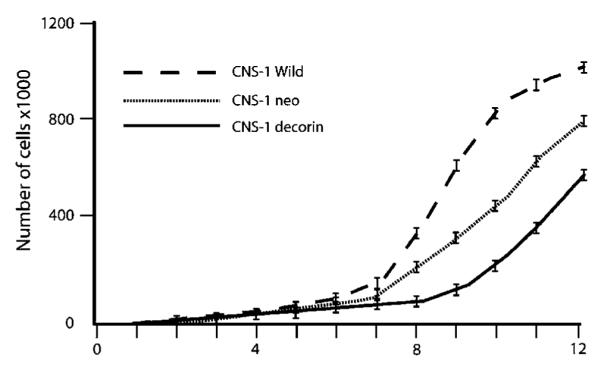
In vitro proliferation of transfected CNS-1 cells. Cultured cells were subjected to daily count in quadruplicate. Growth curves were generated for each of the stably transfected and wild type CNS-1 cells over a 12-day period. Data are represented as the mean of four values (SEM is less than 10%). There was significant difference in cell-doubling times among these cell lines in vitro (P<.0001).
Effects of ectopic decorin expression on CNS-1 glioma growth in Lewis rats
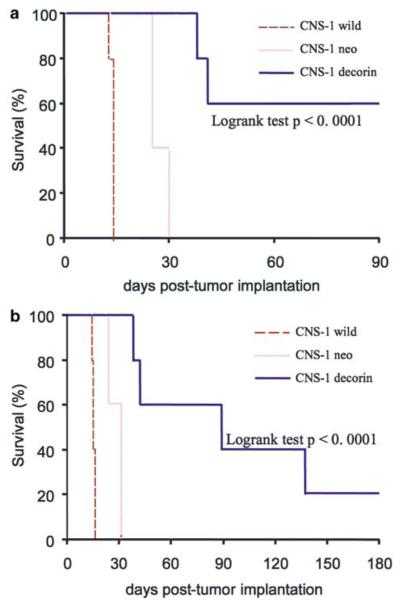
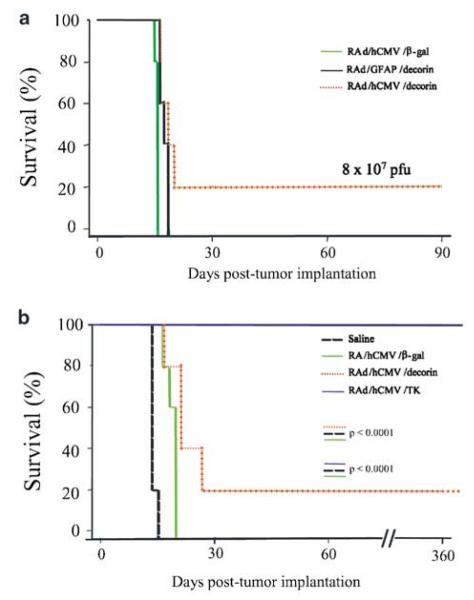
To assess the effect of ectopic expression of decorin on glioma tumor regression in a syngeneic glioma model, stably transfected CNS-1 cells expressing decorin were implanted into the striatum of Lewis rats as described previously.17 Wild-type and neo-transfected CNS-1 cells were used as experimental controls. Animals were monitored daily, and rats that demonstrated signs of distress were perfused-fixed and their brains retained for histological analysis. All control animals implanted with wild-type CNS-1 cells or neo-transfected CNS-1 cells had to be euthanized for progressive tumor growth within 30 days. Lewis rats implanted with CNS-1 cells expressing decorin survived significantly longer than those in the control groups (Fig 3a). The surviving rats (60% of decorin CNS-1 group) were perfused-fixed 90 days post-tumor implantation; upon histopathological analysis one of these animals was shown to still carry remaining tumor tissue indicating that decorin overexpression slows the growth of CNS-1 glioma cells in vivo. In order to assess longer survival of Lewis rats implanted with decorin-transfected cells, the experiment was repeated and surviving animals were allowed to survive for up to 180 days (Figs 3b and 4h). In the second long-term survival experiment (Fig 3b), most animals died at <180 days. Only one rat survived for 180 days and was shown to be tumor free (Fig 4h). Kaplan–Meier survival curves were devised for decorin-expressing CNS-1 tumor-bearing animals and control groups, and significance was determined using the logrank test (P<.0001).
Figure 3.
Kaplan–Meier survival curves for Lewis rats implanted with decorin-transfected CNS-1 cells, observed for up to 90 (a) or 180 (b) days post cell implantation of transfected or wild-type CNS-1 cells into the striatum of Lewis rats. Lewis rats implanted with CNS-1 cells expressing decorin survive significantly longer than those in the control groups (n=5, logrank test P<.0001; in both a and b).
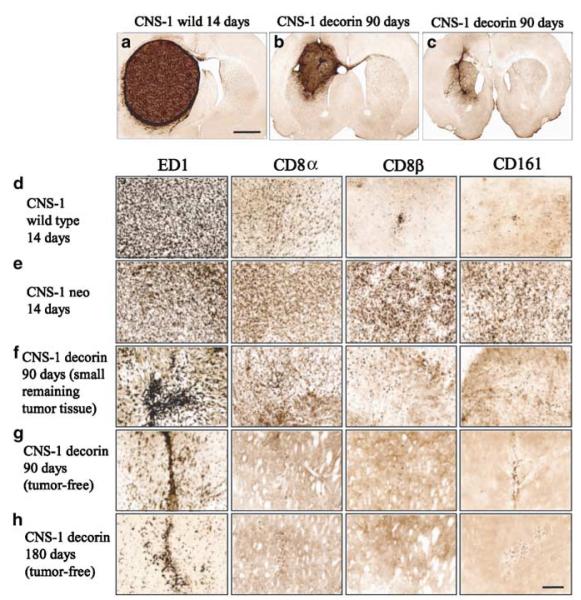
Figure 4.
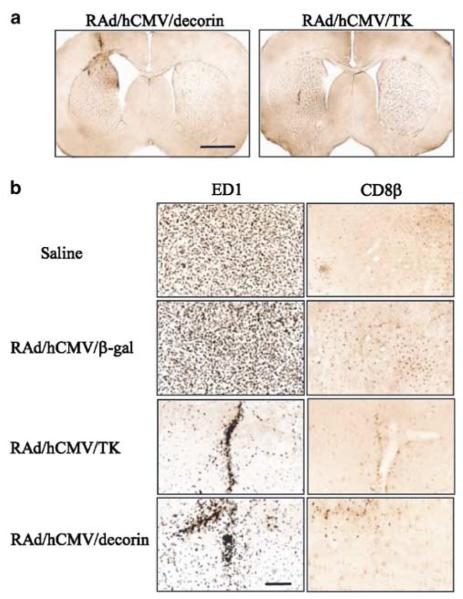
Representative photomicrographs demonstrating brain histology and immunohistochemical staining for ED1, CD8, CD8β and CD161-positive cells in rats implanted with CNS-1 gliomas. (a) At death, all animals had large solid tumours at the site of cell implantation in the striatum. Surviving rats at 90 days post-tumor implantation had a small remaining tumor (b) or were tumor free (c). Scale bars represent 2 mm. (d–h) The distribution of activated ED1 immunoreactive cells was much higher than the distribution of CD8 immunopositive cells in all rats. In the presence of a growing tumor (d–f), the infiltration of tumor and peritumoral tissue with inflammatory immune cells is higher in tumor-bearing animals (d–f) than in animals that eliminated the tumor (g,h). In animals that eliminated the tumor (g,h), only ED1+ cells remained in the visible tissue scar. Infiltration of tumors was higher in animals implanted with CNS-1 neo cells when compared to CNS-1 cells; these animals died at the same time as those implanted with control untransfected CNS-1 cells.
CNS-1 wild-type and CNS-1 neo-implanted animals evaluated at 14 days post-tumor implantation showed tumor masses infiltrated with ED1, CD8α, CD8β and CD161 cells (Fig 4d,e). These animals succumbed to the tumors between 2 and 4 weeks post-tumor implantation (Fig 3a,b), had large solid masses on the left cerebral hemisphere that filled the entire striatum and displaced the cerebral ventricles (Fig 4a). One asymptomatic rat from the CNS-1/decorin group that was killed on day 90, had a small tumor in the striatum infiltrating the cerebral cortex (Fig 4b). Other asymptomatic animals from the same group that were killed on either day 90 or 180 (Fig 4c,h) were tumor free; only a scar remained in the injection site of CNS-1 cells. The number of infiltrating immune cells was inversely correlated with the size of the remaining CNS tumor (Fig 4d–h). Activated macrophages and microglial cells that either infiltrate or surround the tumor were identified using a mouse monoclonal anti-rat ED1. These cells appeared with increased density in the larger tumors (Fig 4d,e). Using a mouse monoclonal anti-rat CD8β antibody, regions of densely staining CD8+ cells were detected within tumors. The density of CD8+ cells in CNS-1 neo-implanted rats that die between 26 and 31 days post-implantation was considerably higher than in wild-type CNS-1-implanted rats which die before day 15. Brain sections were also stained with mouse monoclonal anti-rat CD8β and CD161 antibodies to detect T-lymphocytes and NK cells. Interestingly, the number of CD8β and CD161-positive cells in CNS-1/neo-implanted rats was much higher than in wild-type CNS-1-implanted rats (Fig 4d,e); this could indicate that neo is being recognized as a tumor neo-antigen. If this was the case, however, neo-antigenicity was not enough to lead to tumor rejection.
Assessment of bioactivity of RAds expressing decorin in vitro
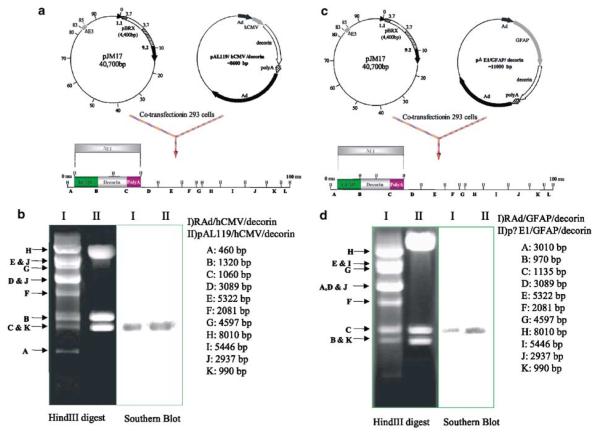
In view of the in vivo effects of decorin expressed within stably transfected and implanted CNS-1 cells, we decided to implement a gene therapy approach to either eliminate or slow glioma growth in vivo using decorin gene transfer. To this end, two first-generation RAd vectors encoding the human decorin, RAd/GFAP/decorin and RAd/hCMV/decorin, were constructed. The presence of decorin in the viral genome was confirmed by Southern blotting HindIII-digested viral DNA with a decorin-specific probe (Fig 5, panels b and d, lane I). The ability of RAds encoding decorin to express biologically active protein and suppress TGF-β bioactivity was evaluated using MLE/PAI/L cells. Following incubation of MLE/PAI/L cells with conditioned medium (containing 0.1 ng/ml recombinant TGF-β3) from HeLa cells infected with RAds encoding the human decorin at MOI of 100 and 300, luciferase activity was measured (Fig 6). Supernatant from HeLa cells infected with a RAd encoding β-galactosidase, RAd/hCMV/β-gal, were used as controls. At MOI of 100, RAd/GFAP/decorin did not significantly suppress the biological activity of TGF-β. However, when a higher MOI of RAd/GFAP/decorin was used, a statistically significant inhibition of TGF-β occurred. Incubation of RAd/hCMV/decorin with MLE/PAI/L cells resulted in a statistically significant inhibition of TGF-β at MOI of 100 and 300 (Fig 6). HeLa cells were used to prepare conditioned media and as a source of decorin after infection with RAd/hCMV/Decorin or RAd/GFAP/Decorin, since they are easily infected by RAds and further, the RAds do not replicate within these cells. Final confirmation that the decorin-expressing RAds were biologically active was performed in the brain glioma model in Lewis rats in vivo.
Figure 5.
(a) Schematic diagram of cotransfection of pJM17 and pAL119/hCMV/decorin to generate the RAd/hCMV/decorin vector genome. (b) Southern blot analysis to verify the correct insertion of the decorin expression cassette within the RAd vector. (c) Schematic diagram of cotransfection of pJM17 and pΔE1/GFAP/decorin to generate the RAd/GFAP/decorin vector genome. (d) Southern blot analysis to confirm the correct insertion of the decorin expression cassette within the RAd vector.
Figure 6.
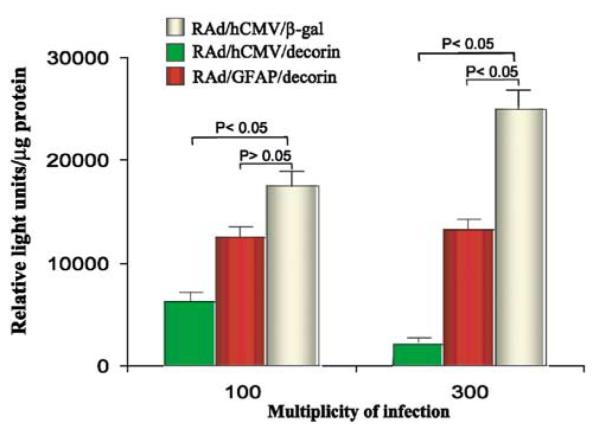
Assessment of the biological activity of RAds encoding decorin. Luciferase activity in MLE/PAI/L cells following incubation with conditioned medium from HeLa cells infected with RAds encoding decorin or β-gal. Both RAd/hCMV/decorin and RAd/GFAP/decorin inhibited TGF-β signaling; RAd/hCMV/decorin was more effective than RAd/GFAP/decorin.
Efficacy of RAd-mediated decorin expression to inhibit rat CNS-1 glioma growth in vivo
Having shown that RAd/GFAP/decorin and RAd/hCMV/decorin express biologically active decorin and suppress TGF-β activity, we then investigated whether the survival observed with decorin expressing CNS-1 cells could be replicated in vivo using RAds expressing the human decorin gene product to transduce established CNS-1 gliomas. We decided to test the efficiency of two RAds expressing decorin, one driven by the pan-cellular hCMV promoter and the other by the glial cell-type specific promoter, GFAP, with the aim of restricting expression of the therapeutic transgene within cells of glial origin. CNS-1 cells are GFAP positive (results not shown). Lewis rats were injected intratumorally with 8 × 107 infectious units (iu) of either of the vectors 3 days post-CNS-1 cell implantation. The survival rate of rats treated with RAd/hCMV/decorin was significantly better compared to the survival rate of rats treated with RAd/hCMV/β-gal (P<.005; Figure 7a). There was no statistically significant difference in survival between the rats treated with RAd/GFAP/decorin or RAd/hCMV/β-gal (P<.12). The long-term efficiency of RAd/hCMV/decorin to inhibit glioma growth was also compared with the efficiency of a RAd/hCMV/HSV1-TK17 and ganciclovir (GCV). The survival of animals treated with either RAd/hCMV/decorin (P<.0001) or RAd/hCMV/HSV1-TK (P<.0001) was better than survival of animals treated with RAd/hCMV/β-gal (Fig 7b). Analysis of rat brains showed that the animals which survived for up to 12 months, were tumor free. Massive enlargement of associated lateral ventricle was observed in the RAd/hCMV/decorin-treated animals that survived for 12 months, but not in RAd/hCMV/HSV1-TK-treated animals (Fig 8a). This enlargement could be due to an ongoing inflammation process in the brains of these animals. All rats treated with RAd/hCMV/HSV1-TK and 20% of the rats treated with RAd/hCMV/decorin were completely tumor free at 12 months. These animals had infiltration of ED1-positive macrophages/microglia in the scar left at the injection site where both the tumor cells and the viruses were delivered, and throughout the striatum (Fig 8b). Rats which were completely tumor free at 12 months also had a small number of CD8β+ cells present along the injection site (Fig 8b). These results indicate that following successful tumor treatment only small amounts of activated macrophages (ED1) or CD8 T-cells (CD8β) remain in the tissue of animals injected with RAd/hCMV/decorin or RAd/hCMV/HSV1-TK when compared to saline or RAd/hCMV/β-gal-treated controls.
Figure 7.
Kaplan–Meier survival curves for CNS-1-implanted Lewis rats treated with RAd/GFAP/decorin or RAd/hCMV/decorin. (a) At the viral dose of 8 × 107, the survival rate of rats treated with RAd/hCMV/decorin was statistically better compared to rats treated with either RAd/hCMV/β-gal or RAd/GFAP/decorin (n=5, log-rank test P<.005). (b) Kaplan Meier survival curves for CNS-1 implanted Lewis rats treated with either RAd/hCMV/decorin or RAd/hCMV/HSV1-TK and ganciclovir. Lewis rats implanted with CNS-1 cells and treated with either RAd/hCMV/decorin or RAd/hCMV/HSV1-TK and ganciclovir survived significantly longer than those in control groups (n=5), with RAd/hCMV/HSV1-TK and ganciclovir being more effective than RAd/hCMV/decorin.
Figure 8.
Representative photomicrographs demonstrating brain histology and immunohistochemical staining for ED1 and CD8β-positive cells in rat CNS-1 gliomas treated with RAd/hCMV/decorin, RAd/hCMV/HSV1-TK and ganciclovir, RAd/hCMV/β-gal or saline. (a) Enlargement of the lateral ventricle observed in an animal treated with RAd/hCMV/decorin and that survived for 12 months; this was not seen in animals that had been treated with RAd/hCMV/HSV1-TK and ganciclovir which had also survived for 12 months. (b) Rats from control groups (saline and RAd/hCMV/β-gal) had high levels of ED1+ and CD8b+ cells throughout the tumor mass, with CD8β immunoreactive cells being higher in animals injected with RAd/hCMV/β-gal. Rats that were completely tumor free at 12 months post-tumor implantation and gene therapy had infiltration of ED1-positive monocytes/macrophages in the scar remaining at the injection site, and scattered ED1 staining throughout the striatum (RAd/hCMV/HSV1-TK and RAd/hCMV/decorin). Scale bar represent 200 μm.
Discussion
The purpose of this study was to examine whether intratumoral expression of decorin can result in tumor regression in an intracranial tumor model and thereby prolong the survival of CNS-1 tumor-bearing Lewis rats. To assess the effect of intratumoral expression of decorin on glioma tumor progression in vivo, we used stably transfected glioma cells expressing decorin. In vivo experiments with CNS-1 cells expressing decorin clearly indicated that sustained expression of decorin from stably transfected tumor cells is able to increase the survival of tumor-bearing Lewis rats, but it is not sufficient to prevent tumor growth in all animals. The improved survival in Lewis rats that received decorin-transfected CNS-1 cells, is similar to results obtained in tumor xenografts and in the C6 glioma model using C6 cells expressing decorin.11,13 However, in the C6 glioma model decorin expression resulted in tumor regression in 100% of experimental Sprague–Dawley (SD) rats. One of the possible explanations for the increased tumor regression is that because C6 cells are not syngeneic in SD rats, tumor regression by decorin could be enhanced by host versus graft rejection.25,28–30
Decorin has the ability to inhibit TGF-β by forming an inactive complex.4,31,32 Additionally, it has also been shown that decorin inhibits TGF-β mRNA transcription and TGF-β protein synthesis.13,33 However, these functions appear to be highly cell-type specific. In certain cellular systems, decorin blocks the activity of TGF-β, whereas in others its binding augments the bioactivity of the cytokine.34 The capacity of CNS-1 cells to produce biologically active decorin and inhibit the action of TGF-β was assessed in vitro using a highly sensitive and specific assay.27 The specificity and sensitivity of the assay are the result of using a truncated PAI-1 promoter which retains the two regions responsible for maximal response to TGF-β. These experiments proved that CNS-1 cells stably transfected with BCMGS/neo/decorin or infected with RAds encoding decorin express and secrete active peptide which can significantly suppress TGF-β biological activity. RAd/GFAP/decorin only displayed a significant inhibitory effect that was detected at MOI of 300 (Fig 6). This could be explained by the weaker transcriptional activity of the GFAP promoter. Cell-type specific promoters are generally weaker than the widely used strong promoters derived from viruses (e.g., CMV), which are able to drive high levels of transgene expression within many cell types. We have also previously demonstrated that the GFAP promoter driving transgenes encoded within recombinant adenovirus vectors exerts a several fold lower translational activity when compared to the hCMV promoter.35
To test the therapeutic effects of adenovirus-mediated decorin gene therapy in our brain tumor model, 3 days after the implantation of tumor cells, adenovirus vectors bearing the therapeutic gene were stereotactically injected into the tumor. The intratumoral delivery of 4 × 107 iu of RAd/GFAP/decorin and RAd/hCMV/decorin resulted in no significant difference in the survival of CNS-1 tumor-bearing rats as compared with RAd/hCMV/β-gal-injected animals (data not shown). However, increasing the dose of inoculated virus two-fold to 8 × 107 iu resulted in a statistically significant increase in the survival time of experimental rats treated with RAd/hCMV/decorin (Fig 7a). Work from our laboratory has demonstrated that the GFAP promoter has restricted glial cell-type specificity; however, transgene expression levels are lower than the hCMV promoter, both in cell lines and in primary cultures.36 Lower expression from the GFAP promoter could explain the failure of RAd/GFAP/decorin to improve the survival of tumor-bearing animals.
To evaluate the reproducibility, the experiment using RAd/hCMV/decorin was repeated on two different occasions (Fig 7b). In these experiments saline-, RAd/hCMV/β-gal- and RAd/hCMV/HSV1-TK-injected animals were evaluated as control groups. We observed significant tumor regression in rats treated with either RAd/hCMV/HSV1-TK or RAd/hCMV/decorin compared with RAd/hCMV/β-gal or saline injected. However, the survival of RAd/hCMV/HSV1-TK and GCV-treated tumor-bearing animals was better than the survival of RAd/hCMV/decorin-treated tumor-bearing animals. A possible explanation is that the rapid growth rate of CNS-1 cells may favor some gene therapy approaches, such as the HSV1-TK/GCV system, that rely on cell division to affect tumor cell killing, while it may be more difficult to see high effectiveness for a strategy like decorin overexpression, that relies on the activation of the immune response and its effector mechanisms.37
Decorin is a secreted protein that has been suggested to act as both an autocrine and paracrine regulator of tumor growth. Decorin has been previously shown to bind the EGFR. This interaction results in activation of the mitogen-activated protein (MAP) kinase pathway, induction of p21, a potent inhibitor of cyclin-dependent kinases, and ultimately cell cycle arrest.8 Recently, it has been determined that decorin causes a sustained down-regulation of the EGFR and the attenuation of EGFR-mediated mobilization of intracellular calcium resulting in a decrease in EGFR kinase activity and inhibition of tumor growth in vivo.10 Further, following adenovirus-mediated transfer of decorin in a mouse lung carcinoma tumor model, tumor volume was decreased and cell apoptosis was revealed in regions surrounding decorin-positive cells in these mice.38 In vivo studies using decorin have also provided evidence that decorin may act through a novel mechanism, where it suppresses vascular endothelial growth factor (VEGF), resulting in inhibition of tumor growth.3 The differences in effectiveness seen in our studies using CNS-1 cells that were stably transfected with decorin, when compared to gene transfer of decorin using RAds, could be due to the fact that in the former, decorin is expressed from the time of tumor cell implantation and therefore its inhibiting effects are seen very early on, while in the latter model, decorin is being delivered using a RAd, which although efficient, does not infect 100% of the tumor mass. Also, delivery was performed 3 days post-tumor implantation, once the tumor is already established, and this might impair the effectiveness of decorin in inhibiting tumor progression.
Enlargements of the lateral ventricle could be seen in the vicinity of the RAd/hCMV/decorin-treated tumors, which is caused by tumor-induced destruction of striatal tissue. Similar effects can be seen in CNS-1 decorin-implanted animals that were tumor free at day 180, but not in RAd/hCMV/HSV1-TK and GCV-treated tumors. This finding suggests that RAd/hCMV/HSV1-TK and GCV can kill tumors before they reach a size that causes striatal tissue destruction.
In conclusion, our results indicate that although decorin has significant potential to slow CNS-1 glioma growth in vivo; it will be necessary to increase its levels of expression, for example, using the murine CMV promoter,39 to enhance its effectiveness further. It will also be necessary to express decorin very early on. Combination of decorin overexpression with conditional cytotoxic strategies, and other antitumor agents, may provide added therapeutic advantages.
Materials and methods
Recombinant adenoviruses
The RAd vectors used are E1/E3-deleted first-generation adenovirus, in which the cassette containing a recombinant transgene and promoter is inserted in place of the E1 region. Four different vectors were used in this study: (I) RAd/GFAP/decorin; (II) RAd/hCMV/decorin; (III) RAd/hCMV/β-gal; (IV) RAd/hCMV/HSV1-TK. Construction of the RAd/hCMV/β-gal and RAd/hCMV/HSV1-TK has been described in detail elsewhere.21,40 To create the shuttle vector pΔE1/GFAP/decorin, which was used to generate RAd/GFAP/decorin, a blunted EcoRI human decorin fragment (1780 bp) was cloned into the blunted BamHI site of the pΔE1/GFAP.36 A blunted EcoRI human decorin fragment (1780 bp) was cloned into the blunted BamHI site of pAL119 (pMV35 in Shering et al40) to create the shuttle vector pAL119/decorin which was used to generate RAd/hCMV/decorin. Recombinant adenoviruses encoding the decorin gene were then generated by homologous recombination in 293 cells following cotransfection of the shuttle vector and pJM17 plasmid (Microbix biosystems, Inc.). Restriction enzyme and Southern blot analysis were carried out to confirm the presence of the human decorin sequences in the viral genome. Production of high-titer stocks, purification by double-cesium chloride density-gradient separation, and titration of viruses were carried out as previously described.41,42 Viral stocks were found to be free of replication-competent adenovirus using a supernatant rescue assay able to detect one replication-competent virus within 109 recombinant viruses.43 Adenovirus preparations were determined to be endotoxin (lipopolysaccharide) free, according to the criteria of Cotton et al44 using the E-TOXATE assay (Sigma-Aldrich, Dorset, UK).
Cell culture
The kidney embryonic 293 cell line was obtained from Microbix biosystems (Ontario, Canada). The HeLa cell line was purchased from the European Collection of Animal Cell Cultures (Salisbury, UK). The mink lung epithelial cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct was a gift from Dr DB Rifkin (Department of Cell Biology, New York University Medical Center, NY). The rat glioma CNS-1 cell line was a generous gift from Professor. W Hickey (Department of Pathology, Dartmouth Medical Centre, Lebanon, NH). The 293 cells were grown in MEME containing 10% FCS and 2 mM glutamine. The HeLa cells, CNS-1 cells and mink lung epithelial cells were grown in DMEM containing 10% FCS and 2 mM glutamine. All growing cells were incubated in a humidified incubator containing 5% CO2 at 37°C.
Generation and evaluation of glioma CNS-1 clonal cell sublines stably expressing decorin
Human decorin cDNA was cloned into the BCMGS/neo plasmid.26 BCMGS/neo/decorin or BCMGS/neo without insert, as a control, was then transfected into CNS-1 glioma cells by electroporation.13 PCR was used to confirm the presence of human decorin sequences in genomic DNA extracted from decorin stably transfected CNS-1 cells. A 944 bp decorin-specific PCR product was amplified using the primers decorin-fwd (5′-CCCAGAAGTTCCTGATGAC-3′) and decorin-rev (5′-CAGAGCGCACGTAGACAC-3′). Two control rat β-actin primers,17 β-actin-fwd (5′-CCAGCCATGTACGTAGCCATCC-3′) and β-actin-rev (5′-GCAGCTCATAGCTCTTCTCCAGG-3′), were used to produce a 340 bp β-actin specific PCR product. In a 50-μl PCR reaction, 10 μl of genomic DNA was used in a solution containing 1 × PCR buffer (Promega), 200 μM dATP, 200 μM dTTP, 200 μM dCTP, 200 μM dGTP, 2 mM MgCl2, 50 pM of each primer oligonucleotide, 2 U Taq polymerase (Promega). PCR conditions were as follows: 45 seconds denaturation, 1-minute annealing, and 1-minute extension for 15 cycles, followed by another 7 minutes of extension. The annealing temperature for all primers was 60°C. The PCR products were separated by 2% agarose gel electrophoresis and were visualized on a UV transilluminator after being stained with ethidium bromide.
Transgene expression was assessed by immunocytochemical analysis. CNS-1 cells were grown directly on glass coverslips in six-well plates, washed twice with PBS and fixed in 4% paraformaldehyde/0.12 M sucrose for 20 minutes. Cells were then permeabilized with 0.5% Triton X-100 for 10 minutes and blocked with 10% normal horse serum in PBS for 30 minutes. A volume of 60 μl of the primary antibody (sheep anti-human decorin, United States Biological) diluted 1:1000 in PBS/1% horse serum, were placed onto coverslips and left at 4°C overnight. The next day, cells were washed three times with PBS and incubated with secondary FITC-labeled antibody (donkey anti sheep), diluted 1:1000 in PBS/1% horse serum, for 2 hours at 4°C. Cells were washed 3 times with PBS, and incubated with 1 × DAPI solution (Sigma) for 10 minutes. Unreacted DAPI was removed with three successive washes in PBS. The cell-coated coverslips were mounted in Mowiol mounting solution (Calbiochem Nottingham, UK) and left to dry at 4°C in dark. Images were acquired using Openlab software (Improvision, Coventry, UK) on an Olympus Corp. (Tokyo, Japan) Vanox microscope.
The ability of decorin-transfected CNS-1 cells to express biologically active protein and suppress TGF-β bioactivity was evaluated using a mink lung epithelial cell line that was stably transfected with a plasminogen activator inhibitor-1 promoter-Luciferase construct (MLE/PAI/L cells). Exposure of MLE/PAI/L cells to TGF-β induces a dose-dependent increase in Luciferase activity.27 The medium was aspirated from 25 cm2 flasks containing CNS-1 cells at 40% confluency and replaced with 3 ml of DMEM supplemented with 0.1% bovine serum albumin and 2 mM l-glutamine. The cells were then incubated in 5% CO2 at 37°C for 48 hours. After 2 days, MLE/PAI/L cells were seeded in a 24-well plate at a density of 8 × 104 cells/well and incubated for 3 hours. After 3 hours the medium was aspirated and replaced with 500 μl supernatant from CNS-1 cells in triplicate. After incubation in 5% CO2 at 37°C for 16 hours, the conditioned medium was removed, the cells washed twice with 500 μl PBS and lysed by addition of 60 μl of cell lysis buffer (1.25 ml 1 M Tris-HCl pH 7.8, 100 μl 0.5 M EDTA pH 8, 15% glycerol, 10 mM MgCl2 and 1% Triton X-100, made up to 50 ml with dH2O). The cell debris was pelleted by centrifugation at 13,000 rpm for 2 minutes and the supernatant was transferred to a clean Eppendorf tube. Luciferase assay reagent (Promega) was prepared immediately prior to use by diluting with an equal volume of distilled water. A volume of 20 μl of cell lysates were added to 100 μl of Luciferase assay reagent (Promega) and Luciferase activity measured over 10 seconds in a luminometer. Luciferase activity was then standardized dividing the RLU by the μg of protein in each sample.
Assessment of the growth rate of decorin-transfected CNS-1 cells in vitro
The growth rate of CNS-1 cells expressing decorin was assessed in vitro and compared with growth rate of wild-type CNS-1 and neomycin control CNS-1 cells. The cells were seeded into 12-well plates at a density of 2500 cells/well in quadruplicates and incubated at 37°C in 5% CO2. During 12 days, every 24 hours, the cells were rinsed with 300 μl of Dulbecco’s PBS and detached using 0.05% Trypsin-EDTA. Then 500 μl of growth medium was added, the cells were dispersed by pipetting up and down, and the number of living cells was determined using trypan blue staining. An improved Neubauer haemocytometer was used to count the cells in quadruplicates.
Bioactivity of RAds encoding decorin in vitro
The medium was aspirated from 25 cm2 flasks containing HeLa cells at 80% confluency and replaced with 3 ml of DMEM supplemented with 0.1% bovine serum albumin and 2 mM glutamine. The cells were then infected with RAds encoding decorin or β-galactosidase at MOI of 100 and 300. The cells were incubated at 37°C in 5% CO2 for 72 hours. After 72 hours conditioned medium was removed from 25 cm2 flasks and TGF-β3 (Promega) was added into the conditioned medium (0.10 ng/ml) and incubated at 37°C for 3 hours. MLE/PAI/L cells were seeded in a 24-well plate at a density of 8 × 104 cells/well and incubated for 3 hours. After incubation in 5% CO2 at 37°C for 16 hours, the conditioned medium was removed. Luciferase activity was determined as described previously.27
Experimental gliomas
All animal experiments were conducted in accordance with the guidelines of the United Kingdom Animal (Scientific Procedures) Act of 1986. The animals used were adult male Lewis rats with a body weight ranging from 250 to 300 g. All animals were housed at constant temperature and humidity, and 12/12-hours light-dark cycle and had free access to food and water. Following anesthesia with 4% halothane (AstraZeneca), the rats were placed in a stereotaxic frame and connected to an anesthetic machine to deliver 1% halothane in 66% medical oxygen and 33% medical nitrous oxide. After making a hole in the skull, using a Hamilton syringe with a 26 gauge needle, 5 × 103 cells (in 3 μl PBS) were injected unilaterally into the right striatum at coordinates 1 mm forward and 3 mm lateral from the bregma and 4 mm vertical from the dura. At 3 days post-tumor implantation, viruses were injected into the tumor site. RAd/hCMV/HSV1-TK-injected animals, received 25 mg/kg GCV (Roche products, Welwyn Garden City, UK) intraperitoneally for 7 days. All animals were monitored daily for temperature and well-being. The tumor-implanted animals were monitored twice daily for any observable signs of distress or discomfort and killed when tumors prevented feeding or ambulation. Rats were monitored daily and unwell rats anesthetized, perfused, and fixed, as described previously.21
Histological analysis
Histological analysis of brain tissue and immunohistochemistry were carried out as previously described.45,46 The primary antibodies and the dilutions at which they were used were anti-decorin (1:1000; US Biological), anti-β-galactosidase (1:1000; Promega), anti-ED1 (1:1000; Serotec), anti-CD8 (1:500; Serotec), anti-CD8b (1:1000; Serotec), and anti-CD161 (1:1000; Serotec). All primary antibodies were mouse monoclonal anti-rat, except for anti-decorin which was sheep polyclonal anti-human. Reacted primary antibodies were labeled with biotinylated anti-mouse or anti-sheep antibodies (Dako) at a dilution of 1:200.
Statistical analysis
Student’s t-test was used to analyze the in vitro experimental results. Survival data were analyzed by Kaplan–Meier estimator analysis, and compared using the logrank test. Statistical analysis was performed using the SPSS program software (version 10).
Acknowledgments
The neurological gene therapy program in the GTRI is funded by NIH Grants 1 RO1 NS42893 (PRL), 1 RO1 NS44556 (MGC), U54 4 NS04-5309 (PRL), R21 NS47298 (PRL), 1 RO3 TW006273-01A1 (MGC), and the Kane Fellowship in Gene Therapy for Cancer Research. PRL holds the Bram and Elaine Goldsmith Chair in Gene Therapeutics. We would also like to thank the Board of Governors at Cedars-Sinai Medical Center for their vision and very generous creation and support of the GTRI. During his PhD work, Dr Ali Biglari was very generously supported by a fellowship from the Government of the Republic of Iran. We would like to thank Dr Shlomo Melmed for his support and academic leadership, Mr Richard Katzman for his first class administrative support, Mrs Semone Muslar for her excellent secretarial skills, and Mr Nelson Jovel for the skillful and top quality editing and preparation of the figures and manuscript for publication.
References
- 1.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 2.Kresse H, Schonherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol. 2001;189:266–274. doi: 10.1002/jcp.10030. [DOI] [PubMed] [Google Scholar]
- 3.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angio-genesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi Y, Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988;336:244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- 5.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci USA. 1995;92:7016–7020. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 7.Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J Clin Invest. 1997;100:149–157. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 10.Csordas G, Santra M, Reed CC, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 11.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 12.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 13.Stander M, Naumann U, Dumitrescu L, et al. Decorin gene transfer-mediated suppression of TGF-beta synthesis abrogates experimental malignant glioma growth in vivo. Gene Therapy. 1998;5:1187–1194. doi: 10.1038/sj.gt.3300709. [DOI] [PubMed] [Google Scholar]
- 14.Kiefer R, Supler ML, Toyka KV, Streit WJ. In situ detection of transforming growth factor-beta mRNA in experimental rat glioma and reactive glial cells. Neurosci Lett. 1994;166:161–164. doi: 10.1016/0304-3940(94)90475-8. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki A, Naganuma H, Satoh E, et al. Secretion of transforming growth factor-beta 1 and -beta 2 by malignant glioma cells. Neurol Med Chir (Tokyo) 1995;35:423–430. doi: 10.2176/nmc.35.423. [DOI] [PubMed] [Google Scholar]
- 16.Platten M, Wild-Bode C, Wick W, Leitlein J, Dichgans J, Weller M. N-[3,4-dimethoxycinnamoyl]-anthranilic acid (tranilast) inhibits transforming growth factor-beta relesase and reduces migration and invasiveness of human malignant glioma cells. Int J Cancer. 2001;93:53–61. doi: 10.1002/ijc.1289. [DOI] [PubMed] [Google Scholar]
- 17.Dewey RA, Morrissey G, Cowsill CM, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 18.Cowsill C, Southgate TD, Morrissey G, et al. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: reduced cytotoxicity of a truncated HSV1-TK. Gene Therapy. 2000;7:679–685. doi: 10.1038/sj.gt.3301147. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 20.Geddes BJ, Harding TC, Lightman SL, Uney JB. Long-term gene therapy in the CNS: reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nat Med. 1997;3:1402–1404. doi: 10.1038/nm1297-1402. [DOI] [PubMed] [Google Scholar]
- 21.Navarro V, Millecamps S, Geoffroy MC, et al. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene Therapy. 1999;6:1884–1892. doi: 10.1038/sj.gt.3301008. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 24.Kruse CA, Molleston MC, Parks EP, Schiltz PM, Kleinschmidt-DeMasters BK, Hickey WF. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neurooncol. 1994;22:191–200. doi: 10.1007/BF01052919. [DOI] [PubMed] [Google Scholar]
- 25.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 26.Karasuyama H, Kudo A, Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990;172:969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 28.Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259:94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- 29.Beutler AS, Banck MS, Aguzzi A. Curing rat glioblastoma: immunotherapy or graft rejection? Science. 1997;276:20–21. doi: 10.1126/science.276.5309.17e. [DOI] [PubMed] [Google Scholar]
- 30.Parsa AT, Chakrabarti I, Hurley PT, et al. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47:993–999. doi: 10.1097/00006123-200010000-00050. discussion 999–1000. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 32.Border WA, Noble NA, Yamamoto T, et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 33.Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi Y, Kodama Y, Matsumoto T. Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. J Biol Chem. 1994;269:32634–32638. [PubMed] [Google Scholar]
- 35.Morelli AE, Larregina AT, Smith-Arica J, et al. Neuronal and glial cell type-specific promoters within adenovirus recombinants restrict the expression of the apoptosis-inducing molecule Fas ligand to predetermined brain cell types, and abolish peripheral liver toxicity. J Gen Virol. 1999;80(Part 3):571–583. doi: 10.1099/0022-1317-80-3-571. [DOI] [PubMed] [Google Scholar]
- 36.Smith-Arica JR, Morelli AE, Larregina AT, et al. Cell-type-specific and regulatable transgenesis in the adult brain: adenovirus-encoded combined transcriptional targeting and inducible transgene expression. Mol Ther. 2000;2:579–587. doi: 10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- 37.Munz C, Naumann U, Grimmel C, Rammensee HG, Weller M. TGF-beta-independent induction of immunogenicity by decorin gene transfer in human malignant glioma cells. Eur J Immunol. 1999;29:1032–1040. doi: 10.1002/(SICI)1521-4141(199903)29:03<1032::AID-IMMU1032>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 38.Tralhao JG, Schaefer L, Micegova M, et al. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer. FASEB J. 2003;17:464–466. doi: 10.1096/fj.02-0534fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol Ther. 2000;2:330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- 40.Shering AF, Bain D, Stewart K. Cell type-specific expression in brain cell cultures from a short human cytomegalovirus major immediate early promoter depends on whether it is inserted into herpesvirus or adenovirus vectors. J Gen Virol. 1997;78(Part 2):445–459. doi: 10.1099/0022-1317-78-2-445. [DOI] [PubMed] [Google Scholar]
- 41.Lowenstein PR, Shering AF, Bain D, Castro MG, Wilkinson GWG. The use of adenovirus vectors to transfer genes to identified target brain cells in vitro. In: P.R.a.E., Lowenstein LW, editors. Protocols for Gene Transfer in Neuroscience. John Wiley and Sons; Chichester: 1996. pp. 93–114. [Google Scholar]
- 42.Southgate T, Kingston P, Castro MG. Gene transer into neural cells in vivo using adenoviral vectors. In: Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley and Sons; New York, NY: 2000. pp. 4.23.1–4.23.40. [Google Scholar]
- 43.Dion LD, Fang J, Garver RI., Jr Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. J Virol Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- 44.Cotten M, Baker A, Saltik M, Wagner E, Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Therapy. 1994;1:239–246. [PubMed] [Google Scholar]
- 45.Thomas CE, Abordo-Adesida E, Maleniak TC, Stone D, Gerdes G, Lowenstein PR. Gene transfer into rat brain using adenoviral vectors. In: Gerfen JN, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley and Sons; New York, NY: 2000. pp. 4.23.1–4.23.40. [DOI] [PubMed] [Google Scholar]
- 46.Stone D, Xiong W, Williams JC, David A, Lowenstein PR, Castro MG. Adenovirus expression of IL-1 and NF-kappaB inhibitors does not inhibit acute adenoviral-induced brain inflammation, but delays immune system-mediated elimination of transgene expression. Mol Ther. 2003;8:400–411. doi: 10.1016/s1525-0016(03)00178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]