Abstract
Molecular imaging aims to assess non-invasively disease-specific biological and molecular processes in animal models and humans in vivo. Apart from precise anatomical localisation and quantification, the most intriguing advantage of such imaging is the opportunity it provides to investigate the time course (dynamics) of disease-specific molecular events in the intact organism. Further, molecular imaging can be used to address basic scientific questions, e.g. transcriptional regulation, signal transduction or protein/protein interaction, and will be essential in developing treatment strategies based on gene therapy. Most importantly, molecular imaging is a key technology in translational research, helping to develop experimental protocols which may later be applied to human patients. Over the past 20 years, imaging based on positron emission tomography (PET) and magnetic resonance imaging (MRI) has been employed for the assessment and “phenotyping” of various neurological diseases, including cerebral ischaemia, neurodegeneration and brain gliomas. While in the past neuro-anatomical studies had to be performed post mortem, molecular imaging has ushered in the era of in vivo functional neuro-anatomy by allowing neuroscience to image structure, function, metabolism and molecular processes of the central nervous system in vivo in both health and disease. Recently, PET and MRI have been successfully utilised together in the non-invasive assessment of gene transfer and gene therapy in humans. To assess the efficiency of gene transfer, the same markers are being used in animals and humans, and have been applied for phenotyping human disease. Here, we review the imaging hallmarks of focal and disseminated neurological diseases, such as cerebral ischaemia, neurodegeneration and glioblastoma multiforme, as well as the attempts to translate gene therapy’s experimental knowledge into clinical applications and the way in which this process is being promoted through the use of novel imaging approaches.
Keywords: Molecular imaging, FIAU, FHBG, PET, Glioma, Parkinson’s disease
Introduction
Over the past decade the clinical neurosciences have increasingly demanded improvements in imaging technologies employed in the diagnostic work-up of neurological disease. These imaging technologies comprise static and functional methods such as cranial computed tomography (CT), magnetic resonance imaging (MRI), spectroscopy (MRS) and angiography (MRA), positron emission tomography (PET), single-photon emission computed tomography (SPECT), ultrasound and invasive angiography.
The first parameter of interest is the precision of imaging of the anatomical location of the lesion causing the symptoms identified as topographical neurological deficits. Due to its high spatial resolution and the possibility of weighting for various tissue components, MRI is the method of choice for localisation of tissue changes at high spatial resolution. Initially, however, it provides only a static anatomical image. Due to its high sensitivity for detection of small amounts of radioactivity emitted by compounds of known chemistry, in addition to the possibility of quantification, PET is being used to characterise disease-specific changes on the basis of altered enzyme activity, changes in the levels of neurotransmitter receptors or even the dynamics of neurotransmitter function. Based on new developments in contrast agents and radiotracers, MRI and PET are being used and further developed for the non-invasive localisation and quantification of gene expression, protein function and profiling of signal transduction pathways in vivo to achieve further insights into the molecular pathophysiology of human diseases and to facilitate the design and implementation of improved therapies, including gene therapy.
The general concept is the establishment of protocols of imaging-guided classification and characterisation of both disease stage and potential therapies. Imaging is being used for the non-invasive characterisation of disease-specific changes, and the resultant disease-specific imaging parameters are then later used as read-outs for the efficiency of individual therapies [1]. It should be pointed out that it is the set of different imaging technologies (e.g. MRI and PET) and their dynamic parameters (e.g. MRI with and without contrast enhancement, diffusion and perfusion weighting; PET employing various radiotracers) that enable accurate, repetitive, non-invasive disease phenotyping [2–6] and the measurement of therapeutic outcomes.
During the past decade, the molecular and genetic causes underlying many neurological diseases have been characterised. Knowledge of the underlying genetic and molecular defects and understanding of related pathophysiological changes are essential to develop novel gene therapeutic approaches intended to offer molecular interventions targeting the disease-causing molecular defects. Gene therapy of neurological diseases aims, for example, to prevent neuronal cell death caused by cerebral ischaemia or neurodegeneration, to restore the function of pathologically altered afferent pathways involved in pain, or even to selectively kill proliferating and migrating glioma and tumour cells [7–20].
As experimental tools, gene therapy vectors are being increasingly used to generate animal models for human disease, through adult transgenesis that does not require the genetic manipulation of the germline [21, 22]. Neurodegenerative gene therapy aims either to restore enzyme protein levels, the lack of which leads to inflammation and brain cell death, or to deliver specific neurotrophic factors to dying nigrostriatal neurons in Parkinson’s disease, or to target anti-apoptotic genes to neurons potentially affected by ischaemia. All these approaches are based on overexpression of individual proteins in the brain. Until recently, reducing or eliminating the expression of genes causing brain cell death, e.g. huntingtin in dominant Huntington’s disease, has been erratic and not very efficient. More recent use of siRNA technology has allowed predictable knocking down of dominant, disease-causing genes by specific mRNA silencing [7, 11, 23–25].
Brain tumour gene therapy relies on (i) the introduction of replicating viruses that will selectively and specifically replicate within brain tumours (oncolytic virus therapy), (ii) the transduction of genes expressing pro-drug activating enzymes to selectively kill brain tumour cells following the administration of a precursor that can only be converted into cytotoxic intermediates within transduced tumour cells, (iii) immuno-modulating molecules to stimulate the systemic anti-tumour immune response, or even (iv) pro-apoptotic proteins or anti-angiogenic proteins to inhibit brain tumour vessel formation [8, 17, 26–33]. Various viral and non-viral vectors have been developed and applied in rodent and non-human primate models of disease. So far, only selected vectors have been successfully used in clinical applications [30, 33–36]. In the clinical application of biologically active agents such as vectors, various issues have to be taken into account, such as efficiency of gene delivery, vector toxicity, stability of transgene expression and choice of promoter, as well as dose, time and route of vector application. It has been shown that gene therapy vectors which can be imaged non-invasively in vivo will greatly enhance the understanding and future development of clinical gene therapy protocols [1].
In general, vectors comprise a capsid or envelope facilitating entry into the cell and the genetic material, which, at least for viral vectors, is delivered directly through the cytoplasm into the cell nucleus. The composition of the capsid or the envelope determines which cells are infected best. By modification of the surface, the virion can be targeted to certain cell types. In the cell nucleus, the genetic material which is carried by the vector is either integrated into the host genome, as for retrovirus- and adeno-associated virus-derived vectors (MoMLV, lentivirus, AAV), or maintained episomally, as for herpes simplex virus type 1 (HSV-1) derived vectors. The transgene capacity varies from low (4.5 kb for AAV vectors) to medium (7–8 kb for retro- and lentiviral vectors) to high [36–150 kb for high-capacity adenovirus (AdV) and HSV-1 amplicon vectors] [37–40]. Use of tissue-specific promoters alone, or combined with regulated promoter elements allows targeted gene expression in specific cell types (e.g. neurons, glia) with or without tight regulation of gene expression levels [14]. Retroviral vectors can only integrate their genetic material during cell division and, therefore, their application in the CNS is confined to the treatment of brain tumours and to the modification of cells in culture for transplantation purposes. It should be pointed out that variants completely devoid of any protein encoding sequences have been constructed for vectors derived from AdV, AAV, LV and HSV-1. In all these cases, vectors express no viral genes necessary for the normal virus life cycle. Therefore, in theory, they were thought to be less toxic and to mediate little to no immune reaction [41], although the reality has provided some fascinating biological surprises [39, 40, 42–52].
Moving away from viruses, synthetic vectors such as cationic liposomes have several attractive features as gene carriers: they are simple to prepare, stable at room temperature, less immunogenic and potentially less toxic [53]. Linked to transferrin, these cationic liposome/DNA complexes (Tf-lipoplex) show efficient transfection of various central nervous system-derived cells [54]. However, plasmid DNA per se is inflammatory, with CpG sequences being recognised by Toll receptors, the transfer of DNA to the nucleus of host cells is relatively inefficient and mechanisms of long-term expression remain unclear. Thus, non-viral vectors still present many of the same challenges as virus-derived ones.
Although there is still some lack of clarity over what should determine the choice of a particular vector, the choice is largely determined by the target tissue, the transgenic construct to be used, the size of transgenic cassette that can be made to fit into different vectors, the need for high volumes and/or concentrations, the intrinsic capacity of different vector systems and even how a particular vector may be perceived by the patient population. Currently, the vectors of choice being used for neurodegenerative diseases are third-generation lentiviral vectors, AAV vectors or even the new high-capacity adenoviral or HSV-1 amplicon vectors [21]. For brain tumour therapy, AdV, replication-conditional AdV and HSV-1 vectors are most promising [30, 35, 36]. Details of individual vectors [37] and how they can be used in various applications for the CNS [7] are summarised in recent reviews [14, 17, 55] cited throughout and in Tables 1, 2 and 3.
Table 1.
Gene transfer vehicles used in gene therapy applications
| Adenovirus | HD-Ad | HSV-1/r | HSV-1/a | AAV | Retrovirus | Vaccinia virus | Microinjection | Transfection | |
|---|---|---|---|---|---|---|---|---|---|
| Size | 36 | 30–36 | 152 | 10–30 | 4.68 | 3.5–9.2 | 186 | Unlimited | Unlimited |
| Cloning capacity (kb) | 7.5 | ~30 | 30 | 10–30 | 2–4.5 | ~8 | 30 | Unlimited | Unlimited |
| Transduction | |||||||||
| In vivo? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| In vitro? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Long-term expression | No | Yes | Yes | Yes | Yes | Yes | No | ? | No |
| Vaccination | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – |
| Vector titres (pfu/ml) | 1012 | 1011 | 108 | 108 | 109 | 107 | 106 –108 | – | – |
HD-Ad gutless helper-dependent adenovirus vector, HSV-1/r herpes simplex type 1 recombinant vector, HSV-1/a herpes simplex type 1 amplicon, pfu/ml plaque-forming units per ml
Table 2.
Advantages and disadvantages of viral vectors for gene therapy
| Virus | Maximum capacity | Advantages | Disadvantages |
|---|---|---|---|
| Adenovirus | 8 kb | Broad cell tropism, infection of dividing and non-dividing cells, easy to produce at high titres |
Inflammatory and immune responses, transient expression |
| HD-Ad | 36 kb | Broad cell tropism, infection of dividing and non-dividing cells, less inflammatory and cellular immune response, longer term transgene expression |
Difficulties in large-scale production |
| AAV | 5 kb, 10 kb (concatamers) |
Broad cell tropism, infection of dividing and non-dividing cells, integration into host genome |
Difficulties in producing pure preparations at high titres, discrete immune response |
| HSV-1 | 30150 kb | Broad cell tropism, latency in neurons, very stable | Highly toxic |
| Lentivirus | 10 kb | Infection of dividing and non-dividing cells, integration into host genome |
Toxic if not packaged as helper virus-free amplicons |
Table 3.
Possible disadvantages among gene transfer vehicles in gene therapy
| Vectors | |
|---|---|
| Adenovirus | Host immune responses: inflammatory and cytotoxic reactions in patients and depletion of transduced cells |
| Host’s humoral immune responses may neutralise adenoviral vector particles during, or even before, the gene transfer processes | |
| Not suitable for long-term expression of the transgene owing to the lack of integration into the host genome | |
| Complicated vector genome | |
| Helper-dependent adenovirus | These vectors can only be grown in the presence of a helper virus and are not economic because of the production of low-level contamination with helper virus |
| In comparison with first-generation adenovirus, virus titres are much lower | |
| AAV | High titres of pure virus are difficult to obtain |
| AAV requires a helper adeno- or herpesvirus | |
| This vector system is still not well characterised | |
| Limited capacity for foreign genes (about 2– 4.5 kb) | |
| Lack of specific integration for recombinant AAV vectors, which may result in cell mutagenesis | |
| HSV-1 | Host immune responses, inflammatory cytopathogenicity and neurotoxicity reactions in patients |
| Complicated vector genome | |
| Difficult to produce | |
| HSV-1-derived vectors could potentially reactivate latent wild-type HSV-1 | |
| Retrovirus | Random insertion of viral genome, which may result in mutagenesis and activate oncogenes |
| Possibility of replication-competent virus formation by homologous recombination. Possible recombination with HERVs | |
| Retroviral vector particles are rapidly degraded by the complement | |
| Infects only dividing cells, small insert capacity (6.5 kb) | |
| Vaccinia virus | Widespread use of vaccinia as a live vaccine; however, depends on improving safety while achieving an even higher immune response to the recombinant protein |
| Microinjection | It is difficult to introduce DNA on a scale large enough for biochemical analysis |
| Transfection (cationic liposomes or DNA protein complexes) |
Targeting is not specific |
| Low transfection efficiency | |
| Only transient expression | |
| Difficult in vivo applications | |
| Host immune responses, inflammatory reactions in patients if they express chimerical cell receptors on their surface or in the presence of unmethylated CpG sequences of bacterial plasmid DNA |
AAV Adeno-associated virus, HSV herpes simplex virus, HERVs human endogenous retroviruses
The target tissue plays an important role in vector selection. Transduction of bone marrow cells will necessitate the use of an integrating vector such as lentiviral or retroviral vectors that indeed infect bone marrow cells. The transgenic construct, and especially its size, guides the next step in the choice. Any construct above 10 kbp will need vectors that accommodate more than the full capacity of a retroviral or lentiviral vector. The need for regulatory sequences will increase the size of the transgenic construct, again reducing the potential choice of vectors. Finally, if a clinical trial is to proceed, patient expectations can play an important role. In spite of the good standing of lentiviral vectors amongst the scientific community, most of these remain derived from, and if not derived from, then related to, HIV. This could create serious concern among patients, given the difficulty of explaining science to a scientifically illiterate community afraid of imponderable dangers like infection by HIV. In practical terms, the choice of vector is to a considerable degree dependent on the technical expertise of the laboratory. Quality control of vector preparations has not been standardised, and frequently even vector preps from commercial sources lack adequate quality control. In fact, the quality of experiments often depends on the quality of vector preps, and ultimately on the laboratory that has prepared them. This has come to the fore in the study of inflammatory responses: a bad vector reputation and adverse inflammatory and immune responses are often due to the inadvertent presence of LPS in the samples rather than to the intrinsic capacity of a vector to induce inflammation.
Cerebral ischaemia
Definition
Cerebral ischaemia causes a complex cascade of cellular events including ATP depletion, membrane depolarisation, calcium influx, and changes in gene expression and cytoskeleton, which finally lead to apoptosis. The core of infarction can be distinguished from the ischaemic penumbra, where hypoperfusion causes disturbance of neuronal function with metabolism of neurons remaining intact [56]. Cells in the core are beyond recovery, while the fate of those in the penumbra depends on the evolution of the disease and/or the treatment.
Imaging
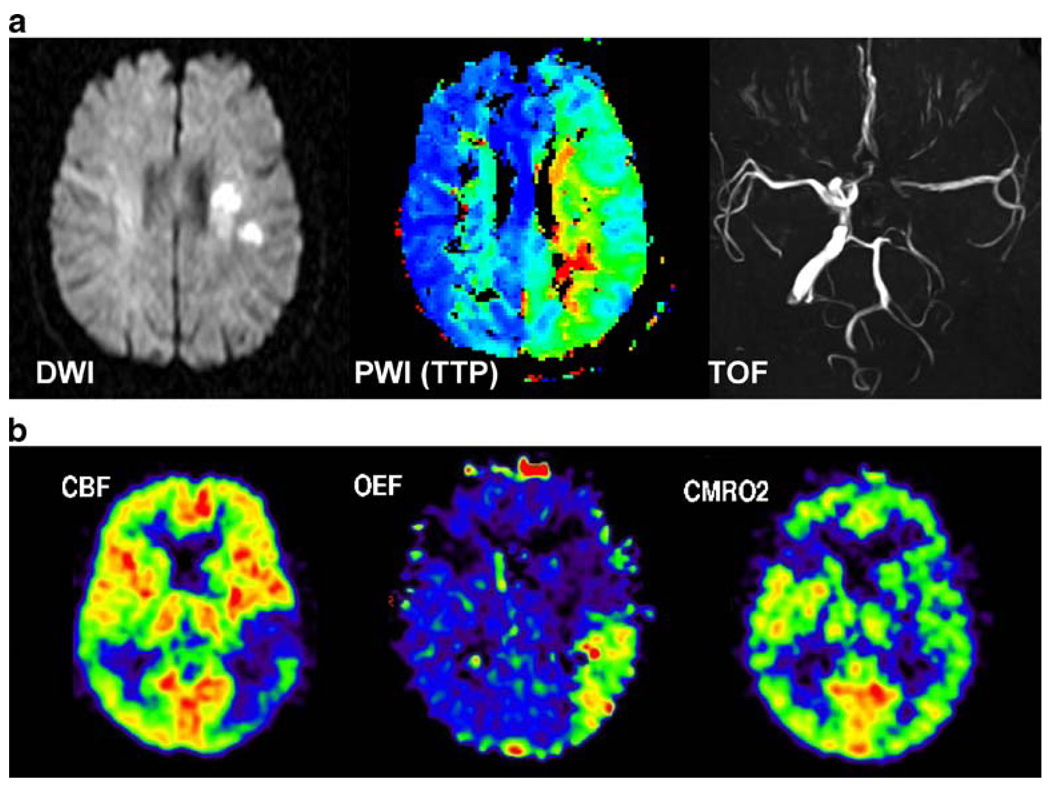
The penumbra is the target tissue for early thrombolysis and can be visualised by various imaging modalities, including PET and MRI [3, 6, 57]. In PET studies, the penumbra is characterised by preserved glucose metabolism, CMRGlc, as measured by 18F-fluorodeoxyglucose (FDG) PET (and expressed as µmol/100 g/min), and preserved oxygen metabolism, CMRO2, as measured by 15O-O2 PET (and given in µmol/100 g/min). CMRO2 is only preserved in these areas of hypoperfusion owing to an increased oxygen extraction fraction (OEF). If hypoperfusion, which can be assessed by 15O-H2O PET (in ml/100 g/min), lasts too long, neurons in the penumbra will die, resulting in extension of the infarct size. 11C-flumazenil binds to central benzodiazepine receptors and, as measured by PET, is an early indicator of preserved cortical neuronal integrity [58]. Moreover, 18F-fluoromisonidazole binds preferentially to viable but hypoxic cells in peri-infarct regions [59]. In MRI studies, the equivalent of the penumbra is called “mismatch”, which can be visualised by a combination of diffusion and perfusion-weighted imaging (DWI, PWI [60]). Early thrombolysis is the only treatment option which has been proven to be efficient in clinical applications: all neuroprotective strategies targeting the cascade of glutamate- and calcium-mediated toxicity have failed to show any benefit in clinical trials. With both MRI and PET, the effect of thrombolysis can be directly visualised in patients. Ongoing research is focussing on attempts to extend the therapeutic window of thrombolysis through imaging guidance and combining thrombolysis with neuroprotective strategies. Figure 1 summarises MRI and PET parameters for the description of the penumbra.
Fig. 1.
MRI and PET parameters used in the assessment of acute cerebral ischaemia. In a, diffusion-weighted imaging (DWI) depicts the area reflecting the core of infarction, perfusion-weighted imaging (PWI) reveals hypoperfusion in the territory of the middle cerebral artery (MCA), which is larger than the DWI lesion (mismatch), and time of flight (TOF) angiography shows a proximal occlusion of the MCA, which is perfused by leptomeningeal anastomoses. In b, cerebral blood flow (CBF) is measured by 15O-H2O PET, demonstrating hypoperfusion in the posterior portion of the MCA territory. By virtue of an increase in the oxygen extraction fraction (OEF), the cerebral metabolic rate of oxygen (CMRO2) as measured by 15O-O2 PET is still intact. This hypoperfused tissue, which is functionally disturbed but still viable, is the classic definition of penumbra [3]
Gene therapy
Gene therapy may be a treatment option for stroke [17]. Experimental studies in rats have shown effects on infarct size after overexpression of bcl-2 [61], interleukin-1 receptor antagonist [62], neuronal apoptosis inhibitory protein (NAIP [63]), sensitive to apoptosis gene (SAG [64]), glial-derived neurotrophic factor (GDNF [65]) and midkine, a heparin-binding growth factor [66]. It should be pointed out that all gene therapy strategies for clinical application in patients with stroke will be complicated by the tight time frame (measured in hours) of the ischaemic cascade, during which therapeutic intervention should take place. Most of the experimental studies have been performed with vector delivery before or during the period (1–2 h) immediately after the induction of ischaemia. Whether this is practically feasible within routine clinical settings is questionable. The development of short-term gene therapy strategies that will target the brain vasculature from a systemic administration site remains an aspiration. However, the use of gene therapy to repair the consequences of stroke is a more viable possibility [67]. The potential of gene therapy to manipulate endogenous stem cells, alone or in combination with stem cell transplantation, is being explored as a novel treatment to promote CNS repair [68–71].
Imaging gene therapy
Infarct size as measured by DWI MRI and impaired oxygen and glucose metabolism (CMRO2, CMRGlc) could be used as read-outs for the efficiency of gene therapy. However, because of the limitations indicated above, clinical gene therapy studies for stroke have not been initiated.
Neurodegeneration
The diagnosis of neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease is based on a set of clinical, laboratory, genetic and neuroradiological criteria. Current treatment regimens for these disorders are mostly symptomatic and have in general shown marginal capacity to prevent progressive cell loss. While in Alzheimer’s disease cell loss is concentrated in the neocortex and hippocampus, in Parkinson’s and Huntington’s diseases it initially affects the basal ganglia. Of these three main neurodegenerative disorders, Parkinson’s disease is the only one where palliative treatment can control the main clinical symptoms for a relatively long period. Newer medications for Alzheimer’s disease may produce some slowing of the memory decline, while no effective therapies are available for Huntington’s disease. The continued, progressive and untreatable neuronal degeneration has now been identified as a target for genetic therapies. Only the development of new treatment strategies, including gene- and cell-based therapies for the transduction of growth factors and modulation of the immune system, will offer novel opportunities to prevent further neuronal loss. In addition, transplantation of neuronal precursor cells has now been tested in two different double-blind controlled clinical trials, with mixed results. While some positive effects could be detected in subgroups of patients with Parkinson’s disease treated with neuronal cell transplantation, in both clinical trials approximately 15% of patients developed significant side-effects [72]. Although uncontrolled clinical trials have been thought to yield generally positive results, the significant and serious side-effects seen in the controlled clinical trials have reduced the general enthusiasm for the transplantation of neuronal precursors. This approach is likely to be replaced by the transplantation of stem cells, raising the issue of how to direct the differentiation of stem cells down the pathway required [73]. It is likely that a combination of stem cells and gene transfer will produce the desired result of differentiation of neurons in the required directions [74, 75].
Alzheimer’s disease
Definition
Alzheimer’s disease (AD) is the most common cause of dementia and is characterised by deposition of amyloid plaques (extracellular), neurofibrillary tangles (intracellular), synaptic loss and neurodegeneration. The amyloid plaques contain insoluble fibrils of the amyloid-beta (Aβ) fragment of a larger amyloid precursor protein (APP). In familial forms of AD, mutations in presenilin and APP genes alter normal processing of APP by proteases (secretases), causing the extracellular accumulation of amyloid plaques. The neurofibrillary tangles represent insoluble polymers of hyperphosphorylated tau protein. Normal phosphorylated tau stabilises microtubules of axonal cytoskeleton. Neurofibrillary tangles induce neuronal dysfunction and destruction of neurons and synapses, first in the limbic system and later in the parietotemporal and prefrontal association cortices. Especially the decrease in the dendritic extent and synaptic depletion of hippocampal projection neurons lead to disruption of the connectivity among various brain regions [76]. The apolipoprotein E (apoE) gene is a risk factor for AD, with the apoE4 allele increasing and the apoE2 allele decreasing the risk of developing AD. The apoE4 allele has a role in the accumulation of Aβ42 and its binding properties to tau, and it operates as a risk modifier by decreasing the age of onset in a dose-dependent manner [77]. Degeneration of noradrenergic neurons arising in the locus ceruleus and of cholinergic neurons arising in the nucleus basalis of Meynert also play a key role in disease pathogenesis. Cholinergic neurons projecting to the hippocampus and cerebral cortex enhance synaptic efficacy and modulate active cortical circuits [78, 79].
Imaging
Imaging by MRI and PET now plays a key role in the diagnosis of AD. Moreover, for the development of new markers and therapeutics, optical imaging technology is being used in experimental models. Neuronal loss in the hippocampus and entorhinal cortex can be quantified by MRI with high reliability [80–82]. There is a quantitative relationship between medial temporal lobe atrophy as assessed by MRI and severity of dementia [83]. Hippocampal volume reduction ranges between 25% and 50% depending on disease severity, and predicts the decline from mild cognitive impairment to AD with high diagnostic accuracy [84]. 18F-FDG PET estimates the local cerebral metabolic rate of glucose consumption, providing evidence of neuronal loss and synapse dysfunction in vivo [85, 86]. In AD, a pathognomonic reduction of glucose metabolism in the posterior cingulate, parietotemporal and prefrontal cortices can be found, with minor reductions in primary visual and motor areas and relative sparing of thalamus, basal ganglia and cerebellum [87, 88]. The decline of 18F-FDG uptake in the posterior cingulate, parietotemporal and prefrontal association cortices allows identification of mild to moderate AD with 93% sensitivity and specificity [89]. The detection of hippocampal hypometabolism [90] relies on new-generation high-resolution PET scanners [91]. Of interest are the results obtained by recent FDG PET studies in normal subjects carrying the apoE4 allele and showing abnormal glucose metabolism in the same brain regions as in AD patients [92]. Frontal alterations of glucose metabolism in apoE4-positive patients with mild cognitive impairment (MCI) were the strongest predictor for the development of AD [93]. In MCI, the degree of cortical deficits of glucose metabolism and the prediction of development of AD are currently under investigation [94, 95]. It seems that already at the stage of MCI, reduced glucose metabolism can be found in a network of limbic regions of interest (hippocampus, amygdala, medial thalamus and posterior cingulate cortex) [90].
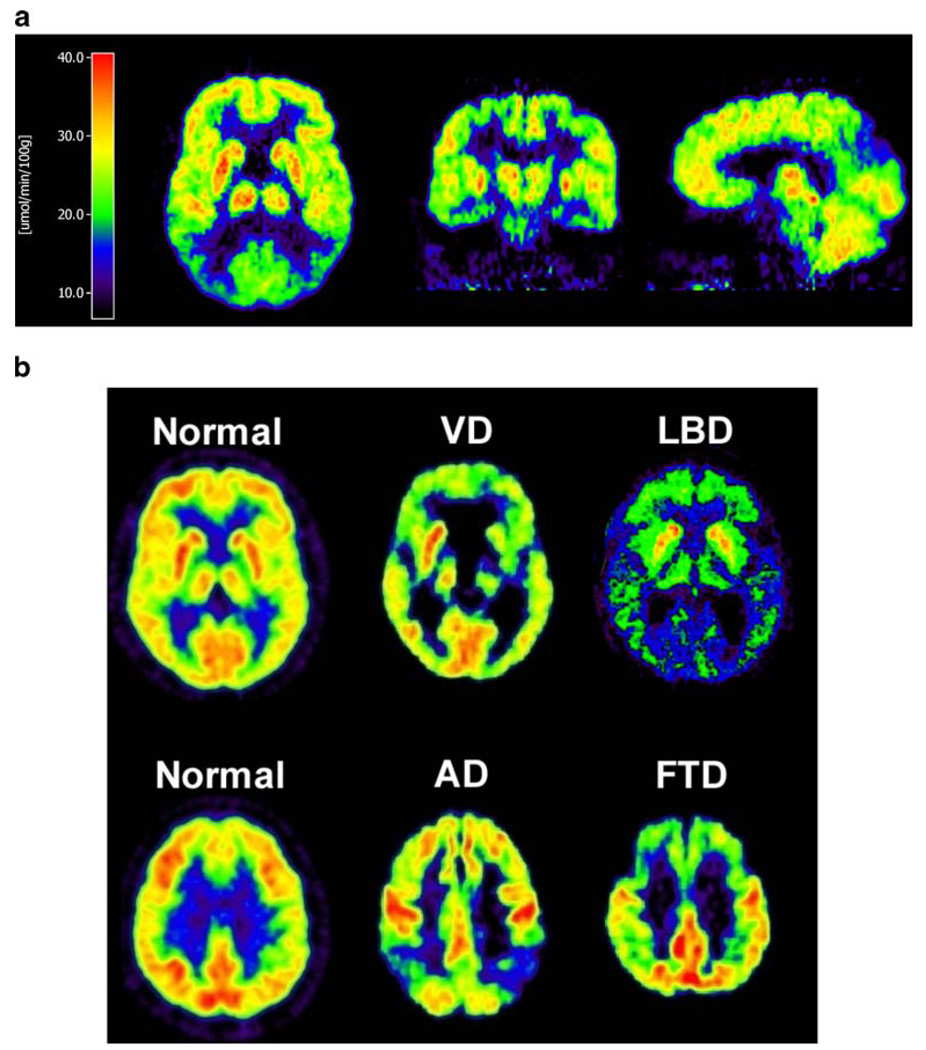
18F-FDG together with 11C-flumazenil has also been shown to be helpful in the diagnosis of other forms of dementia, including variants of Creutzfeld-Jakob disease [96]. The typical distribution of impaired glucose metabolism in AD and other causes of dementia is depicted in Fig. 2.
Fig. 2.
18F-FDG PET in AD and differential diagnosis of AD from other forms of dementia. In AD (a), the typical areas of hypometabolism (posterior cingulate, parietotemporal and temporomesial cortices) are depicted. In b, differentiation from vascular dementia (VD), Lewy body dementia (LBD) and frontotemporal dementia (FTD) is depicted
Besides the assessment of glucose metabolism, degeneration of cholinergic neurons, which originate in the nucleus basalis of Meynert and project to and regulate the function of hippocampal and cortical circuitry [97], causing alteration of acetylcholine esterase (AChE) activity in the amygdala and cerebral cortex in AD, can be assessed by 11C-N-methyl-4-piperidinyl-acetate or -propionate (11C-MP4A; 11C-PMP) and PET [98, 99]. Cortical AChE activity was found to be associated with attention and working memory rather than with performance on primary memory tests in AD [100]. Interestingly, the AChE activity and glucose metabolism are preserved or even increased in the nucleus basalis of Meynert itself, indicating that neocortical and amygdaloid functional changes of the cholinergic system are an early event in AD rather than the consequence of neurodegeneration of the basal nuclei [101]. Given that inhibition of AChE is the only pharmacological therapy with at least partial activity, imaging could help in the optimisation of pharmacological approaches.
18F-FDG and 11C-MP4A PET both reveal information on metabolic and functional consequences of the primary disease process, the disturbed protein processing. Therefore, a further imaging agent is required that will permit direct detection of the disease pathology by binding to Aβ plaques in vivo [102–106]. Based on fluorescent thioflavin derivatives used to image amyloid plaques in transgenic mice in vivo by multiphoton microscopy [103, 107], the neutral benzothiazoles were further investigated as potential PET agents. The basic properties of the prototypical benzothiazole amyloid binding agent, N-methyl-11C2-(4′-methyl-aminophenyl)-6-hydroxybenzothiazole (11C-PIB), include binding to Aβ with low nanomolar affinity, ready passage of the blood-brain barrier and rapid clearance from normal brain tissue [104]. The first imaging results in healthy controls and patients with AD demonstrated retention of 11C-PIB in frontal, parietotemporal and occipital cortices as well as the striatum in patients with AD. In cortical areas, 11C-PIB retention correlated inversely with cerebral glucose metabolism [104]. An Aβ binding compound which can be radiolabelled by 11F has also been developed. 2-(1-(6-[(2-[11F]fluoroethyl)(methyl)amino]-2-naphthyl)ethylidene)malononitrile ([11F]FDDNP) labelled senile plaques in tissue sections from AD brain; however, its in vivo specificity has not yet been clarified owing to its low clearance from white matter [108, 109].
It should be pointed out that activated microglia, which play a key role in the brain’s immune response to neuronal degeneration, are now also assessable by 11C-PK11195, a specific ligand for the peripheral benzodiazepine binding site, and PET. A study in patients with AD revealed increased 11C-PK11195 binding in the entorhinal, temporoparietal and cingulate cortices, suggesting that microglial activation is an early event in the pathogenesis of AD [110]. Although the role of inflammation, non-steroidal non-inflammatory agents and brain immunoreactivity in AD remains under discussion, the possibility of monitoring microglial activation during disease progression and experimental treatments could help clarify this issue of great pathological and therapeutic interest.
In summary, 11C-PIB, 18F-FDG, 11C-MP4A or 11C-PK11195 PET in conjunction with MRI provides an excellent means for multimodal non-invasive assessment of primary and secondary effects in AD pathogenesis which will improve diagnostic security, especially in the early diagnostic phase of disease, and which supply readouts for the potential effects of non-steroidal anti-inflammatory drugs or neutrotrophic factors delivered by gene therapy. Moreover, amyloid imaging in people from families with hereditary forms of AD, including those with chromosomal alterations (APP and PS1 mutations) and carriers of apoE4, is of the greatest interest [111].
Gene therapy
The fact that nerve growth factor (NGF) allows extended survival of basal forebrain cholinergic neurons [112] has led, during the past 20 years, to the experimental investigation of NGF therapy for rescue of degenerating cholinergic neurons arising in the basal forebrain [113–116]. However, in spite of NGF being able to promote cholinergic neuron survival, markers of cholinergic cortical innervation being reduced in AD, and inhibitors of ACHE showing some limited reversal of memory dysfunction in patients, the reasons for the decrease in markers of cholinergic function remain unknown. Cholinergic dysfunction could be a primary defect, or cholinergic lesions could be a secondary effect of primary degeneration of the neocortex. Importantly, lesions of the neocortex induce secondary changes in the basal forebrain, increasing further the difficulty in establishing the pathogenic importance of the basal forebrain cholinergic neurons. Understanding the disease pathophysiology will be important in determining the potential therapeutic benefit of cholinergic drugs in AD.
Intracerebroventricular infusion of recombinant NGF in patients with AD had serious side-effects, such as pain and weight loss [117], and therefore ex vivo NGF gene delivery approaches had to be developed. Transplantation of primary rat fibroblasts producing human NGF was first investigated in fimbria-fornix lesioned rats [115]. After safety and feasibility had been confirmed in primates [118–120], a phase I trial was initiated [121]. Eight patients with mild AD showed no adverse effects 22 months after transplantation of autologous fibroblasts obtained from skin biopsies, infected with an NGF-expressing MoMLV-derived retroviral vector and stereotactically implanted unilaterally (n=2) or bilaterally (n=6) into the nucleus basalis of Meynert. Clinical follow-up indicated an apparent slowing in the rate of cognitive decline, and 18F-FDG PET, which was performed in four patients, showed a significant increase in cortical glucose consumption after treatment. Another phase I trial has been initiated in patients with mild AD based on injection of AAV mediating NGF expression into the basal forebrain (http://www.gemcris.od.nih.gov/). However, the data remain to be replicated in larger double-blind controlled phase III/IV clinical trials in AD patients.
Other gene therapy strategies for AD include transduction of apoE2 [122], which prevents and reduces the Aβ burden and the subsequent development of neuritic plaques in AD mice, as well as active immunisation by vaccination with a plasmid that encodes Aβ42 [123] or by intranasal administration of replication-incompetent AdV carrying both Aβ and GM-CSF genes [124]. These alternative immunisation strategies are currently being developed since, while active immunisation with synthetic Aβ1–42 has been shown to be effective in mouse models, significantly reducing the Aβ burden with an accompanying improvement in cognitive performance, clinical trials had to be stopped after enrolment of about 300 patients owing to the development of T cell-mediated aseptic meningo-encephalitis in 6% of treated patients [125, 126]. Immunisation techniques are clearly double-edged swords. Essentially, in the trials that proceeded, patients were immunised with endogenous brain proteins. Immunisation against brain proteins, whether they are components of myelin or not, have been shown in the past to lead to autoimmune diseases. These experiments have shown that immunisation against Aβ amyloid is no exception. Although a number of strategies are now being tested in the hope that immunogens will be found that do not cause brain inflammation, it is difficult to predict whether this strategy will succeed clinically. The theory is that antibodies to Aβ amyloid, by acting either directly in the brain or systemically, will be able to reduce levels of Aβ and thus restore neuronal function. However, both T cells and antibodies have been shown to be able to mediate neuro-inflammatory disease. Thus, this will have to be tested thoroughly in experimental animals, and any ensuing clinical trials will require the utmost care. The potential to monitor microglial activation in vivo, before any clinical symptoms develop, may be considered a must if such clinical trials are to be allowed to proceed. Indeed, it is difficult to ensure that the experimental models being used are appropriate. Initial immunisations were tested in mouse models of AD. However, none of the mice developed significant brain inflammatory disease. The variation in human MHC haplotypes, compared with the homogeneous H2 of transgenic mice, could explain why inflammation was finally only detected in humans, although it would have been predictable based on our knowledge of how to induce brain inflammation by immunising against brain proteins.
More recently, neprilysin, a major extracellular enzyme that degrades Aβ, has been proposed as an alternative approach for gene therapy, and as such has been tested in a number of different experimental models [127, 128]. Also, IGF-1 has been shown to be effective in experimental models of neuronal degeneration, including mouse models of amyotrophic lateral sclerosis [129]. Even though questions remain on how IGF-1 may be delaying the death of affected mice in this model, the strong experimental results in a disease which is otherwise untreatable have accelerated the development of significant clinical trials. Should IGF-1 prove effective in protecting spinal cord motor neurons from progressive ALS, it may also be tried as a neuroprotectant in AD. Equally, siRNA has now been shown to be effective in reducing the overexpression of pathogenic proteins in experimental models of Huntington’s disease (e.g. huntingtin), dominant inherited ataxias and torsion dystonia [23–25, 130].
Imaging gene therapy
Further imaging methods in clinical protocols as described by Tuszynski et al. [121] should include assessment of the viability of fibroblast grafts as well as direct assessment of improved AChE activity by 11C-MP4A or 11C-PMP PET. Monitoring of microglial activation in some of the novel immunomodulatory strategies could also help prevent the development of untoward clinical responses, and possibly predict the development of inflammatory brain disease in advance of overt behavioural symptoms. Thus, it is envisaged that molecular imaging techniques will not only advance the clinical implementation of gene therapy, but also allow monitoring of aspects of potentially serious adverse side-effects in vivo in human patients, thus helping to make gene therapy safe and effective.
Parkinson’s disease
Definition
The second most common neurodegenerative disorder is Parkinson’s disease (PD), in which a progressive loss of dopaminergic neurons occurs in the substantia nigra and other brain stem nuclei. This neuronal loss is associated with the formation of intracellular Lewy inclusion bodies and leads to dopamine depletion from the striatum, with projections to the putamen being most affected. Later in the disease, other transmitter systems involving serotonergic cells in the median raphe, noradrenergic cells in the locus ceruleus and cholinergic cells in the nucleus basalis of Meynert also become involved in the neurodegenerative process. Therefore, patients with PD have not only the typical motor impairment with resting tremor, bradykinesia and rigidity but also balance problems, autonomic nervous dysfunction, and cognitive and psychiatric features.
The exact mechanisms of dopaminergic neuron degeneration are not fully understood. Genetic factors include mutations in the alpha-synuclein (PARK1) and parkin (PARK2) genes [131, 132]. Parkin functions as an E3 ubiquitin-protein ligase, and a loss of function results in the failure of intracellular protein processing with subsequent accumulation of various proteins to toxic levels [133]. Although sporadic and inherited forms of PD have different causes, they likely intersect in common pathways [134, 135]. The central cause of sporadic PD seems to be a mitochondrial complex I inhibition, and complex I deficiency may cause alpha-synuclein aggregation contributing to the degeneration of dopaminergic neurons [134, 135].
Imaging
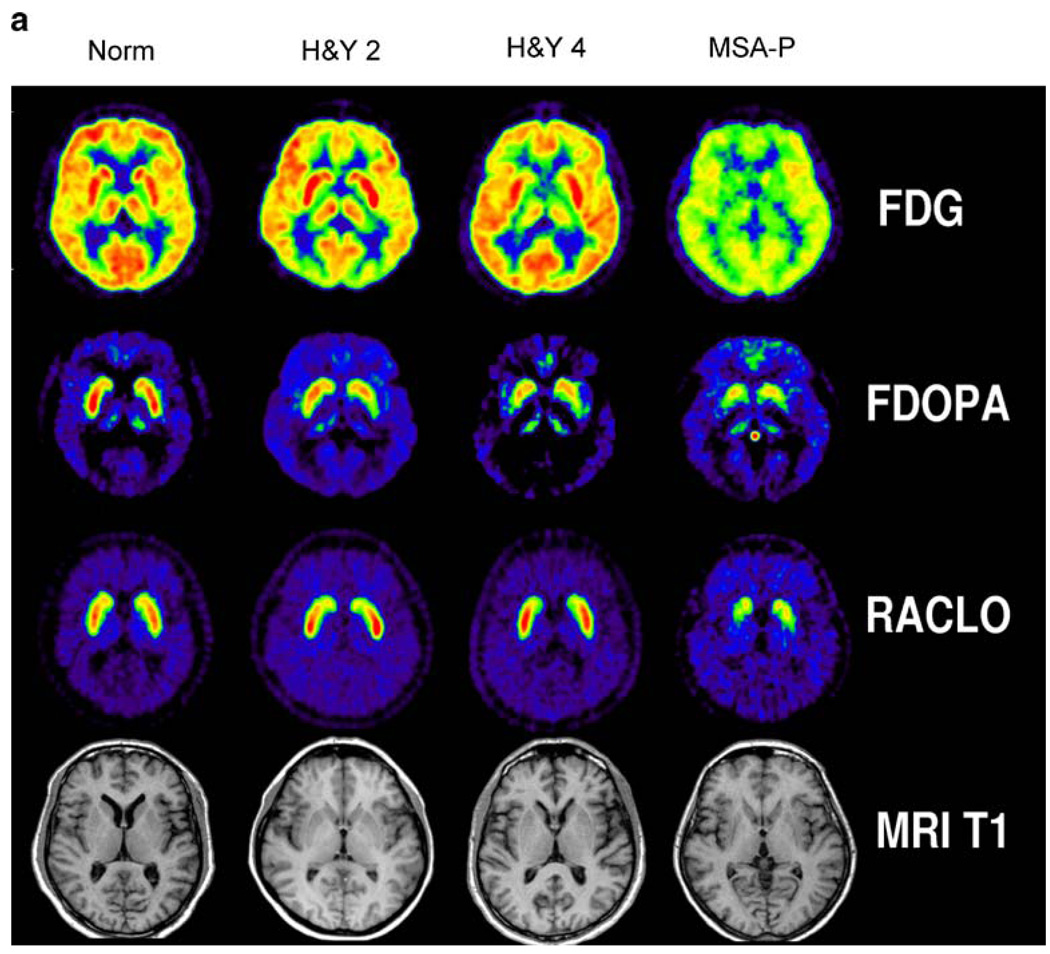
Historically, MRI did not show gross abnormalities in patients with PD whereas PET revealed specific changes in nigrostriatal function by assessing the expression of aromatic amino decarboxylase activity (AADC, the enzyme that converts l-dopa to dopamine) in nigrostriatal neurons and the postsynaptic density of dopamine D2 receptors. With the advent of high-field MRI and improved radiotracer technology, several new imaging results have been obtained in recent years for PD. MRI and even ultrasound may detect structural changes in the pars compacta of the substantia nigra in PD patients [136, 137] and within the putamen, pons, midbrain and cortex in patients with atypical parkinsonian syndromes [138, 139]. PET and SPECT are now able to assess a variety of pre- and postsynaptic processes that reflect the impaired function of the dopaminergic system in the striatum: (i) presynaptic AADC activity is being measured by 18F-6-fluorodopa (18F-DOPA); (ii) vesicle monoamine transporter (VMAT), an indicator of anatomical density of dopaminergic terminals, can be assessed by 11C-dihydro-tetrabenazine (11C-DHTBZ); (iii) expression and activity of presynaptic dopamine transporters are being assessed by 11C- or 18F-labelled 2β-carbomethoxy-3β-(4-fluorophenyl)tropane (11C-/18F-CFT) or 2β-carbomethoxy-3β-(4-123I-iodophenyl)tropane (123I-β-CIT); (iv) finally, expression and availability of postsynaptic D2 receptors are being detected by 11C-raclopride, 11C- or 18F-labelled spiperone and 123I-iodobenzamide (123I-IBZM). Complex methods in combination have also been used to measure dopamine turnover in vivo. Together with MRI and 18F-FDG PET, these tracers can be used to differentiate various stages of PD from multiple system atrophy and normal controls as depicted in Fig. 3. In the early stages, 18F-DOPA is reduced uni- or bilaterally in the posterior putamen, with relatively increased (upregulated) expression of D2 receptors and preserved glucose metabolism [2, 140]. In contrast, in multiple system atrophy, pre- and postsynaptic alterations in 18F-DOPA and 11C-raclopride are accompanied by a reduction in glucose metabolism, indicating pre- and postsynaptic neurodegeneration. In advanced PD, ventral and anterior putamen and dorsal caudate dopaminergic function is altered.
Fig. 3.
Typical features of multi-tracer PET for the differential diagnosis of movement disorders. In early PD (H&Y 2), glucose metabolism is preserved, striatal 18F-DOPA uptake is decreased in the posterior putamen contralateral to the affected side and post-synaptic D2 receptor expression is upregulated. In advanced PD (H&Y 4), both sides are affected, and decreased 18F-DOPA uptake is no longer confined to the posterior putamen. In multiple system atrophy (MSA-P), impaired glucose metabolism, impaired striatal 18F-DOPA uptake and reduced 11C-raclopride (RACLO) binding are suggestive of a generalised neurodegenerative process. MRI does not show gross abnormalities
Besides 18F-DOPA, presynaptic dopamine transport provides an additional measure for the integrity of the nigrostriatal terminals. With the development of 11C- and 18F-labelled CFT and 123I-labelled FP-CIT and altropane, various tropane-based tracers are available for both PET and SPECT imaging [141–143]. Interesting findings have been reported by Lee et al. [144], demonstrating that assessment of presynaptic dopaminergic nerve terminal function by a set of tracers reveals compensatory mechanisms at various stages of PD. They found that 18F-DOPA Ki was reduced less than the binding potential for 11C-DTBZ labelling VMAT2, which in turn was reduced less than the binding potential for 11C-methylphenidate labelling the plasma membrane dopamine transporter. These data indicate that in the striatum of patients with PD, the activity of aromatic amino acid decarboxylase is relatively up-regulated, whereas the plasma membrane dopamine transporter is down-regulated, resulting in an increased dopamine turnover and a decreased re-uptake to compensate for the synaptic dopamine deficiency [144]. Furthermore, the involvement of other transmitter systems and the activation of microglia in the pathogenesis of PD are currently under investigation [145, 146].
In summary, multitracer PET/(SPECT) imaging in conjunction with MRI is being used (i) in the differential diagnosis of PD [2]; (ii) for non-invasive phenotyping of genetic forms of PD [147, 148]; (iii) for the assessment of disease progression [149, 150] and of the effects of potential neuroprotective therapies [151, 152]; and (iv) for the establishment of new forms of therapy, including deep brain stimulation ([153, 154]) as well as gene and cell-based therapies. The value of radiotracer imaging in PD has been summarised in a recent consensus paper [155] and a recent review [156].
Gene therapy
Gene therapy for PD was first developed in rat models using transduction of a single gene encoding tyrosine hydroxylase (TH) [157, 158]. Limitations of this approach included the side-effects of the helper-dependent HSV-1 amplicon vector used at that time, limited long-term expression and expression of TH as the only gene. In the past 10 years, gene therapy approaches for PD have been further developed in three directions: (i) transduction of multiple genes essential in the production of dopamine and dopamine turnover in nigrostriatal nerve terminals, (ii) transduction of genes encoding growth and anti-apoptotic factors for the prevention of further degeneration of nigrostriatal neurons and (iii) vector and promoter systems which are non-toxic and support long-lasting gene expression.
Various studies from recent years have indicated that co-expression of multiple genes is required for the efficient production and release of dopamine in striatal nerve terminals: (i) TH converts tyrosine to l-dopa in the presence of tetrahydrobiopterin (BH4); (ii) GTP cyclohydroxylase I (GCHI) is the rate-limiting enzyme in the biosynthesis of BH4; (iii) aromatic amino decarboxylase (AADC) converts l-dopa to dopamine; (iv) vesicular monoamine transporter type 2 (VMAT-2) transports dopamine into synaptic vesicles. The following gene combinations have been successfully used in rat and primate models of PD using AAV, HSV-1 amplicon or lentiviral vectors: TH and GCHI [159, 160]; TH, GCHI and AADC [161–163]; and TH, GCHI, AADC and VMAT-2 [164]. The latter study demonstrated that transduction of all four genes in comparison to transduction without VMAT-2 supported (i) higher levels of correction of apomorphine-induced rotational behaviour, (ii) extracellular levels of dopamine and dihydroxyphenylacetic acid and (iii) significant K+-dependent release of dopamine [164]. The efficiency of this approach has yet to be confirmed in primate models.
Neuroprotective gene therapy strategies may be especially useful in early PD stages. It has been shown that transduction of glial cell-derived neurotrophic factor (GDNF [165]) and brain cell-derived neurotrophic factor (BDNF [166]), as well as other neurotrophic factors related to GDNF, such as neurturin, can protect nigrostriatal neurons from neurotoxic stress in rat and primate models of PD [167–171]. It should be pointed out that regeneration of nigrostriatal neurons reaching their target in the striatum was promoted only when vectors were injected into the striatum, leading to retrograde transport of GDNF into the substantia nigra. Injection of vectors directly into the substantia nigra promoted uncontrolled sprouting of axons within the vicinity of the substantia nigra, without them growing out to reach their target in the striatum [169]. Prior to potential clinical application, it will be necessary to resolve uncertain consequences of long-term growth factor expression, such as down-regulation of TH [172, 173], and questions regarding timing and regulation of therapy [174] in the disease course. Therefore, alternative growth factor-based gene therapies are being developed which are based on the neurotrophic and neuroprotective properties of Sonic Hedgehog, a secreted neurodifferentiation factor [38, 175]. Additional constraints are imposed by the experimental models, in which the survival or death of the nigrostriatal dopamine neurons depends on the very details used in lesioning the substantia nigra. Thus, while direct infusion of cytotoxins into the nigra or cutting of nigrostriatal axons will lead to the death of nigral neurons, other means of inducing nigral toxicity, such as 6-hydroxydopamine or even MPTP, produce only smaller lesions. That these may be mainly functional, and even reversible over time, further complicates not only the analysis of experimental outcomes, but also their applicability to human disease. Although evolutionary analysis is normally not included amongst the considerations relevant to the development of novel therapies, MPTP produces massive and irreversible lesions of the human but not the rodent substantia nigra, while the introduction of mutations into the mouse germline has induced limited pathological consequences compared with those detected in humans. Thus, it will be important that novel gene therapy strategies, almost unavoidably based on biased models, should include safety procedures for inhibiting gene expression over the long term in case the gene therapies have side-effects or prove inefficient. This raises further complexities, given that regulatory requirements are easier to fulfil, the simpler the novel genetic constructs are. Paradoxically, it thus becomes easier and cheaper for the biotech industry to obtain approval for clinical trials utilising less safe, unregulated genetic constructs!
Other paradigms of gene therapy for PD which are currently being tested in animals models include the transduction of dopaminergic neurons with JNK-interacting protein-1 (JIP-1 [176]), apoptosis protease activating factor-1 (APAF-1) dominant negative inhibitor [177], neuronal apoptosis inhibitor protein (NAIP [178]), Hsp70 [179] and Parkin [180, 181]. Such approaches have been reviewed elsewhere [182].
Another gene therapy paradigm is the conversion of excitatory to inhibitory output neurons arising from the subthalamic nucleus (STN) by transduction of excitatory glutamatergic neurons of the STN with glutamic acid decarboxylase (GAD), the enzyme that catalyses the synthesis of the neurotransmitter GABA [183]. The transduced neurons, when driven by electrical stimulation, produced mixed inhibitory responses associated with GABA release. This phenotypic shift resulted in strong neuroprotection of nigral dopamine neurons and rescue of the parkinsonian behavioural phenotype in rats. It is planned to apply this paradigm in a clinical trial [184]. The hypothesis is that, by AAV-mediated transduction of GAD, increased GABAwill suppress STN overactivity and simulate the good results obtained by deep brain stimulation (DBS). Patients to be included meet the criteria for STN DBS elective surgery. Twenty patients will receive DBS electrodes, and in addition they will be randomised into two groups, to receive either a solution containing rAAV-GAD or a solution which consists just of the vector vehicle. All patients will agree not to have the DBS activated until completion of the study. Patients will be assessed with a core clinical assessment program and in addition will undergo serial PET imaging. At the conclusion of the study, any patient with sufficient symptomatic improvement will be offered DBS removal. Any patients with no benefit will have their stimulators activated, which would normally be the appropriate therapy, requiring no additional operation. If any unforeseen symptoms occur from STN production of GABA, this might be controlled by blocking STN GABA release with DBS, or STN lesioning could be performed using the DBS electrode [184]. This is the first clinical gene therapy trial in humans suffering from PD.
Other clinical gene therapy trials for PD are based on the transduction of AADC and neurturin [185] using AAV vectors, where study specific information can be found under http://www.gemcris.od.nih.gov.
Imaging gene therapy
In a primate model of PD, Kordower et al. non-invasively assessed the therapeutic effect of lentiviral vector-mediated transduction of GDNF by measuring the improvement in endogenous enzymatic activity of AADC by 18F-DOPA PET [170]. The improvement in lenti-GDNF-mediated nigrostriatal function as measured by 18F-DOPA PET in vivo was correlated with independent measures in vivo, such as functional improvements of motor tasks, as well as with positive GDNF expression and an increased number of tyrosine hydroxylase-expressing nigrostriatal neurons (as assessed post mortem). This study indicated the relevance of imaging of an endogenous effector gene (AADC) in vivo for the successful implementation and assessment of this gene therapy paradigm. In a similar approach, efficient AAV vector-mediated transfer of the AADC gene directly has been achieved after convection-enhanced delivery in a non-human primate model of PD [186]. The efficiency of gene transfer was assessed by PET and co-registration of histology/immunohistochemistry. Evaluation of successful gene therapy in clinical applications will require a full set of image parameters assessing pre- and postsynaptic nigrostriatal function before and after intervention and at long-term follow-up.
Gliomas
Definition
Gliomas are the most common primary intracranial neoplasms and are divided into astrocytomas, oligodendrogliomas, oligo-astrocytomas and glioblastomas. The World Health Organisation (WHO) grading of gliomas takes into account the presence of nuclear changes, mitotic activity, presence of endothelial proliferation and necrosis [187]. Glioblastoma is the most fatal and most common primary brain neoplasm, with an incidence of 3–6 in 100,000. Together with all intracranial neoplasms, glioblastoma is the second most common cause of death due to an intracranial disease after stroke. A complex series of molecular changes leads to glioma development, which results in deregulation of the cell cycle, alterations of apoptosis and cell differentiation, neovascularisation and tumour cell migration and invasion. Genetic alterations include a loss or mutation of tumour suppressor genes and other genes involved in the regulation of the cell cycle as well as activation or amplification of growth factors and/or their receptors. During progression from low-grade astrocytoma (WHO grade II) to anaplastic astrocytoma (WHO grade III) and to glioblastoma multiforme (GBM; WHO grade IV), a stepwise accumulation of these genetic alterations occurs. Most importantly, molecular alterations have been identified which indicate therapeutic response of patients and, thus, are prognostically relevant: anaplastic oligodendrogliomas with LOH 1p and/or LOH 19q are characteristically sensitive to PCV chemotherapy, and patients’ survival is significantly prolonged [188].
Imaging
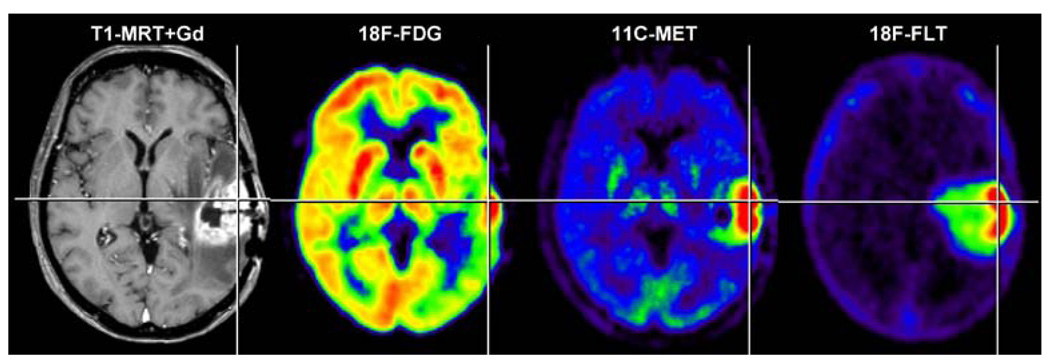
As reviewed recently [189], MRI and PET are highly important in the diagnosis and management of gliomas. Combined imaging by MRI and PET has several aims. The most important parameters of interest are: (i) before therapy: assessment of localisation, biological activity and relation to functionally important neuronal tissue; (ii) after therapy: assessment of the effects of therapy, differentiation of recurrent tumour from radiation necrosis and determination of tumour progression. MRI and PET are therefore instrumental in both the establishment of the diagnosis and the assessment of treatment response. Figure 4 summarises the most important variables which can be non-invasively assessed by MRI and PET in patients with a suspected glioma.
Fig. 4.
Parameters of interest in the non-invasive diagnosis of brain tumours. Alteration of the blood-brain barrier and the extent of peritumoural oedema are detected by MRI. Signs of increased cell proliferation can be observed by means of multitracer PET imaging using 18F-FDG, 11C-MET and 18F-FLT as specific tracers for glucose consumption, amino acid transport and DNA synthesis, respectively. Secondary phenomena, such as inactivation of ipsilat-eral cortical cerebral glucose metabolism, may be observed (18F-FDG) and are of prognostic relevance. Gd gadolinium. (With permission from [257])
Depending on the radiotracer used, various processes can be visualised by PET, most of them relating to increased cell proliferation within gliomas. Radiolabelled 2-18F-fluoro-2-deoxy-d-glucose (18F-FDG), methyl-11C-l-methionine (11C-MET) and 3′-deoxy-3′-18F-fluoro-thymidine (18F-FLT) are the most commonly used radiotracers. They are incorporated into proliferating gliomas depending on their tumour grade, as a reflection of the increased activity of membrane transporters for amino acids (11C-MET) and nucleosides (18F-FLT) as well as the increased expression of cellular hexokinase (18F-FDG) and thymidine kinase (18F-FLT). 18F-FDG monitors the rate of glucose uptake and has been used to detect the metabolic differences between normal brain tissue, low-grade and high-grade gliomas, and radionecrosis. Intratumoural glucose consumption correlates with tumour grade, cell density, biological aggressiveness and patient survival/prognosis. Moreover, 18F-FDG PET is able to guide stereotactic tumour biopsy to identify the most malignant tumour parts, and malignant progression of low-grade gliomas is indicated by newly appearing hypermetabolism. 11C-MET has been shown to be a more specific tracer in tumour detection, delineation and staging owing to its relatively low uptake in normal brain (Fig. 4). The increased 11C-MET uptake is related to increased transport mediated by type L amino acid transporters located at the blood-brain barrier. 11C-MET uptake correlates with cell proliferation in vitro, Ki-67 expression, as well as proliferating cell nuclear antigen expression and microvessel density, indicating its role as a marker for active tumour proliferation. 11C- and 18F-labelled thymidine compounds have been radiosynthesised for non-invasive assessment of tumour proliferation and early response to chemotherapy. Relative 18F-FLT uptake within gliomas is greater than relative 11C-MET uptake owing to very low background activity; however, the sensitivity of 18F-FLT in detecting low-grade gliomas is lower than that of 11C-MET (for details and literature see [189]).
Gene therapy
Originally, the concept of gene therapy for glioblastoma was to transplant fibroblasts genetically engineered to secrete retroviral vectors carrying a pro-drug activating enzyme gene [190]. As retroviruses would only be able to integrate their genetic material into dividing cells, this concept seemed to be safe for the selective transduction of highly proliferating tumour cells. However, clinical application of this approach did not show a clinical benefit for patients [191–193]. The most important limitation of gene therapy for glioblastoma is the hetereogeneity of the tumour tissue, with highly proliferative tumour areas alongside areas of necrosis and non-dividing tumour cells migrating into the surrounding oedema. Therefore, the further development of gene therapy for glioblastoma will need to concentrate on: (i) the combination of different therapeutic genes to achieve a synergistic action; (ii) combination of viral therapy with gene and immunotherapy; (iii) improved methods of vector application based on convection-enhanced delivery; and (iv) imaging-based control of vector application and therapy read-out [1, 194]. The various gene therapeutic strategies which have been studied to treat glioma models have been reviewed recently [8, 26, 195] and include:
Replicating viruses based on replication-conditional HSV-1 and AdV vectors
Prodrug activating enzymes (e.g. thymidine kinase, cytosine deaminase from various bacterial strains, guanine phosphoribosyl transferase, cytochrome P450, deoxycytidine kinase, folylpolyglutamyl synthethase, carboxylesterase)
Cell cycle-regulating proteins (e.g. p53, p16, p21, PTEN, Rb, p300)
Pro-apoptotic genes [caspases, Bax, Fas ligand (FasL)]
Factors inhibiting angiogenesis (endostatin, angiostatin, antisense VEGF, dominant negative VEGF receptors, antisense EGF, dominant negative EGF receptors, antisense basic FGF and IGF1)
Immunomodulation (e.g. IL2, IL4, IL6, IL12, IL13, GM-CSF, TNF-α, interferon-γ, antisense TGF-β, TGF-β soluble receptors, Flt3L)
Some of these systems are currently being evaluated in clinical gene therapy protocols (http://www.gemcris.od.nih.gov) to investigate the safety and efficiency of:
-
Replicating viruses
-
Pro-drug therapy
- HSV-1 thymidine kinase (tk) gene mediated by an AdV vector (H5.020RSVTK) with subsequent ganciclovir or valaciclovir
- HSV-1-tk gene mediated by producer cells (PA317/G1TkSvNa.7) which are stereotactically implanted with subsequent ganciclovir treatment
-
Cell cycle regulation
- p53 gene mediated by a recombinant AdV vector
-
Anti-angiogenesis
- Episome-based antisense cDNA transcription of insulin-like growth factor I
-
Immunomodulation
- IL-4 expressed from autologous tumour
- Irradiated TGF-β2 antisense gene modified autologous tumour cells
- Human interferon-beta (H5.010CMVhIFN-β) mediated by AdV or cationic liposomes [198, 199]
- Allogeneic glioblastoma tumour cell lines (IR850) mixed with allogeneic fibroblasts genetically modified to secrete GM-CSF (IR851)
- Irradiated autologous glioma and dendritic cells admixed with IL-4
- Autologous CD8+ T cells expressing IL13-zetakine and HyTK
- Expanded autologous bone marrow-derived stromal cells expressing IL-12
-
Stem cells
Correction of genetic defects in GBMs
Although the genetic alterations that give rise to a cancer cell are numerous, there are some frequently occurring gene mutations in GBMs. Perhaps, the most common genetic alterations are in the p53 tumour suppressor gene and the EGF receptor, which are mutated in a large percentage of the human tumours. Tumour suppressor genes encode for proteins that suppress cell division; therefore, by introducing an unmutated tumour suppressor gene into cancer cells, apoptosis can be induced and tumour growth inhibited.
Inactivation of p53 occurs early in glial tumorigenesis, and replacement of the mutated p53 gene has been described for many tumour models. Adenoviral delivery of wild-type p53 into glioblastoma cell lines has been attempted, and p53 inhibited proliferation and mutant p53 induced apoptotic cell death. Mutations of the retinoblas-toma (Rb) gene and/or p16 gene are also common. Restoration of p16 expression in p16-deficient glioma cell lines, D-54 M, U-251 MG and U-87 MG, produced growth arrest. In addition, abnormalities in the apoptotic cascade are almost always present in GBMs, and activation of apopotosis-promoting pathways, such as the Fas-FasL system, has been used to induce glioma cell killing.
Inhibiting blood vessel formation
Important and highly active angiogenic factors were initially discovered through tumour resection-induced growth of distant tumour sites. Angiostatin, derived from plasminogen, and endostatin, have been utilised in models of GBM. Growth inhibition was observed and encouraged clinical trials. However, the turnover time of these small peptides is very fast and it has been difficult to deliver enough of these proteins over long-term periods in humans; therefore, long-term, high-level delivery using gene therapy vectors has been proposed. Despite encouraging results from gene therapy, trials of these approaches in human tumours have yet to proceed.
Activation of the immune response
Although it is difficult, if not impossible, to stimulate an immune response from the CNS itself, once an immune response has been stimulated, systemically activated T lymphocytes have relatively few limitations in finding and destroying tumours located within the brain. The blood-brain barrier, the absence of classical lymphatic drainage from the CNS, and the lack of dendritic cells (DCs) in the naive CNS are barriers to the priming, but not to the effector phase of the immune response. Tumour cells can indeed be targets of the immune system. The appearance of progressive multifocal leukoencephalopathy in human patients suffering from multiple sclerosis and Crohn’s disease has demonstrated that the immune system is constantly monitoring the endogenous brain milieu. Therefore, stimulation of the brain immune response is being attempted through systemic vaccination, systemic stimulation of the immune system or even direct intracranial injection of DCs. A particularly promising approach is stimulation of the immune system from within the brain proper. Ali et al. have recently demonstrated that direct injection of Flt3L (a powerful differentiator of DCs) into the brain, in combination with HSV-1-TK, is able to eliminate rather large brain tumours from the CNS of rodents [31, 200].
Enhancement of the immune response using cytokines
The use of cytokines, such as TNF-α, has been attempted, but without encouraging results regarding either preclinical or clinical outcomes. Whether TNF-α might be beneficial in conjunction with other tumour-killing strategies remains to be explored. IL-4 has also been used for glioma treatment due to its induction of a very strong immune response in the brain parenchyma and its antiproliferative effects on glioma cells. Again, combination therapies may be more effective, although trials of IL-4 in experimental models have shown it to be effective; IL-2, IL-12 and IFN-γ have also been tested as potential adjuvants for the treatment of brain tumours. More interesting are the novel approaches in which IL-4 has been expressed fused to the translocation and enzymatic domains of Pseudomonas exotoxin. This has now been tested in a number of clinical trials, and has been claimed to demonstrate rather positive effects in humans. This is now leading to larger clinical trials, and the results thereof are eagerly awaited, as they are the only ones that may indicate the real long-term value of these novel experimental approaches. IFN-β, on the other hand, has now been tested on its own in a number of clinical trials. However, lack of miraculous results has impeded the progression of these therapies into the phase III trials that are needed to demonstrate large-scale effects of novel approaches.
Manipulation of DCs as a tool for brain cancer immunotherapy
An important approach is to increase the number and activity of primary antigen-presenting cells, e.g. DCs. GM-CSF and Flt3 ligand differentiate haematopoietic precursors into DCs and attract them to the tumour site, also activating the DCs to process the tumour antigens, migrate to the local lymph nodes and present antigen to naive T lymphocytes to induce anti-tumour effects.
Antigen-pulsed autologous DCs are also being used to stimulate anti-tumour cytotoxic T cell responses, an approach successfully used experimentally and also in phase I/II human trials.
Given our still poor understanding of the brain’s immune system, the challenge facing immune stimulation against brain tumours is whether clinically effective responses can be induced in the absence of brain autoimmune disease [211]. Importantly, induction of immune responses against melanomas, even in cases causing vitiligo, has never induced brain disease. Vitiligo destroys skin melanocytes that contain melanin, a protein that is also expressed within the nigrostriatal human neurons. On the other hand, non-small cell lung cancer that develops anti-tumour immune responses is frequently accompanied by immune-mediated cerebellar degeneration, evidenced clinically as a paraneoplastic disorder.
Various trials of DC vaccination, in which gene therapy is being used to deliver cytokine genes or antigens, are currently progressing at various centres around the world. A recent resurgence of interest in regulatory T cells that are potentially inhibitory to anti-tumour immune responses is also forcing a rethink on how to prime the subtype of DC that will stimulate cytotoxic T cells without the simultaneous induction of T regulatory cells.
Enzyme/prodrug combinations: HSV-1-TK/ganciclovir
This constitutes the most classical gene therapy approach for GBMs, the thymidine kinase from HSV-1 having been employed both experimentally and clinically. Upon administration of ganciclovir (GCV), phosphorylated GCV is the toxic final product that mainly kills dividing cells—hence its great interest for the treatment of brain tumours.
The HSV-1-TK/GCV system displays important bystander effects that are thought to amplify its cytotoxic activity. The bystander effect has various components that either stimulate the anti-tumour immune response or need cell-to-cell contacts to transfer GCV-triphosphate to un-transduced cells.
HSV-1-TK has been delivered using cationic liposomes, herpes simplex virus (HSV), adenovirus, retroviral vectors and even replicating vectors. When HSV-1-TK is delivered using replicating HSV vectors, GCV itself inhibits the propagation of the vector. This has allowed replicative virus to be used, with the aim of increasing the percentage of tumour cells which can be transduced, using GCV as a useful safety mechanism to prevent uncontrollable viral spread.
As reviewed previously [194], exciting preclinical data for HSV-1-TK/GCV resulted in various phase I–II clinical trials of HSV-1-tk gene therapy mediated by retrovirus in patients with recurrent glioblastoma. Retrovirally transduced fibroblasts expressing TK were administered by intracerebral injection immediately after surgical tumour resection. The data were suggestive, but these were small, uncontrolled trials and statistical significance could therefore not be proven.
The preliminary data highlighted that gene transfer was low and, therefore, that insufficient numbers of tumour cells would have been transduced. Nevertheless, pharmaceutical interests led to the performance of a large multicentre phase III trial with hundreds of patients. Although substantial funds were devoted to this trial, patients on the gene therapy arm were faring worse than those on the control arm, and the trial had to be stopped. The lessons to be extracted from this trial, both for the ethics of clinical trials of novel complex technologies and for the role of the biotechnological industry in advancing such techniques, remain to be fully explored. The failure of the large phase III trial has now consigned the use of retroviral vectors expressing TK to the archives of medical experimental memory. In doing so, an otherwise powerful experimental approach, namely HSV-1-TK itself, has been compromised. This underlines the necessity of including molecular imaging technology in gene therapy approaches, thereby permitting non-invasive assessment of the transduction efficiency of a certain vector and application system and avoiding performance of large phase III trials for gene therapy protocols which demonstrate only limited efficiency in a small patient group [1].
An intelligent alternative to these approaches has been provided by Seppo Yla-Herttuala, who compared side by side the effectiveness of TK when expressed from retroviral vectors or first-generation AdV vectors. This author and co-workers were able to demonstrate in controlled trials that the AdV vectors were more effective than the retroviral vectors [33, 212, 213]. This research has now been expanded and a phase III trial is in the final planning and implementation stages. Larger phase II trials have demonstrated a statistically significant effect of AdV expressing TK plus GCV in patients suffering from GBM. Other alternative conditional cytotoxic approaches, such as cytosine deaminase and 5-fluorouracil, and carboxypeptidase G2 and pro-alkylating agents are also being developed for the treatment of gliomas.
Imaging gene therapy
Imaging-guided follow-up of gene therapy depends on the use of MRI and PET imaging markers for endogenous gene expression, as described previously [189, 214], and on the assessment of vector-mediated (exogenous) gene expression. Non-invasive monitoring and localisation of exogenous genes by PET and MRI relies on the transduction of “marker genes” encoding enzymes [215–217] or receptors [218, 219], leading to regional accumulation of trapped radiolabelled or paramagnetic “marker substrates” or receptor binding compounds which can be detected by PET or MRI.
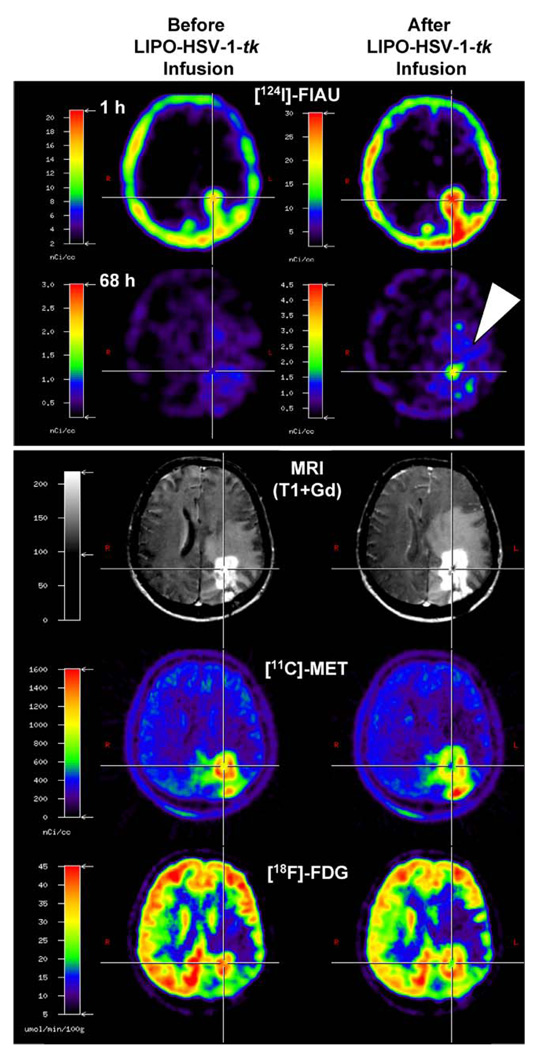
Radiolabelled 2′-fluoro-2′-deoxy-1-β-d-arabinofuranosyl-5-124I-iodo-uracil (124I-FIAU) as well as other specific substrates for HSV-1-TK and PET have been successfully used for the non-invasive PET monitoring of retroviral, adenoviral and herpes viral vector-mediated HSV-1-tk gene expression in various experimental rodent models (reviewed by [214, 220–223]. This method has been used in a phase I HSV-1-tk gene therapy trial investigating the safety and potential therapeutic action of intratumourally infused liposome-plasmid DNA complex followed by GCV administration [1, 224]. After identification of biologically active target tissue by multitracer PET, one or two catheters were stereotactically placed within the tumour and subcutaneously connected to a port. This allowed targeted intratumoural infusion of the cationic liposomal vector [225] containing an HSV-1-tk carrying plasmid. A dynamic 124I-FIAU PET series acquired over 3 days was performed before gene transduction to evaluate the basal state of 124I-FIAU accumulation in and washout from the tumour, and an identical 124I-FIAU PET series was performed after vector application to dynamically investigate whether specific 124I-FIAU accumulation did occur. GCV treatment was carried out starting 4 days after vector infusion. Identification of target tissue before catheter placement and treatment response were recorded by repeated MRI as well as 18F-FDG and 11C-MET PET (Fig. 5). After vector administration, in one out of eight patients, specific 124I-FIAU-derived radioactivity was observed within the vector-infused tumour (Fig. 5). Specific radioactivity was localised at the infusion site within the centre of the tumour and demonstrated a significant increase in the accumulation rate, Ki, of 124I-FIAU, from 0.000047 at baseline to 0.000096 ml/min/g after vector administration (two-compartment model), and an increase in tissue/plasma radioactivity ratios over time (Patlak analysis)—both of these parameters being indicators for specific radiotracer trapping and, hence, HSV-1-tk expression. After GCV treatment, 18F-FDG and 11C-MET PET demonstrated signs of necrosis within the volume of specific 124I-FIAU trapping, this being indicative of the HSV-1-TK-mediated therapeutic response. The overall therapeutic effect was apparently limited to a portion of the tumour. In the seven other patients, no specific FIAU accumulation was observed. In contrast to the patient with specific 124I-FIAU accumulation, histology in these patients exhibited a significantly lower number of proliferating tumour cells per voxel; this indicates that the threshold of PET detection is set by a certain critical number of tk gene-transduced tumour cells per voxel, so that tk gene-related accumulation of 124I-FIAU can be measured and detected by PET.
Fig. 5.
Multimodal imaging for the establishment of imaging-guided experimental treatment strategies. Co-registration of 124I-FIAU, 11C-MET and 18F-FDG PET and MRI before (left column) and after (right column) targeted application (stereotactic infusion) of a gene therapy vector. The region of specific 124I-FIAU retention (68 h) within the tumour after LIPO-HSV-1-tk transduction (white arrowhead) resembles the proposed “tissue dose” of vector-mediated gene expression and shows the signs of necrosis (cross in right column; reduced methionine uptake and glucose metabolism) after GCV treatment. Only such multimodal and multitracer imaging modalities will help us to establish the clinical application of new treatment modalities. (With permission from [1])
Current research on imaging HSV-1-tk gene expression is focussing on: (i) methods for exact quantification of HSV-1-TK expression [226], (ii) improved HSV-1-TK probes for PET [227–229] and SPECT [230], (iii) translation of this imaging paradigm into other vector systems [231], (iv) imaging follow-up in cancer therapy strategies [232–234], (v) imaging transcriptional activation [235–237] and (vi) multimodal imaging by fusing the HSV-1-tk gene with further imaging genes [238–242].
Issues to be resolved in the future
The following issues remain to be addressed with regard to imaging:
Development of improved markers which characterise the disease-specific molecular events in vivo; here the most important challenge is to overcome the intact blood-brain barrier.
Comparison of disease-specific molecular imaging markers in animal models for neurological diseases and in corresponding patients.
Development of lipophilic marker substrates which pass the blood-brain barrier to allow HSV-1-TK imaging in the intact brain.
Alternatively, development of new marker gene/marker substrate combinations [243].
The following issues remain to be addressed in the future with regard to vectors and gene therapy:
-
5
Innate inflammatory and adaptive immune responses to viral vectors in the CNS [16, 55, 211].
-
6
The potential immunogenicity of transgenes and the induction of tolerance in gene therapy [244–248].
-
7
Classification of patients according to unbiased molecular markers of disease when performing clinical trials for GBMs. For example, new microarray studies of GBMs show that there are two populations of patients, one with a rapid ‘typical’ progression and a second long-term survivor group that survives for up to 3 years; the impossibility of differentiating tumours from either group by any other means indicates that tests like these need to be used in the choice of patients for clinical trials [249–251].
-
8
Classification of patients according to biased molecular markers of disease when performing clinical trials for GBMs. For example, new drugs acting on selective signal transduction pathways will require that only patients with alterations in particular pathways are entered into trials testing such new drugs; this will increase the rate of success for the right patients and eliminate useless trials for others [250–254].
-
9
Establishment of proper baseline studies of the natural history of the progression of neurodegenerative diseases [255]: if subgroups of patients progress with different time courses, experimental therapies will provide mixed and unclear results.
-
10
Accept the potential short-term use of immunosuppressants when administering gene therapy to the brain [256].
Frequently asked questions
-
Which molecular imaging technology should I use?
There is no “best” imaging technology. Each of the imaging technologies has certain advantages and disadvantages. In forthcoming years, imaging platforms will be created where all of the imaging technology is available to combine the advantages of each technology (e.g. high spatial resolution for MRI; high sensitivity for PET and optical imaging; direct link to the knowledge of cell biology for optical imaging) for the purpose of multimodal imaging assessment.
-
Will we perform molecular imaging in every patient?
It is not possible to conduct imaging in each individual patient. Multimodal molecular imaging will be performed in the transition from experimental to clinical application. It ideally should be included in phase I/II clinical trials to acquire additional information on the safety and efficiency of a new diagnostic or therapeutic paradigm. In phase III trials only subsets of patients will be able to receive imaging, and once efficiency is established, imaging should not have to be performed on a routine basis. Ideally, it will be possible to correlate disease-specific imaging parameters with soluble biomarkers.
-
Which is the best vector?
There is no ‘best’ vector. There are only very good vectors, and vectors that are either suitable or unsuitable for the questions being addressed.
-
Which is the best transgene to use?
Again, there are no ‘best’ transgenes. There are good transgenes, and the most important issue remains in which model to test them.
-
I was sent a vector and I only see toxicity in the brain: What should I do?
Send the virus back. Ask your collaborators to prepare a novel clean preparation and to provide you with the quality control, such as endotoxin content and presence of replication competent vector and other contaminants.
References
- 1.Jacobs A, Voges J, Reszka R, Lercher M, Gossmann A, Kracht L, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358(9283):727–729. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- 2.Ghaemi M, Hilker R, Rudolf J, Sobesky J, Heiss WD. Differentiating multiple system atrophy from Parkinson’s disease: contribution of striatal and midbrain MRI volumetry and multi-tracer PET imaging. J Neurol Neurosurg Psychiatry. 2002;73(5):517–523. doi: 10.1136/jnnp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heiss WD. Best measure of ischemic penumbra: positron emission tomography. Stroke. 2003;34(10):2534–2535. doi: 10.1161/01.STR.0000092396.70827.28. [DOI] [PubMed] [Google Scholar]
- 4.Hilker R, Klein C, Hedrich K, Ozelius LJ, Vieregge P, Herholz K, et al. The striatal dopaminergic deficit is dependent on the number of mutant alleles in a family with mutations in the parkin gene: evidence for enzymatic parkin function in humans. Neurosci Lett. 2002;323(1):50–54. doi: 10.1016/s0304-3940(01)02529-0. [DOI] [PubMed] [Google Scholar]
- 5.Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C] l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10(21):7163–7170. doi: 10.1158/1078-0432.CCR-04-0262. [DOI] [PubMed] [Google Scholar]
- 6.Sobesky J, Zaro WO, Lehnhardt FG, Hesselmann V, Neveling M, Jacobs A, et al. Does the mismatch match the penumbra? Magnetic resonance imaging and positron emission tomography in early ischemic stroke. Stroke. 2005;36(5):980–985. doi: 10.1161/01.STR.0000160751.79241.a3. [DOI] [PubMed] [Google Scholar]
- 7.Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4(5):353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 8.Castro MG, Cowen R, Williamson IK, David A, Jimenez-Dalmaroni MJ, Yuan X, et al. Current and future strategies for the treatment of malignant brain tumors. Pharmacol Ther. 2003;98(1):71–108. doi: 10.1016/s0163-7258(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 9.Azzouz M, Kingsman SM, Mazarakis ND. Lentiviral vectors for treating and modeling human CNS disorders. J Gene Med. 2004;6(9):951–962. doi: 10.1002/jgm.600. [DOI] [PubMed] [Google Scholar]
- 10.Castro MG, David A, Hurtado-Lorenzo A, Suwelack D, Millan E, Verakis T, et al. Gene therapy for Parkinson’s disease: recent achievements and remaining challenges. Histol Histopathol. 2001;16(4):1225–1238. doi: 10.14670/HH-16.1225. [DOI] [PubMed] [Google Scholar]
- 11.Davidson BL, Paulson HL. Molecular medicine for the brain: silencing of disease genes with RNA interference. Lancet Neurol. 2004;3(3):145–149. doi: 10.1016/S1474-4422(04)00678-7. [DOI] [PubMed] [Google Scholar]
- 12.Fountaine TM, Wood MJ, Wade-Martins R. Delivering RNA interference to the mammalian brain. Curr Gene Ther. 2005;5(4):399–410. doi: 10.2174/1566523054546206. [DOI] [PubMed] [Google Scholar]
- 13.Frampton AR, Jr, Goins WF, Nakano K, Burton EA, Glorioso JC. HSV trafficking and development of gene therapy vectors with applications in the nervous system. Gene Ther. 2005;12(11):891–901. doi: 10.1038/sj.gt.3302545. [DOI] [PubMed] [Google Scholar]
- 14.Goverdhana S, Puntel M, Xiong W, Zirger JM, Barcia C, Curtin JF, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2005;12(2):189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowenstein PR, Suwelack D, Hu J, Yuan X, Jimenez-Dalmaroni M, Goverdhana S, et al. Nonneurotropic adenovirus: a vector for gene transfer to the brain and gene therapy of neurological disorders. Int Rev Neurobiol. 2003;55:3–64. doi: 10.1016/s0074-7742(03)01001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowenstein PR, Castro MG. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10(11):946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein PR, Castro MG. Recent advances in the pharmacology of neurological gene therapy. Curr Opin Pharmacol. 2004;4(1):91–97. doi: 10.1016/j.coph.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandel RJ, Burger C. Clinical trials in neurological disorders using AAV vectors: promises and challenges. Curr Opin Mol Ther. 2004;6(5):482–490. [PubMed] [Google Scholar]
- 19.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 20.Ralph GS, Mazarakis ND, Azzouz M. Therapeutic gene silencing in neurological disorders, using interfering RNA. J Mol Med. 2005;83(6):413–419. doi: 10.1007/s00109-005-0649-1. [DOI] [PubMed] [Google Scholar]
- 21.Deglon N, Hantraye P. Viral vectors as tools to model and treat neurodegenerative disorders. J Gene Med. 2005;7(5):530–539. doi: 10.1002/jgm.707. [DOI] [PubMed] [Google Scholar]
- 22.Kirik D, Bjorklund A. Modeling CNS neurodegeneration by overexpression of disease-causing proteins using viral vectors. Trends Neurosci. 2003;26(7):386–392. doi: 10.1016/S0166-2236(03)00164-4. [DOI] [PubMed] [Google Scholar]
- 23.Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102(16):5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20(10):1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 25.Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10(8):816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs A, Fraefel C, Breakefield XO. HSV-1 based vectors for gene therapy of neurological diseases and brain tumors: Part II: Vector systems and applications. Neoplasia. 1999;1(5):402–416. doi: 10.1038/sj.neo.7900056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs A, Fraefel C, Breakefield XO. HSV-1 based vectors for gene therapy of neurological diseases and brain tumors: Part I: HSV-1 structure, replication and pathogenesis. Neoplasia. 1999;1(5):387–401. doi: 10.1038/sj.neo.7900055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 29.Harsh GR, Deisboeck TS, Louis DN, Hilton J, Colvin M, Silver JS, et al. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: a gene-marking and neuropathological study. J Neurosurg. 2000;92(5):804–811. doi: 10.3171/jns.2000.92.5.0804. [DOI] [PubMed] [Google Scholar]
- 30.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multiinstitutional trial of injection with an E1B–attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulci G, Chiocca EA. Oncolytic viruses for the therapy of brain tumors and other solid malignancies: a review. Front Biosci. 2003;8:e346–e360. doi: 10.2741/976. [DOI] [PubMed] [Google Scholar]
- 33.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10(5):967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs A, Tjuvajev JG, Dubrovin M, Akhurst T, Balatoni J, Beattie B, et al. Positron emission tomography-based imaging of transgene expression mediated by replication-conditional, oncolytic herpes simplex virus type 1 mutant vectors in vivo. Cancer Res. 2001;61(7):2983–2995. [PubMed] [Google Scholar]
- 35.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 36.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 37.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 38.Suwelack D, Hurtado-Lorenzo A, Millan E, Gonzalez-Nicolini V, Wawrowsky K, Lowenstein PR, et al. Neuronal expression of the transcription factor Gli1 using the Talpha1 alpha-tubulin promoter is neuroprotective in an experimental model of Parkinson’s disease. Gene Ther. 2004;11(24):1742–1752. doi: 10.1038/sj.gt.3302377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oehmig A, Fraefel C, Breakefield XO, Ackermann M. Herpes simplex virus type 1 amplicons and their hybrid virus partners, EBV, AAV, and retrovirus. Curr Gene Ther. 2004;4(4):385–408. doi: 10.2174/1566523043346129. [DOI] [PubMed] [Google Scholar]
- 40.Zaupa C, Revol-Guyot V, Epstein AL. Improved packaging system for generation of high-level noncytotoxic HSV-1 amplicon vectors using Cre-loxP site-specific recombination to delete the packaging signals of defective helper genomes. Hum Gene Ther. 2003;14(11):1049–1063. doi: 10.1089/104303403322124774. [DOI] [PubMed] [Google Scholar]
- 41.Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16(6):741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartigan-O’Connor D, Barjot C, Salvatori G, Chamberlain JS. Generation and growth of gutted adenoviral vectors. Methods Enzymol. 2002;346:224–246. doi: 10.1016/s0076-6879(02)46058-2. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Z, Schiedner G, Gilchrist SC, Kochanek S, Clemens PR. CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Ther. 2004;11(19):1453–1461. doi: 10.1038/sj.gt.3302315. [DOI] [PubMed] [Google Scholar]
- 44.Morral N, O’Neal W, Rice K, Leland M, Kaplan J, Piedra PA, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci U S A. 1999;96(22):12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q, et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A. 1998;95(14):7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8(5):846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Palmer DJ, Ng P. Physical and infectious titers of helper-dependent adenoviral vectors: a method of direct comparison to the adenovirus reference material. Mol Ther. 2004;10(4):792–798. doi: 10.1016/j.ymthe.2004.06.1013. [DOI] [PubMed] [Google Scholar]
- 48.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93(24):13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78(12):6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanftner LM, Suzuki BM, Doroudchi MM, Feng L, McClelland A, Forsayeth JR, et al. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther. 2004;9(3):403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Schiedner G, Morral N, Parks RJ, Wu Y, Koopmans SC, Langston C, et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet. 1998;18(2):180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 52.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12(7):839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 53.Reszka RC, Jacobs A, Voges J. Liposome-mediated suicide gene therapy in humans. Methods Enzymol. 2005;391:200–208. doi: 10.1016/S0076-6879(05)91012-4. [DOI] [PubMed] [Google Scholar]
- 54.da Cruz MT, Cardoso AL, de Almeida LP, Simoes S, de Lima MC. Tf-lipoplex-mediated NGF gene transfer to the CNS: neuronal protection and recovery in an excitotoxic model of brain injury. Gene Ther. 2005;12(16):1242–1252. doi: 10.1038/sj.gt.3302516. [DOI] [PubMed] [Google Scholar]
- 55.Lowenstein PR. Virology and immunology of gene therapy, or virology and immunology of high MOI infection with defective viruses. Gene Ther. 2003;10(11):933–934. doi: 10.1038/sj.gt.3302073. [DOI] [PubMed] [Google Scholar]
- 56.Heiss WD, Graf R, Wienhard K, Lottgen J, Saito R, Fujita T, et al. Dynamic penumbra demonstrated by sequential multitracer PET after middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab. 1994;14(6):892–902. doi: 10.1038/jcbfm.1994.120. [DOI] [PubMed] [Google Scholar]
- 57.Sobesky J, Zaro WO, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35(12):2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- 58.Heiss WD, Kracht LW, Thiel A, Grond M, Pawlik G. Penumbral probability thresholds of cortical flumazenil binding and blood flow predicting tissue outcome in patients with cerebral ischaemia. Brain. 2001;124(Pt 1):20–29. doi: 10.1093/brain/124.1.20. [DOI] [PubMed] [Google Scholar]
- 59.Markus R, Reutens DC, Kazui S, Read S, Wright P, Chambers BR, et al. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke. 2003;34(11):2646–2652. doi: 10.1161/01.STR.0000094422.74023.FF. [DOI] [PubMed] [Google Scholar]
- 60.Hjort N, Butcher K, Davis SM, Kidwell CS, Koroshetz WJ, Rother J, et al. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36(2):388–397. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- 61.Linnik MD, Zahos P, Geschwind MD, Federoff HJ. Expression of bcl-2 from a defective herpes simplex virus-1 vector limits neuronal death in focal cerebral ischemia. Stroke. 1995;26(9):1670–1674. doi: 10.1161/01.str.26.9.1670. [DOI] [PubMed] [Google Scholar]
- 62.Betz AL, Yang GY, Davidson BL. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab. 1995;15(4):547–551. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- 63.Xu DG, Crocker SJ, Doucet JP, St-Jean M, Tamai K, Hakim AM, et al. Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nat Med. 1997;3(9):997–1004. doi: 10.1038/nm0997-997. [DOI] [PubMed] [Google Scholar]
- 64.Yang GY, Pang L, Ge HL, Tan M, Ye W, Liu XH, et al. Attenuation of ischemia-induced mouse brain injury by SAG, a redox-inducible antioxidant protein. J Cereb Blood Flow Metab. 2001;21(6):722–733. doi: 10.1097/00004647-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Zhang WR, Sato K, Iwai M, Nagano I, Manabe Y, Abe K. Therapeutic time window of adenovirus-mediated GDNF gene transfer after transient middle cerebral artery occlusion in rat. Brain Res. 2002;947(1):140–145. doi: 10.1016/s0006-8993(02)02923-2. [DOI] [PubMed] [Google Scholar]
- 66.Takada J, Ooboshi H, Ago T, Kitazono T, Yao H, Kadomatsu K, et al. Postischemic gene transfer of midkine, a neurotrophic factor, protects against focal brain ischemia. Gene Ther. 2005;12(6):487–493. doi: 10.1038/sj.gt.3302434. [DOI] [PubMed] [Google Scholar]
- 67.Kitagawa K, Hori M, Matsumoto M. Therapeutic strategy for acute stroke—prologue for an epoch of brain attack. Future aspects of gene therapy in acute ischemic stroke. Intern Med. 2005;44(4):371–374. doi: 10.2169/internalmedicine.44.371. [DOI] [PubMed] [Google Scholar]
- 68.Biffi A, De PM, Quattrini A, Del CU, Amadio S, Visigalli I, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Invest. 2004;113(8):1118–1129. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schouten JW, Fulp CT, Royo NC, Saatman KE, Watson DJ, Snyder EY, et al. A review and rationale for the use of cellular transplantation as a therapeutic strategy for traumatic brain injury. J Neurotrauma. 2004;21(11):1501–1538. doi: 10.1089/neu.2004.21.1501. [DOI] [PubMed] [Google Scholar]
- 70.Snyder EY, Park KI, Flax JD, Liu S, Rosario CM, Yandava BD, et al. Potential of neural “stem-like” cells for gene therapy and repair of the degenerating central nervous system. Adv Neurol. 1997;72:121–132. [PubMed] [Google Scholar]
- 71.Snyder EY, Daley GQ, Goodell M. Taking stock and planning for the next decade: realistic prospects for stem cell therapies for the nervous system. J Neurosci Res. 2004;76(2):157–168. doi: 10.1002/jnr.20033. [DOI] [PubMed] [Google Scholar]
- 72.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001 Mar 8;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 73.Snyder BJ, Olanow CW. Stem cell treatment for Parkinson’s disease: an update for 2005. Curr Opin Neurol. 2005;18(4):376–385. doi: 10.1097/01.wco.0000174298.27765.91. [DOI] [PubMed] [Google Scholar]
- 74.Behrstock S, Svendsen CN. Combining growth factors, stem cells, and gene therapy for the aging brain. Ann N Y Acad Sci. 2004;1019:5–14. doi: 10.1196/annals.1297.002. [DOI] [PubMed] [Google Scholar]
- 75.McKay RD. Stem cell biology and neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci. 2004;359(1445):851–856. doi: 10.1098/rstb.2004.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Lacoste MC, White CL., III The role of cortical connectivity in Alzheimer’s disease pathogenesis: a review and model system. Neurobiol Aging. 1993;14(1):1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- 77.Laws SM, Hone E, Gandy S, Martins RN. Expanding the association between the APOE gene and the risk of Alzheimer’s disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem. 2003;84(6):1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- 78.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 79.Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38(5):819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- 80.Press GA, Amaral DG, Squire LR. Hippocampal abnormalities in amnesic patients revealed by high-resolution magnetic resonance imaging. Nature. 1989;341(6237):54–57. doi: 10.1038/341054a0. [DOI] [PubMed] [Google Scholar]
- 81.Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95(3):721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 82.Jack CR, Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mosconi L, De SS, Rusinek H, Convit A, de Leon MJ. Magnetic resonance and PET studies in the early diagnosis of Alzheimer’s disease. Expert Rev Neurother. 2004;4(5):831–849. doi: 10.1586/14737175.4.5.831. [DOI] [PubMed] [Google Scholar]
- 84.Jack CR, Jr, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999 Apr 22;52(7):1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herholz K. PET studies in dementia. Ann Nucl Med. 2003;17(2):79–89. doi: 10.1007/BF02988444. [DOI] [PubMed] [Google Scholar]
- 86.Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage. 2003;20(3):1894–1898. doi: 10.1016/j.neuroimage.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Friedland RP, Brun A, Budinger TF. Pathological and positron emission tomographic correlations in Alzheimer’s disease. Lancet. 1985;1(8422):228. doi: 10.1016/s0140-6736(85)92074-4. [DOI] [PubMed] [Google Scholar]
- 88.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 89.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17(1):302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 90.Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2003;54(3):343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- 91.Heiss WD, Habedank B, Klein JC, Herholz K, Wienhard K, Lenox M, et al. Metabolic rates in small brain nuclei determined by high-resolution PET. J Nucl Med. 2004;45(11):1811–1815. [PubMed] [Google Scholar]
- 92.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63(12):2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 94.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 95.Chetelat G, Desgranges B, de lS V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 96.Thomas A, Klein JC, Galldiks N, Grond M, Jacobs AH. Multitracer PET imaging in Heidenhain variant of Creutzfeldt-Jakob disease. J Neurol. 2005 Aug 4; doi: 10.1007/s00415-005-0953-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 97.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 98.Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, et al. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer’s disease. Lancet. 1997;349(9068):1805–1809. doi: 10.1016/S0140-6736(96)09124-6. [DOI] [PubMed] [Google Scholar]
- 99.Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60(12):1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 100.Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti B, Davis JG, et al. Cognitive correlates of alterations in acetyl-cholinesterase in Alzheimer’s disease. Neurosci Lett. 2005;380(1–2):127–132. doi: 10.1016/j.neulet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 101.Herholz K, Weisenbach S, Zundorf G, Lenz O, Schroder H, Bauer B, et al. In vivo study of acetylcholine esterase in basal forebrain, amygdala, and cortex in mild to moderate Alzheimer disease. Neuroimage. 2004;21(1):136–143. doi: 10.1016/j.neuroimage.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 102.Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, et al. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7(3):369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- 103.Bacskai BJ, Klunk WE, Mathis CA, Hyman BT. Imaging amyloid-beta deposits in vivo. J Cereb Blood Flow Metab. 2002;22(9):1035–1041. doi: 10.1097/00004647-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 105.Mathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, Hyman BT, et al. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett. 2002;12(3):295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- 106.Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005 Jun 8; doi: 10.1038/sj.jcbfm.9600146. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 107.Bacskai BJ, Hickey GA, Skoch J, Kajdasz ST, Wang Y, Huang GF, et al. Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice. Proc Natl Acad Sci U S A. 2003;100(21):12462–12467. doi: 10.1073/pnas.2034101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, et al. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer’s disease. J Neurosci. 2001;21(24):RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10(1):24–35. [PubMed] [Google Scholar]
- 110.Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358(9280):461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 111.Nordberg A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004;3(9):519–527. doi: 10.1016/S1474-4422(04)00853-1. [DOI] [PubMed] [Google Scholar]
- 112.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 113.Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329(6134):65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 115.Rosenberg MB, Friedmann T, Robertson RC, Tuszynski M, Wolff JA, Breakefield XO, et al. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1988;242(4885):1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- 116.Tuszynski MH, HS U, Amaral DG, Gage FH. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci. 1990;10(11) doi: 10.1523/JNEUROSCI.10-11-03604.1990. 3604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eriksdotter JM, Nordberg A, Amberla K, Backman L, Ebendal T, Meyerson B, et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9(5):246–257. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- 118.Tuszynski MH, Roberts J, Senut MC, HS U, Gage FH. Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Ther. 1996;3(4):305–314. [PubMed] [Google Scholar]
- 119.Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci U S A. 1999;96(19):10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conner JM, Darracq MA, Roberts J, Tuszynski MH. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci U S A. 2001;98(4):1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(5):551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 122.Dodart JC, Marr RA, Koistinaho M, Gregersen BM, Malkani S, Verma IM, et al. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102(4):1211–1216. doi: 10.1073/pnas.0409072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qu B, Rosenberg RN, Li L, Boyer PJ, Johnston SA. Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease. Arch Neurol. 2004;61(12):1859–1864. doi: 10.1001/archneur.61.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim HD, Kong FK, Cao Y, Lewis TL, Kim H, Tang DC, et al. Immunization of Alzheimer model mice with adenovirus vectors encoding amyloid beta-protein and GM-CSF reduces amyloid load in the brain. Neurosci Lett. 2004;370(2–3):218–223. doi: 10.1016/j.neulet.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 125.Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, et al. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8(11):1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 126.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38(4):547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 127.Marr RA, Guan H, Rockenstein E, Kindy M, Gage FH, Verma I, et al. Neprilysin regulates amyloid beta peptide levels. J Mol Neurosci. 2004;22(1–2):5–11. doi: 10.1385/JMN:22:1-2:5. [DOI] [PubMed] [Google Scholar]
- 128.Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23(6):1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301(5634):839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 130.Miller VM, Xia H, Marrs GL, Gouvion CM, Lee G, Davidson BL, et al. Allele-specific silencing of dominant disease genes. Proc Natl Acad Sci U S A. 2003;100(12):7195–7200. doi: 10.1073/pnas.1231012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di Iorio G, et al. Mapping of a gene for Parkinson’s disease to chromosome 4q21–q23 [see comments] Science. 1996;274(5290):1197–1199. doi: 10.1126/science.274.5290.1197. [DOI] [PubMed] [Google Scholar]
- 132.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 133.Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105(7):891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 134.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 135.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 136.Hutchinson M, Raff U, Lebedev S. MRI correlates of pathology in parkinsonism: segmented inversion recovery ratio imaging (SIRRIM) Neuroimage. 2003;20(3):1899–1902. doi: 10.1016/j.neuroimage.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 137.Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D. Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology. 2003;60(1):74–77. doi: 10.1212/wnl.60.1.74. [DOI] [PubMed] [Google Scholar]
- 138.Schulz JB, Skalej M, Wedekind D, Luft AR, Abele M, Voigt K, et al. Magnetic resonance imaging-based volumetry differentiates idiopathic Parkinson’s syndrome from multiple system atrophy and progressive supranuclear palsy. Ann Neurol. 1999;45(1):65–74. [PubMed] [Google Scholar]
- 139.Seppi K, Schocke MF, Esterhammer R, Kremser C, Brenneis C, Mueller J, et al. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of multiple system atrophy. Neurology. 2003;60(6):922–927. doi: 10.1212/01.wnl.0000049911.91657.9d. [DOI] [PubMed] [Google Scholar]
- 140.Morrish PK, Sawle GV, Brooks DJ. Clinical and [18F]dopa PET findings in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1995;59(6):597–600. doi: 10.1136/jnnp.59.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rinne JO, Bergman J, Ruottinen H, Haaparanta M, Eronen E, Oikonen V, et al. Striatal uptake of a novel PET ligand, [18F] beta-CFT, is reduced in early Parkinson’s disease. Synapse. 1999;31(2):119–124. doi: 10.1002/(SICI)1098-2396(199902)31:2<119::AID-SYN4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 142.Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15(4):692–698. doi: 10.1002/1531-8257(200007)15:4<692::aid-mds1014>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 143.Fischman AJ, Bonab AA, Babich JW, Livni E, Alpert NM, Meltzer PC, et al. [11C, 127I]Altropane: a highly selective ligand for PET imaging of dopamine transporter sites. Synapse. 2001;39(4):332–342. doi: 10.1002/1098-2396(20010315)39:4<332::AID-SYN1017>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 144.Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47(4):493–503. [PubMed] [Google Scholar]
- 145.Doder M, Rabiner EA, Turjanski N, Lees AJ, Brooks DJ. Tremor in Parkinson’s disease and serotonergic dysfunction: an 11C-WAY 100635 PET study. Neurology. 2003;60(4):601–605. doi: 10.1212/01.wnl.0000031424.51127.2b. [DOI] [PubMed] [Google Scholar]
- 146.Gerhard A, Banati RB, Goerres GB, Cagnin A, Myers R, Gunn RN, et al. [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology. 2003;61(5):686–689. doi: 10.1212/01.wnl.0000078192.95645.e6. [DOI] [PubMed] [Google Scholar]
- 147.Hilker R, Klein C, Ghaemi M, Kis B, Strotmann T, Ozelius LJ, et al. Positron emission tomographic analysis of the nigrostriatal dopaminergic system in familial parkinsonism associated with mutations in the parkin gene. Ann Neurol. 2001;49(3):367–376. [PubMed] [Google Scholar]
- 148.Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ. The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol. 1999;45(5):577–582. doi: 10.1002/1531-8249(199905)45:5<577::aid-ana5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 149.Nurmi E, Ruottinen HM, Bergman J, Haaparanta M, Solin O, Sonninen P, et al. Rate of progression in Parkinson’s disease: a 6-[18F]fluoro-l-dopa PET study. Mov Disord. 2001;16(4):608–615. doi: 10.1002/mds.1139. [DOI] [PubMed] [Google Scholar]
- 150.Hilker R, Schweitzer K, Coburger S, Ghaemi M, Weisenbach S, Jacobs AH, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005;62(3):378–382. doi: 10.1001/archneur.62.3.378. [DOI] [PubMed] [Google Scholar]
- 151.Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54(1):93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 152.Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287(13):1653–1661. doi: 10.1001/jama.287.13.1653. [DOI] [PubMed] [Google Scholar]
- 153.Hilker R, Voges J, Ghaemi M, Lehrke R, Rudolf J, Koulousakis A, et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003;18(1):41–48. doi: 10.1002/mds.10297. [DOI] [PubMed] [Google Scholar]
- 154.Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. J Cereb Blood Flow Metab. 2004;24(1):7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- 155.Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64(2):208–215. doi: 10.1212/01.WNL.0000149403.14458.7F. [DOI] [PubMed] [Google Scholar]
- 156.Brooks DJ. Neuroimaging in Parkinson’s disease. NeuroRx. 2004;1(2):243–254. doi: 10.1602/neurorx.1.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.During MJ, Naegele JR, O’Malley KL, Geller AI. Long-term behavioral recovery in parkinsonian rats by an HSV vector expressing tyrosine hydroxylase [see comments] Science. 1994;266(5189):1399–1403. doi: 10.1126/science.266.5189.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8(2):148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 159.Mandel RJ, Rendahl KG, Spratt SK, Snyder RO, Cohen LK, Leff SE. Characterization of intrastriatal recombinant adeno-associated virus-mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydrolase I in a rat model of Parkinson’s disease. J Neurosci. 1998;18(11):4271–4284. doi: 10.1523/JNEUROSCI.18-11-04271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kirik D, Georgievska B, Burger C, Winkler C, Muzyczka N, Mandel RJ, et al. Reversal of motor impairments in parkinsonian rats by continuous intrastriatal delivery of l-dopa using rAAV-mediated gene transfer. Proc Natl Acad Sci U S A. 2002;99(7):4708–4713. doi: 10.1073/pnas.062047599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shen Y, Muramatsu SI, Ikeguchi K, Fujimoto KI, Fan DS, Ogawa M, et al. Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-l-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson’s disease. Hum Gene Ther. 2000;11(11):1509–1519. doi: 10.1089/10430340050083243. [DOI] [PubMed] [Google Scholar]
- 162.Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, et al. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic l-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson’s disease. J Neurosci. 2002;22(23):10302–10312. doi: 10.1523/JNEUROSCI.22-23-10302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Muramatsu S, Fujimoto K, Ikeguchi K, Shizuma N, Kawasaki K, Ono F, et al. Behavioral recovery in a primate model of Parkinson’s disease by triple transduction of striatal cells with adeno-associated viral vectors expressing dopamine-synthesizing enzymes. Hum Gene Ther. 2002;13(3):345–354. doi: 10.1089/10430340252792486. [DOI] [PubMed] [Google Scholar]
- 164.Sun M, Kong L, Wang X, Holmes C, Gao Q, Zhang GR, et al. Coexpression of tyrosine hydroxylase, GTP cyclohydrolase I, aromatic amino acid decarboxylase, and vesicular monoamine transporter 2 from a helper virus-free herpes simplex virus type 1 vector supports high-level, long-term biochemical and behavioral correction of a rat model of Parkinson’s disease. Hum Gene Ther. 2004;15(12):1177–1196. doi: 10.1089/hum.2004.15.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 166.Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 167.Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886(1–2):82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- 168.Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275(5301):838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 169.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20(12):4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290(5492):767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 171.Kordower JH. In vivo gene delivery of glial cell line-derived neurotrophic factor for Parkinson’s disease. Ann Neurol. 2003;53 Suppl 3:S120–S132. doi: 10.1002/ana.10485. [DOI] [PubMed] [Google Scholar]
- 172.Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17(2):260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- 173.Georgievska B, Kirik D, Bjorklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyro-sine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004;24(29):6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Georgievska B, Jakobsson J, Persson E, Ericson C, Kirik D, Lundberg C. Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum Gene Ther. 2004;15(10):934–944. doi: 10.1089/hum.2004.15.934. [DOI] [PubMed] [Google Scholar]
- 175.Hurtado-Lorenzo A, Millan E, Gonzalez-Nicolini V, Suwelack D, Castro MG, Lowenstein PR. Differentiation and transcription factor gene therapy in experimental Parkinson’s disease: sonic hedgehog and Gli-1, but not Nurr-1, protect nigrostriatal cell bodies from 6-OHDA-induced neurodegeneration. Mol Ther. 2004;10(3):507–524. doi: 10.1016/j.ymthe.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xia XG, Harding T, Weller M, Bieneman A, Uney JB, Schulz JB. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(18):10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mochizuki H, Hayakawa H, Migita M, Shibata M, Tanaka R, Suzuki A, et al. An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(19):10918–10923. doi: 10.1073/pnas.191107398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Crocker SJ, Wigle N, Liston P, Thompson CS, Lee CJ, Xu D, et al. NAIP protects the nigrostriatal dopamine pathway in an intrastriatal 6-OHDA rat model of Parkinson’s disease. Eur J Neurosci. 2001;14(2):391–400. doi: 10.1046/j.0953-816x.2001.01653.x. [DOI] [PubMed] [Google Scholar]
- 179.Dong Z, Wolfer DP, Lipp HP, Bueler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Mol Ther. 2005;11(1):80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 180.Lo BC, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, et al. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101(50):17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamada M, Mizuno Y, Mochizuki H. Parkin gene therapy for alpha-synucleinopathy: a rat model of Parkinson’s disease. Hum Gene Ther. 2005;16(2):262–270. doi: 10.1089/hum.2005.16.262. [DOI] [PubMed] [Google Scholar]
- 182.Burton EA, Glorioso JC, Fink DJ. Gene therapy progress and prospects: Parkinson’s disease. Gene Ther. 2003;10(20):1721–1727. doi: 10.1038/sj.gt.3302116. [DOI] [PubMed] [Google Scholar]
- 183.Luo J, Kaplitt MG, Fitzsimons HL, Zuzga DS, Liu Y, Oshinsky ML, et al. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science. 2002;298(5592):425–429. doi: 10.1126/science.1074549. [DOI] [PubMed] [Google Scholar]
- 184.During MJ, Kaplitt MG, Stern MB, Eidelberg D. Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. Hum Gene Ther. 2001;12(12):1589–1591. [PubMed] [Google Scholar]
- 185.Fjord-Larsen L, Johansen JL, Kusk P, Tornoe J, Gronborg M, Rosenblad C, et al. Efficient in vivo protection of nigral dopaminergic neurons by lentiviral gene transfer of a modified Neurturin construct. Exp Neurol. 2005;195(1):49–60. doi: 10.1016/j.expneurol.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 186.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164(1):2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 187.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 188.Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 189.Jacobs AH, Kracht LW, Gossmann A, Ruger MA, Thomas AV, Thiel A, et al. Imaging in neurooncology. NeuroRx. 2005;2(2):333–347. doi: 10.1602/neurorx.2.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 191.Klatzmann D, Valery CA, Bensimon G, Marro B, Boyer O, Mokhtari K, et al. A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther. 1998;9(17):2595–2604. doi: 10.1089/hum.1998.9.17-2595. [DOI] [PubMed] [Google Scholar]
- 192.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11(17):2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 193.Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3(12):1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 194.Jacobs AH, Voges J, Kracht LW, Dittmar C, Winkeler A, Thomas A, et al. Imaging in gene therapy of patients with glioma. J Neurooncol. 2003;65(3):291–305. doi: 10.1023/b:neon.0000003658.51816.3f. [DOI] [PubMed] [Google Scholar]
- 195.Lam PY, Breakefield XO. Potential of gene therapy for brain tumors. Hum Mol Genet. 2001;10(7):777–787. doi: 10.1093/hmg/10.7.777. [DOI] [PubMed] [Google Scholar]
- 196.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11(22):1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 197.Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratu-moural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 198.Eck SL, Alavi JB, Judy K, Phillips P, Alavi A, Hackney D, et al. Treatment of recurrent or progressive malignant glioma with a recombinant adenovirus expressing human interferon-beta (H5.010CMVhIFN-beta): a phase I trial. Hum Gene Ther. 2001;12(1):97–113. doi: 10.1089/104303401451013. [DOI] [PubMed] [Google Scholar]
- 199.Yoshida J, Mizuno M, Fujii M, Kajita Y, Nakahara N, Hatano M, et al. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum Gene Ther. 2004;15(1):77–86. doi: 10.1089/10430340460732472. [DOI] [PubMed] [Google Scholar]
- 200.Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10(6):1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 202.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 203.Herrlinger U, Woiciechowski C, Quiones A, Sena-Esteves M, Aboody K, Jacobs A, et al. Neural stem cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 2000;1(4):347–357. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- 204.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. From the cover: neural stem cells display extensive tropism for pathology in adult brain: evidence from intracra-nial gliomas. Proc Natl Acad Sci U S A. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brown AB, Yang W, Schmidt NO, Carroll R, Leishear KK, Rainov NG, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14(18):1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 206.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62(20):5657–5663. [PubMed] [Google Scholar]
- 207.Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62(24):7170–7174. [PubMed] [Google Scholar]
- 208.Ehtesham M, Yuan X, Kabos P, Chung NH, Liu G, Akasaki Y, et al. Glioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4. Neoplasia. 2004;6(3):287–293. doi: 10.1593/neo.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shah K, Bureau E, Kim DE, Yang K, Tang Y, Weissleder R, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57(1):34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 210.Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther. 2003;14(13):1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- 211.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23(1):23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 212.Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9(12):1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 213.Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 214.Jacobs AH, Dittmar C, Winkeler A, Garlip G, Heiss WD. Molecular imaging of gliomas. Mol Imaging. 2002;1(4):309–335. doi: 10.1162/15353500200221392. [DOI] [PubMed] [Google Scholar]
- 215.Gambhir SS, Barrio JR, Wu L, Iyer M, Namavari M, Satyamurthy N, et al. Imaging of adenoviral-directed herpes simplex virus type 1 thymidine kinase reporter gene expression in mice with radiolabeled ganciclovir. J Nucl Med. 1998;39(11):2003–2011. [PubMed] [Google Scholar]
- 216.Tjuvajev JG, Stockhammer G, Desai R, Uehara H, Watanabe K, Gansbacher B, et al. Imaging the expression of transfected genes in vivo. Cancer Res. 1995;55(24):6126–6132. [PubMed] [Google Scholar]
- 217.Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58(19):4333–4341. [PubMed] [Google Scholar]
- 218.MacLaren DC, Gambhir SS, Satyamurthy N, Barrio JR, Sharfstein S, Toyokuni T, et al. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther. 1999;6(5):785–791. doi: 10.1038/sj.gt.3300877. [DOI] [PubMed] [Google Scholar]
- 219.Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6(3):351–355. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 220.Blasberg RG, Tjuvajev JG. Molecular-Genetic Imaging: a nuclear medicine-based perspective. Mol Imaging. 2002;1(2):280–300. doi: 10.1162/15353500200202127. [DOI] [PubMed] [Google Scholar]
- 221.de Vries E, Vaalburg W. Positron emission tomography: measurement of transgene expression. Methods. 2002;27(3):234. doi: 10.1016/s1046-2023(02)00080-4. [DOI] [PubMed] [Google Scholar]
- 222.Gambhir SS, Herschman HR, Cherry SR, Barrio JR, Satyamurthy N, Toyokuni T, et al. Imaging transgene expression with radionuclide technologies. Neoplasia. 2000;2(1–2):118–138. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wiebe LI, Knaus EE. Enzyme-targeted, nucleoside-based radiopharmaceuticals for scintigraphic monitoring of gene transfer and expression. Curr Pharm Des. 2001;7(18):1893–1906. doi: 10.2174/1381612013396817. [DOI] [PubMed] [Google Scholar]
- 224.Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54(4):479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 225.Voges J, Weber F, Reszka R, Sturm V, Jacobs A, Heiss WD, Clinical protocol, et al. Liposomal gene therapy with the herpes simplex thymidine kinase gene/ganciclovir system for the treatment of glioblastoma multiforme. Hum Gene Ther. 2002;13(5):675–685. doi: 10.1089/10430340252837260. [DOI] [PubMed] [Google Scholar]
- 226.Green LA, Nguyen K, Berenji B, Iyer M, Bauer E, Barrio JR, et al. A tracer kinetic model for 18F-FHBG for quantitating herpes simplex virus type 1 thymidine kinase reporter gene expression in living animals using PET. J Nucl Med. 2004;45(9):1560–1570. [PubMed] [Google Scholar]
- 227.Alauddin MM, Shahinian A, Park R, Tohme M, Fissekis JD, Conti PS. Synthesis and evaluation of 2’-deoxy-2’-18F-fluoro-5-fluoro-1-beta-d-arabinofuranosyluracil as a potential PET imaging agent for suicide gene expression. J Nucl Med. 2004;45(12):2063–2069. [PubMed] [Google Scholar]
- 228.Alauddin MM, Shahinian A, Gordon EM, Conti PS. Direct comparison of radiolabeled probes FMAU, FHBG, and FHPG as PET imaging agents for HSV1-tk expression in a human breast cancer model. Mol Imaging. 2004;3(2):76–84. doi: 10.1162/15353500200403160. [DOI] [PubMed] [Google Scholar]
- 229.Kang KW, Min JJ, Chen X, Gambhir SS. Comparison of [14C] FMAU, [3H]FEAU, [14C]FIAU, and [3H]PCV for monitoring reporter gene expression of wild type and mutant herpes simplex virus type 1 thymidine kinase in cell culture. Mol Imaging Biol. 2005 Jul 23; doi: 10.1007/s11307-005-0010-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 230.Choi SR, Zhuang ZP, Chacko AM, Acton PD, Tjuvajev-Gelovani J, Doubrovin M, et al. SPECT Imaging of Herpes simplex virus type1 thymidine kinase gene expression by [123I] FIAU(1) Acad Radiol. 2005;12(7):798–805. doi: 10.1016/j.acra.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 231.Soghomonyan SA, Doubrovin M, Pike J, Luo X, Ittensohn M, Runyan JD, et al. Positron emission tomography (PET) imaging of tumor-localized Salmonella expressing HSV1-TK. Cancer Gene Ther. 2005;12(1):101–108. doi: 10.1038/sj.cgt.7700779. [DOI] [PubMed] [Google Scholar]
- 232.Hackman T, Doubrovin M, Balatoni J, Beresten T, Ponomarev V, Beattie B, et al. Imaging expression of cytosine deaminase-herpes virus thymidine kinase fusion gene (CD/TK) expression with [124I]FIAU and PET. Mol Imaging. 2002;1(1):36–42. doi: 10.1162/15353500200200003. [DOI] [PubMed] [Google Scholar]
- 233.Deng WP, Yang WK, Lai WF, Liu RS, Hwang JJ, Yang DM, et al. Non-invasive in vivo imaging with radiolabelled FIAU for monitoring cancer gene therapy using herpes simplex virus type 1 thymidine kinase and ganciclovir. Eur J Nucl Med Mol Imaging. 2004;31(1):99–109. doi: 10.1007/s00259-003-1269-z. [DOI] [PubMed] [Google Scholar]
- 234.Yaghoubi SS, Barrio JR, Namavari M, Satyamurthy N, Phelps ME, Herschman HR, et al. Imaging progress of herpes simplex virus type 1 thymidine kinase suicide gene therapy in living subjects with positron emission tomography. Cancer Gene Ther. 2005;12(3):329–339. doi: 10.1038/sj.cgt.7700795. [DOI] [PubMed] [Google Scholar]
- 235.Wen B, Burgman P, Zanzonico P, O’donoghue J, Cai S, Finn R, et al. A preclinical model for noninvasive imaging of hypoxia-induced gene expression; comparison with an exogenous marker of tumor hypoxia. Eur J Nucl Med Mol Imaging. 2004;31(11):1530–1538. doi: 10.1007/s00259-004-1673-z. [DOI] [PubMed] [Google Scholar]
- 236.Serganova I, Doubrovin M, Vider J, Ponomarev V, Soghomonyan S, Beresten T, et al. Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res. 2004;64(17):6101–6108. doi: 10.1158/0008-5472.CAN-04-0842. [DOI] [PubMed] [Google Scholar]
- 237.Sundaresan G, Paulmurugan R, Berger F, Stiles B, Nagayama Y, Wu H, et al. MicroPET imaging of Cre-loxP-mediated conditional activation of a herpes simplex virus type 1 thymidine kinase reporter gene. Gene Ther. 2004;11(7):609–618. doi: 10.1038/sj.gt.3302194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jacobs A, Dubrovin M, Hewett J, Sena-Esteves M, Tan C, Slack M, et al. Functional co-expression of HSV-1 thymidine kinase and green fluorescent protein: implications for non-invasive imaging of transgene expression. Neoplasia. 1999;1(2):154–161. doi: 10.1038/sj.neo.7900007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jacobs AH, Winkeler A, Hartung M, Slack M, Dittmar C, Kummer C, et al. Improved HSV-1 amplicon vectors for proportional coexpression of PET marker and therapeutic genes. Hum Gene Ther. 2003;14:277–297. doi: 10.1089/10430340360535823. [DOI] [PubMed] [Google Scholar]
- 240.Kim YJ, Dubey P, Ray P, Gambhir SS, Witte ON. Multimodality imaging of lymphocytic migration using lentiviral-based transduction of a tri-fusion reporter gene. Mol Imaging Biol. 2004;6(5):331–340. doi: 10.1016/j.mibio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 241.Ponomarev V, Doubrovin M, Serganova I, Vider J, Shavrin A, Beresten T, et al. A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear non-invasive imaging. Eur J Nucl Med Mol Imaging. 2004;31(5):740–751. doi: 10.1007/s00259-003-1441-5. [DOI] [PubMed] [Google Scholar]
- 242.Ray P, De A, Min JJ, Tsien RY, Gambhir SS. Imaging trifusion multimodality reporter gene expression in living subjects. Cancer Res. 2004;64(4):1323–1330. doi: 10.1158/0008-5472.can-03-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liang Q, Satyamurthy N, Barrio JR, Toyokuni T, Phelps MP, Gambhir SS, et al. Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther. 2001;8(19):1490–1498. doi: 10.1038/sj.gt.3301542. [DOI] [PubMed] [Google Scholar]
- 244.Bracy JL, Sachs DH, Iacomini J. Inhibition of xenoreactive natural antibody production by retroviral gene therapy. Science. 1998;281(5384):1845–1847. doi: 10.1126/science.281.5384.1845. [DOI] [PubMed] [Google Scholar]
- 245.Darrasse-Jeze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105(12):4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- 246.Forman D, Tian C, Iacomini J. Induction of donor-specific tolerance in sublethally irradiated recipients by gene therapy. Mol Ther. 2005;12(2):353–359. doi: 10.1016/j.ymthe.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 247.Marodon G, Klatzmann D. In situ transduction of stromal cells and thymocytes upon intrathymic injection of lentiviral vectors. BMC Immunol. 2004;5(1):18. doi: 10.1186/1471-2172-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tian C, Bagley J, Cretin N, Seth N, Wucherpfennig KW, Iacomini J. Prevention of type 1 diabetes by gene therapy. J Clin Invest. 2004;114(7):969–978. doi: 10.1172/JCI22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, et al. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64(18):6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 250.Mehrian SR, Reichardt JK, Ya-Hsuan H, Kremen TJ, Liau LM, Cloughesy TF, et al. Robustness of gene expression profiling in glioma specimen samplings and derived cell lines. Brain Res Mol Brain Res. 2005;136(1–2):99–103. doi: 10.1016/j.molbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 251.Mischel PS, Cloughesy TF, Nelson SF. DNA-microarray analysis of brain cancer: molecular classification for therapy. Nat Rev Neurosci. 2004;5(10):782–792. doi: 10.1038/nrn1518. [DOI] [PubMed] [Google Scholar]
- 252.Bryant JA, Finn RS, Slamon DJ, Cloughesy TF, Charles AC. EGF activates intracellular and intercellular calcium signaling by distinct pathways in tumor cells. Cancer Biol Ther. 2004;3(12):1243–1249. doi: 10.4161/cbt.3.12.1233. [DOI] [PubMed] [Google Scholar]
- 253.Mischel PS, Nelson SF, Cloughesy TF. Molecular analysis of glioblastoma: pathway profiling and its implications for patient therapy. Cancer Biol Ther. 2003;2(3):242–247. doi: 10.4161/cbt.2.3.369. [DOI] [PubMed] [Google Scholar]
- 254.Shai R, Shi T, Kremen TJ, Horvath S, Liau LM, Cloughesy TF, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22(31):4918–4923. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- 255.Hilker R, Schweitzer K, Coburger S, Ghaemi M, Weisenbach S, Jacobs AH, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005;62(3):378–382. doi: 10.1001/archneur.62.3.378. [DOI] [PubMed] [Google Scholar]
- 256.Lowenstein PR. The case for immunosuppression in clinical gene transfer. Mol Ther. 2005;12(2):185–186. doi: 10.1016/j.ymthe.2005.06.439. [DOI] [PubMed] [Google Scholar]
- 257.Jacobs AH. PET in gliomas. In: Schlegel U, Weller M, Westphal M, editors. Neuroonkologie. Stuttgart: Thieme; 2003. pp. 72–76. [Google Scholar]