Abstract
Task switching requires executive control processes that undergo age-related decline. Previous neuroimaging studies have identified age-related differences in brain activation associated with global switching effects (dual-task blocks vs. single-task blocks), but age-related differences in activation during local switching effects (switch trials vs. repeat trials, within blocks) have not been investigated. This experiment used functional magnetic resonance imaging (fMRI), and diffusion tensor imaging (DTI), to examine adult age differences in task switching across adjacent trials (i.e., local task switching). During fMRI scanning, participants performed a cued, word categorization task. From interspersed cue-only trials, switch-related processing associated with the cue was estimated separately from the target. Activation associated with task switching, within a distributed frontoparietal network, differed for cue- and target-related processing. The magnitude of event-related activation for task switching was similar for younger adults (n = 20; 18-27 years) and older adults (n = 20; 60-85 years), although activation sustained throughout the on-tasks periods exhibited some age-related decline. Critically, the functional connectivity of switch-related regions, during cue processing, was higher for younger adults than for older adults, whereas functional connectivity during target processing was comparable across the age groups. Further, individual differences in cue-related functional connectivity shared a substantial portion of the age-related variability in the efficiency of target categorization response (drift rate). This age-related difference in functional connectivity, however, was independent of white matter integrity within task-relevant regions. These findings highlight the functional connectivity of frontoparietal activation as a potential source of age-related decline in executive control.
Keywords: Human development, Cognition, Reaction time, Attention, fMRI, DTI
Behavioral studies of cognitive aging have revealed a pattern of both preserved and declining cognitive abilities during normal adult development. Whereas knowledge-based abilities such as semantic memory and vocabulary are typically well-preserved (Burke and Shafto, 2008; Mayr and Kliegl, 2000), processes comprising the on-line storage, updating, and manipulation of new information are usually less efficient for older adults than for younger adults (Salthouse and Miles, 2002; Verhaeghen and Cerella, 2002; Zelazo et al., 2004). These latter abilities are often referred to as executive control processes, reflecting their central role in task coordination and monitoring, but executive functioning is not entirely separable from related processes such as working memory and attentional capacity (Braver and West, 2008; Kramer and Madden, 2008). In addition, tests of elementary perceptual speed, such as digit-symbol coding, share a substantial degree of age-related variance with measures of executive control, and thus age-related effects in executive functioning may be significantly influenced by age-related slowing at a sensory-motor level (Salthouse et al., 2003). When contrasted directly with perceptual speed, however, some tests of executive functioning do exhibit age-related decline, above and beyond the effects of speed (Braver et al., 2001; Keys and White, 2000; Wecker et al., 2000).
Our goal in this research was to investigate age-related differences in the functional neuroanatomy of executive control, in the context of a task-switching paradigm. On each trial, a cue word indicated which one of two categorization decisions should be performed on an upcoming target word. Performance in this type of task is typically slower and less accurate when the categorization on the current trial switches from the previous trial, relative to when it repeats, reflecting many component operations involved in switching from a prepared task to an alternative (Meiran et al., 2000; Monsell, 2003). One interpretation of these component operations is that they are not inherently related to executive control but instead primarily reflect cue encoding, which benefits from repetition across successive trials (Logan and Bundesen, 2003). Other evidence, however, suggests that switching the cue across trials does lead to an endogenous form of executive control involving the reconfiguration of task set (Monsell and Mizon, 2006).
The performance costs of task switching accrue at both a global level and a local level. The global effects represent the additional demands of the task switching context, relative to a single task condition, whereas the local effects represent the switch in task set across individual trials. For example, an increase in reaction time (RT) for task-repeat trials when they are included in a dual-task block, relative to a single-task block, is a global effect, whereas the increase in RT for switch versus repeat trials, within a dual-task block, is a local effect.
Task switching costs are typically more pronounced for older adults than for younger adults, primarily at the global level. Age-related effects also occur at the local level, though less consistently (Kramer et al., 1999; Kray and Lindenberger, 2000; Meiran et al., 2001). Mayr (2001) proposed that age-related increases in global switch costs represent the demands of updating and differentiating internal control settings. Thus, when neither the stimulus information nor the response information is sufficient to keep different task sets apart, older adults need to select the relevant mental set on both switch and non-switch trials, whereas younger adults need to do this only on switch trials.
Functional magnetic resonance imaging (fMRI) studies of younger adults suggest that attentional tasks activate regions within a widely distributed network comprising frontal, diencephalic (e.g., thalamic), and parietal regions (Corbetta and Shulman, 2002; Fan et al., 2005; Kastner and Ungerleider, 2000; Pessoa and Ungerleider, 2004). Particularly important, for top-down regulation of task performance and executive functioning, are regions in dorsolateral prefrontal cortex and superior parietal cortex. Braver, Reynolds, and Donaldson (2003) compared switch and repeat trials in a cued, categorization task for single word targets and found that switch trials led to activation of dorsolateral and ventrolateral prefrontal cortex (Brodmann area [BA] 6/9/45/47) and superior parietal cortex (BA 7), primarily in the left hemisphere. In addition, increasing activation in these prefrontal regions, during the cue interval (i.e., prior to target onset) was associated with faster semantic categorization responses to the target, for both switch and repeat trials. Left superior parietal activation during the cue interval, in contrast, was selectively associated with faster responses on switch trials, indicating that this region may contribute specifically to behavioral switching costs. Using a mixed blocked/event-related design, Braver et al. also estimated the sustained activation occurring throughout the on-task periods. Anterior regions within the frontoparietal network (medial and dorsolateral prefrontal cortex in the right hemisphere, BA 9/10/24/46), exhibited greater sustained activation in dual-task blocks, relative to single-task blocks. Other studies of task switching confirm that activation involves the frontoparietal network, although the regional patterns vary in response to individual task demands (Barber and Carter, 2005; Brass and von Cramon, 2004; Garavan et al., 2000b; Kimberg et al., 2000; Liston et al., 2006; Sohn et al., 2000; Sylvester et al., 2003).
Neuroimaging investigations have also identified age-related differences in several forms of executive control. A general theme, across studies, is that event-related fMRI activation during executive control is greater for older adults than for younger adults in frontoparietal regions, and that older adults’ activation is correlated positively with task performance, suggesting an age-related compensatory recruitment of cortical regions mediating executive processing. This age-related pattern has been reported in tasks ranging from visual monitoring (Cabeza et al., 2004), to inter-limb coordination (Heuninckx et al., 2005), Stroop interference (Langenecker et al., 2004; Milham et al., 2002) and visual letter detection (Madden et al., 2007; Nielson et al., 2002) [but see Hampshire, Gruszka, Fallon, & Owen (2008), and Rosano et al. (2005) for instances of age-related decreases in frontoparietal activation].
In the case of task switching, two studies have compared younger and older adults in the mean levels of fMRI activation. Both DiGirolamo et al. (2001) and Gold, Powell, Xuan, Jicha, and Smith (2010) found that frontoparietal activation was higher during dual-task blocks than during single-task blocks, although the pattern of age-related differences in activation varied across the studies. DiGirolamo et al. found that the increase in frontoparietal activation associated with the dual-task blocks was actually smaller in magnitude for older adults than for younger adults, because older adults activated the frontoparietal network during both single-task and dual-task blocks. Gold et al. reported that the task switching effect in the magnitude of frontoparietal activation did not differ significantly between the age groups, but that the older adults activated some additional cortical regions not activated by younger adults, which is more in line with a compensatory recruitment interpretation. Both of these previous studies used blocked designs, however, and thus the measured activation was not attributable specifically to task switching processes on individual trials.
In this experiment, we used fMRI to investigate three issues associated with age-related differences in executive control during task switching. First, does the pattern of regional brain activation associated with task switching, on individual trials, vary as a function of adult age? Previous studies indicate that age-related effects of task switching occur at a global level, for dual-task relative to single-task blocks (DiGirolamo et al., 2001; Gold et al., 2010), but it has not yet been determined whether local task switching effects vary with adult age. Although behavioral age-related effects in local task switching are typically small in magnitude (Kray and Lindenberger, 2000; Mayr, 2001), older adults may engage a different pattern of cortical activation to achieve a behavioral goal comparable to that of younger adults. We used a mixed blocked/event-related design (Braver et al., 2003; Burgund et al., 2003; Donaldson et al., 2001; Otten et al., 2002; Visscher et al., 2003) to distinguish block-level (sustained) and event-related (transient) activation, during a series of dual-task blocks. Thus, we could obtain some information regarding the global level, in terms of the activation sustained throughout the block, as well as the more critical information regarding event-related activation for local task switching. Although little is known regarding age-related differences in sustained activation, Dennis, Daselaar, and Cabeza (2007) have noted an age-related decline in the sustained activation of prefrontal regions, during a memory encoding task.
To isolate the event-related activity associated specifically with switching, we included a series of cue-only trials, intermixed with trials containing both a cue and a target. That is, the cue-only trials required cue encoding and selection of the relevant categorization task set, but the trials were terminated without target presentation. Thus, from the cue-only trials we could gain a more accurate characterization of event-related activation associated with cue encoding and switching between task sets, independently of activation contributed by target identification and response processes. Neuroimaging studies of task switching, with younger adult participants, have demonstrated that several regions within the frontoparietal attentional network are activated specifically by switching between task sets on cue-only trials (Brass and von Cramon, 2002; Slagter et al., 2006).
The second issue that we investigated was age-related differences in the functional connectivity of regions activated during task switching. Because the network of regions typically activated during attention and executive functioning tasks is distributed widely, across frontal, parietal, and diencephalic regions (Corbetta and Shulman, 2002; Fan et al., 2005; Freiwald and Kanwisher, 2004; Kastner and Ungerleider, 2000; LaBerge, 2000; Pessoa and Ungerleider, 2004), it is important to determine whether age-related differences occur in the functional interdependence of activated regions. Age-related differences in the functional connectivity of task-relevant regions may occur even if the overall level of activation is similar across age groups (Grady, 2005; Stevens, 2009). For younger adults, increasing connectivity of task-related activation, across trials, is associated with better performance in sensory/motor and memory tasks (Daselaar et al., 2006b; Gazzaley et al., 2007; Rissman et al., 2004). Studies of age-related differences in functional connectivity, during episodic memory tasks, have demonstrated significant changes in connectivity across adult age groups. Older adults exhibited lower connectivity among task-relevant cortical regions, and in some instances greater connectivity in regions outside of the memory-relevant network (Daselaar et al., 2006a; Dennis et al., 2008; Grady, 2005; St Jacques et al., 2009). To our knowledge, age-related differences in functional connectivity during executive control have not been reported previously. We predicted that functional connectivity during task switching would be lower for older adults than for younger adults, and that individual differences in functional connectivity would be a significant mediator of age-related variance in behavioral performance.
Third, we investigated the relation between cerebral white matter integrity and task-related, functional connectivity. The current imaging protocol included a diffusion tensor imaging (DTI) sequence, which assessed white matter integrity, in terms of the degree and directionality of diffusivity of molecular water (Basser and Jones, 2002; Beaulieu, 2002; Jones, 2008; Mori, 2007). Age-related decline occurs in the DTI measure of diffusion directionality, fractional anisotropy (FA), which suggests a corresponding decline in the integrity of white matter pathways, due to a number of variables including both a degradation of the axonal myelin and frank loss of neurons (Bennett et al., 2009; Madden et al., 2009a; Sullivan and Pfefferbaum, 2006). In a previous report of the DTI and behavioral data from this experiment, we proposed that individual differences in the integrity of two white matter regions within the frontoparietal network, the genu of the corpus callosum and splenium-parietal fibers in the right hemisphere, mediated age-related variance in the efficiency of behavioral performance in this version of task switching (Madden et al., 2009b; see also Gold et al., 2010; Perry et al., 2009). In the analyses of these fMRI data, we sought to determine whether age-related differences in functional connectivity during task switching were dependent on individual differences in cerebral white matter integrity. Although age-related differences in functional connectivity among the non-task-related components of the fMRI signal (i.e., intrinsic functional connectivity) appear to be dependent on cerebral white matter integrity (Andrews-Hanna et al., 2007; Chen et al., 2009), the relation between task-related functional connectivity and white matter integrity has not, to our knowledge, been investigated in the context of aging. We hypothesized that white matter integrity, within the regions previously identified as relevant for behavioral performance in this task (Madden et al., 2009b), would also mediate age-related effects in task-related functional connectivity.
In summary, we used a task-switching paradigm to investigate adult age differences in executive control. Previous fMRI studies of age-related differences in task switching have focused on the global differences between dual-task and single-task blocks. We used a mixed blocked/event-related design, and interspersed cue-only trials, which allowed us to both a) separate transient from sustained forms of activation; and b) identify switch-related activation during cue processing, independently of target identification and response processes. We were particularly interested in the functional connectivity of regions activated by switching, and we hypothesized that age-related decline in behavioral performance would be related to a corresponding decline in functional connectivity. Finally, we proposed that the degree of cerebral white matter integrity, within regions associated with the frontoparietal attentional network, would influence age-related effects in functional connectivity.
Materials and Methods
Participants
The participants were 20 younger adults (10 women) between 18 and 27 years of age (M = 22.40 years; SD = 2.50) and 20 older adults (10 women) between 60 and 85 years of age (M = 69.60; SD = 6.05). The research procedures were approved by the Duke University Medical Center Institutional Review Board, and all participants provided written informed consent. Participants were healthy, community-dwelling, right-handed individuals who were free of significant disease (e.g., atherosclerosis, hypertension), had corrected visual acuity of 20/40 or better, and did not exhibit atypical neurological findings in T2-weighted structural imaging. The older adults exhibited slower perceptual-motor speed (M = 1836 ms) than younger adults M = 1338 ms), on a computer-administered, two-choice version of digit-symbol coding, t(38) = 5.55, p < .001. The two age groups were comparable in years of education, performance on the vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981), Mini-Mental State Exam (MMSE; Folstein et al., 1975), and Beck Depression Inventory (BDI; Beck, 1978). Mean values on these psychometric tests are reported in Madden et al. (Madden et al., 2009b). The psychometric data were collected in a screening session prior to scanning, and participants also performed a brief, practice version of the task-switching paradigm during this screening session.
Behavioral task
During the scanning session, participants performed a version of task switching, similar to that of Braver et al. (2003). On each trial, a cue word indicated which one of two categorization decisions (manmade/natural or large/small) should be made regarding an upcoming target word. The large/small categorization was defined in terms of whether the referent of the target word was larger or smaller than a computer monitor. The target was a concrete word that was easily categorized along these dimensions (e.g., snail, building). The relevant categorization indicated by the cue either repeated or switched, unpredictably, across trials.
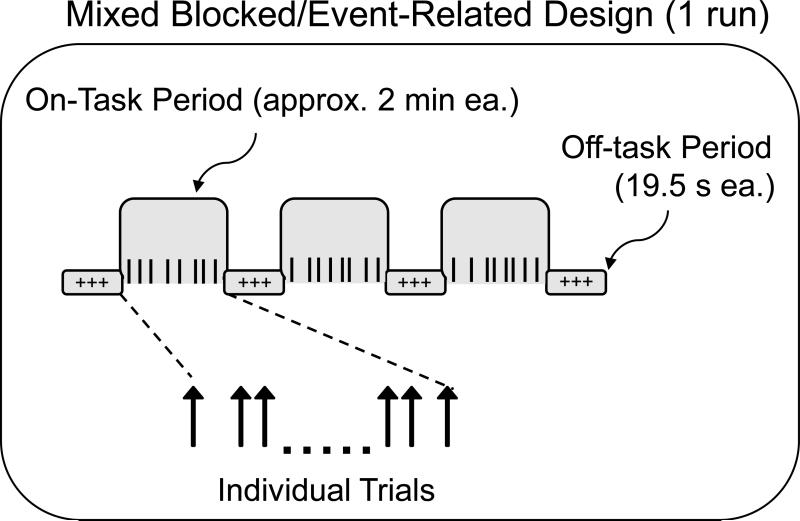
Six blocks of behavioral test trials corresponded to six fMRI runs. Within each of the six fMRI runs, there were three on-task periods (approximately 2 min 7 s each), separated by four off-task periods (19.5 s each). In this mixed blocked/event-related design (Dennis et al., 2007; Donaldson et al., 2001; Visscher et al., 2003), an off-task period always occurred at the beginning, and at the end, of the scanner run (Figure 1).
Figure 1.
Mixed blocked/event-related design. Each on-task period was a mixture of cue-only and cue+target trials.
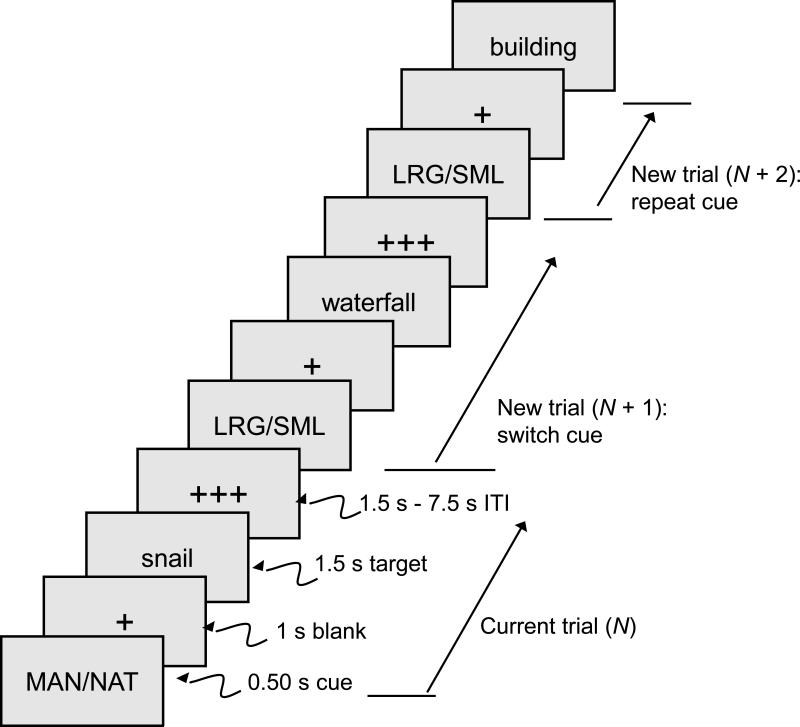
On 75% of the trials, both a cue and a target word were presented (i.e., cue+target trials). The sequence of events on individual cue+target trials is illustrated in Figure 2. To isolate the fMRI activation associated specifically with the cue, 25% of the trials within each trial block were cue-only trials, in which the target was not presented (Brass and von Cramon, 2002; Slagter et al., 2006; Weissman et al., 2005). Participants were instructed that when the target was missing they should just turn their attention to the next trial. In the data analyses, the designation of a trial as a repeat or switch trial (i.e., trial type) referred to the relation between the designated trial and the immediately preceding trial.
Figure 2.
Sequence of events on cue+target trials. The cue-only trials were similar, except that no target was presented, and the inter-trial interval (ITI) was extended by 1.5 s to equate the average durations of cue-only and cue+target trials.
Within each run, there were 36 cue+target trials (12 per on-task period), and 12 cue-only trials (4 per on-task period), yielding a total of 216 cue+target trials and 72 cue-only trials. The number of successive trials with the same cue was limited to five. Across the six runs, the two types of categorization decisions, and the two alternative responses within each category, occurred an approximately equal number of times, on both switch and repeat trials.
Participants viewed the stimuli with MRI-compatible goggles and responded manually (with the index finger of each hand) via a fiber-optic response box (Resonance Technology, Northridge, CA). Participants’ visual acuity prescription was obtained prior to testing, and the goggles were fitted with lenses to match the distance component of the prescription. For each categorization, the two responses were distributed across the response buttons (i.e., both categorizations were represented on each response button). During the 19.5 s off-task period, a row of three white fixation crosses remained in the center of the viewing area. During the on-task periods (Figure 2), each trial began with a 0.50 s cue, followed by a 1.0 s blank interval (single fixation cross) and 1.50 s target word. The target word was presented in white, 56 point Arial font against a black background. Following the offset of the target, a variable, blank inter-trial interval (ITI) occurred for either 1.50 s, 3.0 s, 4.50 s, 6.0 s, or 7.50 s (i.e., 1-5 TR intervals). During target word presentation, the two response options corresponding to the current categorization were presented below the target word, on the left and right sides of the screen, corresponding to the location of the assigned response buttons. The fixation cross was red following the cue but white following the target, during the ITI. On the cue-only trials, 1.5 s was added to the ITI value so that the durations of the cue-only and cue+target trials were (on average) comparable. Within the cue-only and cue+target trials, there was an approximately equal number of trials for each ITI value.
Before performing the test trials, participants completed one block of 51 practice trials while situated in the scanner. The instructions were to read the cue and to respond as quickly as possible to the target while still being correct. The assignment of the two responses (within each categorization) to the response buttons was varied across participants within each age group, as was the presentation order of the six blocks of test trials.
Imaging data acquisition
Imaging was conducted on a 3T GE Signa Excite MRI scanner (GE Healthcare, Waukesha, WI) with an eight-channel head coil. Participants wore ear plugs during scanning to reduce scanner noise, and head motion was minimized with foam pads and headband. The imaging sequence included a sagittal localizer series, followed by T1-weighted, T2-weighted, six runs of T2*-weighted (functional) and two runs of diffusion tensor imaging (DTI). With the exception of the T1-weighted localizer series, slice orientation was near-axial, parallel to the anterior-posterior commissure (AC-PC) plane.
The T1-weighted anatomical images were 104 contiguous slices acquired with a high-resolution, 3D fast inverse-recovery-prepared spoiled gradient recalled (SPGR) sequence, with repetition time (TR) = 7.4 ms, echo time (TE) = 3 ms, field of view (FOV) = 256 mm, 1.2 mm slice thickness, flip angle = 12°, voxel size = 1 × 1 × 1.2 mm, and 256 × 256 matrix.
The T2-weighted anatomical images were 52 contiguous slices acquired with an echo-planar (gradient-echo) sequence, with TR = 4000 ms, TE = 2 ms, FOV = 256 mm, 2.4 mm slice thickness, flip angle = 90°, voxel size = 2 × 2 × 2.4 mm, and 256 × 256 matrix.
The T2*-weighted echo-planar, functional images were 28 contiguous slices acquired using an inverse spiral sequence, with TR = 1500 ms, TE = 27 ms, FOV = 256 mm, 4.8-mm slice thickness, flip angle = 60°, voxel size = 4 × 4 × 4.8 mm, and 64 × 64 matrix.
Each of the DTI sequences was 52 contiguous slices using a dual spin echo base sequence, with TR = 17000 ms, TE = 86.7 ms, FOV = 25.6 cm, flip angle = 90°, 2.4 mm slice thickness, voxel size = 1 × 1 × 1.2 mm, and 128 × 128 matrix. Diffusion was measured from 15 non-collinear directions at a b factor of 1000 s/mm2; one reference image had no diffusion weighting.
Behavioral data analyses
Reaction time (RT) on the cue+target trials was analyzed with a version of the Ratcliff model of two-choice discrimination (Ratcliff, 1978; Ratcliff et al., 2000) as implemented by Wagenmakers et al. (2007). This model uses the distribution of RT for correct responses, across trials, for each participant, to estimate model parameters representing the decisional and nondecisional components of performance. In the present task, the decisional component, drift rate (v), represents the efficiency of the retrieval of semantic information (manmade/natural or large/small) required by the categorization response. The nondecisional component, perceptual-motor response time (T-er), represents the time required for visual feature encoding and response selection.
fMRI analyses
Analyses of functional imaging data were conducted with Statistical Parametric Mapping software (SPM2; Wellcome Department of Cognitive Neurology, London, UK), along with locally developed Matlab (Mathworks, Natick, MA) scripts. The first six volumes of each run, which always occurred during an off-task period, were discarded. Images were corrected for slice-timing and head motion, spatially normalized to the Montreal Neurological Institute (MNI) template, and then spatially smoothed with an 8 mm Gaussian kernel. No participant moved more than 3 mm in any direction, either within or across runs. A high-pass filter was included in every model to correct for scanner drift.
The fMRI data were analyzed within the general linear model (GLM) of SPM2 (Friston et al., 1995), using a mixed blocked/event-related design, which incorporated both event-related (transient) and blocked (sustained) regressors for the hemodynamic response function (HRF; Burgund et al., 2003; Dennis et al., 2007; Donaldson et al., 2001; Otten et al., 2002; Visscher et al., 2003). First-level, subject-specific SPM models contained both transient and sustained regressors. Transient regressors, for both cue-only and cue+target trials, were coded as a stick function (delta) convolved with the HRF+time basis function (Miezin et al., 2000; Woodruff et al., 2006), with the onset corresponding to the onset of the cue. The transient regressors specified whether the trial was cue-only or cue+target, and whether the cue was a repeat or a switch from the previous trial. The sustained regressors were modeled as a fixed response (boxcar) waveform, with onset corresponding to the onset of the first cue in an on-task period and lasting the duration of the on-task period. The implicit baseline was the jittered ITI. The correlation between the transient and sustained regressors did not exceed r = 0.52 for any participant. Trials on which the participant either responded incorrectly, or failed to respond to a target (< 2% of trials within each age group), were modeled separately and eliminated. The first trial of each on-task period was coded separately and deleted from the analyses, because it cannot be designated as either repeat or switch. Head motion and scanner drift were treated as covariates of no interest.
The contrasts of interests were the effects of trial type (repeat, switch) and age group (younger, older). Second-level, random effects analyses were conducted on the first-level contrast images. At the second level, we constructed two omnibus, event-related activation maps, one for cue-only trials and one for cue+target trials, for both age groups combined. These were used as inclusive masks in tests of the contrasts for the trial type and age group effects. For the cue-related contrasts, the omnibus activation map represented the t-statistic per voxel on the cue-only trials, relative to the implicit baseline, across both trial types (repeat, switch) and age groups, with group-variance estimates pooled. This map used a t threshold of p < .001 (uncorrected) and an extent threshold of 10 voxels. For the target-related contrasts, the map was constructed from the contrast of cue+target trials > cue-only trials (p < .001, uncorrected; 10 voxel extent), for both age groups and repeat/switch trial types combined. Thus, target-related activity was identified by subtracting cue-only activity from cue+target activity. To focus specifically on positive activations, this contrast was masked inclusively with all cue+target trials > implicit baseline (p < .05, uncorrected; 10 voxel extent). Thus, the omnibus activation map for the cue-related contrasts represents processes associated with reading the cue and preparing for the target. The map for the target-related contrasts represents additional processing associated with reading the target, selecting the appropriate semantic category, and making the response, beyond the cue-specific processing.
We tested for age group differences in the omnibus activation maps by examining two separate contrasts, older > younger and younger > older, using each omnibus map as an inclusive mask. For each age group contrast, an additional inclusive mask was used to isolate activations above threshold. For example, the older > younger contrast within the omnibus activation map used an inclusive mask of (p < .05, uncorrected; 10 voxel extent) for the older adults’ data (switch and repeat trials combined), to limit the age difference to activation that was above the ITI baseline for the older adults. Similarly, in the younger > older contrast, the younger adults’ data was masked to focus on the above-baseline activation. Each between-group contrast used a significance level of p < .05 and an extent threshold of 10 voxels. The masking procedure yielded a conservative threshold, in that the age group contrast threshold of p < .05 was layered on the initial p < .001 omnibus activation threshold, and both of these were limited to cluster sizes of 10 voxels or greater.
Following this definition of the overall event-related activation, we conducted contrasts that defined the effects of trial type (repeat vs. switch) and age differences in the trial type effect. As in the omnibus map analyses, cue-related and target-related activations were analyzed separately. Using each omnibus activation map as an inclusive mask, a contrast for switch > repeat was defined using a t threshold of p < .01 (uncorrected) and 10 voxel extent. For the switch > repeat contrast, an additional, inclusive mask of switch trials > baseline (p < .05, uncorrected; 10 voxel extent) was used, to limit the results to positive activation on switch trials. The contrast for repeat > switch was constructed similarly, using a p < .01 threshold for the contrast (within the omnibus map) and a p < .05 threshold for the inclusive mask limiting the contrast to positive activation on repeat trials. Age group contrasts for older > younger and younger > older (p < .05; 10 voxel extent) were then added (separately) to these trial type effects, masked to focus on positive activations.
The omnibus map of sustained activation was defined by the voxelwise t statistics for the sustained regressor, for both age groups combined, across the averaged on-task periods within each run, relative to the averaged off-task periods in each run. In preliminary analyses, using a threshold comparable to that of the event-related omnibus map (p < .001; 10 voxel extent), no clusters were significant. We report exploratory analyses of the sustained activation using a less conservative threshold of p < .05 (uncorrected; 10 voxel extent) for the omnibus activation map. Age group contrasts (p < .05; 10 voxel extent) were constructed as in the event-related case, with additional masking to limit the age effects to positive activations.
Functional connectivity analyses
In analyses of functional connectivity of event-related activation, we first identified the local maxima of the switch > repeat contrast, from the random effects, SPM analyses conducted for both age groups combined. (As noted in the Results section, no event-related activation was significant for repeat > switch.) The switch-related activation was analyzed separately within the cue-related and target-related omnibus activation maps. For these local maxima, we calculated the percent signal change on each trial, for each participant, using locally developed Matlab scripts. We created a GLM in which each individual trial was modeled by a separate covariate, yielding parameter estimates for each individual trial and for each individual participant. Activity levels for each voxel (local maximum), on individual trials, were derived from the parameter estimates, scaled to the session mean per voxel, and converted to percent signal change. The validity of this approach has been confirmed in previous studies (Daselaar et al., 2006a; Daselaar et al., 2006b; Rissman et al., 2004). For the set of local maxima associated with each switch > repeat contrast, correlations were computed for each pairwise combination of local maxima, across all trials (within participants). Correlations were transformed to Fisher z values (Fisher, 1921) for further analysis. Thus, an increasing z value, for these variables, represents increasing connectivity. For each participant, a composite connectivity measure was constructed by averaging the z values across all pairs of local maxima, within the set of local maxima identified in the switch > repeat contrast.
DTI analyses
The two DTI runs were merged to a single data set for each participant, to improve the signal-to-noise ratio. We calculated diffusion tensors from the 15 diffusion weighted images, based on a least squares fit of the tensor model to the diffusion data (Basser and Jones, 2002). Diagonalization of the tensor yielded three voxel-specific eigenvalues (λ1 > λ2 > λ3), which represent diffusivities along the three principal directions of the tensor. White matter pathways were estimated using DTI tractography (Corouge et al., 2006; Mori and Zhang, 2006), and fractional anisotropy values within pathways are reported in Madden et al. (2009b).
Results
Behavioral data
The mean values for reaction time, error rate, and the reaction time distribution parameters for the quality of evidence accumulation (drift rate, v) and perceptual encoding and response time (nondecision time, T-er) are presented in Table 1. Our primary interest was in the reaction time distribution parameters. Analysis of variance (ANOVA) of these latter values, with age group as a between-subjects variable and trial type (repeat, switch) as a within-subjects variable (reported previously, Madden et al., 2009b), yielded significant main effects of age group for both drift rate, F(1, 38) = 10.74, p < .01, and nondecision time, F(1, 38) = 6.84, p < .05, representing less efficient semantic retrieval and longer nondecision time for older adults than for younger adults. Trial type was also associated with a significant main effect for both drift rate, F(1, 38) = 36.52, p < .0001, and nondecision time, F(1, 38) = 5.80, p < .05, reflecting increased difficulty in both the decisional and nondecisional components for switch trials relative to repeat trials. The Age Group x Trial Type interaction was not significant.
Table 1.
Behavioral Performance as a Function of Age Group and Trial Type
|
M |
SD |
|||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Repeat Trials | ||||
| Reaction time | 0.897 | 1.038 | 0.105 | 0.125 |
| Error | 0.045 | 0.065 | 0.022 | 0.037 |
| Drift rate (v) | 0.239 | 0.199 | 0.350 | 0.042 |
| Nondecision time (T-er) | 0.641 | 0.711 | 0.106 | 0.078 |
| Switch Trials | ||||
| Reaction time | 0.899 | 1.046 | 0.115 | 0.118 |
| Error | 0.077 | 0.086 | 0.029 | 0.027 |
| Drift rate (v) | 0.209 | 0.181 | 0.033 | 0.031 |
| Nondecision time (T-er) | 0.645 | 0.729 | 0.109 | 0.078 |
Note. n = 20 per age group. Reaction time = mean value in seconds; error = mean proportion of incorrect responses; drift rate = quality of information accumulation; nondecision time = encoding and response time.
Cue-related activation
The event-related activation for cue-only trials, relative to the implicit baseline, is presented in Table 2. From the SPM analyses, the MNI coordinates were converted to Talairach space (Talairach and Tournoux, 1988), using the method of Lacadie et al. (2008), and the Talairach coordinates are listed in the table. The omnibus activation map included a cluster of 3169 voxels comprising extensive activation posteriorly, throughout the occipital and parietal lobes, and extending into the cerebellum. A second cluster of 1143 voxels extended through the middle frontal, superior frontal, and precentral gyri. Within this combined map of repeat and switch trials, several regions exhibited higher activation for older adults than for younger adults, with the largest clusters (136-201 voxels) in the occipitoparietal areas, bilaterally, and smaller clusters in the cerebellar, temporal, and prefrontal regions. One cluster in the right occipital lobe (61 voxels) exhibited higher activation for younger adults than for older adults.
Table 2.
Event-Related Activation Associated with Cue Processing
| Region | Hem | BA | Voxels | Talairach Coordinates | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Omnibus Activation | |||||||
| Superior Occipital Gyrus | R | 19 | 3169 | 37 | -73 | 24 | 9.83 |
| Fusiform Gyrus | L | 19 | 3169 | -31 | -68 | -7 | 9.50 |
| Inferior Occipital Gyrus | R | 19 | 3169 | 45 | -76 | -3 | 9.48 |
| Middle Frontal Gyrus | R | 6 | 1143 | 40 | -1 | 45 | 9.87 |
| Superior Frontal Gyrus | R | 6 | 1143 | 4 | 7 | 52 | 8.26 |
| Precentral Gyrus | L | 6 | 1143 | -48 | -5 | 49 | 7.45 |
| Thalamus | L | — | 103 | -7 | -21 | 0 | 5.12 |
| Thalamus | R | — | 103 | 7 | -25 | 0 | 4.86 |
| Thalamus | R | — | 103 | 15 | -13 | 7 | 4.17 |
| Claustrum | L | — | 59 | -27 | 17 | 7 | 6.31 |
| Omnibus Activation, Older > Younger | |||||||
| Precuneus | L | 19 | 201 | -28 | -68 | 36 | 5.33 |
| Precuneus | L | 7 | 201 | -12 | -68 | 39 | 3.20 |
| Supramarginal Gyrus | L | 40 | 201 | -36 | -46 | 36 | 2.77 |
| Inferior Parietal Lobule | R | 40 | 141 | 37 | -49 | 36 | 4.02 |
| Precuneus | R | 19 | 141 | 37 | -68 | 36 | 3.96 |
| Middle Occipital Gyrus | R | 19 | 141 | 37 | -85 | 9 | 1.87 |
| Middle Frontal Gyrus | R | 9 | 136 | 39 | 12 | 29 | 3.19 |
| Precentral Gyrus | R | 9 | 136 | 47 | 5 | 37 | 2.46 |
| Precentral Gyrus | L | 6 | 83 | -43 | 0 | 32 | 3.61 |
| Cerebellum (Culmen) | L | — | 79 | -23 | -44 | -8 | 3.06 |
| Cerebellum (Declive) | L | — | 79 | -27 | -61 | -21 | 2.86 |
| Fusiform Gyrus | L | 20 | 79 | -30 | -41 | -17 | 1.97 |
| Inferior Temporal Gyrus | R | 37 | 76 | 56 | -61 | -7 | 3.29 |
| Cerebellum (Declive) | R | — | 76 | 43 | -54 | -20 | 2.91 |
| Cerebellum (Culmen) | R | — | 76 | 27 | -45 | -20 | 2.05 |
| Middle Temporal Gyrus | L | 37 | 38 | -49 | -52 | 0 | 3.02 |
| Inferior Temporal Gyrus | L | 19 | 38 | -50 | -64 | -3 | 2.35 |
| Middle Temporal Gyrus | L | 21 | 38 | -53 | -44 | 9 | 2.11 |
| Middle Frontal Gyrus | L | 6 | 31 | -24 | 0 | 60 | 2.60 |
| Omnibus Activation, Younger > Older | |||||||
| Lingual gyrus | R | 19 | 61 | 24 | -70 | 4 | 2.64 |
| Fusiform gyrus | R | 37 | 61 | 35 | -52 | -3 | 2.34 |
| Cuneus | R | 18 | 61 | 16 | -81 | 16 | 1.90 |
| Switch > Repeat, Both Groups | |||||||
| Supramarginal Gyrus | R | 40 | 526 | 56 | -46 | 25 | 4.71 |
| Precuneus | R | 39 | 526 | 37 | -65 | 32 | 4.53 |
| Middle Temporal Gyrus | R | 37 | 526 | 54 | -49 | -7 | 4.42 |
| Middle Frontal Gyrus | R | 8 | 442 | 43 | 34 | 41 | 4.30 |
| Insula | R | 13 | 442 | 31 | 14 | 15 | 3.86 |
| Precentral Gyrus | R | 6 | 442 | 31 | 0 | 28 | 3.57 |
| Cerebellum (Uvula) | L | — | 292 | -15 | -76 | -25 | 5.97 |
| Fusiform Gyrus | L | 19 | 292 | -46 | -65 | -11 | 4.31 |
| Fusiform Gyrus | L | 37 | 292 | -34 | -49 | -12 | 3.75 |
| Thalamus | R | — | 61 | 15 | -13 | 12 | 3.53 |
| Thalamus | R | — | 61 | 11 | -28 | 4 | 3.52 |
| Thalamus | L | — | 61 | -7 | -21 | 3 | 3.44 |
| Inferior Parietal Lobule | L | 40 | 42 | -36 | -49 | 40 | 3.03 |
| Middle Frontal Gyrus | L | 6 | 18 | -44 | 2 | 53 | 3.17 |
| Switch >Repeat, Older > Younger | |||||||
| No significant clusters | |||||||
| Switch > Repeat, Younger > Older | |||||||
| No significant clusters | |||||||
| Repeat > Switch, Both Groups | |||||||
| No significant clusters | |||||||
Note. Omnibus activation = cue-only, switch and repeat trials combined and both age groups combined, relative to the implicit baseline (inter-trial interval; ITI). Hem = hemisphere; L = left; R = right; BA = Brodmann area; Voxels = number of voxels in cluster; Talairach Coordinates = location in standard stereotaxic space (Talairach and Tournoux, 1988); T = T value of cluster peak. For the switch > repeat contrast, the local maximum of each cluster, presented in bold, was selected for analyses of functional connectivity (Figure 3).
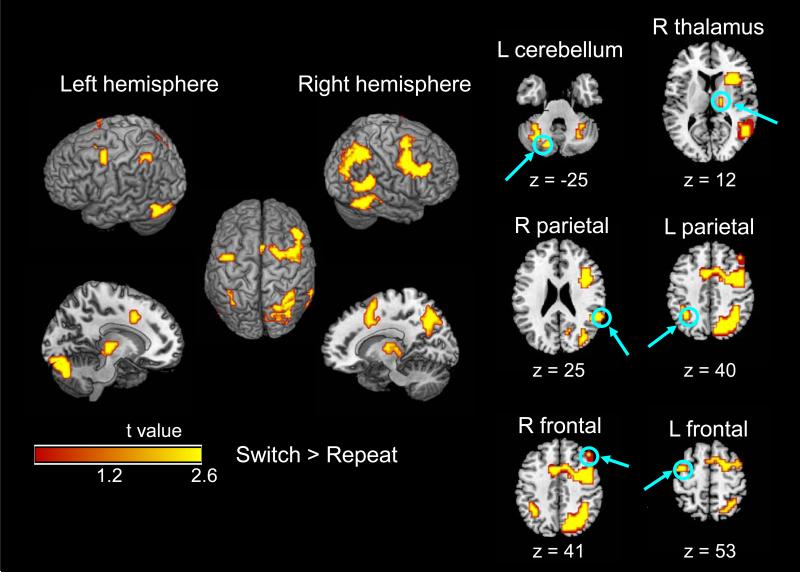
Within this omnibus map, six clusters exhibited higher activation on switch trials than on repeat trials (Table 2, Figure 3). The prefrontal and occipitoparietal activations were bilateral but more extensive in the right hemisphere, whereas the cerebellar/occipital activation was limited primarily to the left hemisphere. The two age groups did not differ significantly in the magnitude of the switch-related activation. No significant activation was observed for the repeat > switch contrast.
Figure 3.
Event-related activation for switch > repeat on the cue-only trials, within the omnibus map (Table 2). No age group difference was significant for the switch > repeat contrast. Individual slices represent local maxima of the switch > repeat contrast for both age groups combined (Table 2, bold values).
Target-related activation
The event-related, omnibus activation map for cue+target trials (repeat and switch combined) is presented in Table 3. As noted previously (Materials and Methods), this map was thresholded to represent activation associated with the target, above that associated with the cue. A single cluster of 5105 voxels represented widespread, target-related activation, which did not differentiate into smaller clusters. The local maxima were highest in the occipital lobes bilaterally, but activation in the frontal, parietal, and cerebellar regions was also clearly evident. The age group contrast yielded seven clusters of higher activation for older adults than for younger adults, most prominently in the prefrontal cortex bilaterally but also including basal ganglia and cerebellum. One cluster in the left fusiform gyrus exhibited higher activation for younger adults than for older adults.
Table 3.
Event-Related Activation Associated with Target Processing
| Region | Hem | BA | Voxels | Talairach Coordinates | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Omnibus Activation, Both Groups | |||||||
| Inferior Occipital Gyrus | R | 17 | 5105 | 29 | -94 | -7 | 10.09 |
| Inferior Frontal Gyrus | L | 45 | 5105 | -42 | 22 | 15 | 9.65 |
| Inferior Frontal Gyrus | L | 9 | 5105 | -42 | 7 | 24 | 9.46 |
| Omnibus Activation, Older > Younger | |||||||
| Postcentral Gyrus | R | 3 | 918 | 33 | -36 | 48 | 4.36 |
| Postcentral Gyrus | R | 2 | 918 | 47 | -26 | 28 | 3.45 |
| Middle Frontal Gyrus | R | 6 | 918 | 37 | -3 | 61 | 3.25 |
| Cerebellum (Culmen) | L | — | 621 | -15 | -45 | -12 | 3.35 |
| Posterior Cingulate | L | 31 | 621 | -11 | -62 | 16 | 3.24 |
| Diencephalic | R | — | 621 | 7 | -25 | -8 | 2.87 |
| Insula | R | 13 | 128 | 38 | -9 | 16 | 2.95 |
| Claustrum | R | — | 128 | 27 | 10 | 12 | 2.46 |
| Inferior frontal gyrus | R | 47 | 128 | 30 | 20 | -5 | 2.31 |
| Lentiform nucleus | L | — | 74 | -23 | 16 | -1 | 3.12 |
| Inferior frontal gyrus | L | 47 | 74 | -35 | 19 | -9 | 2.26 |
| Insula | L | — | 74 | -46 | 0 | 3 | 2.20 |
| Cerebellum (Declive) | L | — | 41 | -36 | -77 | -20 | 3.09 |
| Inferior frontal gyrus | L | 9 | 22 | -54 | 19 | 23 | 2.34 |
| Cerebellum (Declive) | R | — | 21 | 50 | -77 | -16 | 2.18 |
| Cerebellum (Declive) | R | — | 21 | 45 | -69 | -16 | 2.04 |
| Cerebellum (Tuber) | R | — | 21 | 45 | -66 | -25 | 1.76 |
| Omnibus Activation, Younger > Older | |||||||
| Fusiform gyrus | L | 37 | 21 | -38 | -56 | 0 | 2.51 |
| Switch > Repeat, Both Groups | |||||||
| Precuneus | L | 7 | 552 | -28 | -53 | 48 | 4.47 |
| Inferior Parietal Lobule | L | 40 | 552 | -32 | -45 | 40 | 4.43 |
| Precuneus | L | 7 | 552 | -24 | -68 | 32 | 4.35 |
| Postcentral Gyrus | R | 3 | 164 | 36 | -25 | 44 | 4.58 |
| Postcentral Gyrus | R | 40 | 164 | 54 | -23 | 24 | 2.74 |
| Posterior Cingulate | L | 31 | 64 | -15 | -66 | 16 | 3.80 |
| Posterior Cingulate | R | 31 | 64 | 16 | -66 | 16 | 3.40 |
| Posterior Cingulate | R | 30 | 64 | 20 | -66 | 8 | 3.27 |
| Inferior Occipital Gyrus | R | 19 | 30 | 33 | -75 | 0 | 3.65 |
| Middle Occipital Gyrus | R | 19 | 30 | 45 | -79 | 0 | 2.81 |
| Middle Temporal Gyrus | L | 37 | 13 | -57 | -52 | 0 | 3.21 |
| Switch > Repeat, Older > Younger | |||||||
| No significant clusters | |||||||
| Switch > Repeat, Younger > Older | |||||||
| No significant clusters | |||||||
| Repeat > Switch, Both Groups | |||||||
| No significant clusters | |||||||
Note. Omnibus activation = contrast for cue+target > cue-only, switch and repeat trials combined and both age groups combined, masked inclusively for all cue+target trials greater than the implicit baseline (inter-trial interval; ITI). Hem = hemisphere; L = left; R = right; BA = Brodmann area; Voxels = number of voxels in cluster; Talairach Coordinates = location in standard stereotaxic space (Talairach and Tournoux, 1988); T = T value of cluster peak. For the switch > repeat contrast, the local maximum of each cluster, presented in bold, was selected for analyses of functional connectivity (Figure 4).
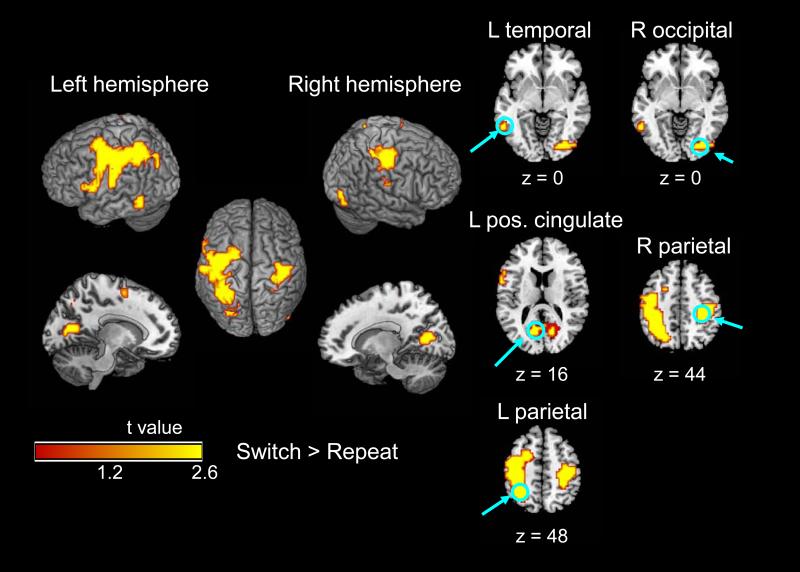
Five clusters in the target-related omnibus map exhibited higher activation for switch trials than for repeat trials (Table 3, Figure 4). The most extensive cluster (552 voxels) spanned the lateral regions of the parietal and frontal lobes, primarily in the left hemisphere. Other clusters, in the right hemisphere, were located more posteriorly, with local maxima in the postcentral gyrus, posterior cingulate, and inferior occipital gyrus. The magnitude of the switch-related activation associated with the target did not exhibit significant age-related change. The repeat > switch contrast did not yield significant activation on these trials.
Figure 4.
Event-related activation for switch > repeat on the cue+target trials (cue-related activation subtracted), within the omnibus map (Table 3). No age group difference was significant for the switch > repeat contrast. Individual slices represent local maxima of the switch > repeat contrast for both age groups combined (Table 3, bold values).
Sustained activation
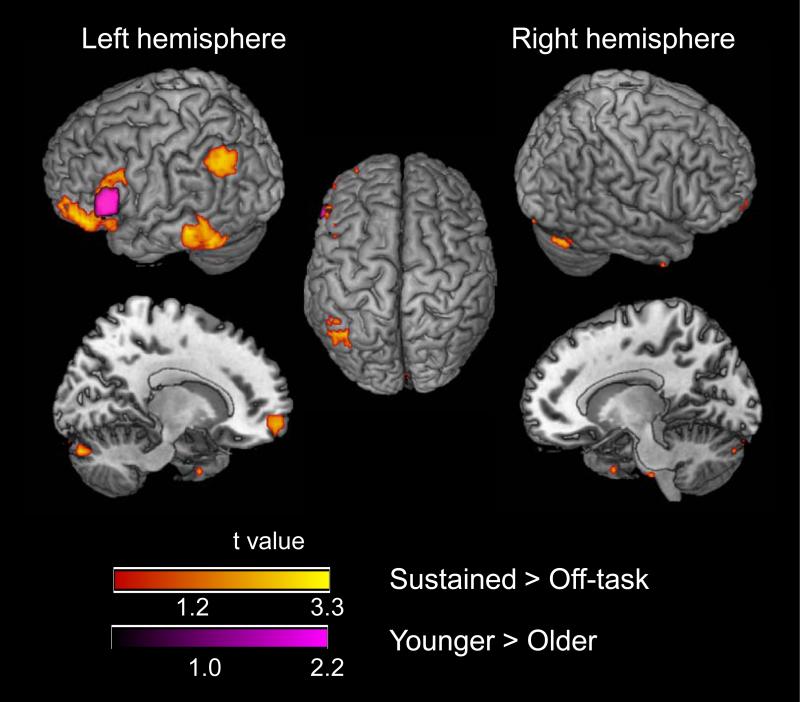
The magnitude of the sustained activation during the on-task periods, relative to the off-task baseline, for both age groups combined, is presented in Table 4 and Figure 5. This activation was located primarily in the left hemisphere, in the parietal, inferior temporal, and inferior prefrontal regions. Less extensive activation occurred in the middle frontal gyrus and cerebellum of the right hemisphere. The age group contrasts indicated that activation in the left inferior frontal gyrus was greater for younger adults than for older adults. No region of increased activation for older adults was observed.
Table 4.
Sustained Activation
| Region | Hem | BA | Voxels | Talairach Coordinates | T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Both Groups | |||||||
| Middle Temporal Gyrus | L | 21 | 140 | -56 | -37 | -4 | 3.30 |
| Inferior Temporal Gyrus | L | 20 | 140 | -52 | -49 | -12 | 2.79 |
| Inferior Frontal Gyrus | L | 45 | 139 | -54 | 26 | 11 | 3.08 |
| Middle Frontal Gyrus | L | 10 | 139 | -36 | 52 | -4 | 2.97 |
| Inferior Frontal Gyrus | L | 47 | 139 | -47 | 40 | -7 | 2.91 |
| Inferior Parietal Lobule | L | 40 | 77 | -48 | -53 | 41 | 2.68 |
| Angular Gyrus | L | 39 | 77 | -36 | -57 | 33 | 2.61 |
| Middle Frontal Gyrus | R | 11 | 32 | 23 | 51 | -9 | 2.49 |
| Cerebellum (Declive) | R | — | 29 | 9 | -82 | -20 | 3.15 |
| Cerebellum (Declive) | R | — | 14 | 45 | -73 | -20 | 2.48 |
| Cerebellum (Uvula) | R | — | 14 | 29 | -72 | -25 | 1.92 |
| Younger > Older | |||||||
| Inferior Frontal Gyrus | L | 45 | 37 | -54 | 22 | 11 | 3.29 |
| Older > Younger | |||||||
| No significant clusters | |||||||
Note. Hem = hemisphere; L = left: R = right; BA = Brodmann area; Voxels = number of voxels in cluster; Talairach Coordinates = location in standard stereotaxic space (Talairach and Tournoux, 1988); T = T value of cluster peak.
Figure 5.
Sustained activation during the on-task periods, relative to the off-task periods (see Materials and Methods). The red-yellow color scale represents active voxels for both age groups combined. The purple color scale represents greater activation for younger adults than for older adults (Table 4). No activation was significantly greater for older adults than for younger adults.
In the mixed blocked/event-related design, event-related activation may contribute to the estimated sustained effects (Braver et al., 2003; Burgund et al., 2003; Donaldson et al., 2001; Otten et al., 2002; Visscher et al., 2003). In the present data, all of the correlations between the event-related and sustained effects (per participant, within each run), were positive and ranged from r = 0.26 to r = 0.52. We therefore analyzed the age difference in the sustained activation as percent signal change, covaried for the corresponding event-related effect. At the local maximum in the left inferior frontal gyrus (BA 45), which was the region exhibiting the age difference in sustained activation, the percent signal change in the sustained activation was 23.77 (SD = 22.77) for younger adults and 1.45 (SD = 23.71) for older adults. Two covariates were constructed, one for switch-related activation on cue-only trials and one for the switch-related activation on cue+target trials. For each participant, the mean event-related, percent signal change, across all trials, was obtained from the local maxima associated with switching on the cue-only trials (Table 2) and on the cue+target trials (Table 3). The age-related decline in sustained activation, at the left inferior frontal gyrus, was significant when covaried for the event-related activation, F(1, 37) = 7.58, p < .01.
Functional connectivity of switch-related activation on cue-only trials
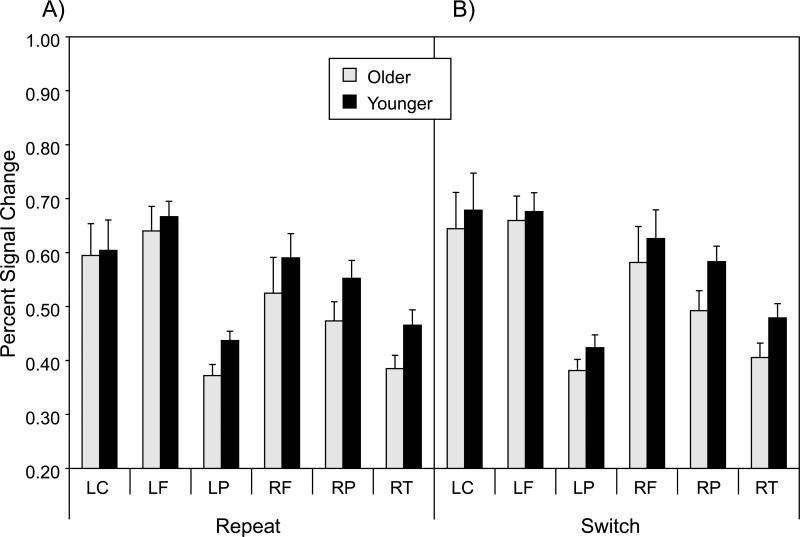
As a measure of functional connectivity of switch-related activation on cue-only trials, we first isolated the percent change in the fMRI signal, on each trial, for each participant, at the six voxels corresponding to the local maxima for the switch > repeat contrast, in the omnibus activation map for cue-only trials (Table 2; Figure 3). The percent signal change values are presented in Figure 6. A multivariate analysis of variance (MANOVA) on these six values, with age group as a between-subjects variable, and trial type (repeat, switch) as a within-subjects variable, yielded only a significant main effect of trial type, F(6, 33) = 6.15, p < .01, reflecting the higher fMRI signal for switch trials relative to repeat trials, across the six local maxima.
Figure 6.
Mean percent signal change for each of the six local maxima of switch-related activation in the omnibus activation map for cue-only trials (Table 2, bold values). Panel A = repeat trials; Panel B = switch trials; LC = left cerebellum; LF = left frontal; LP = left parietal; RF = right frontal; RP = right parietal; RT = right thalamus.
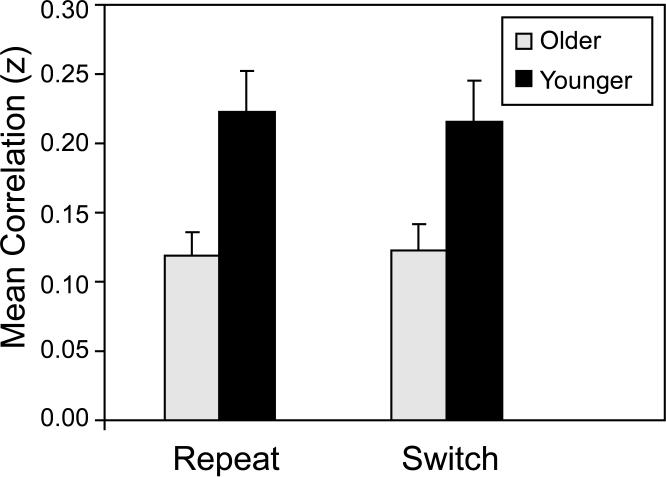
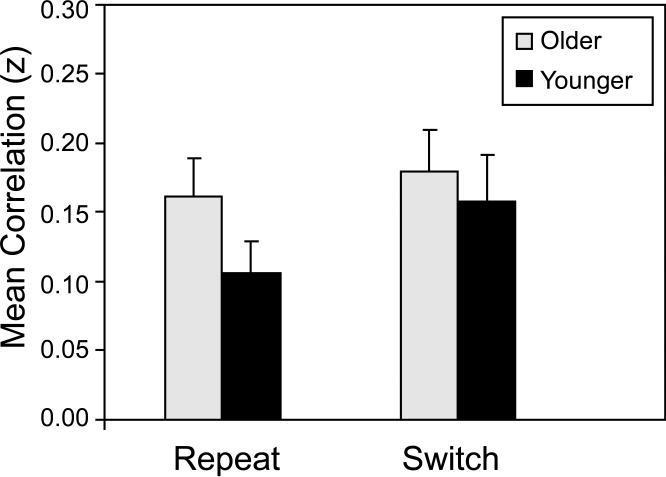
For each participant and trial type (repeat and switch), we computed all possible bivariate correlations (15 total), across trials, among the percent signal change values at the six local maxima. The Pearson r values, averaged across participants, ranged from -0.01 to 0.36 for younger adults, and from -0.06 to 0.28 for older adults. We then averaged the 15 Fisher z-transformed correlations, yielding a single summary measure for each participant within each trial type. Mean values for this summary measure are presented in Figure 7. An ANOVA of these data indicated that the mean correlation was significantly higher for younger adults than for older adults, F(1, 38) = 11.67, p < .001, but that neither the trial type main effect nor the Age Group × Trial Type interaction was significant (F < 1.0 in each case). This summary measure of cue-related, functional connectivity was significantly greater than zero for each combination of age group and trial type, with t(19) > 6.0, p < .0001, in each case.
Figure 7.
Functional connectivity on cue-only trials, as a function of age group and trial type. Across trials for each participant, Fisher z-transformed correlations were obtained for each of the bivariate correlations of percent signal change, among the six local maxima associated with the switch > repeat contrast (Table 2, bold values; Figure 6). Data are the mean values of all 15, z-transformed correlations. Higher z values represent increasing connectivity.
Functional connectivity of switch-related activation on cue+target trials
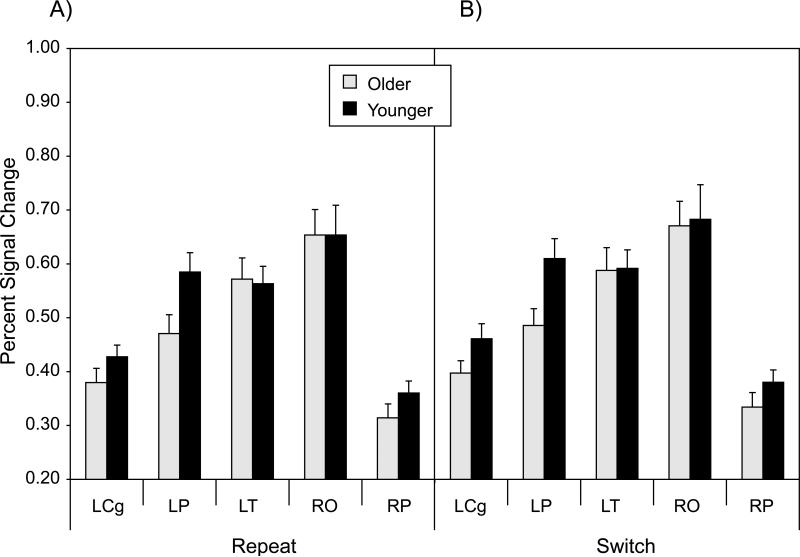
We used a similar approach to define functional connectivity on the cue+target trials, using the percent signal change at the five local maxima for the switch > repeat contrast in the omnibus activation map (Table 3; Figure 4). The percent signal change values at these local maxima are presented in Figure 8. A MANOVA of these values yielded significant main effects of age group, F(5, 34) = 2.83, p < .05, and trial type, F(5, 34) = 4.01, p < .01, reflecting relatively higher activation for younger adults than for older adults, and for switch trials than for repeat trials, across the five local maxima.
Figure 8.
Mean percent signal change for each of the five local maxima of switch-related activation in the omnibus activation map for cue+target trials (Table 3, bold values). Panel A = repeat trials; Panel B = switch trials; LCg = left anterior cingulate; LP = left parietal; LT = left temporal; RO = right occipital; RP = right parietal.
There were ten possible bivariate correlations among the five local maxima. The Pearson r values, averaged across participants, ranged from 0.01 to 0.31 for younger adults and from -0.06 to 0.33 for older adults. We performed an ANOVA of these data, using the mean of the Fisher z-transformed values of these ten correlations, for each participant and trial type, as a summary measure (Figure 9). This analysis indicated that connectivity did not vary significantly across age group, but that connectivity was higher on switch trials than on repeat trials, F(1, 38) = 4.06, p < .05. The interaction term was not significant. The summary measure of target-related, functional connectivity was significantly greater than zero for each combination of age group and trial type, with t(19) > 4.50, p < .001, in each case.
Figure 9.
Functional connectivity on cue+target trials, as a function of age group and trial type. Across trials for each participant, Fisher z-transformed correlations were obtained for each of the bivariate correlations of percent signal change, among the five local maxima associated with the switch > repeat contrast (Table 3, bold values; Figure 8). Data are the mean values of all 10, z-transformed correlations. Higher z values represent increasing connectivity.
Functional connectivity, cognitive performance, and white matter integrity
We examined whether the functional connectivity measures were mediators of age differences in cognitive performance, that is, whether the age-related variance in drift rate and nondecision time is attenuated when controlled for functional connectivity. To qualify as a mediator, a variable (e.g., functional connectivity) must exhibit both a significant age group difference and an age-independent relation to performance (Baron and Kenny, 1986). Because the age group difference in the summary measure of functional connectivity was significant for cue-related activation, but not for target-related activation (Figures 7 and 9), the mediational analyses were limited to the cue-related data.
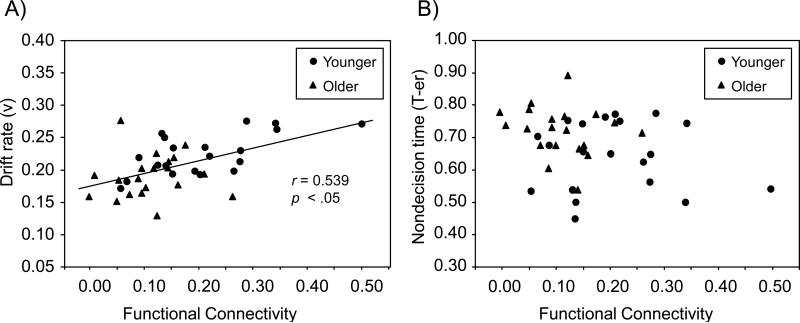
For the cue-only trials, we first examined whether the summary measure of functional connectivity exhibited an age-independent relation to the RT distribution measures of drift rate and nondecision time. The age group difference in the drift rate and nondecision time measures did not vary as a function of trial type (Table 1), and thus we averaged over trial type in creating mean drift rate and nondecision time variables. With age group included in the regression model, functional connectivity on cue-only trials was a significant predictor of mean drift rate, t(37) = 2.68, SE = 0.055, p < .01, reflecting an age-independent effect. As illustrated in Figure 10, increasing functional connectivity was associated with higher drift rate (i.e., more efficient semantic retrieval). Functional connectivity did not exhibit an age-independent relation to nondecision time.
Figure 10.
Relation between functional connectivity on cue-only trials and target categorization. Panel A = connectivity (x-axis) versus the efficiency of semantic retrieval (drift rate) in the categorization response (y-axis); Panel B = connectivity (x-axis) versus nondecision time (T-er) in the categorization response (y-axis). Connectivity is the mean of all correlations, of event-related activation for the switch > repeat contrast on cue-only trials (Figure 7), averaged across trial type (repeat, switch).
To examine the mediation of age-related effects in drift rate, we used hierarchical regression analyses in which age group was entered, as a predictor of drift rate on cue-only switch trials, both alone and following functional connectivity (Salthouse, 1992). These analyses demonstrated that functional connectivity was a powerful mediator and accounted for 74% of the age-related variance in drift rate, on cue-only trials (Table 5).
Table 5.
Mediation of Drift Rate by Functional Connectivity on Cue-Only Trials
| B | SE B | r2 | Δ r2 | F | Percentage Attenuation | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Age Group | -0.017 | 0.005 | 0.220 | 10.74** | ||
| Model 2 | ||||||
| Functional Connectivity | 0.196 | 0.050 | 0.291 | 15.58*** | ||
| Age Group | -0.010 | 0.006 | 0.347 | 0.057 | 3.21 | 74.09 |
Note. B = parameter estimate; SE = standard error; r2 = cumulative r2 for current and preceding steps; Δ r2 = unique effect of age.
p < .01
p < .001
Madden et al. (2009b) reported that, in these data, white matter integrity, as assessed from DTI, was a significant mediator of age-related differences in drift rate. Specifically, fractional anisotropy (FA) in two regions, the central portion of the genu and the spleniumparietal fibers of the right hemisphere, satisfied the tests of mediation and led to significant attenuation of age-related variance in drift rate. The possibility thus exists that the mediation of age-related differences in drift rate by functional connectivity (Table 5) is influenced by white matter integrity in the genu and splenium-parietal regions. With age group included in the regression model, however, the FA values associated with these regions were not significant predictors of functional connectivity (parameter estimate t[37] = 0.27, p > 0.50 for central genu, and t[36] = 1.59, p > 0.10, for right splenium-parietal). The FA variables did not exhibit an age-independent relation to functional connectivity, and thus the mediating effects of functional connectivity, on drift rate, were independent of the mediating effects of white matter integrity.
To examine potential mediation of the cognitive measures by sustained activation, we analyzed the relation between sustained activation at the local maximum of the age group difference (left inferior frontal gyrus; Table 4) and the drift rate and nondecision time measures. No age-independent relation was evident between the sustained activation and the cognitive performance measures.
Discussion
Our goal in this experiment was to investigate adult age differences in the functional neuroanatomy of one form of executive control—task switching—specifically, the pattern of event-related and sustained task-related activation, the functional connectivity of activated regions, and the role of white matter integrity in relation to functional connectivity and task performance. By including cue-only trials, we were able to distinguish the preparatory processes of task switching associated with the cue, independently of target identification and response processes (Brass and von Cramon, 2002; Slagter et al., 2006). For both age groups combined, the switch-related activation on cue-only trials comprised a broadly distributed network, with six clusters in dorsolateral prefrontal, parietal, diencephalic, and occipital/cerebellar regions (Table 2, Figure 3), consistent with previous studies of task switching (Barber and Carter, 2005; Brass and von Cramon, 2004; Garavan et al., 2000b; Kimberg et al., 2000; Liston et al., 2006; Sohn et al., 2000; Sylvester et al., 2003). In this experiment, the switch-related activation on cue-only trials was largely bilateral but more extensive in the right hemisphere, a laterality effect that has been noted in some imaging studies of visual attention (Imaruoka et al., 2003; Kim et al., 1999; Nobre et al., 1997). In contrast, the switch-related activation for target processing, beyond that associated with the cue, was more left-lateralized, especially in the parietal lobe (Table 3, Figure 4), which may represent the increased contribution of semantic retrieval during target identification (Binder et al., 2003; Price, 2000).
Adult age differences in event-related and sustained activation
We found that the pattern of event-related activation for task switching was similar for younger and older adults. In contrast to our initial expectation, the magnitude of event-related switching effects, for both cue and target processing, was comparable for the two age groups (Tables 2 and 3; Figures 3 and 4). Previous studies reporting age-related differences in task switching used a blocked design (DiGirolamo et al., 2001; Gold et al., 2010), rather than our mixed blocked/event-related design. Thus, our findings suggest that, at the level of individual trials, the magnitude of frontoparietal activation associated with switching between tasks does not vary significantly as a function of age group. The behavioral data (Table 1) also exhibited task switching effects that were constant across age group, which has been noted in some previous behavioral studies (Mayr, 2001; Verhaeghen and Cerella, 2002). Overall, our data suggest that age-related differences associated with task switching occur across most or all of the component processes (Salthouse et al., 2003), beyond the level of task-specific processing on individual trials.
The event-related activation in the omnibus maps, however, to which both switch and repeat trials contributed equally, did exhibit significant age-related differences (Tables 2 and 3). The pattern of these age-related effects in the omnibus maps resembled the previous studies of task switching and aging (DiGirolamo et al., 2001; Gold et al., 2010), in that several regions of the frontoparietal network (particularly prefrontal regions), for both cue and target processing, were greater in magnitude for older adults than for younger adults. A more limited number of regions in the event-related, omnibus activation maps, primarily in occipital cortex, exhibited greater activation for younger adults than for older adults. Because these maps do not represent specific, task-related contrasts, the relevant cognitive operations cannot be defined. These results are valuable for demonstrating that the age similarity observed for the task switching contrasts was not due to an inability to detect an age-related difference in the data. In addition, the trend of the age-related effect in the event-related, omnibus activation maps, with younger adults exhibiting higher activation in occipital cortex, and the older adults exhibiting higher activation in prefrontal cortex, illustrates the posterior-anterior shift in aging (PASA) pattern described by Davis, Dennis, Daselaar, Fleck, and Cabeza (2008). Overall, the age-related increase in prefrontal activation in the omnibus activation maps, combined with the age similarity in activation for task switching, support a nonselective recruitment model (Logan et al., 2002). That is, older adults activate multiple prefrontal regions during task performance, rather than limiting activation to those regions specifically supporting the executive control of task switching.
Sustained activation, for both age groups combined, occurred in several prefrontal, parietal, and ventral temporal regions, primarily in the left hemisphere (Table 4 and Figure 5). Because the sustained activation is defined as occurring throughout the on-task period, the relevant cognitive processing is difficult to define; and the sustained analyses used a less conservative threshold than the event-related analyses. The pattern of regional activation is consistent with the engagement of linguistic and semantic memory retrieval processes necessary for the categorization of the target words (Binder et al., 2003; Price, 2000). This sustained activation differs from the sustained activation of right prefrontal regions, during task switching, reported by Braver et al. (2003). The present experiment differs in several respects from that of Braver et al., specifically in our use of cue-only trials. Also, Braver et al. used a single-task baseline for the estimation of sustained activation, which would subtract many of the linguistic processes contributing to the sustained effect here.
In comparing age groups, we found that sustained activation within the left inferior frontal gyrus (BA 45), covaried for event-related activity, was lower for older adults than for younger adults (Table 4 and Figure 5). Imaging studies have yielded evidence for left inferior frontal gyrus involvement in several forms of cognitive processing relevant for word categorization and task switching, including semantic retrieval (Demb et al., 1995; Fiez, 1997), the selection of response alternatives (Thompson-Schill et al., 1997), and inhibitory control (Brass and von Cramon, 2004; Swick et al., 2008). Thus, older adults’ nonselective prefrontal activation, at the individual trial level, is associated with a decrease in sustained activation during the on-task periods. These findings are broadly similar to those of Dennis et al. (2007), who reported an age-related decline in sustained prefrontal activation during memory encoding, combined with an age-related increase in transient, prefrontal activation.
Adult age differences in functional connectivity
The analyses of functional connectivity demonstrated that although the magnitude of event-related activation for task switching was similar for younger and older adults, age-related differences were evident in the temporal coherence of switch-related regions.1 From the omnibus activation maps of all participants combined, we calculated percent signal change at the local maxima of switch-related activation for both cue and target processing (Figures 6 and 8). The average correlation of event-related activation, across trials, associated with the cue, was significantly higher for younger adults than for older adults (Figure 7). This finding supported our initial hypothesis, based on previous findings of age-related decline in functional connectivity during episodic memory performance (Daselaar et al., 2006a; Dennis et al., 2008; Grady, 2005). These data suggest that age-related decline occurs in the functional connectivity of the preparatory processes associated with the cue. For target-related regions, in contrast, functional connectivity was similar for the two age groups (Figure 9). Note that although the local maxima were defined by the switch-related activation, the degree of functional connectivity, for both cue and target processing, did not vary significantly across switch and repeat trials (Figures 7 and 9). Thus, these frontoparietal networks were engaged consistently across all trials and were not limited to switch trials.
Individual differences in the degree of functional connectivity associated with cue processing mediated age-related differences in the categorization responses to the target. From a model of the reaction time distributions (Wagenmakers et al., 2007), we separated the decisional component of categorization (e.g., semantic retrieval) from the nondecisional component (e.g., target encoding and response selection). As reported in previous analyses of the behavioral data (Madden et al., 2009b), older adults exhibited both less efficient decisional processing (drift rate) and slower nondecisional processing, relative to younger adults (Table 1). We adopted the Baron and Kenny (1986) criteria and required that an age-independent relation exist, between functional connectivity and behavioral performance, to qualify as a mediating effect. An age-independent relation was present for drift rate, but not for nondecision time. As illustrated in Figure 10, increasing functional connectivity of cue-related activation was associated with higher drift rate, that is, more efficient retrieval of target-relevant, semantic information. Further, including cue-related, functional connectivity as a predictor, in the regression model for drift rate, attenuated the age-related variance in drift rate by 74% (Table 5), demonstrating a substantial degree of mediation.
Thus, our initial hypothesis that functional connectivity would mediate age-related effects in cognitive performance held for the activation associated with the cue, in relation to decision processes associated with the target. The age-related decline in drift rate for target categorization is in some respects surprising, because performance in semantic tasks is often well preserved for older adults (Burke and Shafto, 2008; Mayr and Kliegl, 2000). In this particular task, however, participants held in mind different categorization criteria and switched between them as instructed by the cue, which required more executive control processing than typical measures of semantic retrieval (e.g., lexical decision). By adding a single-task condition, it would be possible to test this interpretation of the age-related difference in drift rate.
The role of white matter integrity
In a previous analysis of the relation between these cognitive performance data and the DTI data (Madden et al., 2009b), we demonstrated that individual differences in white matter integrity within two regions, the genu of the corpus callosum and splenium-parietal fibers in the right hemisphere, mediated the age-related decline in drift rate (see also Gold et al., 2010; Perry et al., 2009). This finding suggests that age-related decrease in white matter integrity, within the frontoparietal network, leads to a disconnection among task-relevant cortical regions (Madden et al., 2009a; Sullivan and Pfefferbaum, 2006). Given the observed mediation of age-related differences in drift rate, by cue-related functional connectivity, we hypothesized that white matter integrity, within the two previously identified regions, contributed significantly to the relation between functional connectivity and drift rate. Our analyses, however, did not detect the hypothesized relation. Individual differences in white matter integrity (FA) did not exhibit an age-independent relation to the functional connectivity associated with cue processing. Although other studies have reported a correlation between white matter integrity and resting state (i.e., intrinsic) functional connectivity (Andrews-Hanna et al., 2007; Chen et al., 2009), we did not observe the relation for task-dependent activation. Thus, in this form of executive control, both white matter integrity and cue-related functional connectivity are mediators of age-related variability in the efficiency of target categorization (drift rate), but these mediating effects appear to be independent of each other. Although this negative result should be interpreted with caution, it is possible that intrinsic functional connectivity is related more closely to anatomical integrity than is task-dependent functional connectivity (Madden et al., 2009a).
Limitations
The design and analysis of this research are limited in several respects. In the analyses of both brain activation and cognitive performance, we relied on comparisons between two age categories, younger and older adults. This approach maximizes the sensitivity of the age group effect, but without intermediate age values, the trajectory of the age-related function cannot be defined. A related limitation is that the comparisons are cross-sectional, and thus differences between age groups reflect the differences between cohorts of participants, as well as chronological age. Estimation of true age-related change requires a longitudinal assessment, within participants. Our results point to task set reconfiguration processes (Meiran et al., 2000; Monsell, 2003; Monsell and Mizon, 2006), as a source of age-related difference in functional connectivity, but cue encoding time may also contribute, independently of executive control (Logan and Bundesen, 2003). In addition, given the variety of analyses we conducted, we limited the scope of our inquiry to positive activations (i.e., changes in the fMRI signal above baseline), and deactivations may also occur (i.e., priming effects), which we have not addressed (Dobbins et al., 2004; Lustig and Buckner, 2004; Persson et al., 2007). The activation of the frontoparietal network associated with executive control may also change significantly as a function of practice (Erickson et al., 2007; Garavan et al., 2000a; Kelly and Garavan, 2005). Finally, in our analyses of white matter integrity we limited the analysis to white matter regions that had been identified previously as mediators of age-related variability in the behavioral data. Without this constraint, it may be possible to identify white matter regions that are related significantly to age-related effects for functional connectivity.
Conclusion
In this experiment we provide the first evidence, to our knowledge, of an age-related difference in functional connectivity during executive control. In a cued word categorization task, the requirement to switch between categorization tasks, across trials, led to changes in behavioral performance (decrease in drift rate and increase in nondecision time) that were similar for younger and older adults. From cue-only trials, we isolated activation associated with switch-related preparatory processing, within a widely distributed frontoparietal network, that differed from switch-related activation occurring during target categorization. Younger and older adults were similar in the magnitude of event-related, frontoparietal activation associated with task switching, for both cue and target processing, although the omnibus activation maps exhibited several clusters of increased activation for older adults, relative to younger adults, suggesting a less selective pattern of activation on the part of older adults.
More critically, the functional connectivity of activation within switch-related regions, during cue processing, was higher for younger adults than for older adults. Further, individual differences in functional connectivity of these regions shared a substantial portion of the age-related variability in the efficiency of the categorization response to the target (drift rate). However, the functional connectivity of switch-related regions, during target processing, was comparable for the two age groups. Thus, functional connectivity of frontoparietal regions associated with preparatory processing is a significant mediator of age-related differences in the efficiency of target categorization. Further studies should address the individual components of executive control and the potential relation between functional connectivity and white matter integrity.
Acknowledgments
This research was supported by research grants R01 AG011622 (DJM), R01 AG019731 and R01 AG23770 (RC), and T32 AG000029 (MCC, NAD, BB) from the National Institute on Aging. We thank Susanne Harris, Scott Huettel, Sara Moore, Daniel Weissman, James Kragel, and Allen Song for assistance.
Footnotes
The percent signal change in the local maxima associated with task switching exhibited a trend of age-related decline (Figures 6 and 8), which was statistically significant for target-related processing (Figure 8). Thus, although the voxelwise, SPM analyses indicated age constancy in switch-related activation (Figures 3 and 4), the most highly activated voxels for the switch > contrast (local maxima) exhibited some degree of age-related decline.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb. Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: Theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Beck depression inventory. Psychological Corporation; New York: 1978. [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Jr., Howard DV. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20872. DOI: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. J. Cogn. Neurosci. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb. Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. J. Cogn. Neurosci. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Janowsky JS, Taylor SF, Yesavage JA, Mumenthaler MS, Jagust WJ, Reed BR. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. J. Exp. Psychol. Gen. 2001;130:746–763. [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Braver TS, West R. Working memory, executive control, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Psychology Press; New York: 2008. pp. 311–372. [Google Scholar]
- Burgund ED, Lugar HM, Miezin FM, Petersen SE. Sustained and transient activity during an object-naming task: A mixed blocked and event-related fMRI study. Neuroimage. 2003;19:29–41. doi: 10.1016/s1053-8119(03)00061-2. [DOI] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Language and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Psychology Press; New York: 2008. pp. 373–443. [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Chen N.-k., Chou Y.-h., Song AW, Madden DJ. Measurement of spontaneous signal fluctuations in fMRI: Adult age differences in intrinsic functional connectivity. Brain Struct. Funct. 2009;213:571–585. doi: 10.1007/s00429-009-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corouge I, Fletcher PT, Joshi S, Gouttard S, Gerig G. Fiber tract-oriented statistics for quantitative diffusion tensor MRI analysis. Med. Image Anal. 2006;10:786–798. doi: 10.1016/j.media.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cereb. Cortex. 2006a;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Prince SE, Cabeza R. The medial temporal lobe distinguishes old from new independently of consciousness. J. Neurosci. 2006b;26:5835–5839. doi: 10.1523/JNEUROSCI.0258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb. Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J. Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol. Aging. 2007;28:1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. Learn. Mem. Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E. General and task-specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF. Training-induced functional activation changes in dual-task processing: An fMRI study. Cereb. Cortex. 2006;17:192–204. doi: 10.1093/cercor/bhj137. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum. Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Fisher RA. On the “probable error” of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freiwald WA, Kanwisher NG. Visual selective attention: Insights from brain imaging and neurophysiology. In: Gazzaniga MS, editor. The cognitive neurosciences III. The MIT Press; Cambridge, MA: 2004. pp. 575–588. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc. Res. Tech. 2000a;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Li SJ, Stein EA. A parametric manipulation of central executive functioning. Cereb. Cortex. 2000b;10:585–592. doi: 10.1093/cercor/10.6.585. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex. 2007;17(Suppl 1):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol. Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Functional connectivity during memory tasks in healthy aging and dementia. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford University Press; Oxford: 2005. pp. 286–308. [Google Scholar]
- Hampshire A, Gruszka A, Fallon SJ, Owen AM. Inefficiency in self-organized attentional switching in the normal aging population is associated with decreased activity in the ventrolateral prefrontal cortex. J. Cogn. Neurosci. 2008;20:1670–1686. doi: 10.1162/jocn.2008.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: The penetration of cognition into action control. J. Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaruoka T, Yanagida T, Miyauchi S. Attentional set for external information activates the right intraparietal area. Brain Res. Cogn. Brain Res. 2003;16:199–209. doi: 10.1016/s0926-6410(02)00274-4. [DOI] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb. Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Keys BA, White DA. Exploring the relationship between age, executive abilities, and psychomotor speed. J. Int. Neuropsychol. Soc. 2000;6:76–82. doi: 10.1017/s1355617700611098. [DOI] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M. Modulation of task-related neural activity in task-switching: An fMRI study. Brain Res. Cogn. Brain Res. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychol. (Amst) 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Psychology Press; New York: 2008. pp. 189–249. [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychol. Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- LaBerge D. Networks of attention. In: Gazzaniga MS, editor. The new cognitive neurosciences. 2nd ed. MIT Press; Cambridge, MA: 2000. pp. 711–723. [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Logan GD, Bundesen C. Clever homunculus: is there an endogenous act of control in the explicit task-cuing procedure? J. Exp. Psychol. Hum. Percept. Perform. 2003;29:575–599. doi: 10.1037/0096-1523.29.3.575. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol. Rev. 2009a;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J. Cogn. Neurosci. 2009b;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiol. Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychol. Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Mayr U, Kliegl R. Complex semantic processing in old age: Does it stay or does it go? Psychol. Aging. 2000;15:29–43. doi: 10.1037//0882-7974.15.1.29. [DOI] [PubMed] [Google Scholar]
- Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cognit. Psychol. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Meiran N, Gotler A, Perlman A. Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2001;56:P88–102. doi: 10.1093/geronb/56.2.p88. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn. Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Monsell S, Mizon GA. Can the task-cuing paradigm measure an endogenous task-set reconfiguration process? J. Exp. Psychol. Hum. Percept. Perform. 2006;32:493–516. doi: 10.1037/0096-1523.32.3.493. [DOI] [PubMed] [Google Scholar]
- Mori S. Introduction to diffusion tensor imaging. Elsevier; Amsterdam: 2007. [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol. Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RSJ, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. State-related and item-related neural correlates of successful memory encoding. Nat. Neurosci. 2002;5:1339–1344. doi: 10.1038/nn967. [DOI] [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ, Jr., Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47:2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: A link to cognitive control? J. Cogn. Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Top-down mechanisms for working memory and attentional processes. In: Gazzaniga MS, editor. The cognitive neurosciences III. The MIT Press; Cambridge, MA: 2004. pp. 919–930. [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional neuroimaging. J. Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychol. Rev. 1978;85:59–108. [Google Scholar]
- Ratcliff R, Spieler D, McKoon G. Explicitly modeling the effects of aging on response time. Psychon. Bull. Rev. 2000;7:1–25. doi: 10.3758/bf03210723. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman AB, Kuller LH, Lopez OL, Carter CS. Functional neuroimaging indicators of successful executive control in the oldest old. Neuroimage. 2005;28:881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mechanisms of age-cognition relations in adulthood. Erlbaum; Hillsdale: NJ: 1992. [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J. Exp. Psychol. Gen. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Miles JD. Aging and time-sharing aspects of executive control. Mem. Cognit. 2002;30:572–582. doi: 10.3758/bf03194958. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Weissman DH, Giesbrecht B, Kenemans JL, Mangun GR, Kok A, Woldorff MG. Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn. Affect. Behav. Neurosci. 2006;6:175–189. doi: 10.3758/cabn.6.3.175. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: The role of prefrontal cortex and posterior parietal cortex in task switching. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of functional magnetic resonance imaging data. Psychol. Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain Cogn. 2009;70:1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci. Biobehav. Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: A review of meta-analyses. Neurosci. Biobehav. Rev. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, van der Maas HL, Grasman RP. An EZ-diffusion model for response time and accuracy. Psychon. Bull. Rev. 2007;14:3–22. doi: 10.3758/bf03194023. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wecker NS, Kramer JH, Wisniewski A, Delis DC, Kaplan E. Age effects on executive ability. Neuropsychology. 2000;14:409–414. doi: 10.1037//0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb. Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Uncapher MR, Rugg MD. Neural correlates of differential retrieval orientation: Sustained and item-related components. Neuropsychologia. 2006;44:3000–3010. doi: 10.1016/j.neuropsychologia.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Craik FI, Booth L. Executive function across the life span. Acta Psychol. (Amst) 2004;115:167–183. doi: 10.1016/j.actpsy.2003.12.005. [DOI] [PubMed] [Google Scholar]