Successful neurosurgical procedures hinge on the accurate targeting of regions of interest. Resection of brain tumors is enhanced by the surgeon's ability to accurately define margins. Epileptic foci are identified by coregistration of functional and antatomic information, and stereotactic targets must be pinpointed with submillimetric accuracy for surgical efficacy. Specialized neuronavigational tools have been developed over the last 20 years to assist surgeons in these endeavors; the development of MRI-guided navigation systems represents a significant improvement in the surgical treatment of various intracranial lesions. The ability for most intraoperative image guidance systems to remain faithful to the anatomy once the cranium has been opened remains problematic, however. “Brain shift,” the term applied to the dynamic change that intracranial anatomy undergoes after craniotomy, burr hole placement, drainage of cerebrospinal fluid, or resection of a lesion, compromises the localization of neural structures in space relative to where they were when preoperative images were acquired (Fig. 1).1–8 Gliomas also pose a particular challenge to surgeons because many of these tumors (particularly low-grade gliomas) do not possess distinct capsules. As a result, even well-trained human eyes are incapable of discerning where the border of the lesion ends and viable brain begins. This uncertainty leads to two problems: (1) inadequate resection secondary to the surgeon stopping at what appears to be grossly abnormal tissue (so as to avoid neurologic damage) and (2) neurologic damage caused by aggressive surgery in which resection ends only when clearly normal brain tissue is visualized.
Fig. 1.
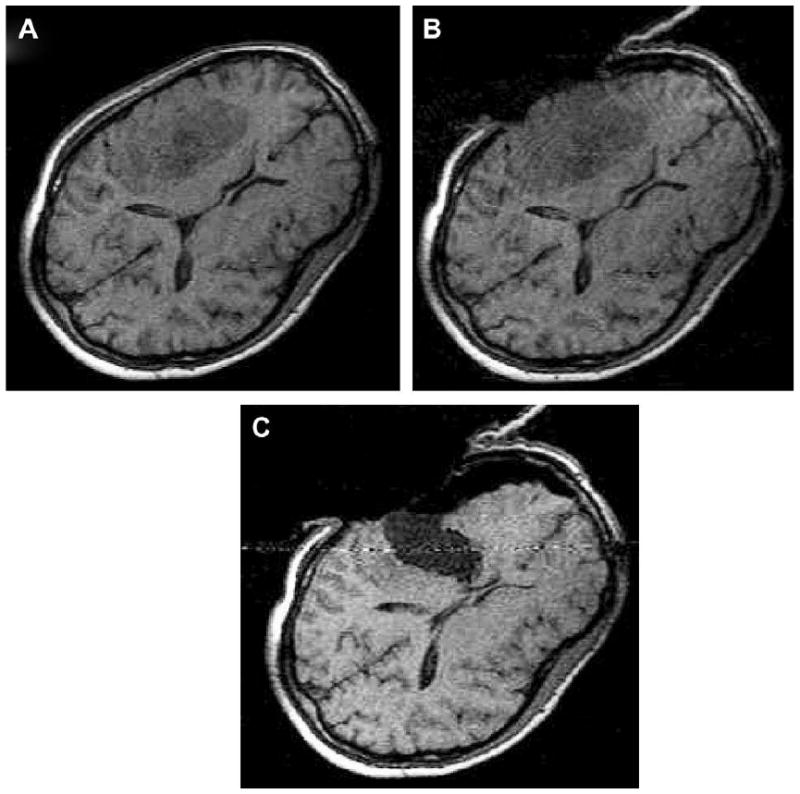
Brain shift. (A) T1-weighted axial MRI before craniotomy. (B) Same axial plane MRI after craniotomy performed. (C) MRI after lesion resection. Note the significant shift of intracranial contents after craniotomy, cerebrospinal fluid drainage, and lesion resection.
Only intraoperatively acquired images can provide neurosurgeons with the information needed to perform real-time, image-guided surgery. Uncertainty is reduced significantly when the surgeon places an instrument at the edge of what is believed to be the resection cavity, and a small nodule of tumor is immediately identified by intraoperative imaging. Avoidance and preservation of eloquent cortex such as motor, speech, and visual areas depend on precise identification of these regions during the procedure. The boundary between tumor and viable neural tissue is often difficult to see with the naked eye, so the superimposition of functional MRI, diffusion tensor imaging, and awake cortical mapping images eliminates a surgeon's uncertainty in determining tumor boundary and shifting brain structures. This leads to surgeons achieving maximal lesion resection while minimizing untoward neurologic sequelae. Maximal lesion resection is a principal goal in tumor resection because abundant evidence indicates that a more complete resection directly impacts the survival time of patients with low- and high-grade gliomas.9–18
Origins of Intraoperative MRI: 0.5T Open-Configuration Prototype
The origin of iMRI for neurosurgery was the Magnetic Resonance Therapy (MRT) Unit at Brigham and Women's Hospital (BWH) in Boston, Massachusetts. It began as a collaborative project among four groups: Ferenc Jolesz of the Department of Radiology at BWH, engineers at General Electric Medical Systems (Milwaukee, Wisconsin), the neurosurgical service at BWH with Dr. Peter Black as head, and the department of otorhinolaryngology with Marvin Fried as director. Throughout the late 1980s, these physicians and scientists collaborated in the development of an open-configuration MRI scanner that allowed surgery to be performed with concurrent intraoperative image guidance. At the time of inception, the closed-configuration of conventional MRI systems precluded direct access to the patient; therefore, fundamental changes in magnet and coil design and display methods were necessary to fully realize the concept of iMRI. This concept was a radical departure from MR physics of the time, with the magnetic field highest in the space between the double donut.
Early interventional procedures in an open MRI system were performed in a low-field imager by Gronemeyer and colleagues.19,20 This system provided access to patients through a horizontal gap in its magnet. Access was significantly limited, however, and open surgeries that required full access to patients were impractical. Based on this information, after discussing several alternative designs, a “double donut” magnet system that would allow free access to patients within the magnetic field was chosen by BWH for development.21 The initial research and development phase came to fruition in 1994 with the completion and installation of a prototype midfield intraoperative MRI system (GE 0.5-T Signa SP) unit at the BWH (Fig. 2).22–24
Fig. 2.
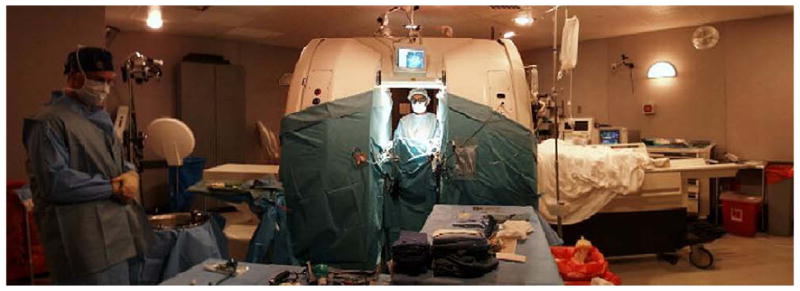
The MRT unit at BWH. The General Electric Signa 0.5T iMRI is an open-configuration “double donut” system that allows the surgeon to operate between each superconductive magnet coil (pictured).
Direct access to patients was achieved by the construction of two vertically oriented superconducting magnets with coils in separate but communicating cryocoolers. This design results in a vertical gap between the coils through which patients can be fully accessed during image acquisition. Niobium tin, which has a maximum superconducting transition at higher temperatures than the more common niobium titanium, allows for sufficient cooling of the coils and thermal shield with cryocooler assembly, which eliminates the need for liquid helium coolant. This design resulted in a significantly increased area of patient access: the modified magnet provides a spherical imaging volume 30 cm in diameter and a 56-cm wide area of patient access, allowing surgeons and first assistants to be positioned on either side of patients.21 In addition to the wide patient access area, the configuration of the “double-donut” magnet allows the position of patients within the imager to be flexible; the table can be inserted into the magnet along two orthogonal axes (“end docked” and “side docked”), which allows convenient access to different areas of anatomy. Because of the open configuration of the MRT, surgeons can perform various percutaneous, interventional, endoscopic, or open surgical procedures while standing or sitting and simultaneously viewing intraoperatively obtained MRI displayed on monitors placed in the gap of the magnet.
Many challenges needed to be met during the original implementation of iMRI, including the development of MRI-compatible equipment, instruments, and various tools along with the integration of the intraoperative display of images, the audiovisual communication among the team members, and the interactive manipulation of image data.
The initial phase of the iMRI project was slowed down by the unavailability of MR-compatible surgical instruments. In many cases, extensive changes were required to adapt instruments and equipment to the unique electromagnetic environment.25–28 Many ferromagnetic surgical instruments were replaced by titanium, providing the essential capabilities required for craniotomies without becoming a ballistic hazard. Several metallic instruments that were not ferromagnetic still caused a substantial artifact when placed near a target within the imaging field of view and could not be used. An early problem was the headholder, which had to be firm, nonferromagnetic, and flexible. It was possible to create a headholder similar to the Mayfield device made of high performance plastic, but it took many months. The next challenge was the power drill; for more than a year, the only procedures that could be done were biopsies because there was no way to turn a craniotomy flap. The Midas Rex Corporation (Medtronic, Minniapolis, Minnesota) finally was able to create a nonferromagnetic drill. The operating microscope was the third major device to be created; it was possible to create a plastic microscope with nonferromagnetic joints that, although simple, was adequate. Finally, we were able to develop a bipolar coagulator whose current would not interfere with the magnetic field. Each of these technologic developments took 6 to 12 months to complete, but gradually it was possible to do surgery in the intraoperative GE Signa system just as readily as in a routine operating room. Anesthesia and patient monitoring systems that did not emit any electronic noise and could function during a scan were developed and installed within the MRT suite29; fortunately, they had been created for performing pediatric MR imaging under anesthesia.
The development of imaging during surgery led to the “Surgical Planning Laboratory” at BWH. The occurrence of surgically induced volumetric deformations known as brain shift has been well established. There were no detailed analyses, however, of the changes that occur during surgery. As a result, Gering and colleagues30 at BWH developed a volumetric display software (3D Slicer; www.slicer.org) that allowed quantitative analysis of the degree and direction of brain shift (Fig. 3). For 25 patients, multiple intraoperative volumetric image acquisitions were extensively evaluated. It was found that brain shift is a continuous dynamic process that evolves differently in distinct brain regions. The authors concluded that only serial imaging or continuous data acquisition can provide consistently accurate image guidance.30,31 Further refinements in tracking intraoperative brain deformation were performed in a pilot study by Archip and colleagues in 2008.32
Fig. 3.
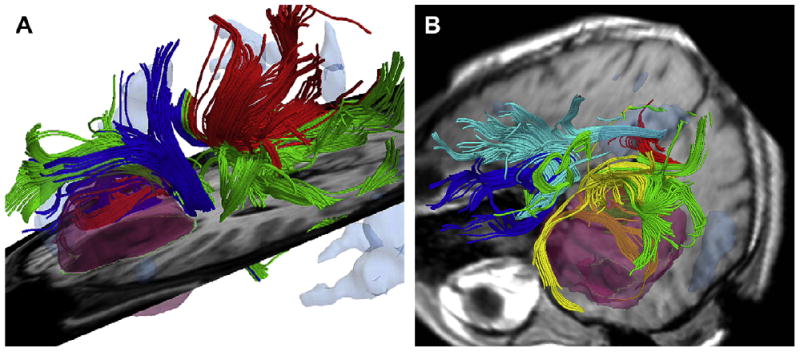
Intraoperative colocalization using iMRI images and 3-D Slicer software. (A) Tumor, functional MRI, and diffusion tensor imaging are colocalized with standard MRI. (B) After craniotomy is performed, 3-D Slicer compensates for brain shift, allowing surgeons to visualize not only shift of gross neuroanatomy but also functional regions and white matter tracts.
The combination of the 0.5-T iMRI and three-dimensional slicer transformed the MRT into an exceptionally effective tool for neurosurgeons. Since the first craniotomy for brain tumor resection in 1996, more than 1000 craniotomies for intracranial tumor resection have taken place in the MRT (40% low-grade gliomas, 50% high-grade gliomas, and 10% other intracranial lesions such as metastases, meningiomas, and vascular malformations).33,34 In most patients with brain tumor operated on in the MRT, resection rates of 80% or more were achieved.10 This percentage reinforces the value of iMRI in extension of lifespan of patients, because patients with subtotal tumor resection are at a higher risk of recurrence and death compared with patients with gross total tumor removal.
Expanding the Scope of Open-Configuration Intraoperative MRI
The BWH MRT was the original iMRI system but it was by no means the last. Because of the specialized nature of the equipment, the entire MRT suite needed to be custom-made to accommodate the scanner. Room shielding, coolant, and power consumption were only a few of the expensive and high-maintenance aspects of the MRT. iMRI had proven itself to be a powerful tool in the hands of neurosurgeons attempting to achieve maximal tumor resection while preserving neurologic function, but how could institutions and practitioners take advantage of this technology without embarking on the expense of a major remodeling of their operative suite?
There were several answers, all driven by neurosurgeons. A major center was Erlangen, where Rudolph Fahlbusch helped to develop multiple concepts of Siemens for intraoperative imaging. This system moved from a side-opening low field to a system in which a table rotated into and out of a 1.5-T closed-bore magnet. Fahlbusch showed that for pituitary tumors and low-grade gliomas this system had a major advantage over other systems. A second answer was driven by the Israeli surgeon Moshe Hadani and his group.35 Initially introduced in 2001 by Hadani and collagues,35 the PoleStar (Medtronic Navigation, Louisville, Colorado) N-10 iMRI offered an open-configuration, portable 0.12-T magnet that required only modest remodeling of the operative suite. The device was stored in what amounted to a small garage within the operating theater. The N-10's compact size and low magnetic footprint allowed units to be integrated into multiple institutions' conventional operating rooms. Despite a slightly increased time for induction of anesthesia and intubation, the units made a significant and positive impact on the safety and completeness of tumor resection in adult and pediatric intracranial procedures.36–41 PoleStar recently introduced a higher-field (0.15 T) N-20 (Fig. 4), and initial evaluations confirm that the accuracy, versatility, and quality of this new-generation iMRI scanner are at least as good as the N-10. Clearly further clinical analysis of the accuracy on clinical cases using the N-20 is needed to confirm that these results will bear out in surgical reality.36,37,42
Fig. 4.
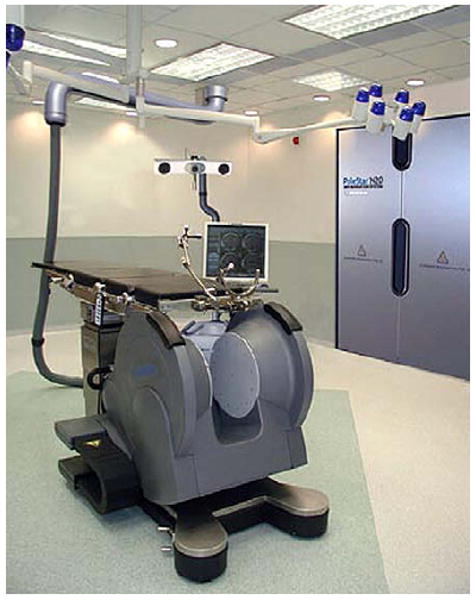
The PoleStar iMRI. The open-bore configuration PoleStar N-20, despite its low-field 0.15T magnet, has allowed many institutions to take advantage of iMRI without completely remodeling their operative suite to accommodate a larger, high-field stationary iMRI. The Polestar is compact enough to be stored in a shielded room (pictured on the right side of the figure) when not in use. If a smaller room cannot be dedicated to storage, an in-suite “hangar” can be set up to shield the magnet when not in use. (Courtesy of Medtronic Navigation, Louisville, CO; with permission.)
Other open-configuration iMRIs have since been developed. A 0.3-T horizontal iMRI by Hitachi at the University of Cincinnati43,44 is a customized diagnostic iMRI, but the draping configuration does not lend itself to the sterile nature of neurosurgical interventions.
Origins of Closed-Configuration Intraoperative MRI
A significant shortfall of the BWH MRT was the relatively low field offered by such a specialized system: the 0.5-T field did not yield image resolution comparable to contemporary diagnostic 1.5-T and 3-T MRI scanners. Initially, the basis of the double-donut design was that sacrifice of high-field imaging was acceptable if the patient did not have to be moved. This paradigm of imaging on demand proved to be efficient and effective over more than a decade. The field moved toward a paradigm of “in and out” imaging, however, primarily to enable the use of more off-the-shelf scanners. Surgical teams have developed protocols for moving patients in and out of the scanner that are relatively rapid and efficient. Methods to maintain patient registration data throughout the procedure were developed using an integrated overhead navigation camera or fixed markers on patients.45,46 As a result of these findings, it was felt that the next iteration that should evolve from the original open-configuration MRT would be developed from a high-field, closed-bore system. Consequently, several static closed-bore 1.5-T and 3-T systems have been installed in a growing number of institutions.46,47 They are essentially hybrid systems that can be used for imaging or surgery.
The IMRIS system was developed by a neurosurgeon, Dr. Garnette Sutherland of Calgary, Alberta, Canada. This system offers a unique intraoperative rail-mounted system in which the scanner is brought to patients (Fig. 5). By enabling the MR system to move to patients, the system allows for improved surgical work-flow and enhanced patient safety in the surgical environment. The 70-cm bore 1.5-T magnet is able to move from room to room via a ceiling-mounted rail system, which allows the system to be shared between two operating rooms. A magnet room that is separated from the operating room via sliding radiofrequency- and sound-shielded doors allows the magnet to be used for diagnostic studies when not used in the surgical theater. The suite is designed around the IMRIS magnet and features an MR-compatible operating room table, application-specific 8-channel intraoperative radiofrequency coils, and head fixation devices specifically designed to fit with the IMRIS operating room table and radiofrequency coils. Nine systems have been installed worldwide, with more than 1000 surgeries performed with these systems. Twelve additional systems are currently in stages of installation. The next generation IMRIS suites will feature a 3-T magnet with capacity for biplanar angiography within the magnet room.
Fig. 5.

The IMRIS iMRI. The IMRIS iMRI suite features a high-field, closed-bore magnet. (A) The IMRIS magnet in its storage room, shielding doors opened to show its relation to the operative suite when not in use. (B) The magnet has moved on its ceiling-mounted rails to its position over the region of the patient's head. The 1.5-T magnet can be brought from its park position in the storage room to a fully operational position within the operating room in less than 90 seconds, allowing for efficient scanning while still remaining unobtrusive. (C) An example of the IMRIS magnet serving two separate operating rooms. (The magnet can swivel 180° in the storage room to orient the working end toward the appropriate operating room.) (Courtesy of IMRIS, Inc., Winninpeg, Manitoba, Canada.)
Future Horizons of Intraoperative MRI
Intraoperative MRI Robotics
In the iMRI suite, manual manipulation of instruments limits the precision and repeatability of placement, particularly when surgeons have their attention divided between multiple surgical team members, image displays, and the surgical field. As closed-bore systems predominate, the ability to manipulate tools remotely becomes increasingly important. For this reason, several groups have considered the use of instrument manipulation via robotics.48,49 In the late 1990s, Chinzei and colleagues50 developed one of the first MRI-compatible robotic manipulators, and the resulting positioning device was integrated into the MRT to form an MRI-guided interventional system.51–53 Despite its revolutionary concept, this robot is designed exclusively for the original open-bore 0.5-T MRT, so its use is limited to few facilities. An MRI-compatible surgical robot suited for the new generation of closed-bore iMRI systems may offer more applicability and versatility in the neurosurgical community at large.
To enhance a surgeon's ability within the closed-bore iMRI environment, Sutherland and colleagues54 developed an MRI-compatible neurosurgical robot, the NeuroArm (Fig. 6). The robot is compact enough to function within the confines of the 70-cm bore MRI, is entirely MRI compatible, and features haptic feedback. The latter feature is a significant step in the progression of neurosurgical assistive technologies, because real-time tactile feedback is known to reduce error and increase efficiency.49,55 Haptic data may be recorded in a real case and replayed off-line. This feature represents a powerful educational tool for neurosurgeons-in-training, allowing them to develop an understanding of tactile experience of manipulating delicate neural tissue.
Fig. 6.
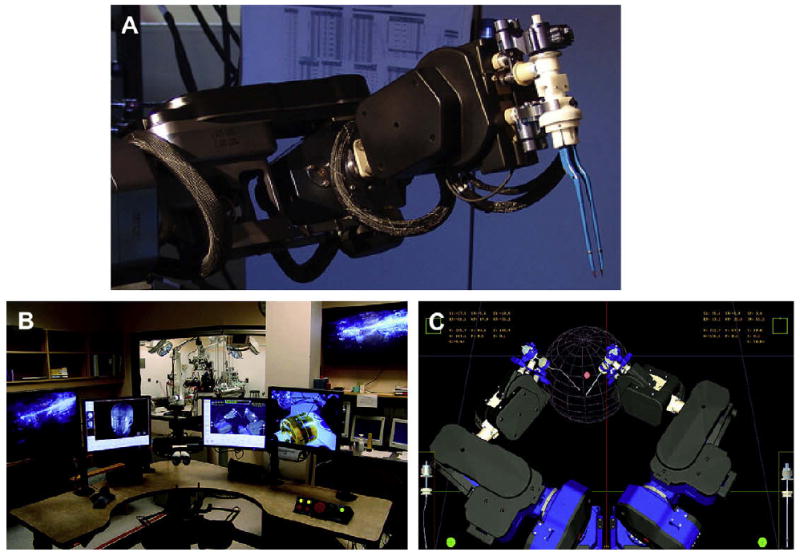
The NeuroArm MRI-compatible neurosurgical robot. (A) Detailed picture of one of the NeuroArm's two operative limbs, with bipolar cautery attachment. (B) Command center for NeuroArm robot, where surgeon is seated and driving the movements of the robot through haptic-feedback controllers. (C) Real-time virtual reality display of the robot's position is delivered to the surgeon. Other virtual reality displays feature colocalization of MRI, functional MRI, and diffusion tensor imaging. (Courtesy of NeuroArm, Calgary, Alberta, Canada.)
Future Intraoperative MRI Suites
Since the first biopsy was performed in the BWH MRT in 1995, researchers, engineers, and surgeons have been collaborating on developing the next-generation iMRI. In 2009, the National Center for Image Guided Therapy at BWH will open the Advanced Multimodality Image Guided Operating (AMIGO) suite, a multimodality image-guided operating suite dedicated to intraoperative guidance (Fig. 7).56 The suite will allow neurosurgeons to use 3-T MRI scans, positron emission tomographic (PET)/CT scans, ultrasound, radiographic fluoroscopy, and microscopy to update preoperative plans. The seamless unification of multiple intraoperative images promises the effective delivery of superior care for a wide range of medical conditions.
Fig. 7.
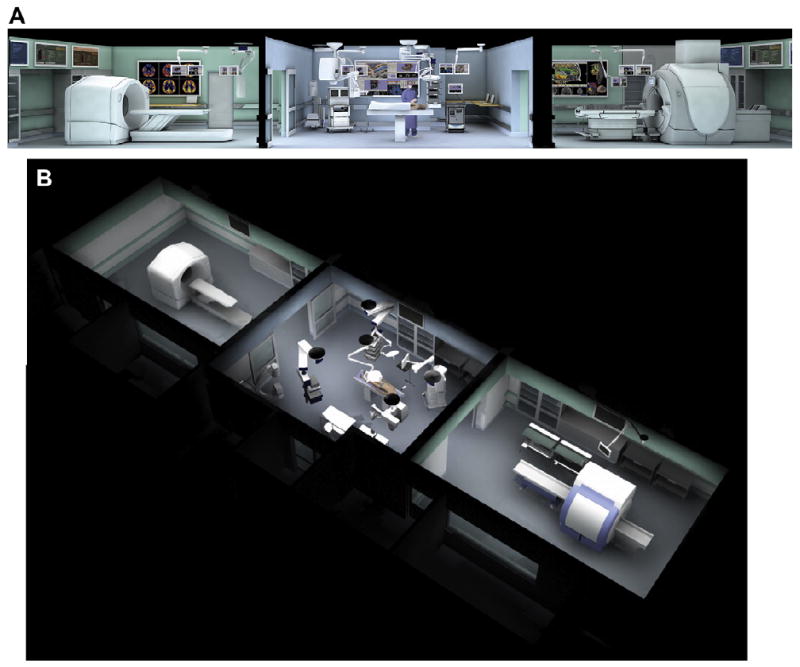
The Advanced Multimodality Image Guided Operative (AMIGO) suite at the National Center for Image Guided Therapy, BWH and the Harvard Medical School. In AMIGO, real-time anatomic imaging modalities such as radiography and ultrasound are combined with the cross-sectional digital imaging systems of PET-CT and MRI. (A, B) Cross-sectional and oblique views of the three-room suite composed of a PET-CT (left room), state-of-the-art operating room, and 3.0T MRI (right room). (Courtesy of GE Healthcare, Wauwatosa, WI; with permission.)
The AMIGO will be a three-room, 5700-sq-ft interventional suite with an operating room and, on opposing sides of it, a GE (Waukesha, Wisconsin) Discovery STE 64-slice PET-CT scanner and a GE 3T 750 DVMR scanner. Staff will move patients under general anesthesia via a specialized surgical table through the operating room, PET suite, and MR suite using the table's wheels or between the patient beds of the PET and MR suite on a mobile transfer tabletop. The seamless nature of patient transfer helps to address the concern over patient safety prompted by previous generations of static closed-bore iMRI systems. Other imaging devices will include ultrasound, radiographic fluoroscopes, and surgical navigation equipment. All components are designed to function in an integral manner.
As a state-of-the-art suite, the AMIGO is designed for the implementation and further development and refinement of multimodal imaging in diagnosis and therapy, such as enabling biopsies and the removal of any unwanted tissue to occur with enhanced accuracy during the same treatment session. Within the AMIGO, imaging modalities will be used in conventional and novel ways. For example, in addition to being used as it customarily is, ultrasound will be tested under research protocols within the AMIGO for its potential to monitor brain shift in real-time during neurosurgery.56 The new understandings and techniques emerging from within the AMIGO will enable clinicians to better understand areas of interest; plan, monitor, or change treatment; navigate through a procedure or operation; and know how, where, and when to best apply a novel therapy.
Summary
In this article, we present a comprehensive framework describing the motivation, development, and evolution of contemporary iMRI in neurosurgery. We describe several key iMRI systems that have developed over the last 15 years, several of which have been evaluated in clinical cases at BWH and others that remain in developmental stages. Although the origins of iMRI can be traced to open-bore MRI at the BWH MRT, the framework for future growth and refinement will be applicable in closed-MRI systems and multimodal operating rooms that are currently on the cusp of operational capacity. Image-guided surgical navigation systems and robotics will be invaluable for applications in closed, high-field MRI magnets, particularly for applications in which real-time imaging is critical. Because targeting with submillimetric precision is required for functional neurosurgical procedures such as deep brain stimulation, gene therapy, and cell transplantation therapeutic strategies, iMRI suites will be of great importance for the future success of functional neurosurgery as a field.
The iMRI suite is a hybrid, combining elements of an interventional radiology unit, an MRI facility, and operating room. In this setting, the respective role and communication among team members (eg, surgeons, radiologists, MR technologists, nurses, anesthesiologists, computer scientists, and engineers) is of paramount importance. In the increasingly technologically driven field of medicine and science, the human factor remains the most critical.
Acknowledgments
This article was supported by the following grants: NIH F32-NS061483-01A1 (JM). NIH P01-CA67165, U41-RR 019,703, K08 NS48063-01, and the Brain Science Foundation (AG).
References
- 1.Tronnier VM, Wirtz CR, Knauth M, et al. Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery. Neurosurgery. 1997;40(5):891–900. doi: 10.1097/00006123-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Wirtz CR, Tronnier VM, Bonsanto MM, et al. Image-guided neurosurgery with intraoperative MRI: update of frameless stereotaxy and radicality control. Stereotact Funct Neurosurg. 1997;68(1–4 Pt 1):39–43. doi: 10.1159/000099900. [DOI] [PubMed] [Google Scholar]
- 3.Maurer CR, Jr, Hill DL, Martin AJ, et al. Investigation of intraoperative brain deformation using a 1.5-T interventional MR system: preliminary results. IEEE Trans Med Imaging. 1998;17(5):817–25. doi: 10.1109/42.736050. [DOI] [PubMed] [Google Scholar]
- 4.Nimsky C, Ganslandt O, Hastreiter P, et al. Intraoperative compensation for brain shift. Surg Neurol. 2001;56(6):357–64. doi: 10.1016/s0090-3019(01)00628-0. discussion: 64–5. [DOI] [PubMed] [Google Scholar]
- 5.Ferrant M, Nabavi A, Macq B, et al. Serial registration of intraoperative MR images of the brain. Med Image Anal. 2002;6(4):337–59. doi: 10.1016/s1361-8415(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 6.Soza G, Grosso R, Labsik U, et al. Fast and adaptive finite element approach for modeling brain shift. Comput Aided Surg. 2003;8(5):241–6. doi: 10.3109/10929080309146059. [DOI] [PubMed] [Google Scholar]
- 7.Hastreiter P, Rezk-Salama C, Soza G, et al. Strategies for brain shift evaluation. Med Image Anal. 2004;8(4):447–64. doi: 10.1016/j.media.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Clatz O, Delingette H, Talos IF, et al. Robust nonrigid registration to capture brain shift from intraoperative MRI. IEEE Trans Med Imaging. 2005;24(11):1417–27. doi: 10.1109/TMI.2005.856734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger MS, Deliganis AV, Dobbins J, et al. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–91. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–33. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 11.Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52(4):371–9. doi: 10.1016/s0090-3019(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 13.Berger MS, Rostomily RC. Low grade gliomas: functional mapping resection strategies, extent of resection, and outcome. J Neurooncol. 1997;34(1):85–101. doi: 10.1023/a:1005715405413. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Hidalgo OA, Vanaclocha V, Vieitez JM, et al. High-dose BCNU and autologous progenitor cell transplantation given with intra-arterial cisplatinum and simultaneous radiotherapy in the treatment of high-grade gliomas: benefit for selected patients. Bone Marrow Transplant. 1996;18(1):143–9. [PubMed] [Google Scholar]
- 15.Johannesen TB, Langmark F, Lote K. Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99(5):854–62. doi: 10.3171/jns.2003.99.5.0854. [DOI] [PubMed] [Google Scholar]
- 16.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2008;110:156–62. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 17.Sakata K, Hareyama M, Komae T, et al. Supratentorial astrocytomas and oligodendrogliomas treated in the MRI era. Jpn J Clin Oncol. 2001;31(6):240–5. doi: 10.1093/jjco/hye052. [DOI] [PubMed] [Google Scholar]
- 18.Wirtz CR, Knauth M, Staubert A, et al. Clinical evaluation and follow-up results for intraoperative magnetic resonance imaging in neurosurgery. Neurosurgery. 2000;46(5):1112–20. doi: 10.1097/00006123-200005000-00017. discussion: 20–2. [DOI] [PubMed] [Google Scholar]
- 19.Gronemeyer D, Seibel R, Erbel R, et al. Equipment configuration and procedures: preferences for interventional microtherapy. J Digit Imaging. 1996;9(2):81–96. doi: 10.1007/BF03168861. [DOI] [PubMed] [Google Scholar]
- 20.Gronemeyer DH, Seibel RM, Schmidt A, et al. Two- and three-dimensional imaging for interventional MRI and CT guidance. Stud Health Technol Inform. 1996;29:62–76. [PubMed] [Google Scholar]
- 21.Schenck JF, Jolesz FA, Roemer PB, et al. Superconducting open-configuration MR imaging system for image-guided therapy. Radiology. 1995;195(3):805–14. doi: 10.1148/radiology.195.3.7754014. [DOI] [PubMed] [Google Scholar]
- 22.Alexander E, 3rd, Moriarty TM, Kikinis R, et al. The present and future role of intraoperative MRI in neurosurgical procedures. Stereotact Funct Neurosurg. 1997;68(1–4 Pt 1):10–7. doi: 10.1159/000099896. [DOI] [PubMed] [Google Scholar]
- 23.Alexander E, 3rd, Moriarty TM, Kikinis R, et al. Innovations in minimalism: intraoperative MRI. Clin Neurosurg. 1996;43:338–52. [PubMed] [Google Scholar]
- 24.Moriarty TM, Kikinis R, Jolesz FA, et al. Magnetic resonance imaging therapy: intraoperative MR imaging. Neurosurg Clin N Am. 1996;7(2):323–31. [PubMed] [Google Scholar]
- 25.Kanal E. An overview of electromagnetic safety considerations associated with magnetic resonance imaging. Ann N Y Acad Sci. 1992;649:204–24. doi: 10.1111/j.1749-6632.1992.tb49610.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanal E, Borgstede JP, Barkovich AJ, et al. American College of Radiology White Paper on MR Safety: 2004 update and revisions. AJR Am J Roentgenol. 2004;182(5):1111–4. doi: 10.2214/ajr.182.5.1821111. [DOI] [PubMed] [Google Scholar]
- 27.Kanal E, Borgstede JP, Barkovich AJ, et al. American College of Radiology White Paper on MR Safety. AJR Am J Roentgenol. 2002;178(6):1335–47. doi: 10.2214/ajr.178.6.1781335. [DOI] [PubMed] [Google Scholar]
- 28.Kettenbach J, Kacher DF, Kanan AR, et al. Intraoperative and interventional MRI: recommendations for a safe environment. Minim Invasive Ther Allied Technol. 2006;15(2):53–64. doi: 10.1080/13645700600640774. [DOI] [PubMed] [Google Scholar]
- 29.Black PM, Moriarty T, Alexander E, 3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41(4):831–42. doi: 10.1097/00006123-199710000-00013. discussion: 42–5. [DOI] [PubMed] [Google Scholar]
- 30.Gering DT, Nabavi A, Kikinis R, et al. An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imaging. 2001;13(6):967–75. doi: 10.1002/jmri.1139. [DOI] [PubMed] [Google Scholar]
- 31.Nabavi A, Black PM, Gering DT, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48(4):787–97. doi: 10.1097/00006123-200104000-00019. discussion: 97–8. [DOI] [PubMed] [Google Scholar]
- 32.Archip N, Clatz O, Whalen S, et al. Compensation of geometric distortion effects on intraoperative magnetic resonance imaging for enhanced visualization in image-guided neurosurgery. Neurosurgery. 2008;62(3 Suppl 1):209–15. doi: 10.1227/01.neu.0000317395.08466.e6. discussion: 15–6. [DOI] [PubMed] [Google Scholar]
- 33.Oh DS, Black PM. A low-field intraoperative MRI system for glioma surgery: is it worthwhile? Neurosurg Clin N Am. 2005;16(1):135–41. doi: 10.1016/j.nec.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Jolesz FA, Talos IF, Schwartz RB, et al. Intraoperative magnetic resonance imaging and magnetic resonance imaging-guided therapy for brain tumors. Neuroimaging Clin N Am. 2002;12(4):665–83. doi: 10.1016/s1052-5149(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 35.Hadani M, Spiegelman R, Feldman Z, et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery. 2001;48(4):799–807. doi: 10.1097/00006123-200104000-00021. discussion: 9. [DOI] [PubMed] [Google Scholar]
- 36.Gerlach R, du Mesnil de Rochemont R, Gasser T, et al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery. 2008;63(2):272–84. doi: 10.1227/01.NEU.0000312362.63693.78. discussion: 84–5. [DOI] [PubMed] [Google Scholar]
- 37.Ntoukas V, Krishnan R, Seifert V. The new generation Polestar N20 for conventional neurosurgical operating rooms: a preliminary report. Neurosurgery. 2008;62(3 Suppl 1):82–9. doi: 10.1227/01.neu.0000317376.38067.8e. discussion: 89–90. [DOI] [PubMed] [Google Scholar]
- 38.Samdani AF, Schulder M, Catrambone JE, et al. Use of a compact intraoperative low-field magnetic imager in pediatric neurosurgery. Childs Nerv Syst. 2005;21(2):108–13. doi: 10.1007/s00381-004-1008-1. discussion: 14. [DOI] [PubMed] [Google Scholar]
- 39.Levivier M, Wikler D, De Witte O, et al. PoleStar N-10 low-field compact intraoperative magnetic resonance imaging system with mobile radiofrequency shielding. Neurosurgery. 2003;53(4):1001–6. doi: 10.1227/01.neu.0000084167.18475.ba. discussion: 7. [DOI] [PubMed] [Google Scholar]
- 40.Schulder M, Sernas TJ, Carmel PW. Cranial surgery and navigation with a compact intraoperative MRI system. Acta Neurochir Suppl. 2003;85:79–86. doi: 10.1007/978-3-7091-6043-5_11. [DOI] [PubMed] [Google Scholar]
- 41.Kanner AA, Vogelbaum MA, Mayberg MR, et al. Intracranial navigation by using low-field intraoperative magnetic resonance imaging: preliminary experience. J Neurosurg. 2002;97(5):1115–24. doi: 10.3171/jns.2002.97.5.1115. [DOI] [PubMed] [Google Scholar]
- 42.Salas S, Brimacombe M, Schulder M. Stereotactic accuracy of a compact intraoperative MRI system. Stereotact Funct Neurosurg. 2007;85(2–3):69–74. doi: 10.1159/000097921. [DOI] [PubMed] [Google Scholar]
- 43.Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery. 2001;48(4):731–42. doi: 10.1097/00006123-200104000-00007. discussion: 42–4. [DOI] [PubMed] [Google Scholar]
- 44.Nimsky C, Ganslandt O, Fahlbusch R. 1.5 T: intraoperative imaging beyond standard anatomic imaging. Neurosurg Clin N Am. 2005;16(1):185–200. vii. doi: 10.1016/j.nec.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Lipson AC, Gargollo PC, Black PM. Intraoperative magnetic resonance imaging: considerations for the operating room of the future. J Clin Neurosci. 2001;8(4):305–10. doi: 10.1054/jocn.2000.0833. [DOI] [PubMed] [Google Scholar]
- 46.Hushek SG, Martin AJ, Steckner M, et al. MR systems for MRI-guided interventions. J Magn Reson Imaging. 2008;27(2):253–66. doi: 10.1002/jmri.21269. [DOI] [PubMed] [Google Scholar]
- 47.Hall WA, Truwit CL. Intraoperative MR-guided neurosurgery. J Magn Reson Imaging. 2008;27(2):368–75. doi: 10.1002/jmri.21273. [DOI] [PubMed] [Google Scholar]
- 48.Louw DF, Fielding T, McBeth PB, et al. Surgical robotics: a review and neurosurgical prototype development. Neurosurgery. 2004;54(3):525–36. doi: 10.1227/01.neu.0000108638.05274.e9. discussion: 36–7. [DOI] [PubMed] [Google Scholar]
- 49.McBeth PB, Louw DF, Rizun PR, et al. Robotics in neurosurgery. Am J Surg. 2004;188(4A Suppl):68S–75S. doi: 10.1016/j.amjsurg.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Chinzei K, Miller K. Towards MRI guided surgical manipulator. Med Sci Monit. 2001;7(1):153–63. [PubMed] [Google Scholar]
- 51.Hata N, Tokuda J, Hurwitz S, et al. MRI-compatible manipulator with remote-center-of-motion control. J Magn Reson Imaging. 2008;27(5):1130–8. doi: 10.1002/jmri.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimaio SP, Archip N, Hata N, et al. Image-guided neurosurgery at Brigham and Women's Hospital. IEEE Eng Med Biol Mag. 2006;25(5):67–73. doi: 10.1109/memb.2006.1705749. [DOI] [PubMed] [Google Scholar]
- 53.DiMaio SP, Pieper S, Chinzei K, et al. Robot-assisted needle placement in open-MRI: system architecture, integration and validation. Stud Health Technol Inform. 2006;119:126–31. [PubMed] [Google Scholar]
- 54.Sutherland GR, Latour I, Greer AD. Integrating an image-guided robot with intraoperative MRI: a review of the design and construction of neuroArm. IEEE Eng Med Biol Mag. 2008;27(3):59–65. doi: 10.1109/EMB.2007.910272. [DOI] [PubMed] [Google Scholar]
- 55.Rizun PR, McBeth PB, Louw DF, et al. Robot-assisted neurosurgery. Semin Laparosc Surg. 2004;11(2):99–106. doi: 10.1177/107155170401100206. [DOI] [PubMed] [Google Scholar]
- 56.Advanced Multimodality Image Guided Operating (AMIGO) Suite. [November 2, 2008];2008 Available at: http://www.ncigt.org/pages/AMIGO.


