Abstract
Previously, we reported that the nucleoside analog/transcriptional inhibitor ARC was able to induce p53-independent apoptosis in multiple cancer cell lines of different origin. This occurred at least in part by the suppression of short-lived, pro-survival member of the Bcl-2 family, Mcl-1. In contrast, we show here that treatment of human cancer cells with the pan-Bcl-2 inhibitor ABT-737 alone led to up-regulation of Mcl-1 protein expression. Combination of sub-apoptotic concentrations of ABT-737 and ARC induced mitochondrial injury and potent caspase-3/caspase-9-dependent apoptosis in wide variety of human cancer cell lines. These data suggest that ABT-737/ARC combination that simultaneously targets Bcl-2 and Mcl-1 may be efficient against human cancer.
Keywords: ARC, ABT-737, apoptosis, Mcl-1, Bcl-2
INTRODUCTION
Previously, we identified the nucleoside analog named ARC (4-amino-6-hydrazino-7-β-D-ribofuranosyl-7H-Pyrrolo[2,3-d]-pyrimidine-5-carboxamide), which is an inhibitor of P-TEFb kinase (CDK9/CyclinT1 complex) and acts as a transcriptional inhibitor [1, 2]. We showed that ARC induced potent apoptosis in transformed and cancer, but not in normal cells and exhibited potent anti-angiogenic activity in vitro [1, 3–5]. In addition, we found that ARC targets labile antiapoptotic protein, Mcl-1 [3–5] and overexpression of Mcl-1 protects cells from ARC-induced apoptosis [3, 5].
Abbott laboratories recently synthesized pan-Bcl-2 inhibitor, ABT-737, a BH3 mimetic developed by structure-based drug design [6]. ABT-737 competes with BAD for docking to the hydrophobic groove of Bcl-2 family proteins, thus promoting Bax and Bak activation [6]. At the same time, ABT-737 has a low affinity for another member of the Bcl-2 family protein, Mcl-1 [6], which is a critical survival factor for various malignancies [7]. Cancer cells with high levels of Mcl-1 expression have been associated with resistance to ABT-737 [8, 9], while down-regulation of Mcl-1 significantly augmented ABT-737-induced apoptosis in human cancer cells [8, 10, 11]. As a single agent, ABT-737 was efficient against some small-cell lung cancer cell lines [12] and leukemia cells [11], but largely ineffective at promoting cell death in prostate and renal cancer cells [13]. We show here that combination of sub-apoptotic concentrations of ARC with ABT-737 resulted in synergistic induction of cell death in numerous human cancer cell lines of different origin. Our data suggest that down-regulation of Mcl-1 by ARC may contribute to its synergy with ABT-737.
MATERIALS AND METHODS
Cell Culture and Reagents
The melanoma cell lines, DM366 and DM833 were grown in IMDM (Invitrogen) medium. The osteosarcoma cell line U2OS-C3, the colon cancer cells LIM1215 and SW480, the liver cancer cell lines Huh7 and HepG2 were all grown in DMEM medium (Invitrogen). The neuroblastoma cell lines SKNAS and IMR32 were grown in RPMI1640 medium (Invitrogen). HPAC pancreatic cell line was grown in DME/F-12 medium (Invitrogen). All the media were supplemented with 10% fetal bovine serum (Atlanta Biologicals), 2mM L-glutamine and 1% penicillin-streptomycin and the cells were grown at 37° C in 5% CO2. ARC was obtained from NCI and ABT-737 from Abbott Laboratories. All these drugs were dissolved in DMSO and stored as 10 mM stock solutions. Specific inhibitor to caspase-3 (Z-DEVD-FMK) catalog no. 550378, caspase-9 (Z-LEHD-FMK) catalog #550381 and general/pan-caspase inhibitor (Z-VAD-FMK) catalog #550377 were purchased from BD Pharmigen. Specific inhibitor to caspase-8 (Granzyme B; catalog #218773) was purchased from EMD Biosciences. Solutions for the caspase inhibitors were made according to manufacturer’s instructions.
Annexin V-PE staining and FACS analysis
Aliquots of cells were stained using Annexin V-PE apoptosis detection kit (Cat# 559763, BD-Pharmingen) according to the manufacturer’s recommendations. Briefly, the cells were trypsinized, washed in PBS and resuspended in binding buffer (1×106 per ml). 5µl of AnnexinV-PE and 5µl of 7-AAD (7-aminoactinomycin-D) were added and incubated for 15 minutes at room temperature in the dark and analyzed by flow cytometry.
Measurement of Combination Index Value
The synergy between ARC and ABT-737 in human cancer cells was quantitatively evaluated by the median effect plot method formulated by Chou-Talalay [14]. Cells were treated with varied doses of ARC alone, ABT-737 alone or ARC and ABT-737 in combination. In our experiments, the IC30, IC50, IC70, IC80 and IC90 value (i.e., the drug concentration needed to cause 30, 50, 70, 80 and 90% reduction in cell viability) was chosen for comparison. Percent viability of cells was determined using standard MTT assay. Combination index (CI) values were calculated using the formula
| [14] |
CA,x and CB,x are the concentrations of drug A and drug B used in combination to achieve x% drug effect. ICx,A and ICx,B are the concentrations for single agents to achieve the same effect. CI values of <1 indicate synergy, a value of 1 indicates additive effects and a value of >1 indicates antagonism.
Immunoblot Analysis
The cells were harvested and lysed in IP buffer (20mM HEPES, ph7.4, 1% Triton X-100, 150mM NaCl, 1mM EDTA, 1mM EGTA, 100mM NaF, 10mM Na4 P2 O7, 1mM sodium orthovanadate, 0.2mM PMSF supplemented with protease inhibitors and the protein concentrations were measured by the Bio-Rad method. Fifty micrograms of the cell lysates were separated by electrophoresis on a 12% SDS-polyacrylamide mini gel and transferred polyvinylidine difluoride (PVDF) membrane. Immunoblotting was performed as described [15] with specific antibodies for Mcl-1 (MS-683: Lab Vision), Bax (2774, Cell Signaling), Bcl-2 (7382 Santa Cruz), cleaved caspase-3 (9664; Cell Signaling) and β-actin (AS441, Sigma).
Nuclear-ID Green Chromatin Condensation detection
Cells were stained using in vitro apoptosis detection kit (Cat# ENZ-51021-K200 Enzo Life Sciences), according to the manufacturer’s recommendations. Briefly 3-4×104 cells were plated in 96-well black wall clear bottom plate and allowed to grow overnight. Cells were pretreated with apoptosis inhibitors for 2 hours following which they were treated with either DMSO or ARC/ABT-737 combination and incubated for 24hrs. Cells were washed with assay buffer and stained with 1µM nuclear-ID green dye. The plate was read in a fluorescence microplate reader with excitation wavelength 488 nM and emission wavelength 520 nM.
Clonogenic Assay
HepG2 and SW480 cells were grown in RPMI1640 medium to 50–70% confluence and treated with various combinations of ARC and ABT-737 for 24hrs.The cells were then trypsinized, re-suspended in the media and counted. The cells were re-seeded (2000 cells per dish) into 100mm new tissue culture dishes and incubated for 10 days. Fresh media was added on the fifth day. On the tenth day, media was removed from the dishes and washed once with ice-cold PBS. The colonies were stained with 2 ml each of 0.25% 1,9-dimethyl-methylene blue in 50% ethanol for 45 minutes on a rocking platform. The dishes were rinsed 3 times with PBS, air-dried and the colonies were counted.
Mitochondrial Injury
106 cells were re-suspended in fresh RPMI640, treated with tetramethyl rhodamine methyl ester (TMRE) to a final concentration of 25 nM and incubated at 37° for 20 minutes. The cells were centrifuged and resuspended in 25 nm TMRE in PBS. The mitochondrial membrane potential was measured by flow cytometry.
RESULTS AND DISCUSSION
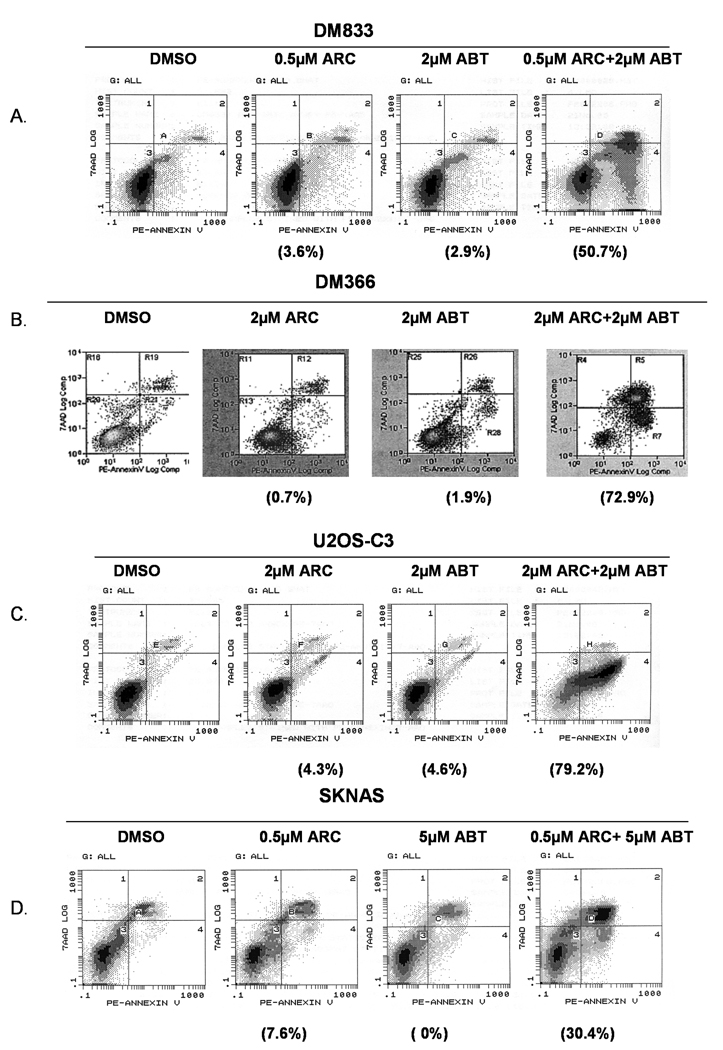
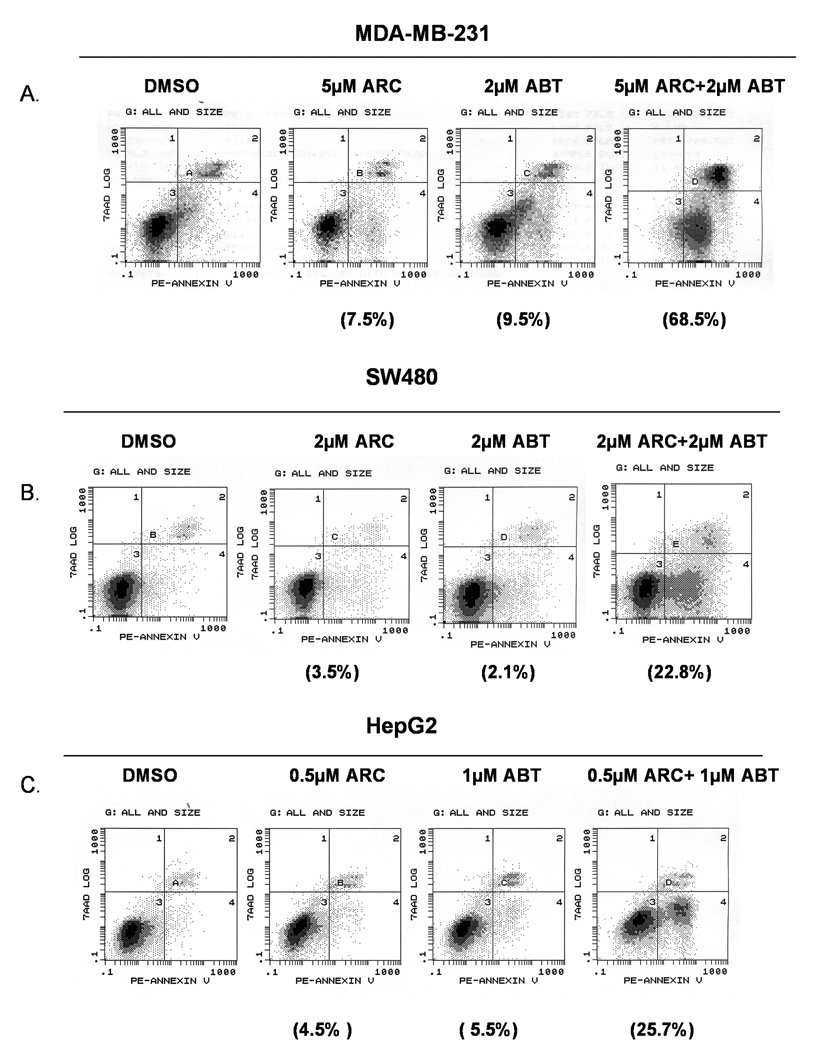
We showed earlier that ARC inhibited the growth and induced apoptosis in melanoma, neuroblastoma, liver, breast and colon cancer cell lines [1, 3–5]. To determine whether ARC may synergize with ABT-737 against human cancer cell lines of different origin we treated melanoma, osteosarcoma, neuroblastoma, breast, pancreatic, liver and colon cancer cells with either sub-apoptotic concentrations of ARC or ABT-737 alone or with combinations of the two for 24 hours and used annexin V-PE/7AAD staining and flow cytometry to determine the percent of apoptotic cells (Fig 1, 2). As shown in Fig 1A, treatment of DM833 cells with 0.5 µM ARC or 2 µM ABT-737 induced apoptosis of only 3.6% cells and 2.9% cells respectively over the control, while treatment with both drugs at the same doses caused 50.7% of cells to undergo apoptosis (Fig. 1A). Similarly, in osteosarcoma cells, treatment with 2 µM ARC or 2 µM ABT-737 induced only 4.3% and 4.6% of apoptosis over the control, whereas combined treatment with both drugs resulted in 79.2% of cell death (Fig. 1C). In addition, enhanced apoptotic effects of ARC/ABT-737 combinations were also seen in other cell types such as neuroblastoma (Fig. 1D), breast cancer (Fig. 2A), colon cancer (Fig. 2B) and liver cancer (Fig. 2C). All these data suggest that combination of ARC with ABT-737 resulted in synergistic programmed cell death in human cancer cell lines of different origin.
Fig. 1. Annexin V-PE staining after combination treatment of human tumor cells with ARC and ABT-737.
A, B. DM833 and DM366 melanoma cells were treated with sub-apoptotic concentrations of ARC, ABT-737 or both as shown for 24 hrs, stained with annexin V-PE/7-AAD and analyzed by flow cytometry. C. U2OS-C3 osteosarcoma cells were treated with ARC, ABT-737 or combination of ARC/ABT-737 for 24 hrs, stained with AnnexinV-PE and analyzed by flow cytometry. D. SKNAS neuroblastoma cells were treated with ARC, ABT-737 and co-treated with ARC and ABT-737 stained with annexin V-PE and 7-AAD and analyzed by flow cytometry. The net percentages of apoptotic cells relative to untreated control are shown in parentheses.
Fig. 2. Combination treatment of ARC and ABT-737 induces apoptosis in human tumor cell lines.
A. MDA-MB-231, breast cancer cells treated with sub-apoptotic concentrations of ARC, ABT-737 and ARC/ABT-737 combination for 24 hrs, stained with AnnexinV-PE and 7-AAD and analyzed by flow cytometry. B. SW480, colon cancer cells were treated with sub-apoptotic concentrations of ARC, ABT-737 or combination of ARC and ABT-737 stained with annexin V-PE/7-AAD and analyzed by flow cytometry. C. HepG2, liver cancer cells treated with sub-lethal concentration of ARC alone and ABT-737 alone and combination of ARC and ABT-737 as shown and analyzed after annexin V-PE/7-AAD staining by flow cytometry. The net percentages of apoptotic cells relative to untreated control are shown in parentheses.
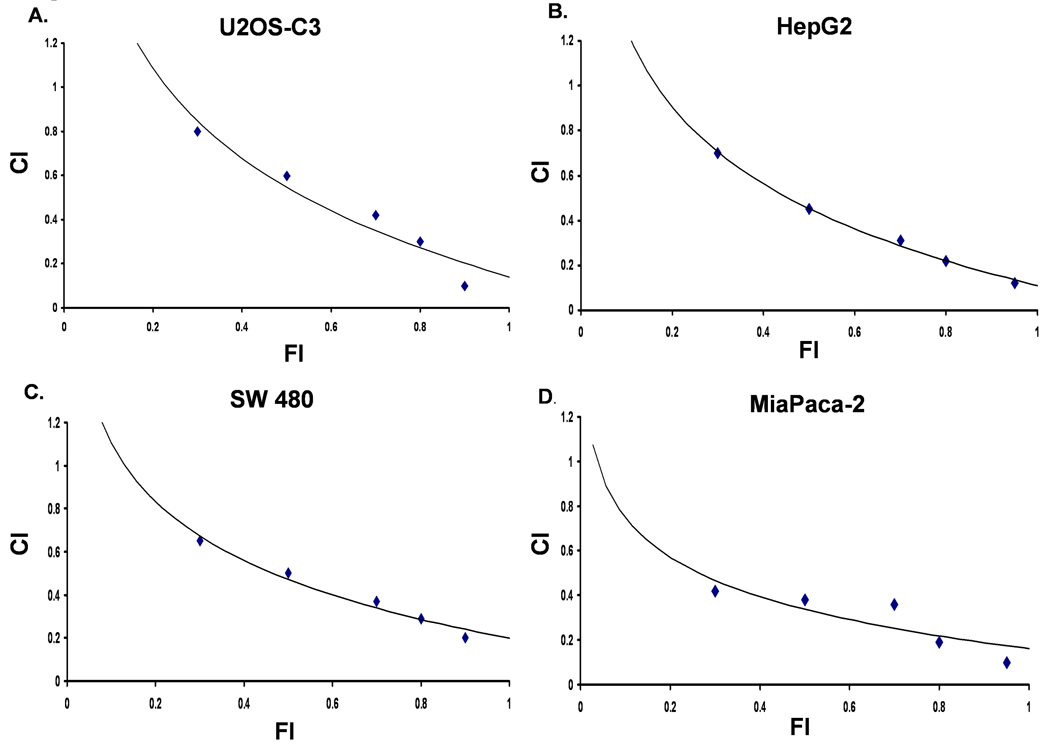
To quantitatively validate the synergistic nature of the interaction between ARC and ABT-737, we examined the cell viability after single and combination drug treatments by using the Chou/Talalay median-effect equation method [14]. The combination index (CI) values below 1 indicates synergistic anti-proliferative effect and the CI range values for the combined treatments with ARC/ABT-737 in four different human cancer cell lines were 0.1–0.8 (Fig 3) for fractional effect corresponding to 0.3–0.9, suggesting strong synergistic effect.
Fig. 3. ARC and ABT-737 display synergistic combination index values in human cancer cells.
Combination index (CI) chart for the ARC/ABT-737 drug combination was plotted with CI on y axis and fractional effect (FI) on x-axis. Percentage inhibition of proliferation was determined following treatment with a single agent or the ARC/ABT-737 combination. Combination index (CI) values of the two agents plotted for U2OS-C3 (A), HepG2 (B), SW480 (C) and Mia Paca-2 (D) cells.
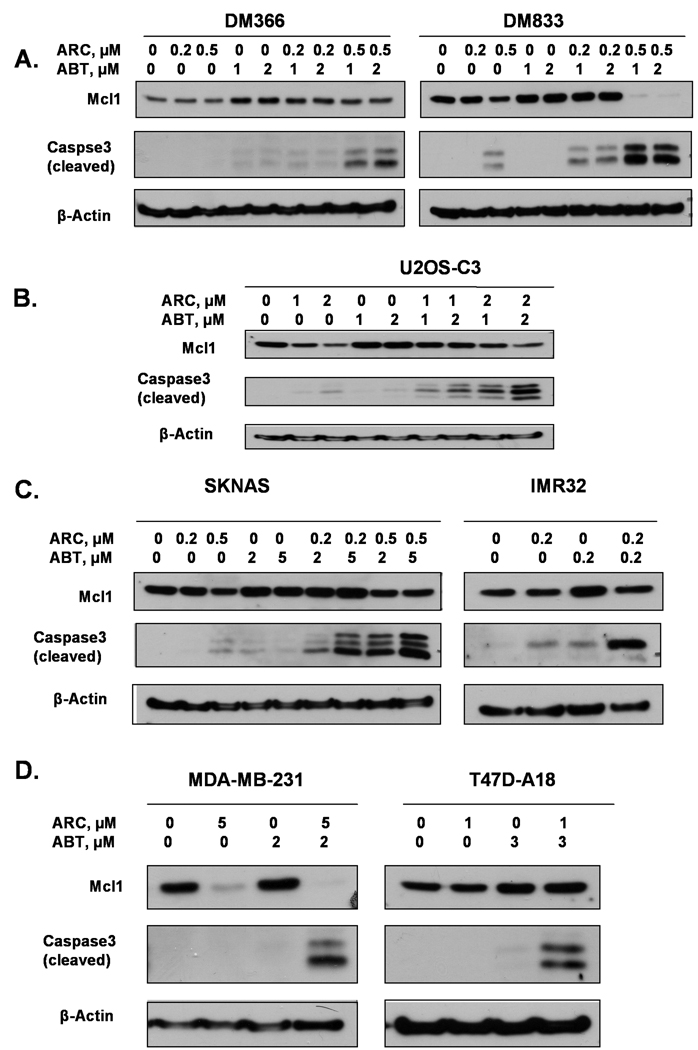
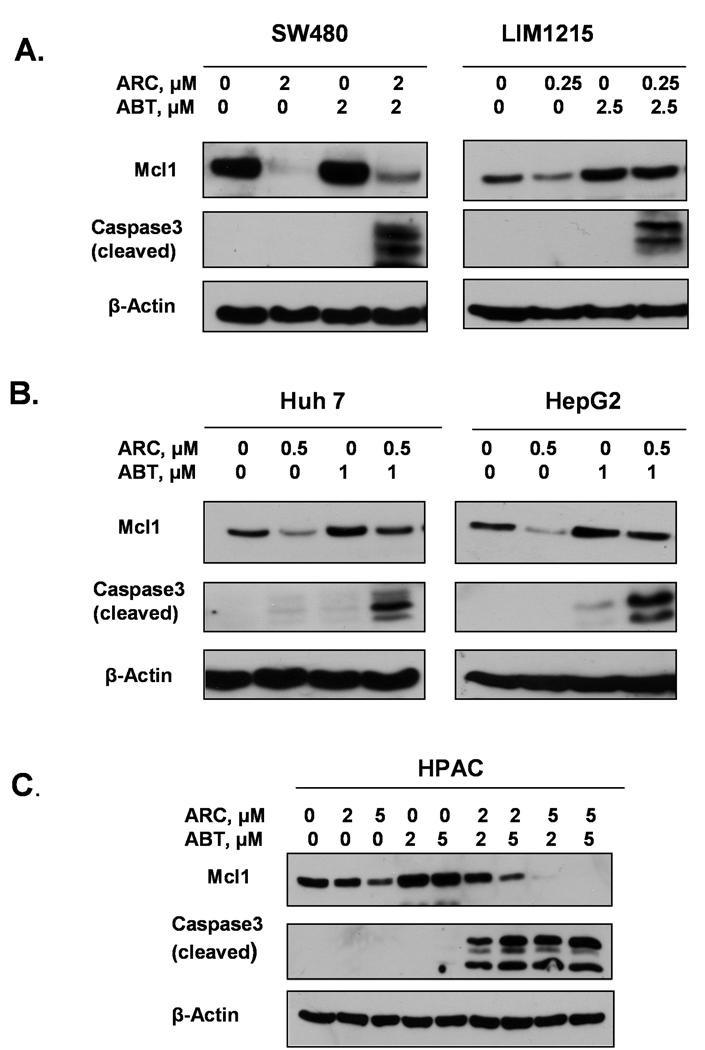
To further confirm that combination treatment of ARC and ABT-737 induces synergistic apoptosis, we used caspase-3 cleavage in drug-treated and control cells to serve as an indicator of apoptotic cell death. We treated DM366 and DM833 melanoma cells with ARC, ABT-737 or both as indicated for 24 hrs and performed immunoblotting for cleaved caspase-3 (Fig. 4A). While treatment with ARC or ABT-737 alone induced little or no caspase-3 cleavage in these cells, treatment with several different combinations of these drugs showed potent caspase-3 cleavage. Similar synergistic effects of ARC/ABT-737 combinations on caspase-3 cleavage were seen in osteosarcoma (U2OS-C3, Fig. 4B), neuroblastoma (SKNAS and IMR32) (Fig. 4C), breast (MDA-MB-231 and T47D-A18, Fig. 4D), colon (SW480 and LIM1215 Fig. 5A), liver (Huh-7 and HepG2, Fig. 5B) and pancreatic (HPAC, Fig. 5C) cancer cells.
Fig. 4. ARC and ABT-737 induce caspase-3 cleavage in human melanoma, osteosarcoma, neuroblastoma and breast cancer cells.
A. Melanoma DM366 and DM 833 cells were treated with ARC, ABT-737 or both as indicated for 24 hrs, total cell lysates were extracted and immunoblotted with antibodies for Mcl-1, cleaved caspase-3 and β-actin B. U2OS-C3 osteosarcoma cells were treated with the drugs as shown for 24 hrs and the cell lysates were immunoblotted for Mcl-1, cleaved caspase-3 and β–actin. C. Neuroblastoma cells, SKNAS and IMR32 were treated with ARC, ABT-737 or both as shown for 24 hrs and immunoblotted with specific antibodies for Mcl-1, cleaved caspase-3 and β-actin. D. Breast cancer cell lines, MDA-MB-231 and T47D-A18 were treated with ARC, ABT-737 and both for 24 hrs and immunoblotted for Mcl-1, cleaved caspase-3 and β-actin.
Fig. 5. ARC synergizes with ABT-737 in inducing caspase-3 cleavage in human colon, liver and pancreatic cancer cells.
A. SW480 and LIM1215 colon cancer cells were treated as indicated for 24 hrs and analyzed by immunoblotting. B. Huh-7 and HepG2 liver cancer cells were treated with ARC, ABT-737 and combination of ARC/ABT-737 as shown and immunoblotted for the proteins as indicated. C. Pancreatic cancer cells, HPAC were treated with ARC, ABT-737 or both and analyzed for the expression of Mcl-1, cleaved caspase-3 and β-actin by immunoblotting.
To determine how down-regulation of Mcl-1 by ARC/ABT-737 treatment correlates with cell death we measured Mcl-1 protein levels in cells treated with either ARC or ABT-737 or with their combination by immunoblotting with specific Mcl-1 antibody (Fig. 4, 5). In accordance with our previous results [3–5], treatment with ARC alone attenuated Mcl-1 protein levels in all the cell lines in a dose-dependent manner. In contrast, treatment with ABT-737 up-regulated Mcl-1 in all the cell lines, although to varying degree. However, the effect of treatment with the drug combinations on Mcl-1 expression was variable in the different cell lines, but in all cases these levels were lower than after treatment by ABT-737 alone. These data suggest that proapoptotic synergy between ABT-737 and ARC is partially based on suppression of Mcl-1 protein by ARC.
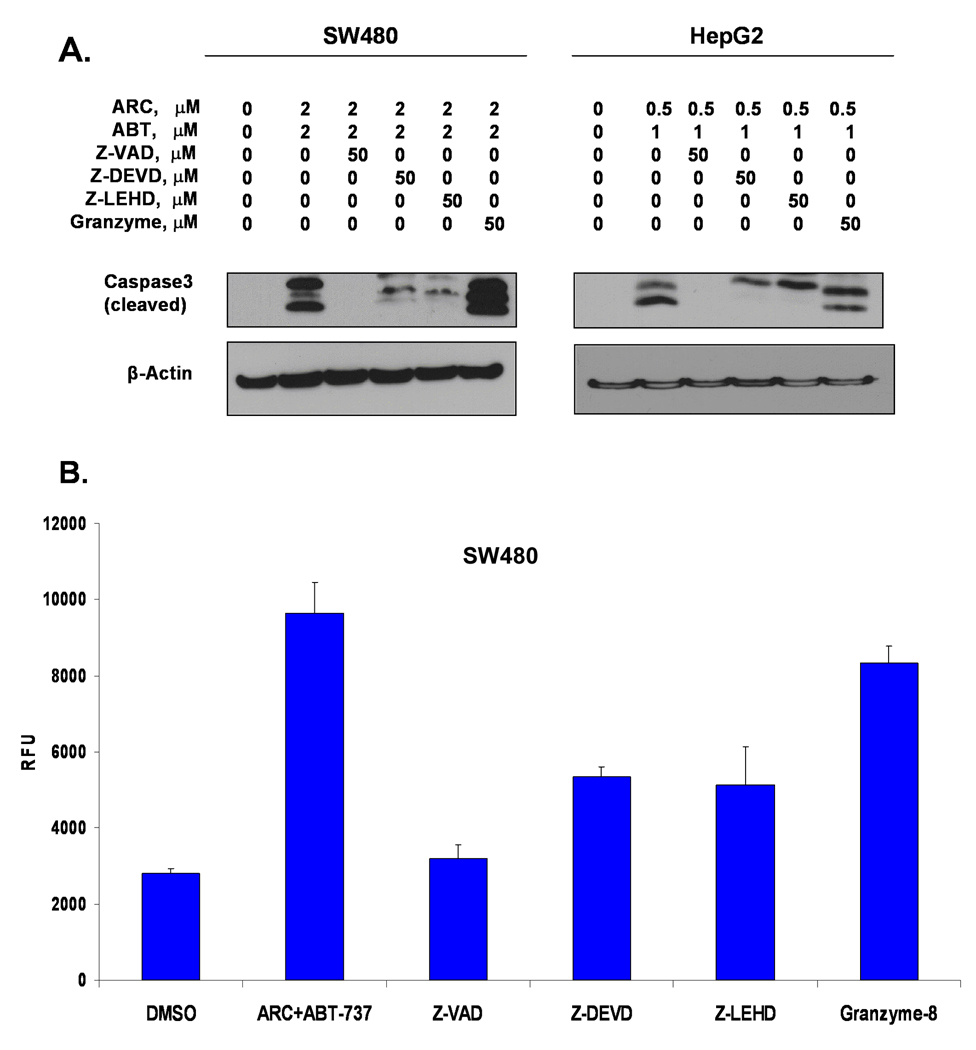
To determine the roles of different caspases in ARC/ABT-737-induced apoptosis we treated SW480 colon cancer and HepG2 liver cancer cell line with this drug combination in presence of caspase inhibitors and evaluated apoptosis by western blot with antibodies specific for cleaved caspase-3 or by using in vitro chromatin condensation detection assay (Fig 6). Using western blot we found that caspase-3 and caspase-9 inhibitors, but not caspase-8 inhibitor inhibit caspase-3 cleavage after treatment with ARC/ABT-737 (Fig 6A). Similarly, using a fluorescent green probe (chromatin condensation detection kit from Enzo Life Biosciences, see M&M) we measured the DNA condensation in cells induced by ARC/ABT-737. Cells pre-treated with specific inhibitors to caspase-3 and -9 lowered the fluorescence induced by ARC/ABT-737 combination (Fig 6B). In contrast, pre-incubation with specific inhibitor to caspase-8 did not affect the ARC/ABT-737-induced DNA condensation and apoptosis. Therefore, apoptosis induced by combination of ARC/ABT-737 in human cancer cells is dependent on caspases-3 and -9, but not on caspase-8, which is required for extrinsic apoptosis (Fig 6). Our findings contradict the data of Keuling et al. that caspase-8 is required for combination treatment of ABT-737 and Mcl-1 inhibitors of melanoma cells [16]. Additional experiments are needed to resolve these differences.
Fig. 6. ARC/ABT-737-induced apoptosis in human cancer cells is dependent on caspase-3 and –9, but not on caspase-8 activity.
A. Specific inhibitor for caspase-3, caspase-9 and pancaspase inhibitor reduce ARC/ABT-737 induced apoptosis, while specific inhibitor for caspase-8 demonstrates no reduction in apoptosis detected by immunoblotting for cleaved caspase-3 in SW480 and HepG2 cells. B. Quantitation of apoptotic cells by using in vitro chromatin condensation detection kit. Combination of ARC/ABT-737 enhance fluorescence intensity in SW480 cells, pan-caspase inhibitor, caspase-3 and -9 inhibitors, but not caspase-8 inhibitor lower the fluorescence intensity of SW480 cells (mean ± SD of 3 separate determinations).
To confirm that the combination of these drugs may induce intrinsic apoptosis, we investigated whether the drugs cause mitochondrial injury one of the hallmarks of the intrinsic pathway. We stained treated and control cells with the mitochondria staining dye, tetramethylrhodamine ethyl ester (TMRE) and analyzed the mitochondrial membrane potential by flow cytometry. The results show that combined treatment of ARC and ABT-737 caused depolarization of the mitochondrial membrane of melanoma (Fig 1A-Suppl.) and osteosarcoma (Fig 1B- Suppl.) cells, while treatment with either drug alone had little effect. These data suggest that mitochondrial injury induced by ARC/ABT-737 in human cancer cells correlated with cell death (Fig 1 A, C) after combination treatment.
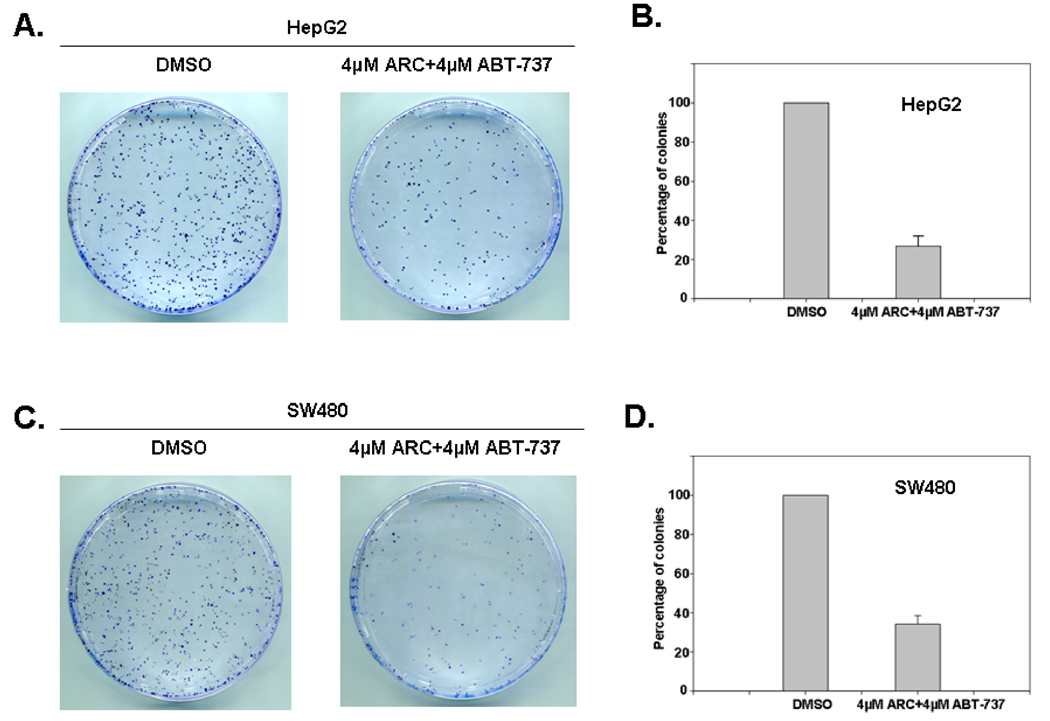
We also tested the levels of other proteins that play important role in apoptosis such as, Bcl-2 and Bax after treatment with ARC alone, ABT-737 alone or with ARC/ABT-737 combination in a few human cancer cell lines (Fig 2-Suppl.) and we found that in contrast to Mcl-1, these treatments did not change expression of Bax or Bcl-2. The long-term effects of combination treatment with ARC and ABT-737 were assessed by clonogenic assay. We found that colony formation in colon and liver cancer cells treated with these drugs for 24 hours was suppressed 3–4 fold (Fig 7).
Fig. 7. Clonogenic assay demonstrates the long-term effects of combination treatment of human cancer cells with ARC and ABT-737.
A. Clonogenic assay of HepG2 cells treated with DMSO or ARC-ABT-737 combination for 24hrs as detailed; Photographs of Petri dishes in a representative experiment are shown. B. Bar graph showing percentage of inhibition of colony formation by treatment with the drug combination (mean ± SD of 3 separate experiments). C. Clonogenic assay of SW480 cells treated with DMSO or ARC/ABT-737 combinations for 24 hrs. D. Bar graph showing percentage inhibition of colony formation by treatment with the drug combinations (mean ± SD of 3 separate determinations).
It has been shown in preclinical studies that ABT-737 synergizes with Mcl-1 inhibitors against leukemia [8, 17–19], melanoma [16], multiple myeloma [20], lymphoma [10], prostate [21] and small cell lung cancer [12]. In this paper, we showed the same effect for osteosarcoma, neuroblastoma, breast, colon, pancreatic and liver cancer cells suggesting that synergy of ABT-737 used in combination with Mcl-1 inhibitors to induce cell death in human cancer cells is a very general phenomenon. Our results suggest that it will be important to investigate the efficacy of ABT-737 in combination with ARC or with other transcriptional inhibitors against human solid tumors.
Supplementary Material
ACKNOWLEDGMENTS
Grant support: 1RO1CA1294414 and 1R21CA134615 from NIH to ALG.
We thank Ms Marianna Halasi for valuable suggestions and proofreading of the manuscript.
Abbreviations
- ARC
4-amino-6-hydrazino-7-β-D-ribofuranosyl-7H-Pyrrolo[2,3-d]-pyrimidine-5-carboxamide
- TMRE
tetramethylrhodamine ethyl ester
Footnotes
No conflict of interest.
REFERENCES
- 1.Radhakrishnan SK, Gartel AL. A novel transcriptional inhibitor induces apoptosis in tumor cells and exhibits antiangiogenic activity. Cancer Res. 2006;66:3264–3270. doi: 10.1158/0008-5472.CAN-05-3940. [DOI] [PubMed] [Google Scholar]
- 2.Radhakrishnan SK, Bhat UG, Halasi M, Gartel AL. P-TEFb inhibitors interfere with activation of p53 by DNA-damaging agents. Oncogene. 2008;27:1306–1309. doi: 10.1038/sj.onc.1210737. [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan SK, Halasi M, Bhat UG, Kurmasheva RT, Houghton PJ, Gartel AL. Proapoptotic compound ARC targets Akt and N-myc in neuroblastoma cells. Oncogene. 2008;27:694–699. doi: 10.1038/sj.onc.1210692. [DOI] [PubMed] [Google Scholar]
- 4.Bhat UG, Gartel AL. Differential sensitivity of human colon cancer cell lines to the nucleoside analogs ARC and DRB. Int J Cancer. 2008;122:1426–1429. doi: 10.1002/ijc.23239. [DOI] [PubMed] [Google Scholar]
- 5.Bhat UG, Zipfel PA, Tyler DS, Gartel AL. Novel anticancer compounds induce apoptosis in melanoma cells. Cell Cycle. 2008;7:1851–1855. doi: 10.4161/cc.7.12.6032. [DOI] [PubMed] [Google Scholar]
- 6.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 7.Adams JM, Huang DC, Strasser A, et al. Subversion of the Bcl-2 life/death switch in cancer development and therapy. Cold Spring Harb Symp Quant Biol. 2005;70:469–477. doi: 10.1101/sqb.2005.70.009. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 9.Lin X, Morgan-Lappe S, Huang X, et al. 'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 10.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 13.Song JH, Kandasamy K, Kraft AS. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem. 2008;283:25003–25013. doi: 10.1074/jbc.M802511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 15.Radhakrishnan SK, Feliciano CS, Najmabadi F, et al. Constitutive expression of E2F-1 leads to p21-dependent cell cycle arrest in S phase of the cell cycle. Oncogene. 2004;23:4173–4176. doi: 10.1038/sj.onc.1207571. [DOI] [PubMed] [Google Scholar]
- 16.Keuling AM, Felton KE, Parker AA, Akbari M, Andrew SE, Tron VA. RNA silencing of Mcl-1 enhances ABT-737-mediated apoptosis in melanoma: role for a caspase-8-dependent pathway. PLoS One. 2009;4:e6651. doi: 10.1371/journal.pone.0006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat UG, Gartel AL. Nucleoside analog ARC targets Mcl-1 to induce apoptosis in leukemia cells. Leukemia. 2010 doi: 10.1038/leu.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroda J, Kimura S, Andreeff M, et al. ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drugresistance mechanisms. Br J Haematol. 2008;140:181–190. doi: 10.1111/j.1365-2141.2007.06899.x. [DOI] [PubMed] [Google Scholar]
- 19.Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP. Mechanism of synergy of N-(4-hydroxyphenyl) retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst. 2008;100:580–595. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan D, Velankar M, Brahmandam M, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26:2374–2380. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 21.Pandit B, Gartel AL. New potential anti-cancer agents synergize with bortezomib and ABT-737 against prostate cancer. Prostate. doi: 10.1002/pros.21116. Published Online: 7 Jan 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.