Abstract
Store-operated Ca2+ entry through the plasma membrane Ca2+ release–activated Ca2+ (CRAC) channel in mammalian T cells and mast cells depends on the sensor protein stromal interaction molecule 1 (STIM1) and the channel subunit ORAI1. To study STIM1-ORAI1 signaling in vitro, we have expressed human ORAI1 in a sec6-4 strain of the yeast Saccharomyces cerevisiae and isolated sealed membrane vesicles carrying ORAI1 from the Golgi compartment to the plasma membrane. We show by in vitro Ca2+ flux assays that bacterially expressed recombinant STIM1 opens wild-type ORAI1 channels but not channels assembled from the ORAI1 pore mutant E106Q or the ORAI1 severe combined immunodeficiency (SCID) mutant R91W. These experiments show that the STIM1-ORAI1 interaction is sufficient to gate recombinant human ORAI1 channels in the absence of other proteins of the human ORAI1 channel complex, and they set the stage for further biochemical and biophysical dissection of ORAI1 channel gating.
Ca2+ influx through the CRAC channel in mammalian T cells and mast cells is essential for transcriptional responses and other effector responses to physiological stimuli1–4. Gating of the CRAC channel is a classical instance of store-operated Ca2+ entry, where an initial release of Ca2+ from internal cellular stores, by depleting the stores, triggers sustained Ca2+ signaling due to opening of plasma membrane Ca2+ channels5–7. RNA interference (RNAi) screens and determination of the genetic basis of a human severe combined immunodeficiency (SCID) syndrome have identified two proteins required for CRAC channel function: STIM1, a protein anchored in the endoplasmic reticulum (ER) that senses depletion of ER Ca2+ stores8–10, and ORAI1, a pore subunit of a plasma-membrane Ca2+ channel that is gated directly or indirectly by STIM1 (refs. 11–16).
The early steps of STIM1-ORAI1 signaling have been worked out in studies using engineered fluorescent STIM proteins. STIM1 senses a reduction in the ER luminal Ca2+ concentration, when Ca2+ dissociates from its luminal EF hand 1, a canonical helix-loop-helix Ca2+-binding motif9,10. Dissociation of Ca2+ leads to oligomerization of STIM1, followed by a local redistribution within the ER by which STIM1 becomes enriched at sites of ER–plasma membrane apposition, termed “puncta”9,10,17–22. Subsequently, STIM1 recruits ORAI1 to ER–plasma membrane contacts, where Ca2+ enters the cell through the opened ORAI1 channels23–27. Structural and biochemical studies with recombinant ER-luminal portions of STIM1 and STIM2 have illuminated the molecular mechanism by which STIM proteins sense Ca2+ changes in the ER lumen28,29.
Despite these insights, it remains unclear whether STIM1 directly gates ORAI1 channels. Overexpressed ORAI1 seems to be part of a larger channel complex30, and RNAi screens have identified other proteins that contribute measurably to store-operated Ca2+ entry12,13 (S. Sharma, P.G.H. and A.R., unpublished data), leaving open the possibility that proteins in addition to STIM and ORAI have a direct role in channel opening. The observation that overexpression of STIM with ORAI is sufficient for large store-operated Ca2+ currents12,18,31,32 has been taken as an indication that STIM by itself can gate ORAI. However, the cells used for expression of STIM and ORAI normally possess a store-operated Ca2+ entry pathway and may supply other proteins necessary to gate the overexpressed ORAI channels.
To dissect the essential steps in STIM1-ORAI1 signaling, we have expressed ORAI1 in the yeast Saccharomyces cerevisiae and isolated membrane vesicles containing functional ORAI1 channels. The yeast cells do not use the classical store-operated Ca2+ entry pathway that has been defined in mammalian cells—S. cerevisiae has no appreciable reservoir of Ca2+ in the ER33, does not possess orthologs of the ER Ca2+-ATPase (ATP2A1–ATP2A3) or IP3 receptor (ITPR1–ITPR3)34,35 and has no STIM or ORAI homologs—and therefore they are not likely to contribute proteins dedicated to this pathway. Here we report that recombinant STIM1 cytoplasmic fragments gate the ORAI1 channel in vitro.
RESULTS
Expression of recombinant ORAI1 and STIM1
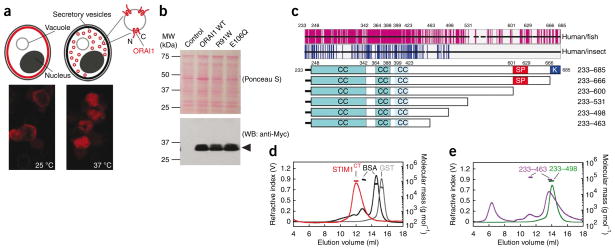
We set out to express properly assembled recombinant ORAI1 channels in the yeast S. cerevisiae. S. cerevisiae strains carrying the temperature-sensitive sec6-4 mutation have a defect in fusion of vesicles trafficking from the Golgi compartment to the plasma membrane at the restrictive temperature, 37 °C, and newly synthesized plasma-membrane proteins accumulate in vesicles in the cell cytoplasm36,37 (Fig. 1a, above). The isolated vesicles have been used in flux assays to investigate transport mediated by several plasma-membrane proteins38–41, and they seemed particularly suited to our planned experiments because recombinant ORAI1 would be oriented with its cytoplasmic face accessible for interaction with recombinant STIM1 (Fig. 1a, above). Myc-tagged human ORAI1 expressed in a sec6-4 strain of S. cerevisiae seemed to be correctly targeted to the plasma membrane at the permissive temperature, 25 °C, as indicated by a circumferential pattern of immunocytochemical staining of the Myc tag in most cells, and was retained in the cell interior at the restrictive temperature, 37 °C (Fig. 1a, below). Secretory vesicles isolated from cells that had been incubated at the restrictive temperature contained Myc-ORAI1 as demonstrated by western blotting (Fig. 1b).
Figure 1.
Recombinant ORAI1 and STIM1 proteins used in the present study. (a) Above, ORAI1 (red shading) is incorporated into the plasma membrane of sec6-4 yeast at the permissive temperature, 25 °C, but accumulates in vesicles within the cell at the nonpermissive temperature, 37 °C. Cytoplasmic portions of ORAI1 in isolated vesicles face the external solution. Below, immunocytochemical localization of Myc-ORAI1 in sec6-4 cells grown at 25 °C and at 37 °C, respectively. (b) Western blot (WB) for Myc-ORAI1 in vesicles isolated from control yeast or from yeast expressing wild-type ORAI1, ORAI1R91W or ORAI1E106Q. (c) Above, sequence conservation in the STIM C-terminal region. Each horizontal black bar represents the human STIM1 sequence, with gaps introduced to maintain alignment with fish or insect orthologs. Vertical magenta lines indicate identity between human STIM1 and at least four of five fish orthologs; vertical blue lines indicate identity with at least two of three insect Stim proteins. Below, STIM1 cytoplasmic fragments with predicted coiled-coil (CC), Ser-Pro-rich (SP) and polybasic (K) regions indicated. (d) SEC-MALLS analysis of STIM1CT. Recombinant STIM1CT (red) migrated as a single symmetrical peak, with no evidence of aggregated protein in the void volume at ~5–8 ml. Plotted molecular mass estimates refer to the axis at right. STIM1CT experimental molecular weight (MW), 110.5 kDa; theoretical monomer MW, 54.7 kDa. Standards were BSA monomer and dimer (black) and glutathione S-transferase dimer (GST, gray). (e) SEC-MALLS profiles of STIM1233–498 (experimental MW, 70.1 kDa; theoretical monomer MW, 34.8 kDa) and STIM1233–463 (experimental MW, 87.5 and 119.6 kDa; theoretical monomer MW, 30.9 kDa). Standards (omitted from the plot for clarity) were the same as in d.
To investigate the interaction by which STIM1 gates ORAI1 channels, we have focused on the cytoplasmic region of STIM1 (STIM1CT), which is sufficient in cells to trigger activation of ORAI1 (refs. 26,42–45). Sequence alignments of human STIM1 with its vertebrate orthologs show that the region of pronounced conservation ends around residue 531 of human STIM1; additional alignments with insect Stim proteins and with STIM2 show a shorter region of conservation, ending around residue 498 (Fig. 1c and Supplementary Fig. 1). On the premise that interaction with either ORAI itself or other proteins of the ORAI channel complex is a basic function of STIM proteins that will be reflected in sequence conservation, we expressed and purified STIM1 C-terminal proteins truncated at residue 498, at residue 531 and at other sites suggested by sequence conservation. The anchoring of STIM1 in the ER and the measured ER–plasma membrane distance, 17 ± 10 nm (ref. 19), render it unlikely that the initial part of the STIM1 coiled coil interacts directly with ORAI1, but we have retained the entire coiled coil in our constructs because of its possible role in proper STIM1 multimer assembly. The STIM1 proteins used in this study support store-operated Ca2+ influx when expressed in mammalian cells (Supplementary Data and Supplementary Figs. 2 and 3).
Analysis of recombinant STIM1CT by size exclusion chromatography coupled with multiangle laser light scattering (SEC-MALLS) showed that the freshly prepared protein is dimeric under our conditions in vitro (Fig. 1d). STIM1233–498 also formed dimers; STIM1233–463 was a heterogeneous mixture of trimers and smaller material, tetramers and large aggregates (Fig. 1e). STIM1CT and its fragments eluted earlier than expected for compact globular proteins of comparable mass, consistent with an elongated shape, and in particular consistent with the predicted STIM1 coiled coil. CD spectroscopy of STIM1CT, STIM1233–498 and STIM1233–463 indicated α-helical contents of 49%, 54% and 57%, respectively (Supplementary Fig. 4). The α-helical contents and the [θ]222/[θ]208 ratios, ~1, of the latter fragments provide strong experimental support for the predicted coiled coil.
Recombinant STIM1 binds to ORAI1
We used a membrane-flotation assay to evaluate binding of these recombinant STIM1 proteins to ORAI1. For these experiments, we expressed ORAI165–301, an N-terminally truncated ORAI1 protein that forms functional Ca2+ channels in mammalian cells (Supplementary Fig. 5), in the yeast Pichia pastoris. We prepared microsomal membranes from P. pastoris expressing ORAI165–301, layered these membranes at the bottom of a discontinuous sucrose gradient and centrifuged them. After centrifugation, the recombinant ORAI1 was recovered near the top of the gradient in the fraction visually identified as containing membranes (Fig. 2a), as expected for an integral membrane protein. STIM1CT centrifuged together with control membranes from yeast not expressing ORAI1 remained at the bottom of the gradient, as expected for a soluble protein. However, when we mixed STIM1CT with membranes containing ORAI1 and then centrifuged them, a substantial fraction of STIM1CT rose with the membranes into the upper part of the gradient, demonstrating its interaction with ORAI1. STIM1233–498 and all the longer STIM1 fragments tested in this assay also clearly bound ORAI1, whereas STIM1233–463 did not bind detectably.
Figure 2.
STIM1 cytoplasmic fragments interact with ORAI1 assembled in yeast membranes and with two cytoplasmic fragments of ORAI1. (a) Above, the part of ORAI1 expressed as a membrane protein in P. pastoris is highlighted in red. Below, P. pastoris membranes containing Flag-ORAI165–301 E106Q or control membranes, with or without His6-STIM1 protein, were loaded at the bottom of a discontinuous sucrose density gradient and subjected to centrifugation. Flag-ORAI1 and His6-STIM1 in individual gradient fractions were detected by western blotting (WB). The fraction of unbound STIM1 remaining at the bottom of the gradient is probably due to the presence of a moderate excess of STIM1 over ORAI1 in the assay. (b) Above, the C-terminal cytoplasmic tail of ORAI1 expressed as a GST fusion protein is highlighted in red. Below, the indicated STIM1 fragments were incubated with immobilized GST-ORAI1259–301 or with GST. Bound proteins were analyzed by SDS-PAGE and staining with Coomassie Brilliant Blue R-250. Samples on the input gel correspond to 20% of protein in the binding assay. (c) Above, the segment ORAI165–87 is highlighted in red. Below, STIM1 fragments were incubated with immobilized GST-ORAI165–87 or with GST and analyzed as in Figure 2b. Samples on the input gel correspond to 5% of protein in the binding assay.
Recruitment of ORAI1 to puncta by full-length STIM1 depends on a direct or indirect interaction of STIM1 with the C-terminal cytoplasmic tail of ORAI1 (refs. 26,46). Therefore, we next examined the direct interaction of recombinant STIM1 proteins with a bacterially expressed fusion protein, GST-ORAI1CT, containing the cytoplasmic tail of ORAI1, residues 259–301. STIM1CT and the other STIM1 fragments, except for STIM1233–463, bound to GST-ORAI1CT immobilized on resin (Fig. 2b). Binding was dependent on recognition of ORAI1CT, as there was no binding to GST alone. Because we visualized all input and bound proteins on the gel with Coomassie Brilliant Blue staining and detected no other proteins, the current experiment provides unambiguous proof of a direct protein-protein interaction between STIM1CT and ORAI1CT.
A conserved region of ORAI1 immediately preceding, and extending into, ORAI1 transmembrane segment 1 is implicated in channel opening11,46–48, and GFP-ORAI148–91 expressed in mammalian cells co-immunoprecipitates with STIM1342–448 (ref. 47). Here again, the protein-protein interaction is direct, because purified recombinant STIM1CT bound to purified GST-ORAI165–87 (Fig. 2c). This experiment required a large amount of input protein, and the fraction of input STIM1 retained by immobilized GST-ORAI1 peptide was small, indicating that the interaction is weaker than the STIM1CT-ORAI1CT interaction.
STIM1-ORAI1 interaction is sufficient for gating
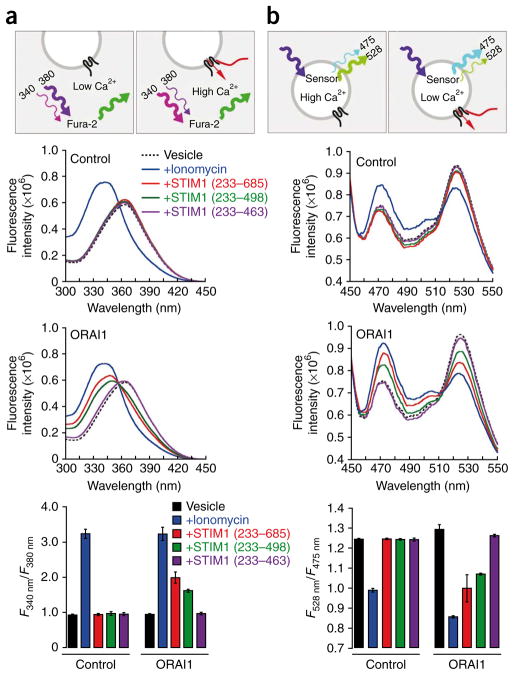
We next asked whether STIM1-ORAI1 protein-protein interactions are sufficient to open the ORAI1 Ca2+ channel. We obtained vesicles from sec6-4 S. cerevisiae expressing ORAI1 and incubated them under conditions in which Fura-2 was the principal Ca2+ buffer in the extravesicular solution, to monitor Ca2+ efflux49. Treatment with the Ca2+ ionophore ionomycin increased the prominence of the peak of the Fura-2 excitation spectrum near 340 nm (Ca2+–dye complex) relative to the peak near 365 nm (free dye), indicating release of Ca2+ to the extravesicular solution (Fig. 3a). Addition of STIM1CT or STIM1233–498 also elicited efflux of Ca2+ from vesicles with ORAI1, whereas addition of STIM1233–463 had little effect (Fig. 3a). The effects of STIM1CT and STIM1233–498 required ORAI1, because neither STIM1 fragment was effective in releasing Ca2+ from control vesicles lacking ORAI1 (Fig. 3a).
Figure 3.
STIM1 triggers ORAI1-dependent Ca2+ efflux from membrane vesicles of S. cerevisiae. (a) Above, principle of the Ca2+ flux assay using Fura-2. Initially, the external Ca2+ concentration is low and the peak of the free Fura-2 excitation spectrum is ~365 nm. When STIM1 gates the ORAI1 channel, Ca2+ is released from the vesicles, Fura-2 binds Ca2+ and the excitation peak is shifted to ~340 nm. Middle, Fura-2 excitation spectra of control and ORAI1-containing vesicles, with no addition or after the addition of ionomycin (20 μM) or STIM1 cytoplasmic fragments (2 μM). Below, graph of the fluorescence intensity ratio of Fura-2 (F340nm/F380nm) in each condition. Error bars indicate the range of duplicate measurements. (b) Above, principle of the Ca2+ flux assay using the FRET-based Ca2+ sensor cameleon D4cpV. In unstimulated vesicles, internal Ca2+ concentration is sufficient for binding to a fraction of the sensor, and cyan fluorescent protein-yellow fluorescent protein (CFP-YFP) FRET is evident in the peak at ~528 nm. When STIM1 gates the ORAI1 channel, Ca2+ is released from the vesicles, Ca2+ dissociates from the sensor, and there is a decline in the YFP peak at ~528 nm accompanied by an increase in the CFP peak at ~475 nm. Middle, D4cpV fluorescence emission spectra of control and ORAI1-containing vesicles, with no addition or after the addition of ionomycin (20 μM) or STIM1 cytoplasmic fragments (2 μM). Below, graph of the fluorescence intensity ratio of D4cpV (F528nm/F475nm) in each condition. Error bars indicate the range of duplicate measurements.
In complementary measurements, we monitored vesicular Ca2+ directly by coexpressing ORAI1 with the Ca2+ sensor D3cpV or D4cpV (ref. 50) fused to the α-mating–factor secretion signal to target the sensor to the vesicles. We isolated vesicles that had incorporated ORAI1 from sec6-4 yeast incubated at the restrictive temperature, and we diluted the vesicles into assay buffer. The intravesicular sensor gave a stable FRET signal at ~528 nm due to the internal Ca2+, indicating that the vesicles were not allowing Ca2+ to leak out. Treatment with ionomycin reduced the FRET signal at ~528 nm and increased the donor signal at ~475 nm, corresponding to depletion of vesicular Ca2+ (Fig. 3b). Addition of either STIM1CT or STIM1233–498 similarly triggered substantial loss of vesicular Ca2+ (Fig. 3b). For control vesicles lacking ORAI1, only ionomycin was effective in releasing Ca2+ (Fig. 3b).
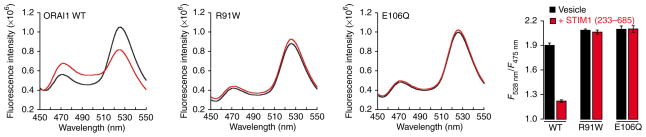
We sought confirmation that Ca2+ release from the vesicles was due to gating of the ORAI1 channel. Two mutant ORAI1 proteins that do not support Ca2+ influx in mammalian cells—ORAI1 R91W, which cannot be activated by STIM1 (ref. 11), and ORAI1 E106Q, which is disabled in the ion-conducting pore15—were expressed individually in the sec6-4 strain for control experiments. Although each mutant ORAI1 protein was present in isolated vesicles at levels comparable to those of wild-type ORAI1 (Fig. 1b), STIM1CT did not elicit Ca2+ release from vesicles containing either mutant protein (Fig. 4). We conclude that the in vitro assay reflects STIM1-dependent gating of ORAI1 and Ca2+ flux through the ORAI1 channel pore.
Figure 4.
STIM1 activates ORAI1 channels in membrane vesicles from S. cerevisiae. The experiment was carried out as in Figure 3b except for the use of D3cpV sensor. Fluorescence emission spectra were obtained from vesicles containing wild-type ORAI1, ORAI1 R91W or ORAI1 E106Q, either with no addition or following addition of STIM1CT (2.6 μM). The bar graph plots mean fluorescence intensity ratio F528nm/F475nm ± s.e.m. for each condition. STIM1CT changes the ratio significantly for wild-type ORAI1 (P < 0.001, two-tailed Welch’s t-test), but not for ORAI1 R91W and ORAI1 E106Q.
DISCUSSION
We have taken advantage of the absence of STIM-ORAI signaling in the yeast S. cerevisiae to show that recombinant STIM1 gates ORAI1 directly, without assistance from other proteins that have a dedicated role in the mammalian store-operated Ca2+ entry pathway. It seems certain from RNAi studies that additional proteins in mammalian cells can modulate Ca2+ influx through ORAI1 channels, but these proteins are not essential to channel function.
Our work implies that native STIM1 in cells interacts directly with ORAI1 across the ~17-nm distance19 that separates the ER and plasma membrane. The store-operated channels formed by ORAI165–301 (Supplementary Fig. 5) and ORAI174–301 (ref. 46) have short cytoplasmic portions that cannot span this distance. Rather, as we have shown here, STIM1 forms a coiled coil of sufficient length to position the central region of the STIM1 cytoplasmic domain near the cytoplasmic face of the plasma membrane. The latter finding complements evidence that the region of STIM1 encompassing residues 344–442 causes constitutive activation of ORAI1 channels when expressed in mammalian cells47,51–53.
Major questions remain about how specific STIM1-ORAI1 interactions participate in the gating conformational change, and about the conformational change itself. Our development of in vitro functional assays, with defined protein reagents, to probe the STIM1-ORAI1 interaction and Ca2+ flux through the ORAI1 channel is an essential step toward the rigorous biochemical characterization of STIM1-ORAI1 gating.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/nsmb/.
Supplementary Material
Acknowledgments
We are grateful to H. Li for guidance on protein expression and purification. We thank R. Rao for S. cerevisiae strain NY17 and for advice on membrane protein expression in that sec6-4 strain, R.Y. Tsien for plasmids encoding the calcium sensors D3cpV and D4cpV, J. Cregg for advice on protein expression in P. pastoris, the Department of Neurobiology, Harvard Medical School, for use of their spectrofluorimeter and T. Rapoport for access to SEC-MALLS instrumentation. This work was supported by US National Institutes of Health grants AI40127, GM075256 and AI084167 (to A.R. and P.G.H.) and by an Irvington Fellowship from the Cancer Research Institute and a Postdoctoral Fellowship from the Leukemia and Lymphoma Society (to Y.Z.).
Footnotes
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
AUTHOR CONTRIBUTIONS
P.G.H. set overall goals for the project and coordinated the work; Y.Z., P.M., A.R. and P.G.H. designed experiments and wrote the manuscript; Y.Z. prepared and characterized the STIM1 reagents, measured STIM-ORAI interactions and conducted the assays using mammalian cells; P.M. prepared and characterized sec6-4 ORAI1 vesicles; Y.Z. and P.M. conducted the in vitro Ca2+ flux assays; D.M. and H.T.K. developed the P. pastoris membrane-flotation assay; M.O., with Y.Z., carried out reconstitution and Ca2+-imaging experiments using STIM1−/− T cells; J.Z., with Y.Z., carried out SEC-MALLS analyses; Y.H., with Y.Z., contributed confocal microscopy; A.S. helped P.M. with construction of S. cerevisiae expression plasmids.
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/nsmb/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 2.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba Y, et al. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 4.Vig M, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 6.Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32:235–245. doi: 10.1016/j.tibs.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+ store depletion–triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 16.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spassova MA, et al. STIM1 has a plasma membrane role in the activation of tore-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer JC, et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong HL, et al. Relocalization of STIM1 for activation of store-operated Ca2+ entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca2+ store. J Biol Chem. 2007;282:12176–12185. doi: 10.1074/jbc.M609435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu P, et al. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 24.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muik M, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Borelly L, et al. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol (Lond) 2008;586:5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 29.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Várnai P, Tóth B, Tóth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 31.Peinelt C, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soboloff J, et al. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 33.Strayle J, Pozzan T, Rudolph HK. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 1999;18:4733–4743. doi: 10.1093/emboj/18.17.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol Cell Biol. 2000;20:6686–6694. doi: 10.1128/mcb.20.18.6686-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman-Gavrila LB, Lew RR. An IP3-activated Ca2+ channel regulates fungal tip growth. J Cell Sci. 2002;115:5013–5025. doi: 10.1242/jcs.00180. [DOI] [PubMed] [Google Scholar]
- 36.Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 37.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamoto RK, Rao R, Slayman CW. Expression of the yeast plasma membrane [H+]ATPase in secretory vesicles. A new strategy for directed mutagenesis. J Biol Chem. 1991;266:7940–7949. [PubMed] [Google Scholar]
- 39.Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994;77:1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 40.Laizé V, et al. Functional expression of the human CHIP28 water channel in a yeast secretory mutant. FEBS Lett. 1995;373:269–274. doi: 10.1016/0014-5793(95)01060-r. [DOI] [PubMed] [Google Scholar]
- 41.Coury LA, et al. Reconstitution of water channel function of aquaporins 1 and 2 by expression in yeast secretory vesicles. Am J Physiol. 1998;274:F34–F42. doi: 10.1152/ajprenal.1998.274.1.F34. [DOI] [PubMed] [Google Scholar]
- 42.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 43.Zhang SL, et al. Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. J Biol Chem. 2008;283:17662–17671. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penna A, et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji W, et al. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc Natl Acad Sci USA. 2008;105:13668–13673. doi: 10.1073/pnas.0806499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, et al. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 47.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derler I, et al. Increased hydrophobicity at the N terminus/membrane interface impairs gating of the severe combined immunodeficiency-related ORAI1 mutant. J Biol Chem. 2009;284:15903–15915. doi: 10.1074/jbc.M808312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer T, Wensel T, Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990;29:32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- 50.Palmer AE, et al. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muik M, et al. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.