Summary
The complexity of immunoregulation has focused attention on the CD4+ T “suppressor” regulatory cell (Treg), which helps maintain balance between immunity and tolerance. An immunoregulatory T cell population that upon activation amplifies cellular immune responses was described in murine models more than thirty years ago. However, no study has yet identified a naturally occurring T “inducer” cell type. Here, we report that the ectoenzyme CD39/NTPDase1 (ecto-nucleoside triphosphate diphosphohydrolase 1) helps to delineate a novel population of human “inducer” CD4+ T cells (TInd) that significantly increases the proliferation and cytokine production of responder T cells in a dose-dependent manner. Furthermore, this unique TInd cell subset produces a distinct repertoire of cytokines in comparison to the other CD4+ T cell subsets. We propose that this novel CD4+ T cell population counterbalances the suppressive activity of suppressor Treg cells in peripheral blood and serves as a calibrator of immunoregulation.
Keywords: CD4, CD39, Regulatory T cells, FOXP3, CD127, CD25, proliferation, Inducer, cytokine, IFN-γ, IL-6, IL-10, TNF-α, GM-CSF, ATP
Introduction
Naturally occurring regulatory T cells (Treg) constitute a suppressor T cell population that play a pivotal role in maintaining the balance between tolerance and immunity. Regulatory T cells can suppress effector T cell responses, and dampen immune activation in various autoimmune, transplant and infectious disease states[1–11]. The existence of a reciprocal pathway of immune “contrasuppression” was first reported by seminal reports more than thirty years ago [12–15]. The description of a natural T “inducer” cell population, that upon activation amplified cellular immune responses was suggested by clonal studies in mice [16], however, such a natural population of T cells has not been clearly identified in mice or humans.
The CD4+ T cell pool is diverse and plays important immunomodulatory roles in inflammation and infection. Naïve CD4+ T cells are induced to differentiate towards Th1, Th2, Tr1, Th17 or regulatory T cells, each under a distinct transcriptional control, and dependent on the influence of mitogenic stimulation or a cytokine directed milieu[10, 17, 18]. Two recent studies highlight the importance of CD39/NTPDase1 as a useful biomarker for the identification of a CD4+ regulatory suppressor T (Tsup) cell population with potent immunosuppressive activity in humans and mice [19, 20]. CD39 is an endothelial cell membrane-associated nucleotidase initially described on B cell lines[21], and reported to play a dominant role in thromboregulation and vascular homeostasis[22, 23].
In this study, we characterize a novel CD4+ inducer T cell subset in humans, expressing CD39, which potently enhances T cell immune responses that may be involved in the counter-regulation of regulatory T cells.
Results and Discussion
Expression of the CD39 molecule on CD3+ T cell subsets
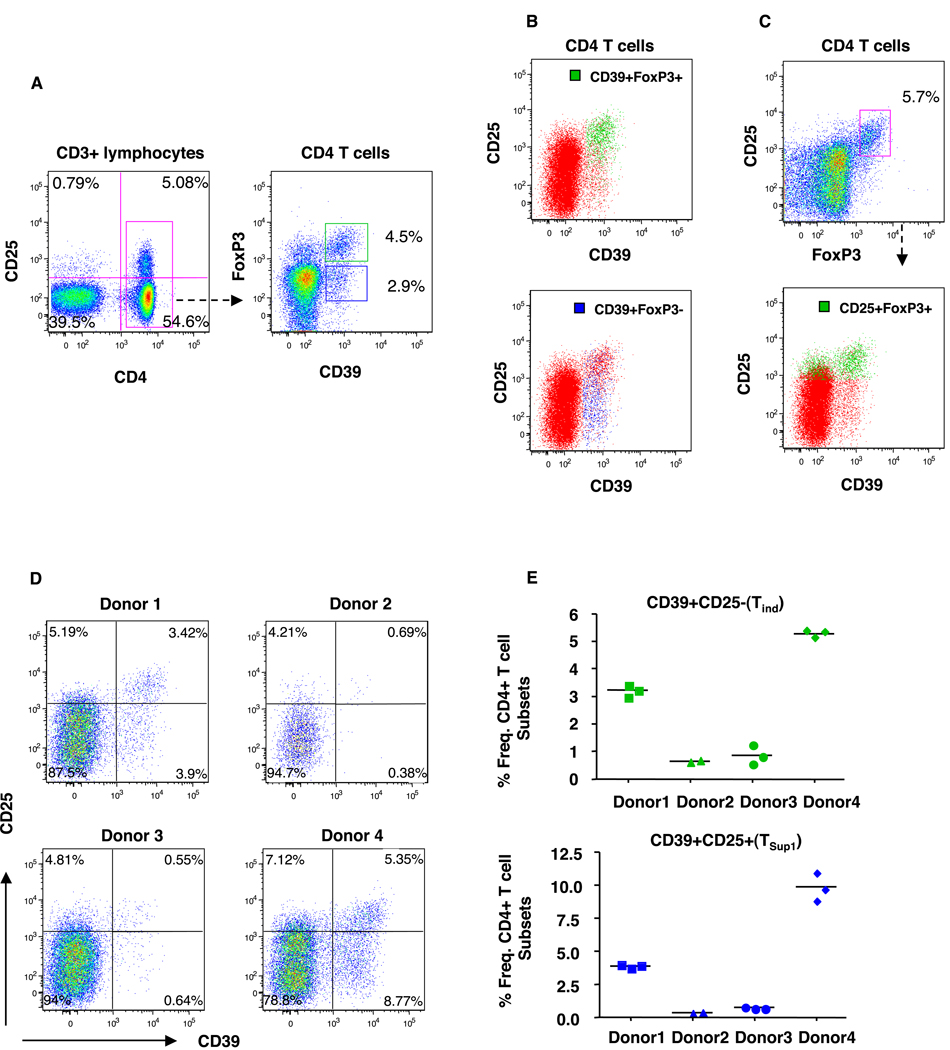
In order to investigate the expression of CD39 on lymphocyte subsets, we stained human PBMC and peripheral lymphoid cells from healthy individuals. We observed that the expression of CD39 was restricted to CD4+ T cells (Fig 1A). CD39 expression has recently been used to delineate CD4+ regulatory T cell subsets [19, 20]. To further characterize the CD39-expressing T cells we stained for CD25 and for intracellular FOXP3 expression and identified two CD4+ T subsets: CD39+FOXP3+CD25+ and CD39+FOXP3-CD25- T cells in peripheral blood (Fig. 1 A, B) as well as lymph node cells (data not shown). CD25+FOXP3+ CD4+ T cells are considered the canonical CD4+ regulatory T cell population, and we observed this subset to be represented primarily in the CD39+CD25high/+ fraction, although they were also present amongst the CD39-CD25+ T cells (Fig 1C). Similar results were observed using CD25+CD4+CD127low T cells to define regulatory T cells (data not shown).
Figure 1.
Differential expression of CD39 on CD4+ T cell subsets. PMBC from healthy donors were surface stained for CD4, CD3, CD25, CD127 and CD39 followed by intracellular staining for FoxP3. (A) Representative dot plots showing expression of CD39 as assessed by flow cytometry on live CD3 gated lymphocytes. The frequency (%) of CD4 T cells gated is shown according to their dual expression of CD39 and FOXP3. (B) Two distinct CD39-expressing CD4 T cell subsets are identified and further delineated by CD25 and CD39 expression: CD39+FOXP3+CD25hi/+(green) and CD39+FoxP3-CD25- T cells (blue). (C) FoxP3+CD25+ gated CD4 T cells (green) were further characterized by CD25 and CD39 expression. (D) Plot shows the frequency of CD4+ T cell subsets defined by CD39 and CD25 in four representative healthy donors. (E) Stability of CD39+CD25- and CD39+CD25+ T cells as a percentage of total CD4 T cells among 4 representative healthy donors at 2–3 time points approximately 4–6 months apart. At least 200,000 lymphocyte events were counted and collected for each sample.
The frequency of both the CD39+FOXP3+ and CD39+FOXP3-CD4+ T cell ranged from 1-5% each of the total CD4+ T cells in healthy subjects (n=10) (representative subject shown in Fig 1B,C). Interestingly, the frequencies of the CD39+CD25+ and CD39+CD25- CD4+ T cell subsets varied widely from individual to individual (Fig. 1D), but were relative stable within each healthy individual over time (Fig. 1E). Additional immune surface biomarkers were used to help delineate the two subsets, including CD73, CD57, CD27, HLA-DR, CD45RA, CCR5, CD7, Ki-67 and CD69, but these did not uniquely distinguish the CD39+CD25- bearing CD4+ T cells from the other CD4+ T cell subsets (data not shown).
Functional analysis of CD39+ CD4+ T cells
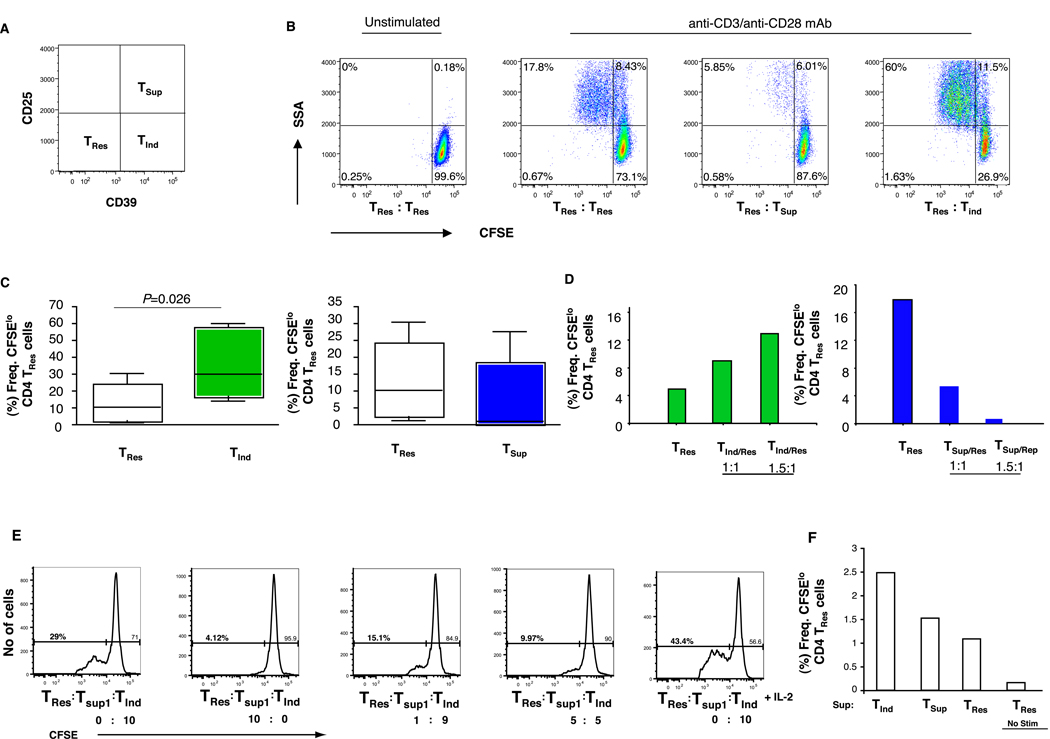
To address the functional capacity of these distinct CD4+ T cell populations, we first sorted CD4+ T cells from PBMC from healthy donors into CD39+CD25+, CD39+CD25- and CD39-CD25- T cell subsets, to a purity of >95%. We then assessed the ability of these three CD4+ T cell populations to independently influence the proliferative capacity and cytokine secretion of CFSE-labeled CD39-CD25- “responder” (TRes) CD4+ T cells. In co-culture experiments with anti-CD3 and anti-CD28, we observed that CD39+CD25+ CD4+ T cells from all donors markedly suppressed the proliferative capacity of TRes cells when compared to control unlabeled TRes cells to varying degrees (Fig. 2A, B). This was not surprising, as CD39+CD25+ T “suppressor” (TSup) CD4+ T cells were of a FOXP3+CD127lo phenotype, which is consistent with a suppressive regulatory T cell population[11, 24, 25]. Unexpectedly, the CD39+CD25-CD4+ T cell subset that expressed no FOXP3, dramatically increased the proliferation of responder CD4+ TRes cells, by as much as 4-fold in some donors, as compared to either of the other CD4+ T cell subsets (Fig. 2B,C). The proliferative responses elicited by the “inducer” (TInd) subset as well as suppressor (TSup) cells were dose-dependent (Fig. 2 C, D). The addition of TInd cells partially rescued the proliferation of responder CD4+ TRes cells in co-culture with Tsup cells, but this was over-ridden at higher ratios of TInd and TSup cells (Fig. 2 E). We further observed that while the addition of IL-2 markedly increased the proliferation of the TRes cells in the presence of TInd (Fig. 2 E), blockade of the effects of IL-2 using anti-CD122 or anti-CD25 mAbs in the cultures did not alter TRes cell responses (data not shown).
Figure 2.
Differential function of CD39 expressing CD4+ T cells. Purified CD4+ T cell subsets were isolated from PBMC from healthy donors by cell sorting to a purity >95% as demonstrated in Fig 1A. (A, B) Representative plots shows 50,000 CFSE-labeled CD39-CD25- responder (TRes) T cells co-cultured in duplicate wells together with an equal number of CD39+CD25- (TInd), CD39+CD25+ (TSup) or unlabeled control CD39-CD25- (TRes) T cells for 4–5 days in the presence of soluble anti-CD3/anti-CD28. Plots show proliferation of responder TRes cells as determined by CFSE dilution. (C) Similar results were obtained in six separate experiments from six different donors. The statistical difference was deemed significant using a Mann Whitney U test analysis if p<0.05. (D, E) In some experiments TRes cells were co-cultured at different ratios with TInd cells or TSup cells or a combination of both in the absence or presence of IL-2. Data in D, E are of a representative donor from two separate experiments with two different donors. (F) Graph shows the co-culture of Tres cells with supernatants derived from 4 day stimulated (anti-CD3/anti-CD28 mAb mixture) individual CD4+ T cells subsets from a representation donor. Similar results were observed in three separate experiments from three different donors.
We next explored the mechanisms of immune stimulation by the CD39+ CD25- TInd cells, and assessed whether the stimulatory effect can be mediated by soluble factors. We obtained supernatants from 4 day cultures of the individual CD4+ T cell subsets and added an equal amount of supernatants to CFSE labeled responder CD4+ TRes cells and assessed for T cell proliferation. In the presence of anti-CD3/CD28 stimulation the supernatants from TInd cells were able to induce significant stimulation of TRes cells compared to supernatants obtained from either TRes or TSup CD4+ T cell subsets (Fig. 2F).
Cytokine Profile of CD39+CD4+ T cell subsets
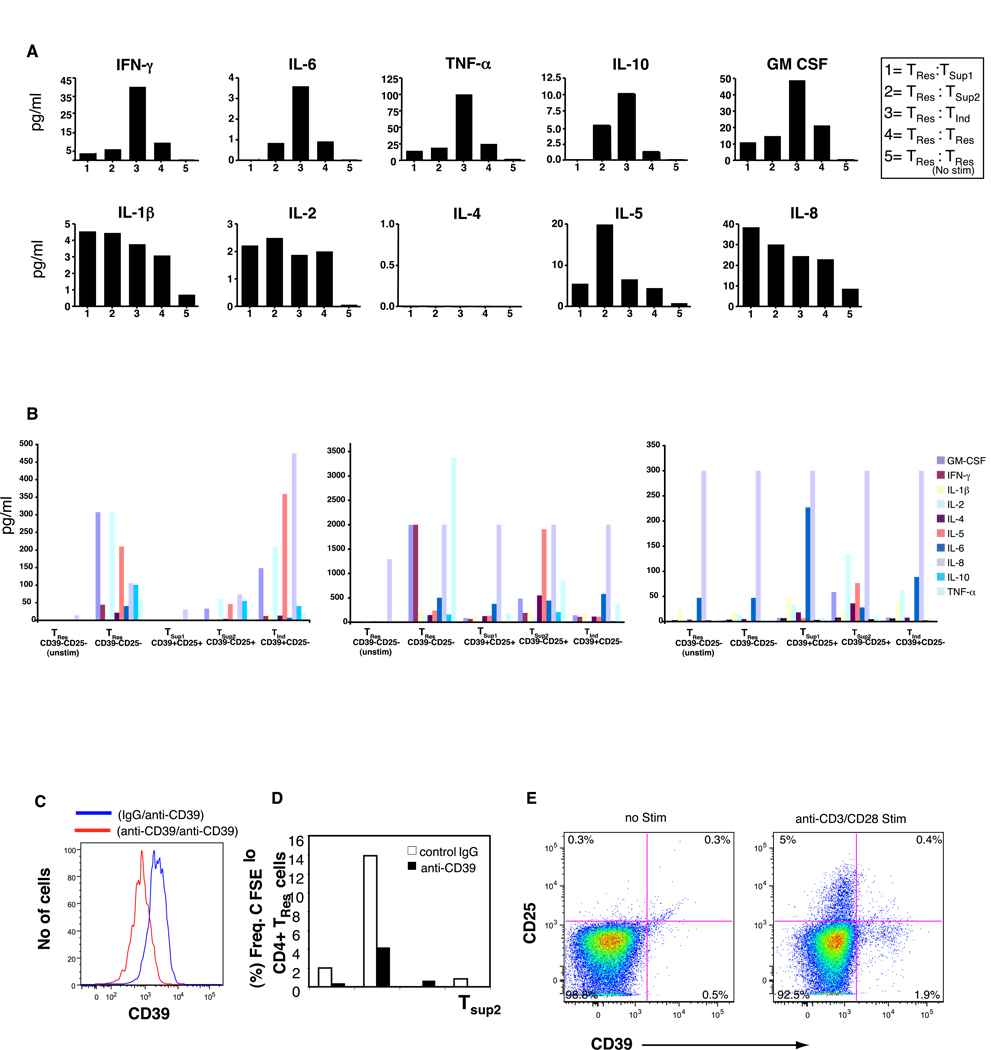
To explore the influence of these CD4+ T cell subsets on cytokine production, we performed multiplex 10-cytokine bead arrays on the co-culture supernatants. We saw a marked increase in the production of IFN-γ, TNF-α, GM-CSF, IL-6 and IL-10 in co-cultures with inducer TInd cells when compared to all other CD4+ T cell subsets examined (Fig. 3A). By contrast, these cytokines were produced in much lower quantities in co-cultures with either of the CD39+CD25+ (TSup1) or the CD39-CD25+ T cells (refered to as TSup2 cells based on their partial suppressive effects), as compared to TRes cells alone (Fig. 3A).
Figure 3.
Detection of human cytokines in cell supernatants in anti-CD3/anti-CD28 stimulated (A) co-cultures of responder TRes cells with either 1= CD39+CD25+ (TSup1); 2= CD39-CD25+ (TSup2), 3= CD39+CD25- (TInd); 4= CD39-CD25- (TRes); or 5= unstimulated CD39-CD25- (TRes) T cells. Data is presented from a single donor with background subtracted and is a representation of three separate experiments (from three separate donors). Standard curves were performed for each biomarker using the mixed standards provided with the kit. (B) Cell sorted, purified individual CD4+ T cell subsets alone from three donors is presented. Data is the average of duplicate wells. (C) Specificity of a purified anti-human CD39 (clone BU61) mAb. PBMC from a healthy donor were incubated with either isotype control IgG antibody (blue line) or purified anti-human CD39 (clone BU61) mAb (red line) prior to staining with a conjugated anti-CD39 mAb (clone eBioA1) antibody. (D) CFSE labeled CD39-CD25- responder (TRes) T cells co-cultured in duplicate wells together with an equal number of indicated CD4 T cell subsets in the presence of either purified anti-CD39 (clone BU61) mAb or isotype IgG control. Cells were stimulated with anti-CD3/CD28 antibody mixture and cultured for 4 days before assessment of CFSE dilution. Data is a representation of two separate experiments from two different donors. (E) Plot shows expression of CD39 and CD25 by purified cell sorted CD39-CD25- responder (TRes) obtained from a representative healthy donor following anti-CD3/anti-CD28 stimulation for 12 hours. Similar results were obtained in 4 day cultures.
To determine the direct ex vivo activity of each individual CD4+ T cell subset, we cell sorted each subset and evaluated the cytokine profile following stimulation with anti-CD3/CD28. We demonstrated that TInd and TSup1 cells produced varied overall cytokine quantities compared to TRes cells (Fig 3B). The TInd cells produced slightly higher cytokines levels compared to TSup1 cells, however no specific cytokine profile distinct to TInd cells was noticable. Interestingly we observed that TSup2 cell produced a unique cytokine profile that was dominant for the Th2 cytokines IL-4 and IL-5 in two of three individuals assayed.
Influence of anti-CD39MAb on CD39-expressing CD4+ T cell responses
CD39 has been shown to be responsible for the degradation of ATP to AMP. Activated regulatory T (CD4+CD25+) cells appear to abrogate ATP-related events through CD39 [20]. To test whether the biological activity of CD39 on CD39+CD25- (TInd) and CD39+CD25+ (TSup1) T cells was retained and functional, we performed co-culture experiments of all four purified CD4+T cell subsets with responder TRes cells in the presence of either a purified anti-CD39 MAb that diminished the expression of CD39 on PMBC (Fig 3C), or with an isotype control, and assessed for both CFSE dilution and cytokine release. We observed no reversal of function but instead reduced proliferation (Fig. 3D), and a reduction of cytokine responses (data not shown) in cultures with anti-CD39 MAb, with the only residual responses preserved in cultures with TInd cells. Interestingly, following anti-CD3/CD28 stimulation, surface expression of CD39 was partially upregulated on purified cell sorted CD39-CD25- (TRes) CD4+ Tcells, demonstrating the responsiveness to the anti-CD39MAb (Fig. 3E).
The mechanisms by which the inducer T cell subset acts remains unclear, as our studies do not distinguish whether CD39 directly contributes to this phenomenon or simply acts as a biomarker. ATP metabolites have paradoxical effects on the immune response and can mediate both immunostimulatory and inhibitory effects. Although experiments performed with the anti-CD39 MAb did not interfere with the inductive capacity of the TInd cells, we cannot completely disregard CD39 as a component of the inductive machinery. Notably, CD39 can exist in two different functional states based on its two transmembrane domains, and these two forms can present with distinct quantitative and qualitative enzymatic properties[26, 27]. It is likely that investigation into the structural composition of CD39 on different CD4+ T cell subsets will improve our understanding of this ectoenzyme.
Concluding Remarks
The major observation from this study is the identification of a novel population of human “inducer” CD4+ T cells that significantly increased the proliferation and cytokine production of responder T cells from healthy donors in vitro. Phenotypically, the inducer T cells are CD25 and FOXP3 negative, but express CD39. The CD39 molecule has recently been identified as a marker for suppressive regulatory T cells, and has been suggested to exert immunosuppressive functions in part by hydrolyzing both ATP, ADP, and other di- and triphosphate nucleotides. Here, we identify and characterize a subset of CD4+ T cells that express CD39, yet induce and potentiate T cell responses. The diversity of outcomes of CD4+ T cell fate has garnered much interest, with CD4+ T cells differentiating into TH1, TH2 , TH17 or T regulatory cells, each with distinct functions [28]. Here, we add a new dimension to this T cell pathway, and identify a potentially new paradigm in CD4+ T cell differentiation. Based on the novel functional capacity of TInd cells, we believe this reflects a novel distinct functional subset of CD4+ T cells. However, it remains possible that the TInd cell subset is not a distinct lineage but part of the TH1, TH2 or TH17 subpopulations. Furthermore, any overlap with the transcriptional patterns of T-bet, GATA-3 or RORγt, which are essential for development of TH1, TH2 and TH17 cells respectively, or if distinct specific lineage factors exist, will need to be determined.
It has been proposed that the suppressive function of CD39 is coupled to the presence of CD73, that would convert AMP to adenosine (from the enzymatic degradation of ATP by CD39 [19]. Adenosine would then act on adenosine A2a receptors on responder T cells to increase the intracellular signal molecule cAMP, which has a well known immunosuppressive function [29, 30]. It is intriguing to speculate that the absence or presence of CD73 could determine whether CD39 expressing CD4+T cells would act as suppressor or inducer T cells. Although CD39 helps to identify the inducer T cell population, the identification of additional cell surface antigens which could delineate the TInd subset further, will be needed. While our results highlight a distinct functional difference among the CD4+ CD39+ T cells based on the coexpression of CD25, this was not considered in other recent studies [19, 20]. Since some of our donors clearly presented with very low frequencies of CD39+ CD25- CD4+ T cells, we presume this TInd CD4+ T cell population may not been considered in these studies.
This study brings another dimension to the field of immune regulation, by introducing TInd cells as a newly defined inductive T cell population. As suggested here, the interplay between TInd and TSup1 cell subsets may be important to immune homeostasis. The observed donor variability in both T suppressor and T inducer cell numbers – and in their ratios in vivo – should be an interesting vantage point for future studies. Further studies elucidating the precise mechanism by which these cells function and evaluating the frequency and function of this CD4+ TInd cell subset in various immune-mediated pathologies are warranted.
Materials and Methods
Human subjects
Freshly blood was obtained from healthy blood donors and from the Stanford Blood Bank. Peripheral lymphoid tissues were obtained from donors with tonsillar hypertrophy from the Karolinska University Hospital under IRB approved protocols. Samples were processed with Ficoll-Paque PLUS (Amersham Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation and peripheral-blood mononuclear cells (PBMC) were isolated and cyropreserved in 10 % DMSO in FBS. This study was approved by the institutional review boards and ethical committees of University of California, San Francisco, Committee on Human Research and the Karolinska Institutet.
Flow cytometry
Frozen PBMCs and peripheral lymph node cells were rapidly thawed and incubated with a combination of the following conjugated anti-human monoclonal antibodies: CD4, CD25, CD8, CD45RA, CD73, CD27, CD57, HLA-DR, CCR5, CD7, Ki-67, CD69 (BD Biosciences, San Jose, CA), CD39 (e-bioscience, San Diego, CA and Ancell, Bayport, MN), CD3, (Beckman Coulter, Fullerton, CA), and CD127 (Biolegend, San Diego, CA). For dead cell exclusion we used a live dead amine aqua dye (Invitrogen, Carlsbad, CA). Intracellular staining for FoxP3 (BD) was performed using the protocol as recommended by the manufacturer. Cells were then washed in FACS buffer (PBS in 2mM EDTA and 1% bovine serum albumin) and then fixed in 1% paraformaldehyde before being run on an LSRII flow cytometer (BD). Data was analyzed using Flowjo Software version 6.4 (Treestar Inc, Ashland, OR). In additional functional assays, purified anti-human CD39 (clone BU61) mAb (Ancell, Bayport, MN), conjugated anti-CD39 mAb (clone eBioA1) antibody (Ebisocience, San Diego,CA), purified mouse anti-human IgG (Ebisocience), purified anti-CD122 mAb and anti CD25 mAb (R&D Systems) were used.
Suppression assay
PBMC were labeled with a combination of antibodies to delineate CD4+ T cell subsets and sorted using a FACS ARIA (BD) to purity levels in excess of 95%. The fluorescent intracellular dye, 5- (and 6-) carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) was used to track cell division. In brief, autologous defined responder CD4+ T cell subsets were labeled with 1mM of CFSE in PBS and mixed periodically for 10 minutes at 37°C. Labeling was quenched by addition of an equal volume of complete medium (15% FBS in RPMI) for 2 minutes. The labeled cells were then washed twice, counted and resuspended in cell culture media and used as responder cells. Cell cultures were stimulated with purified azide free anti-CD3 and anti-CD28 MAb mixture (BD) in the presence or absence of sorted T cell subsets at the indicated ratio of suppressors to responders.
Multiplex human cytokine measurement
Supernatants from individual and co-culture experiments were removed from the wells and assayed in duplicates using a highly sensitive bead-based sandwich immunoassay for the simultaneous detection of multiple cytokines using the Biosource human cytokine 10-plex Panel kit (Invitrogen), simultaneously measuring the following 10 human biomarkers: TNF-α, IFN-γ, GM-CSF, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, and IL-10. The beads were analyzed with a Luminex 100 system (Luminex Inc, Austin, Texas) according to the manufacturer’s protocol. Standard curves were performed for each biomarker using the mixed standards provided with the kit.
Statistical measurements
A paired Student's t-test was performed for comparison of changes within subject and correlations were assessed using the Spearman rank test for non-parametric data. All statistical analyses were conducted using Prism Graphpad release 4a (Graphpad Software, San Diego, CA) and the statistical significance of the findings was set at a p value of less than 0.05.
Acknowledgements
This research was supported by funds the National Institutes of Health, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763), the UCSF AIDS Biology Program of the AIDS Research Institute (ARI), NIH grants (AI060379, AI068498, and AI042590), the Fogarty International Center of the National Institutes of Health, University of California, Berkeley, School of Public Health, Division of Epidemiology, Berkeley, California 94720-7360.
Footnotes
There are no financial/commercial conflicts of interests.
References
- 1.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol. 2003;3:223–232. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 2.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 3.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 5.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 6.Nixon DF, Aandahl EM, Michaelsson J. CD4+CD25+ regulatory T cells in HIV infection. Microbes Infect. 2005;7:1063–1065. doi: 10.1016/j.micinf.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 8.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 11.Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. FOXP3 expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J Leukoc Biol. 2008;83:254–262. doi: 10.1189/jlb.0507281. [DOI] [PubMed] [Google Scholar]
- 12.Green DR, Chue B, Gershon RK. Discrimination of 2 types of suppressor T cells by cell surface phenotype and by function: the ability to regulate the contrasuppressor circuit. J Mol Cell Immunol. 1983;1:19–30. [PubMed] [Google Scholar]
- 13.Gershon RK, Eardley DD, Durum S, Green DR, Shen FW, Yamauchi K, Cantor H, Murphy DB. Contrasuppression. A novel immunoregulatory activity. J Exp Med. 1981;153:1533–1546. doi: 10.1084/jem.153.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehner T. Special regulatory T cell review: The resurgence of the concept of contrasuppression in immunoregulation. Immunology. 2008;123:40–44. doi: 10.1111/j.1365-2567.2007.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athanassakis I, Vassiliadis S. T-regulatory cells: are we re-discovering T suppressors? Immunol Lett. 2002;84:179–183. doi: 10.1016/s0165-2478(02)00182-7. [DOI] [PubMed] [Google Scholar]
- 16.Nabel G, Greenberger JS, Sakakeeny MA, Cantor H. Multiple biologic activities of a cloned inducer T-cell population. Proc Natl Acad Sci U S A. 1981;78:1157–1161. doi: 10.1073/pnas.78.2.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 19.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'cqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 21.Rowe M, Hildreth JE, Rickinson AB, Epstein MA. Monoclonal antibodies to Epstein-Barr virus-induced, transformation-associated cell surface antigens: binding patterns and effect upon virus-specific T-cell cytotoxicity. Int J Cancer. 1982;29:373–381. doi: 10.1002/ijc.2910290403. [DOI] [PubMed] [Google Scholar]
- 22.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 24.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grinthal A, Guidotti G. CD39, NTPDase 1, is attached to the plasma membrane by two transmembrane domains. Why? Purinergic Signal. 2006;2:391–398. doi: 10.1007/s11302-005-5907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musi E, Islam N, Drosopoulos JH. Constraints imposed by transmembrane domains affect enzymatic activity of membrane-associated human CD39/NTPDase1 mutants. Arch Biochem Biophys. 2007;461:30–39. doi: 10.1016/j.abb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Reiner SL. Decision making during the conception and career of CD4+ T cells. Nat Rev Immunol. 2009;9:81–82. doi: 10.1038/nri2490. [DOI] [PubMed] [Google Scholar]
- 29.Aandahl EM, Moretto WJ, Haslett PA, Vang T, Bryn T, Tasken K, Nixon DF. Inhibition of antigen-specific T cell proliferation and cytokine production by protein kinase A type I. J Immunol. 2002;169:802–808. doi: 10.4049/jimmunol.169.2.802. [DOI] [PubMed] [Google Scholar]
- 30.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]