Abstract
Trials of anemia correction in chronic kidney disease have found either no benefit or detrimental outcomes of higher targets. We did a secondary analysis of patients with chronic kidney disease enrolled in the Correction of Hemoglobin in the Outcomes in Renal Insufficiency trial to measure the potential for competing benefit and harm from achieved hemoglobin and epoetin dose trials. In the 4 month analysis, significantly more patients in the high-hemoglobin compared to the low-hemoglobin arm were unable to achieve target hemoglobin and required high-dose epoetin-α. In unadjusted analyses, the inability to achieve a target hemoglobin and high-dose epoetin-α were each significantly associated with increased risk of a primary endpoint (death, myocardial infarction, congestive heart failure or stroke). In adjusted models, high-dose epoetin-α was associated with a significant increased hazard of a primary endpoint but the risk associated with randomization to the high hemoglobin arm did not suggest a possible mediating effect of higher target via dose. Similar results were seen in the 9 month analysis. Our study demonstrates that patients achieving their target had better outcomes than those who did not; and among subjects who achieved their randomized target, no increased risk associated with the higher hemoglobin goal was detected. Prospective studies are needed to confirm this relationship and determine safe dosing algorithms for patients unable to achieve target hemoglobin.
Keywords: anemia, chronic kidney disease, epoetin-α, dose, epidemiology and outcomes
Recombinant erythropoietin revolutionized the care of patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD)1,2 reducing blood transfusions and complications like iron overload. Although initial therapy was aimed at partial anemia correction, observational studies suggested that treatment with erythropoietin-stimulating agents (ESA) to higher targets was associated with improved survival.3,4 Three randomized trials of anemia correction in CKD and ESRD patients, however, failed to demonstrate a benefit of higher hemoglobin targets.5–8 In fact, the final analyses of one trial unexpectedly demonstrated harm among subjects randomized to higher targets.5
Two factors that may contribute to worse outcomes with higher targets include failure to attain target and higher doses of ESAs. Higher doses of ESAs are required with higher hemoglobin targets,5,7 and the failure to achieve a hemoglobin target leads to further increases in ESA dose. In an observational study among patients with ESRD, a lesser response to therapy as well as higher doses of ESA were associated with increased mortality.9
This secondary analysis of subjects with CKD enrolled in the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial was undertaken to examine the potential for competing benefit and harm from achieved dose of hemoglobin and epoetin. Specifically, this analysis examines the associations between ESA dose, inability to achieve hemoglobin target, and clinical outcomes.
RESULTS
Four-month landmark analysis
Baseline characteristics including markers of inflammation, such as albumin and ferritin, were similar between groups analyzed in the 4-month landmark analysis (Table 1, n =1290). Hemoglobin at baseline and at 3 weeks (before which subjects all received the same ESA dose) were similar between groups. Subjects randomized to the high-hemoglobin arm had a higher prevalence of self-reported hypertension and coronary artery bypass graft (both P =0.03).
Table 1.
Baseline characteristics of subjectsa
| Four-month landmark analysis population |
Nine-month landmark analysis population |
|||||
|---|---|---|---|---|---|---|
| High-hemoglobin group (N=627) | Low-hemoglobin group (N=633) | P-value | High-hemoglobin group (N=519) | Low-hemoglobin group (N=538) | P-value | |
| Age, year | 65.9 (14.2) | 66.6 (13.2) | 0.37 | 65.7 (14.3) | 66.4 (13.2) | 0.41 |
| Female sex (%) | 56.5% | 54.0% | 0.39 | 57.4% | 54.1% | 0.28 |
| Race (%) | 0.74 | 0.53 | ||||
| White | 62.7% | 61.6% | 63.5% | 60.8% | ||
| Black | 28.0% | 29.5% | 28.6% | 30.5% | ||
| American-Indian or Alaskan Native | 0.2% | 0.5% | 0.2% | 0.6% | ||
| Asian or Pacific Islander | 3.7% | 2.8% | 3.1% | 2.2% | ||
| Other | 5.4% | 5.5% | 4.6% | 5.9% | ||
| Hispanic ethnic background (%) | 12.3% | 13.1% | 0.67 | 12.6% | 13.4% | 0.69 |
| History of smoking tobacco (%) | 46.6% | 43.8% | 0.31 | 45.1% | 43.3% | 0.57 |
| Cause of CKD (%) | 0.50 | 0.66 | ||||
| Diabetes mellitus | 46.4% | 49.7% | 45.8% | 48.6% | ||
| Hypertension | 30.2% | 28.3% | 30.9% | 29.1% | ||
| Other | 23.4% | 22.1% | 23.3% | 22.3% | ||
| Cardiovascular history (%) | ||||||
| Hypertension | 95.8% | 92.9% | 0.03 | 95.2% | 92.4% | 0.07 |
| Myocardial infarction | 14.8% | 14.6% | 0.92 | 14.6% | 13.6% | 0.66 |
| CABG | 17.9% | 13.2% | 0.03 | 16.7% | 12.5% | 0.05 |
| PCI | 9.4% | 10.8% | 0.43 | 9.6% | 10.9% | 0.50 |
| Congestive heart failure | 22.0% | 20.7% | 0.58 | 21.0% | 18.9% | 0.41 |
| Atrial fibrillation | 8.1% | 8.8% | 0.66 | 8.2% | 7.8% | 0.81 |
| Stroke | 9.5% | 9.0% | 0.74 | 9.8% | 9.2% | 0.75 |
| Lower-extremity amputation | 3.2% | 2.8% | 0.71 | 3.2% | 2.5% | 0.53 |
| MI, CABG, or PCI | 26.3% | 25.0% | 0.59 | 26.1% | 23.7% | 0.37 |
| Body Mass Index (kg/m2) | 30.5 (7.8) | 30.4 (7.5) | 0.85 | 30.5 (7.6) | 30.7 (7.7) | 0.69 |
| GFR (ml/min/m2) | 27.1 (8.7) | 27.6 (9.1) | 0.30 | 27.5 (8.7) | 28.3 (9.1) | 0.14 |
| Baseline hemoglobin (g/100 ml) | 10.1 (0.86) | 10.1 (0.85) | 0.78 | 10.1 (0.85) | 10.1 (0.84) | 0.28 |
| Week 3 hemoglobin (g/100 ml) | 10.7 (0.94) | 10.6 (0.94) | 0.10 | 10.7 (0.95) | 10.6 (0.94) | 0.50 |
| Baseline albumin (g/10 ml) | 3.8 (0.51) | 3.8 (0.46) | 0.35 | 3.8 (0.47) | 3.8 (0.45) | 0.94 |
| Baseline phosphorus (mg/100 ml) | 4.1 (0.73) | 4.1 (0.74) | 0.36 | 4.1 (0.73) | 4.0 (0.73) | 0.12 |
| Baseline cholesterol (mg/100 ml) | 184.6 (50.2) | 183.5 (47.9) | 0.70 | 184.5 (48.9) | 183.9 (47.7) | 0.83 |
| Ratio of total protein/creatinine in urine | 1.5 (2.10) | 1.4 (2.10) | 0.36 | 1.3 (1.84) | 1.2 (1.84) | 0.38 |
| Ferritin (ng/ml) | 167.8 (157.2) | 178.5 (173.1) | 0.25 | 165.9 (158.1) | 172.5 (157.0) | 0.50 |
| Transferrin saturation (%) | 25.3 (11.7) | 24.6 (10.1) | 0.29 | 25.2 (11.8) | 24.7 (10.2) | 0.43 |
| Transferrin saturation <20% (%) | 36.1% | 33.9% | 0.40 | 36.1% | 33.9% | 0.44 |
| Medications (%) | ||||||
| ACE inhibitor or ARB | 75.8% | 75.3% | 0.84 | 77.5% | 76.8% | 0.80 |
| Beta blocker | 45.0% | 47.9% | 0.30 | 44.9% | 47.7% | 0.36 |
| HMG CoA reductase inhibitor | 52.3% | 53.0% | 0.79 | 54.4% | 55.0% | 0.85 |
| Iron | ||||||
| Intravenous | 2.9% | 1.8% | 0.18 | 3.1% | 2.1% | 0.28 |
| Oral | 27.1% | 25.8% | 0.60 | 26.8% | 24.1% | 0.32 |
| Not specified | 3.4% | 1.6% | 0.04 | 3.3% | 2.4% | 0.06 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CKD, chronic kidney disease; GFR, glomerular filtration rate; HMG CoA, 3-hydroxy-3-methyl-glutaryl coenzyme A; PCI, percutaneous coronary intervention; MI, myocardial infarction.
Continuous variables reported as means (s.d.) unless noted otherwise.
In both treatment groups, subjects not achieving their target hemoglobin within 4 months experienced events at a higher rate (Table 2). During this first 4 months, a greater proportion of subjects in the high-hemoglobin as compared with the low-hemoglobin group were unable to achieve their target (37.5 vs 4.7%, P<0.001).
Table 2.
Description of achieved hemoglobin, epoetin-α dose, and outcomes in 4- and 9-month landmark analysis
| Four-month landmark analysis population |
Nine-month landmark analysis population |
|||
|---|---|---|---|---|
| High-hemoglobin (n=627) | Low-hemoglobin (n=633) | High-hemoglobin (n=519) | Low-hemoglobin (n=538) | |
| Achieved hgb<11.1, N (%) | 36 (5.7%) | 30 (4.7%) | 4 (0.8%) | 4 (0.7%) |
| Max hgb g/100 ml, mean (s.d.) | 10.2 (0.80) | 10.3 (0.68) | 10.9 (0.10) | 10.9 (0.19) |
| Composite events (%) | 9 (25.0%) | 3 (10.0%) | 2 (50.0%) | 0 |
| High-dose ESA (%) | 30 (83.3%) | 19 (63.3%) | 4 (100.0%) | 3 (75.0%) |
| Achieved hgb 11.1–13.1, N (%) | 199 (31.7%) | 443 (70.0%) | 73 (14.1%) | 351 (65.2%) |
| Max hgb g/100 ml, mean (s.d.) | 12.2 (0.57) | 12.3 (0.47) | 12.3 (0.46) | 12.5 (0.38) |
| Composite events (%) | 34 (17.1%) | 49 (11.1%) | 13 (17.8%) | 32 (9.1%) |
| High-dose ESA (%) | 121 (60.8%) | 35 (7.9%) | 63 (86.3%) | 57 (16.2%) |
| Achieved hgb≥13.1 | 392 (62.5%) | 160 (25.3%) | 442 (85.2%) | 183 (24.0%) |
| Max hgb g/100 ml, mean (s.d.) | 14.2 (0.70) | 13.6 (0.58) | 14.6 (0.69) | 13.6 (0.59) |
| Composite events (%) | 49 (12.5%) | 15 (9.4%) | 47 (10.6%) | 9 (4.9%) |
| High-dose ESA (%) | 69 (17.6%) | 7 (4.4%) | 230 (52.0%) | 23 (12.6%) |
| High-dose ESA, N (%) | 220 (35.1%) | 61 (9.6%) | 297 (57.2%) | 83 (15.4%) |
| Max dose ESA, mean (s.d.) | 20,123 (769.5) | 20,033 (256.1) | 20,088 (766.1) | 20,024 (219.5) |
| Composite events (%) | 40 (18.2%) | 10 (16.4%) | 41 (13.8%) | 6 (7.2%) |
| Max hgb g/100 ml | 12.4 (1.39) | 11.5 (1.26) | 14.0 (1.16) | 12.7 (0.89) |
| Low-dose ESA (%) | 407 (64.9%) | 572 (90.4%) | 222 (42.8%) | 455 (84.6%) |
| Max dose ESA, mean (s.d.) | 13,162 (2988.2) | 11,533 (2577.8) | 14,185 (2917.4) | 11,933 (2615.7) |
| Composite events (%) | 52 (12.8%) | 57 (10.0%) | 21 (9.5%) | 35 (7.7%) |
| Max hgb g/100 ml, mean (s.d.) | 13.9 (1.08) | 12.6 (0.79) | 14.6 (0.76) | 12.9 (0.69) |
hgb, hemoglobin.
Bold-faced numbers represent the patients who did not achieve their hgb target within their randomized group.
Within each arm, subjects receiving high-dose epoetin-α experienced events at a higher rate (18.2 vs 12.8% in the high-hemoglobin and 16.4 vs 10.0% in the low-hemoglobin groups). A larger proportion of subjects in the high-hemoglobin as compared with the low-hemoglobin group required high-dose epoetin-α (35.1 vs 9.6%, P<0.001). Among subjects not achieving their target, 64.2% received high-dose epoetin-α as compared with 11.2% among subjects achieving their target (P<0.001).
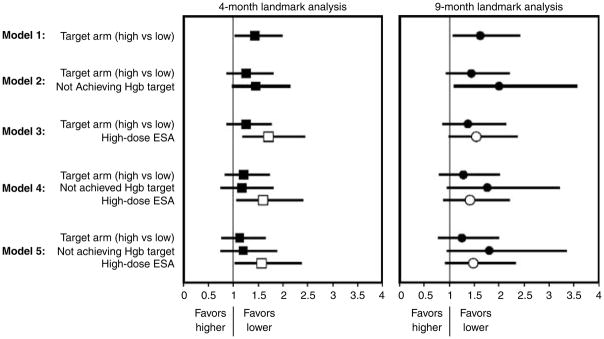
The high-hemoglobin as compared with low-hemoglobin group in the 4-month landmark analysis had an increased hazard of the primary end point (1.44, CI 1.05–1.97, P =0.02) (Table 3, PFigure 1). When inability to achieve target hemoglobin and use of high-dose epoetin-α were added to the model with treatment arm separately, treatment arm was not significant, and inability to achieve target or use of high-dose epoetin-α were significant predictors ( =0.05 and 0.003). When dose, target, and inability to achieve target were entered, only high-dose epoetin-α remained significant (HR =1.60, CI 1.08–2.38, P =0.02). In the adjusted model, high-dose epoetin-α was associated with a 57% increased hazard of the primary composite end point (HR 1.57, CI 1.04–2.36, P =0.03) (Table 3, PFigure 1). As treatment assignment to higher target is significantly associated with inability to achieve target and use high-dose epoetin-α by the landmark time point, this suggests that detrimental outcome of higher target in CHOIR trial may be mediated through the use of high dose. No significant interactions were present between achieved hemoglobin, high-dose ESA, or treatment arm (all >0.10).
Table 3.
Cox proportional hazards models for the primary composite endpoint of death, coronary heart failure hospitalization, stroke, or MI
| Four-month landmark analysis N=1260 |
Nine-month landmark analysis N=1057 |
|||
|---|---|---|---|---|
| Variable | HR, 95% CI | P-value | HR, 95% CI | P-value |
| Model 1 | ||||
| Target arm (high vs low) | 1.44, 1.05–1.97 | 0.02 | 1.62, 1.09–2.40 | 0.02 |
| Model 2 | ||||
| Target arm (high vs low) | 1.26, 0.89–1.78 | 0.20 | 1.44, 0.95–2.18 | 0.09 |
| Not achieving hemoglobin target | 1.46, 1.00–2.13 | 0.05 | 1.99, 1.12–3.55 | 0.02 |
| Model 3 | ||||
| Target arm (high vs low) | 1.26, 0.90–1.75 | 0.18 | 1.37, 0.89–2.11 | 0.15 |
| High-dose ESA | 1.71, 1.20–2.43 | 0.003 | 1.54, 1.00–2.35 | 0.05 |
| Model 4 | ||||
| Target arm (high vs low) | 1.21, 0.85–1.71 | 0.29 | 1.28, 0.82–2.00 | 0.27 |
| Not achieving hemoglobin target | 1.17, 0.76–1.79 | 0.47 | 1.76, 0.97–3.20 | 0.06 |
| High-dose ESA | 1.60, 1.08–2.38 | 0.02 | 1.40, 0.90–2.19 | 0.13 |
| Model 5 | N=1192 | N=1016 | ||
| Target arm (high vs low) | 1.17, 0.81–1.68 | 0.41 | 1.25, 0.80–1.97 | 0.33 |
| Not achieving hemoglobin target | 1.21, 0.78–1.89 | 0.39 | 1.80, 0.97–3.34 | 0.06 |
| High-dose ESA | 1.57, 1.04–2.36 | 0.03 | 1.48, 0.94–2.32 | 0.09 |
| Self-reported hypertension | 0.94, 0.48–1.85 | 0.86 | 0.66, 0.32–1.37 | 0.27 |
| Previous CABG | 2.44, 1.70–3.49 | <0.01 | 1.75, 1.08–2.86 | 0.02 |
| Use of IV iron | 0.47, 0.12, 1.90 | 0.29 | 0.36, 0.05, 2.63 | 0.32 |
CABG, coronary artery bypass graft; ESA, erythropoietin-stimulating agent.
Figure 1.
Cox proportional hazards models for the primary composite end point of death, coronary heart failure hospitalization, stroke, or MI.
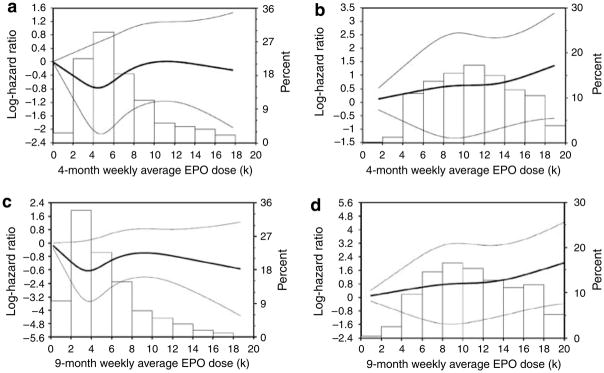
A restricted cubic spline Cox regression model was fitted to examine the relationship of average dose before the 4-month landmark time point with outcome. In the low-hemoglobin group (median average epoetin dose 5623, 25–75% IQR 3959–8376 U), the relationship between dose and log hazard surrounding the majority of observations appear to be J-shaped (Figure 2a), although the confidence interval widens at higher dosing levels. In the high-hemoglobin group (median epoetin dose 10,786, IQR 7803–14,114 U), the relationship between dose and log hazard surrounding the majority of observations appears to be linear (Figure 2b).
Figure 2. Association between epoetin-α dose and primary end point.
(a) Among subjects randomized to the low-hemoglobin group in 4-month landmark analysis. (b) Among subjects randomized to the high-hemoglobin group in the 4-month landmark analysis. (c) Among subjects randomized to the low-hemoglobin group in 9-month landmark analysis. (d) Among subjects randomized to the high-hemoglobin group in the 9-month landmark analysis. (The line indicates the log hazard. The bar graph indicates the percent of treatment arm that fell within each dosing interval.)
Nine-month landmark analysis
Findings of the 9-month landmark analysis were similar to those of the 4-month analysis. Among subjects included in the 9-month landmark analysis (n =1057), factors reflecting inflammation were similar between treatment arms (Table 1).
Subjects not achieving their target hemoglobin within the first 9 months of the trial experienced events at a higher rate (Table 2). During these first 9 months, a greater proportion of subjects in the high-hemoglobin, as compared with the low-hemoglobin group, were unable to achieve their target hemoglobin (14.8 vs 0.7%, P<0.001).
Within the high-hemoglobin group, subjects receiving high-dose epoetin-α experienced events at a higher rate (13.8 vs 9.5%). Event rates for high- vs low-dose epoetin-α were similar in the low-hemoglobin group (7.2 vs 7.7%). A larger proportion of subjects in the high-hemoglobin as compared with the low-hemoglobin group required high-dose epoetin-α (57.2 vs 15.4%, P<0.001). Among subjects not achieving their target, 86.4% required high-dose epoetin-α as compared with 31.8% among subjects achieving their target (P<0.001).
Randomization to the high-hemoglobin as compared with the low-hemoglobin group was associated with an increased hazard of primary end point (1.62, CI 1.09–2.40, P =0.02) (Table 3, PFigure 1). When added to models containing treatment arm, inability to achieve target and high-dose epoetin-α separately were predictors of worse outcomes ( =0.02 and 0.05). When dose, target, and inability to achieve target were entered, inability to achieve target trended toward a significant association with outcomes (HR =1.76, CI 0.97–3.20, P =0.06), and high-dose epoetin-α therapy held a similar point estimate as was seen in the 4-month landmark, however, no longer reaching statistical significance (HR =1.40, CI 0.90–2.19, P =0.13). In the adjusted model, relationships were similar (Table 3, Figure 1).
Again, the restricted cubic spline Cox regression model shows that in the low-hemoglobin group (median epoetin dose 4513, IQR 2949–7026 U), the relationship between dose and log hazard surrounding the majority of observations may be J-shaped (Figure 2c), although the confidence interval widens at higher dosing levels. In the high-hemoglobin group (median epoetin dose 10,692, IQR 7821–14,410 U), the relationship between dose and log hazard surrounding the majority of observations was linear (Figure 2d).
DISCUSSION
Although complete correction of anemia of kidney disease with epoetin-α has been associated with increased mortality compared with partial correction,5,8 the underlying mechanisms are not clear. This post hoc analysis of CHOIR generates the hypothesis that toxicities related to high-dose epoetin-α may contribute to worse outcomes among subjects with higher targets particularly among those who do not achieve their targeted hemoglobin. Further, this analysis demonstrates that subjects achieving their target had better outcomes than those who did not, and among subjects who achieved their randomized target, no increased risk associated with the higher hemoglobin goal was detected.
Similar to CHOIR, the Normal Hematocrit study demonstrated a relative risk of 1.28, 95% CI 0.92, 1.78, favoring the low-hematocrit group.8 Subjects achieving the higher target had a lower mortality rate than subjects in the lower-hematocrit group.7 Higher hemoglobin values alone in both the Normal Hematocrit study and CHOIR were not associated with worse outcomes. Rather, lower achieved values appeared to be associated with higher mortality. Additionally, in the Normal Hematocrit study, following cessation of the target intervention, subjects randomized to the higher arm had ‘near-identical’ rates of mortality as those randomized to the lower target.8 Thus, although being randomized to the higher hemoglobin treatment arm in each study that resulted in higher rates of adverse outcomes overall, achieving the higher hemoglobin target was associated with lower mortality. And following discontinuation of the intervention to achieve the higher target, differences in outcomes are lost. This implies that another factor must be responsible for these outcomes differences. The analysis presented here suggests that factor may be high-doses of epoetin-α.
Epoetin-α requirements are variable among anemic patients.1,7,9,10 This variability has been attributed to multiple etiologies, including iron deficiency, infection, and inflammation.9–11 Hyporesponsiveness to epoetin requires higher doses.12 Among those in the higher target hemoglobin group, the high doses of epoetin were associated with poorer outcomes, and when higher epoetin doses were considered in multivariate analyses, treatment to the higher hemoglobin target was no longer associated with increased risk, suggesting possible mediating effect of higher target via dose.
Higher doses of epoetin have been demonstrated to be an independent predictor of mortality in United States Renal Data Service data of hemodialysis patients.9 Across all hematocrit categories, significant direct relationships between dose and mortality were observed. The steepest increases in risk were found above the 72.5th dose percentile, corresponding to 18,800–29,300 U, similar to the analyses presented here. However, because of the observational nature of the United States Renal Data Service data set, the possibility that relationship between dose and outcome may reflect confounding due by comorbidity and inflammation cannot be excluded.
Defining a relationship between dose and outcomes must attempt to separate the potential contribution of increased dose requirement as a marker of comorbidity. Two observational studies have demonstrated relationships between epoetin dose requirements and clinical factors such as age, diabetes mellitus, and serum ferritin.12,13 This potential for confounding limits the ability of a data set with a single hemoglobin target or dosing strategy to discern whether the risk detected is associated with dose or with clinical factors necessitating the dose. However, a trial randomizing to two targets can take advantage of the benefits of randomization. If randomization is successful in equally distributing factors between treatment arms, by definition, factors that are reflective of inflammation and epoetin-α responsiveness will also be equally distributed at the baseline. Supporting the assertion that such factors may be still equally distributed between arms in CHOIR at the landmark time, key parameters between treatment groups were similar. To the extent that albumin and ferritin reflect inflammation, no difference between treatment groups was detected. Additionally, as a more functional marker, hemoglobin at baseline and at 3 weeks (before which both treatment groups received the same dose of epoetin-α) were also similar between groups. Together, this supports the assertion that there was a relatively equal distribution of factors reflecting epoetin-α resistance between arms. In this setting, rates of adverse events were higher among those treated to the higher hemoglobin target. Subjects in the high-hemoglobin group required the use of higher doses of epoetin, which may have predisposed these patients to a greater dose effect.
The specific mechanisms by which high-doses of epoetin-α may be associated with a greater risk of adverse outcomes remain unclear. Erythropoietin receptors have been demonstrated on human endothelial cells and multiple other sites.14,15 Additionally, receptors have been found on tumor cells, suggesting potential roles of erythropoietin as angiogenic.16–18 Therapy with large episodic doses of erythropoietin do not reflect normal erythropoietin biology and have unknown effects on erythropoietin receptors.19 Finally, the nature of the relationship as suggested by these data may not be linear as seen in previous studies11,20,21 Greater epoetin resistance or requirements in the ESRD population may alter key thresholds in this relationship and deserves additional scrutiny. However, future translational research to investigate this should allow for the potential that smaller doses may provoke differential responses or that the beneficial association with higher hemoglobin may mask the relationship with dose within certain ranges.
The Normal Hematocrit trial and other observational studies have raised concern in the renal community over potential risks associated with the use of intravenous iron.7,22–24 Although contradictory studies exist that argue the presence of this risk,25 it is noteworthy that in the Normal Hematocrit study more subjects in the higher hemoglobin arm received intravenous iron and those who received intravenous iron had a greater odds of mortality compared with those who did not receive intravenous iron. Although 990 of the 1233 subjects reported in the primary publication of the Normal Hematocrit study received intravenous iron, its use was reported in far fewer subjects in CHOIR (n =29). To fully explore the potential confounding that may exist between intravenous iron and the relationship presented here, the use of intravenous iron was included in a multivariable model revealing stability of all point estimates.
Although this study suggests a relationship between dose and outcomes, it has limitations. It is a secondary analysis of a trial designed to test the effect of target but not dose on outcomes. The ability to generalize dose thresholds to other populations should be carefully considered given the volunteerism in trial enrollment. Landmark analyses minimize biases created by differential dropout of subjects and intervening events between the time of randomization and the inception time for the outcome measurement. However, the impact of later hemoglobin levels and doses received after the landmark time cannot be examined using this methodology. Hemoglobin target, actual hemoglobin, and dose are closely related in CHOIR due to the design. Dose is a consequence of failure to respond. Associations between outcomes and hemoglobin, dose, or both may be confounded by factors not available in the CHOIR database. Their interplay on outcome cannot be definitely isolated without future, properly designed confirmation study. The conclusions of this analysis should therefore be considered hypothesis generating. And although hyporesponsiveness and high-dose requirements for epoetin significantly attenuated the increased risk associated with a higher hemoglobin target in CHOIR, these factors do not fully explain the increase in risk. Finally, while increased parathyroid hormone levels have been associated with an increased mortality risk as well as an increased risk of ESA resistance among patients with CKD,26,27 PTH measurements were not performed as a part of the CHOIR trial and will need to be the subject of further investigation.
This secondary analysis of the CHOIR trial demonstrates a relationship between epoetin-α dose and poorer outcomes beyond the relationship previously appreciated between dose as a marker of resistance conferred by comorbidity. Current Food and Drug Administration guidance provides a goal for epoetin-α therapy focused on a target hemoglobin. Considerable discussion has recently focused on the target that balances the quality-of-life benefit1,28,29 and the poorer outcomes associated with targeting a higher hemoglobin goal in CKD and ESRD populations.5,7 These data suggest that the dose of epoetin-α should play an increasing role in discussions to determine best policy-maximizing safety. Although future investigations on the impact of dose on outcomes may suggest a maximum dose for all patients or separate maximum doses for specific subgroups, these data suggest that among patients who do not achieve their targeted goal for hemoglobin, consideration should be given toward limiting dose escalation. For patients who do not respond to lower doses of epoetin-α, the final hemoglobin achieved may not be as important as the maximum doses required.
MATERIALS AND METHODS
Study subjects
The Correction of Hemoglobin and Outcomes in Renal Insufficiency was a randomized trial comparing the effect of treatment with epoetin-α to one of two hemoglobin targets on the composite end point of death, congestive heart failure, stroke, and myocardial infarction in CKD patients. Methods, baseline characteristics, and results of CHOIR have been reported.5 Inclusion criteria were hemoglobin <11.0 g/100 ml and modification of diet in renal disease glomerular filtration rate of 15–50 ml/min/1.73 m2 (refs. 30,31)
Two landmark analyses were performed. To be included in either analysis, subjects needed to be free of the composite event at the landmark, receive epoetin-α, and have ≥1 post-baseline hemoglobin measurement. Of the 1432 subjects randomized, 25 were excluded because they had not received epoetin-α or obtained post-baseline hemoglobin measurement. In the 4- and 9-month landmark analyses, 147 and 350 subjects were excluded, respectively, because they had events or terminated the study before this landmark. The populations for these analyses were 1260 and 1057 subjects.
Measurements
Subjects in CHOIR were randomized to hemoglobin targets of 11.3 or 13.5 g/100 ml utilizing different dosing algorithms and were administered weekly or biweekly epoetin-α subcutaneously. Information on ESA dose and hemoglobin were collected at least biweekly.
Definitions
Three categories of achieved hemoglobin (gm/100 ml) were considered: <11.1, ≥11.1 and <13.1, and ≥13.1. These categories were chosen on the basis of CHOIR’s dosing algorithm dictating no change or decrease in dose if hemoglobin exceeded 11.1 in the low-hemoglobin or 13.1 gm/100 ml in the high-hemoglobin group. Therefore, these values defined a range in which subjects functionally met target. Subjects achieved their respective targets if their maximum hemoglobin (within the first 4 or 9 months for each analysis) was ≥13.1 gm/100 ml in high-hemoglobin or ≥11.1 gm/100 ml in low-hemoglobin group.
Erythropoietin-stimulating agent use was categorized as high- or low-dose if the maximum dose (within the first 4 or 9 months of the study for each landmark analysis) was ≥20,000 or <20,000 U (high vs low dose). This threshold was chosen because CHOIR limited the dose of epoetin-α to 20,000 U/week.
Statistical analysis
Landmark analyses32,33 were performed to examine associations between achieved hemoglobin, epoetin-α dose, and outcomes. Analyses were performed using both the composite end point and death alone. As conclusions were similar, only analyses using the composite end point are presented. Landmark analyses are used to test for effects of a direct treatment arm and associations between post-baseline variables and outcomes. In landmark analyses, subjects surviving to the ‘landmark’ time are included. Post randomization but ‘pre-landmark’ values of potential predictors are summarized and tested for association with outcomes.
The association between independent variables (that is, data obtained post-baseline but prelandmark) and survival are estimated from the landmark time point through the end of follow-up using time-to-event analyses. This approach eliminates biases introduced by defining early event as no-response (hyporesponsiveness) and by including the time before response as part of the survival time for responders (lead-time bias). The interpretation of results is conditional on a subject being free from the composite event before the landmark. No hemoglobin or dose values after the landmark time are utilized.
The landmarks of 4 and 9 months were chosen on the basis of the qualitative observations that epoetin-α dose and hemoglobin measurements stabilized at 4 months and outcomes curves comparing treatment arms began to separate at 9 months.5 The P-values of comparing the treatment groups for time to composite event in the first 4 or 9 months are 0.59 and 0.44, respectively. Data before each landmark were summarized to categorize subjects on the basis of their ability to achieve hemoglobin target and use of high-dose ESA using the above definitions.
Multivariable Cox hazards regression analyses were performed. Initial models tested the additive association of achieved hemoglobin, high-dose ESA, or both to the effect of treatment arm. Subsequently, an adjusted model including variables that significantly different between groups was developed. Interactions between achieved hemoglobin, high-dose ESA, and treatment arm were tested.
A proportional hazards Cox model was also fit to assess the descriptive effect of average dose on the outcome. Restricted cubic splines for average dose were used in the Cox regression model within each group without adjusting other variables.34,35 Average epoetin-α dose for each patient for both the 4- and 9-month landmark analyses were calculated using all values preceding each landmark time point. The log-hazard ratios with their confidence intervals for increasing average epoetin-α dose were plotted against average dose for each target group and landmark analysis separately. As a complement to the confidence intervals (also plotted on a log scale), frequency distributions of patients with average dose requirements within each treatment group were overlaid on the curves to assist in the interpretation of the curves.
All statistical analyses were performed using SAS (version 8.2, SAS Institute, Cary, NC, USA).
Acknowledgments
This work was presented at the Federal Drug Advisory Meeting held on September 11, 2007, in Washington, DC and at the American Society Nephrology Annual Meeting on November 4, 2007 in San Francisco, CA.
Footnotes
DISCLOSURE
The original CHOIR trial was supported by Ortho Biotech Clinical Affairs and Johnson & Johnson Pharmaceutical Research and Development, both subsidiaries of Johnson & Johnson. This analysis was supported by Duke Clinical Research Institute. Dr Szczech reports receiving consulting fees from Ortho Biotech Clinical Affairs, Nabi Pharmaceuticals, Gilead, Fresenius Medical Care, Kureha, Affymax, and Acologix; lecture fees from Nabi Biopharmaceuticals, Fresenius Medical Care, GlaxoSmithKline, Gilead, Genzyme, Abbott, Amgen, and Ortho Biotech; and grant support from Ortho Biotech Clinical Affairs, GlaxoSmithKline, Pfizer, and Genzyme. Dr Barnhart reports receiving consulting fees and grant support from Ortho Biotech Clinical Affairs. Dr Inrig reports support by NIH Grant 1KL2 RR024127 and the R Snyderman Clinical Research Fellowhip Grant. She has also received investigator-initiated research support from Genzyme. Dr Reddan reports receiving consulting fees from Ortho Biotech Clinical Affairs and Shire Pharmaceuticals; lecture fees from Amgen, Novartis, Pfizer, AstraZeneca, and General Electric; and grant support from Ortho Biotech Clinical Affairs, Amgen, and Novartis. Ms Sapp has no disclosures. Dr Califf reported having received research grants and contracts from Merck, Novartis Pharmaceutical, Shering Plough, and Scios Pharma. He also reports receiving consulting fees from Avalere Health, Bayer, Biogen Idec, Grandeis Univ, Bristol Myers Squibb/Sanofi, Eli Lilly, Five Prime, Heart.org/Conceptis, Kowa Research Institute, Nitrox LLC, Sanofi-Aventis, and Vertex. Dr Patel reports support by NIH Grant K23DK075929 and investigator-initiated research support from Abbott Laboratories. Dr Singh reports receiving consulting fees from Ortho Biotech Clinical Affairs, Amgen, Roche, Merck (Germany), Abbott, Watson, and Horizon Blue Cross Blue Shield and lecture fees from Ortho Biotech Clinical Affairs, Roche, Amgen, Abbott, Watson, Scios, Pfizer, and Genzyme; serving on advisory boards for Ortho Biotech Clinical Affairs, Roche, Acologix, Watson, Advanced Magnetics, and Amgen; and receiving grant support from Ortho Biotech Clinical Affairs, Dialysis Clinic, Roche, Baxter, Johnson & Johnson, Amgen, Watson, and Aspreva. No other potential conflict of interest relevant to this article was reported.
References
- 1.Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. doi: 10.7326/0003-4819-111-12-992. [DOI] [PubMed] [Google Scholar]
- 2.Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98:708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 3.Ma JZ, Ebben J, Xia H, et al. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol. 1999;10:610–619. doi: 10.1681/ASN.V103610. [DOI] [PubMed] [Google Scholar]
- 4.Xia H, Ebben J, Ma JZ, et al. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol. 1999;10:1309–1316. doi: 10.1681/ASN.V1061309. [DOI] [PubMed] [Google Scholar]
- 5.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 6.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 7.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 8.Besarab A, Goodkin DA, Nissenson AR. The Normal Hematocrit Study—follow-up. N Eng J Med. 2008;358:433–434. doi: 10.1056/NEJMc076523. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Thamer M, Stefanik K, et al. Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis. 2004;44:866–876. [PubMed] [Google Scholar]
- 10.IV. NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease. Update 2000. Am J Kidney Dis. 2001;37:S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 11.Eschbach JW. Anemia management in chronic kidney disease: role of factors affecting epoetin responsiveness. J Am Soc Nephrol. 2002;13:1412–1414. doi: 10.1097/01.asn.0000016440.52271.f7. [DOI] [PubMed] [Google Scholar]
- 12.Coladonato JA, Frankenfield DL, Reddan DN, et al. Trends in anemia management among US hemodialysis patients. J Am Soc Nephrol. 2002;13:1288–1295. doi: 10.1097/01.asn.0000013294.11876.80. [DOI] [PubMed] [Google Scholar]
- 13.Regidor DL, Kopple JD, Kovesdy CP, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 14.Anagnostou A, Liu Z, Steiner M, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 16.Leo C, Horn LC, Rauscher C, et al. Expression of erythropoietin and erythropoietin receptor in cervical cancer and relationship to survival, hypoxia, and apoptosis. Clin Cancer Res. 2006;12:6894–6900. doi: 10.1158/1078-0432.CCR-06-1285. [DOI] [PubMed] [Google Scholar]
- 17.Mohyeldin A, Lu H, Dalgard C, et al. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia. 2005;7:537–543. doi: 10.1593/neo.04685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribatti D, Marzullo A, Gentile A, et al. Erythropoietin/erythropoietin-receptor system is involved in angiogenesis in human hepatocellular carcinoma. Histopathology. 2007;50:591–596. doi: 10.1111/j.1365-2559.2007.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fishbane S. Recombinant human erythropoietin: has treatment reached its full potential? Semin Dial. 2006;19:1–4. doi: 10.1111/j.1525-139X.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 20.Klassen PS, et al. Oral presentation. FDA Advisory Committee; [accessed on June 17, 2008]. http://www.fda.gov/ohrms/dockets/ac/cder07.htm#CardiovascularRenal. [Google Scholar]
- 21.Zhang Y, et al. Oral Presentation. FDA Advisory Committee Meeting; [accessed on June 17, 2008]. http://www.fda.gov/ohrms/dockets/ac/cder07.htm#CardiovascularRenal. [Google Scholar]
- 22.Feldman HI, Santanna J, Guo W, et al. Iron administration and clinical outcomes in hemodialysis patients. J Am Soc Nephrol. 2002;13:734–744. doi: 10.1681/ASN.V133734. [DOI] [PubMed] [Google Scholar]
- 23.Hoen B, Kessler M, Hestin D, et al. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant. 1995;10:377–381. [PubMed] [Google Scholar]
- 24.Hoen B, Paul-Dauphin A, Hestin D, et al. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9:869–876. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 25.Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol. 2004;15:1623–1632. doi: 10.1097/01.asn.0000128009.69594.be. [DOI] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- 27.Hsu SP, Peng YS, Pai MF, et al. Influence of relative hypoparathyroidism on the responsiveness to recombinant human erythropoietin in hemodialysis patients. Blood Purif. 2003;21:220–224. doi: 10.1159/000070693. [DOI] [PubMed] [Google Scholar]
- 28.Lundin AP, Akerman MJ, Chesler RM, et al. Exercise in hemodialysis patients after treatment with recombinant human erythropoietin. Nephron. 1991;58:315–319. doi: 10.1159/000186443. [DOI] [PubMed] [Google Scholar]
- 29.Evans RW, Rader B, Manninen DL. The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. Cooperative Multicenter EPO Clinical Trial Group. JAMA. 1990;263:825–830. [PubMed] [Google Scholar]
- 30.Levey AS, Greene T, Kusek J, et al. A simplified equation to predict glomerular filtration rate from serum creatinine [abstract] J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Marubini E, Valsecchi MG. Analysing Survival Data from Clinical Trials and Observational Studies. John Wiley & Sons; Chichester, West Sussex, England: 2004. [Google Scholar]
- 33.Anderson J, Cain K, Gelber R. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 34.Stone CJ, Koo CY. Proceedings of the Statistical Computing Section. American Statistical Association; 1985. Additive splines in statistics; pp. 45–48. [Google Scholar]
- 35.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. Springer; New York: 2001. [Google Scholar]