Abstract
Objective
To assess the effects of decreased antiretroviral therapy exposure on body fat and metabolic parameters.
Design
Sub-study of the SMART study in which participants were randomized to intermittent CD4-guided (DC group) or to continuous (VS group) antiretroviral therapy.
Methods
Participants at 33 sites were co-enrolled in the SMART Body Composition Sub-study. Regional fat was assessed annually by whole-body dual-energy X-ray absorptiometry and abdominal computed tomography. Fasting metabolic parameters were assessed at months 4, 8, and annually. Treatment groups were compared for changes in fat and metabolic markers using longitudinal mixed models.
Results
Two hundred seventy-five patients were randomized to the DC (n=142) or VS (n=133) groups, and followed for a median of 2.0 years. By month 12, limb fat (DC-VS difference 9.8%, 95% confidence interval [CI] 3.5 to 16.1; P=0.003) and subcutaneous abdominal fat (DC-VS difference 14.3 cm2, 95% CI −0.1 to 28.7; P=0.05) increased in the DC group. There was no treatment difference on visceral abdominal fat (DC-VS difference −2.1%, 95% CI −13.5 to 9.4; P=0.72). Lipids significantly decreased in the DC group by month 4 and treatment differences persisted throughout follow-up (P≤0.001). By 12 months, hemoglobin A1C increased in the DC (+0.3%) and remained stable in the VS group (P=0.003); the treatment difference remained significant through follow-up (P=0.02).
Conclusions
After 12 months, intermittent antiretroviral therapy increased subcutaneous fat, had no effect on visceral abdominal fat, decreased plasma lipids, and increased hemoglobin A1C compared with continuous antiretroviral therapy.
Keywords: Intermittent antiretroviral therapy, Body Composition, Metabolism
Introduction
Lipodystrophy is a frequent problem in HIV-infected patients receiving antiretroviral therapy (ART), with a reported prevalence of 30–50% (1–4). Lipodystrophy stigmatizes, reduces quality of life, puts at risk adherence to ART, and is commonly associated with dyslipidemia, insulin resistance, hyperlactatemia, and sexual dysfunction (5–9). Although HIV and host-dependent factors are involved, exposure to ART is a key factor in the development of lipodystrophy. Thymidine nucleoside analogue reverse transcriptase inhibitors (NRTIs; stavudine more so than zidovudine), particularly in combination with first-generation protease inhibitors (PIs), have been consistently associated with an increased risk for lipodystrophy (10–15).
Discontinuation of thymidine NRTIs is the only proven intervention for lipoatrophy, but the resulting improvement is slow and incomplete (16–18). Switching ART has no clear effect on visceral fat accumulation (9). Intermittent ART has been associated with objective limb fat increases in small, non-randomized studies (19–22) as well as subjective improvement of lipodystrophy in larger, randomized trials (23, 24).
Using objective measurement techniques, we evaluated body composition and metabolic parameters in a subset of patients enrolled in a large randomized trial of intermittent, CD4-guided versus continuous ART, the Strategies for Management of Anti-Retroviral Therapy (SMART) trial (25). Because ART may be associated with subcutaneous lipoatrophy, relative central fat accumulation, increased total cholesterol and triglycerides, and decreased insulin sensitivity (9), we hypothesized that intermittent ART would have a favorable impact on these abnormalities relative to continuous ART.
Methods
Study Population
The SMART study enrolled HIV-infected patients older than 13 years with CD4+ lymphocyte counts >350 cells/mm3. Participants could be ART-naïve or ART-experienced, and currently receiving ART or not. Pregnant or breastfeeding women were excluded. All eligible SMART participants at 33 clinical sites in the United States, Australia and Spain were offered co-enrollment in the Body Composition Sub-study. Sub-study sites were selected based on their access to study-certified dual-energy X-ray absorptiometry (DEXA) and computed tomography (CT) equipment. The institutional review board at each site approved the study and each participant gave written, informed consent.
Study Design
The design of the SMART study has been described elsewhere (25). Briefly, participants were randomized 1:1 to intermittent ART (Drug Conservation [DC] group) or continuous ART (Viral Suppression [VS] group). Participants randomized to the DC group deferred or stopped ART until their CD4+ lymphocyte count declined to below 250 cells/mm3, then re-initiated therapy, and stopped again when the CD4+ count had increased to above 350 cells/mm3. Participants in the VS group were prescribed continuous ART with the goal of sustained viral suppression. Randomization was stratified by site.
The primary endpoint of the Body Composition Sub-study was to compare the DC versus VS groups for changes in limb fat measured by DEXA and in visceral fat measured by abdominal CT. DEXA and CT scans were obtained at baseline and annually. It was planned to co-enroll 300 participants into the sub-study and follow them for at least 5 years. The sample size was calculated to detect a treatment difference of 0.5 kg in limb fat and 25 cm2 in visceral fat with 80% power at a 5% significance level.
Due to increased risk of AIDS, death, and serious cardiovascular, liver and renal complications in the DC group, enrollment in the SMART study was stopped on 11 January 2006 per recommendation of an independent Data and Safety Monitoring Board, and ART-experienced participants were advised to receive continuous ART.
Data Collection
Imaging
Total body DEXA (limb fat, trunk fat, total body fat, and lean mass) and abdominal CT (visceral [VAT] and subcutaneous [SAT] adipose tissue) scans were obtained at baseline and annually. For women of childbearing potential, a negative serum or urine pregnancy test result was required within 14 days before each scan. Baseline scans were performed within 60 days before randomization.
Imaging was performed at 10 radiology sites. Standardized scanning protocols based on each manufacturer’s imaging specifications were used across all sites, and all scans were analyzed at a central reading center (Bio-Imaging Technologies, Newtown, PA, USA). In particular, scanning protocols specified that follow-up scans were obtained on the same X-ray machines with the same parameters as the baseline scans. Development of the scanning protocols, certification of radiology equipment and personnel prior to the study, and ongoing quality assurance were undertaken by Bio-Imaging Technologies.
For DEXA scans, patients were positioned straight on the table, with all body parts in the scan field, palms down and separated from the thighs and legs rotated inward 25 degrees. Six of the 10 radiology sites used Lunar (GE Healthcare Lunar, Madison, WI, USA), and four Hologic (Bedford, MA, USA) x-ray equipment. At the beginning of the study and whenever a shift in calibration was suspected, a variable composition phantom (VCP 027, Abbott Plastics, Rockford, IL, USA) was scanned to mimic a range of percent fat values to confirm the accuracy of DEXA body composition measurements.
The methodology and the quality assurance procedures for CT scanning have been described previously (26). Three axial-oblique CT slices of the abdomen were obtained at the levels of the L2-3, L3-4, and L4-5 inter-vertebral disk spaces. SAT and VAT were evaluated at each inter-vertebral location based on pixel intensity. The average values across the three inter-vertebral spaces were used for calculations.
Metabolic Laboratory Markers and Other Assessments
Blood samples were obtained after a minimum 8-hour fast, at baseline (within 45 days prior to randomization), at months 4, 8, and 12, and annually thereafter. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and glucose were measured using automated enzymatic assays, and low-density lipoprotein (LDL) cholesterol using spectrophotometry, all on an Olympus AU600 chemistry analyzer (Olympus America, Center Valley, PA, USA). Insulin was measured by chemiluminescent immunometric assay using monoclonal murine anti-insulin antibody and polyclonal sheep anti-insulin antibody, and C-peptide using an immuno-chemiluminescence assay; both on a Siemens DPC Immulite 2000 (Siemens Medical Solutions, Flanders, New Jersey, USA). Venous lactate (Vitros DT60 II, Rochester, NY, USA) and hemoglobin A1C (Immunoturbidimetric Roche Integra 700 assay, Rotkreuz, Switzerland) were measured at baseline and annually only. All samples were frozen at −70°C until they were batch-tested at a central laboratory (Quest Diagnostics, Inc., Baltimore, MD, USA). Insulin resistance was estimated using the Homeostasis Model Assessment (HOMA) equation (27).
For each participant, the provider assessed presence and severity of lipoatrophy and abnormal fat accumulations (2). Patient satisfaction with body image was recorded using a visual analogue scale from 0–100. Medical and ART history, CD4+ cell count and HIV RNA levels were obtained at baseline and through follow-up as previously described (25).
Statistical Analysis
Baseline characteristics were summarized. Median time to first ART initiation in the DC arm was assessed through Kaplan-Meier estimates. Follow-up time was censored on the last sub-study visit prior to 12 January 2006.
All comparisons between treatment groups were done by intention to treat. The DC and VS groups were compared for changes in fat, lean mass, and laboratory markers from baseline through follow-up using longitudinal mixed models, and to specific follow-up visits using linear regression. By chance, more women were randomized to the VS group, and the proportion of blacks was higher among women. Therefore, we adjusted treatment group comparisons for race, sex, and the race-by-sex interaction, as body composition and many laboratory values differ by sex and race. Covariate-adjusted estimates for mean changes in both treatment groups, estimated treatment differences with 95% confidence intervals [CI], and p-values were calculated. Changes in limb fat were assessed both as absolute and percent changes relative to baseline. Variability in absolute change in limb fat was higher in those with more limb fat, and the model assumption of homoscedasticity was better met when evaluating percent changes. Therefore, results on the percent scale are more reliable. We present results on both scales, because the study protocol referred to absolute changes.
The DC and VS groups were compared for changes in fat within subgroups defined at baseline, using separate models. Homogeneity of the treatment difference across subgroups was assessed by testing for an interaction between the subgroup factor (continuous where possible) and treatment group indicators in a joint model. Subgroup analyses were performed by age, gender, race/ethnicity, baseline CD4 and nadir CD4, HIV RNA level (less or greater than 400 copies/mL), smoking, hepatitis B or C, prior AIDS, BMI, presence of lipoatrophy or fat accumulation, limb fat by DEXA, and use of ART, PIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), and thymidine NRTIs at baseline. Subgroups by gender and baseline ART use were pre-specified in the protocol.
Statistical analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC, USA). All statistical tests were two-sided. P-values ≤ 0.05 were considered significant.
Results
Characteristics of participants
From May 2002 to January 2006, 275 (DC group, n=142; VS group, n=133) participants were co-enrolled into the Body Composition sub-study of the SMART study. Baseline characteristics are summarized in Table 1. At study entry, the proportions of participants who were male, on ART and virologically suppressed were higher in the DC group than in the VS group. Among men, the proportion of blacks was lower (18% versus 61% among women). Otherwise, the two treatment groups were similar. Compared to the parent SMART study, fewer participants were female (19 versus 27%), fewer were using ART at baseline (74 versus 84%), and fewer had HIV-RNA ≤ 400 copies/mL (58 versus 72%) (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | Body Composition Substudy | SMART Study | ||
|---|---|---|---|---|
| Overall (n=275) | Drug Conservation Group (n=142) | Viral Suppression Group (n=133) | Overall (n=5472) | |
| Demographics | ||||
| Age (years) (median. IQR) | 44.0 (39.0, 50.0) | 45.0 | 43.0 | 43 |
| Female (%) | 18.5 | 14.1 | 23.3 | 27.2 |
| Race/ethnicity | ||||
| Black or African American (%) | 25.8 | 23.9 | 27.8 | 29.1 |
| Latino or Hispanic (non-black) (%) | 17.1 | 18.3 | 15.8 | 21.1 |
| White (%) | 55.3 | 56.3 | 54.1 | 43.6 |
| Other or Unknown (%) | 1.8 | 1.4 | 2.3 | 6.2 |
| Probable HIV Transmission Modes | ||||
| Male-Male Sexual Contact (%) | 62.9 | 68.3 | 57.1 | 49.9 |
| Heterosexual Contact (%) | 33.1 | 31.0 | 35.3 | 45.0 |
| IV Drug Use (%) | 13.8 | 14.1 | 13.5 | 9.7 |
| Other (%) | 6.9 | 4.9 | 9.0 | 8.1 |
| Years Since Known HIV-infected [median (IQR)] | 8.5 (5.0, 13.6) | 8.7 | 7.9 | |
| Clinical Characteristics | ||||
| Prior AIDS Diagnosis (%) | 22.2 | 24.6 | 19.5 | 23.9 |
| Hepatitis B (%) | 3.7 | 2.9 | 4.6 | 2.3 |
| Hepatitis C (%) | 16.1 | 16.4 | 15.8 | 14.8 |
| Current Smoker (%) | 45.8 | 41.5 | 50.4 | 40.5 |
| On Lipid-lowering Drugs (%) | 22.2 | 22.5 | 21.8 | |
| CD4+ (cells/mm3) [median (IQR)] | 554 (437, 783) | 590 | 525 | 597 |
| Nadir CD4+ (cells/mm3) [median (IQR)] | 260 (150, 377) | 256 | 270 | 250 |
| HIV RNA ≤ 400 copies/mL (%) | 57.5 | 64.8 | 49.6 | 71.7 |
| ART History | ||||
| ART Naïve (%) | 7.6 | 5.6 | 9.8 | 4.6 |
| On ART at baseline (%) | 74.2 | 79.6 | 68.4 | 83.9 |
| On PI (%) | 35.6 | 38.7 | 32.3 | 45.2 |
| On NNRTI (%) | 36.7 | 40.1 | 33.1 | 49.0 |
| On Thymidine NRTI (%) | 52.0 | 56.3 | 47.4 | 58.7 |
| Years Since Start of ART [median (IQR)] | 6.0 (3.4, 8.1) | 5.9 | 6.2 | 6.0 |
| Years on a PI [median (IQR)] | 2.5 (0.0, 4.5) | 2.5 | 1.5 | 2.5 |
| Abnormal Body Fat | ||||
| Peripheral LipoatrophyA (%) | 19.3 | 20.4 | 18.0 | - |
| Abnormal Fat AccumulationA (%) | 20.7 | 22.5 | 18.8 | - |
| LipodystrophyA,B (%) | 8.4 | 8.5 | 8.3 | - |
| Body Composition | ||||
| Body mass index (kg/m2) [median (IQR)] | 25.6 (23.3, 28.8) | 25.7 | 25.6 | 25.9 |
| Limb fat (kg) [median (IQR)] | 6.6 (4.0, 9.4) | 6.5 | 6.7 | - |
| Limb fat (% of limb mass) [median (IQR)] | 19.5 (13.9, 27.3) | 19.6 | 19.4 | - |
| Lean body mass (kg) [median (IQR)] | 57.8 (49.0, 63.5) | 57.8 | 58.2 | - |
| Total body fat (kg) [median (IQR)] | 18.0 (12.9, 24.8) | 18.4 | 17.5 | - |
| VAT (cm2) [median (IQR)] | 117 (69, 181) | 119 | 117 | - |
| SAT (cm2) [median (IQR)] | 143 (88, 207) | 139 | 146 | - |
| Satisfaction with Body Image (0–100)C (%) | 70 (50, 80) | 70 | 70 | 75 |
| Metabolic Values (≥ 8 hours fasting)D | ||||
| Total cholesterol (mg/dL) [median (IQR)] | 183 (159, 217) | 193 | 181 | 191 |
| LDL cholesterol (mg/dL) [median (IQR)] | 109 (84, 133) | 112 | 107 | 112 |
| HDL cholesterol (mg/dL) [median (IQR)] | 39 (31, 47) | 39 | 38 | 41 |
| Total:HDL cholesterol ratio [median (IQR)] | 4.7 (3.7, 6.0) | 4.8 | 4.6 | 4.6 |
| Triglycerides (mg/dL) [median (IQR)] | 167 (114, 260) | 185 | 156 | 163 |
| Lactate (mmol/L) [median (IQR)] | 1.4 (1.1, 1.7) | 1.3 | 1.4 | - |
| C-Peptide (ng/mL) [median (IQR)] | 2.8 (1.9, 3.7) | 2.8 | 2.8 | - |
| Insulin (μU/mL) [median (IQR)] | 11 (7, 18) | 11 | 12 | - |
| Glucose (mg/dL) [median (IQR)] | 93 (86, 101) | 94 | 92 | - |
| Hemoglobin A1C (% of total hemoglobin) [median (IQR)] | 5.1 (4.8, 5.5) | 5.0 | 5.2 | - |
| HOMAE [median (IQR)] | 2.6 (1.7, 4.5) | 2.5 | 2.6 | - |
Most baseline characteristics remained stable with respect to date of enrolment, including demographics, body fat and metabolic parameters, and the proportion of participants using ART at baseline. The proportion with prior AIDS decreased from 26% among those enrolled in the first two years of the study (n=172) to 17% among those enrolled later (n=103). Similarly, the proportion of participants who used PIs and thymidine NRTIs at baseline decreased from 38% to 32% and from 56% to 44%, respectively.
Physician-assessed, moderate or severe
Participants with both peripheral lipoatropy and abnormal fat accumulation.
Patient self-report using a visual analogue scale from 0–100, with 0 being the worst and 100 the best.
The fasting requirement applied only to the Body Composition substudy, not the parent SMART study.
HOMA = (fasting plasma glucose [mmol/l]) * (serum insulin [μU/l])/22.5
Abbreviations: CT, computed tomography; HOMA, Homeostasis Model Assessment of Insulin Resistance; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
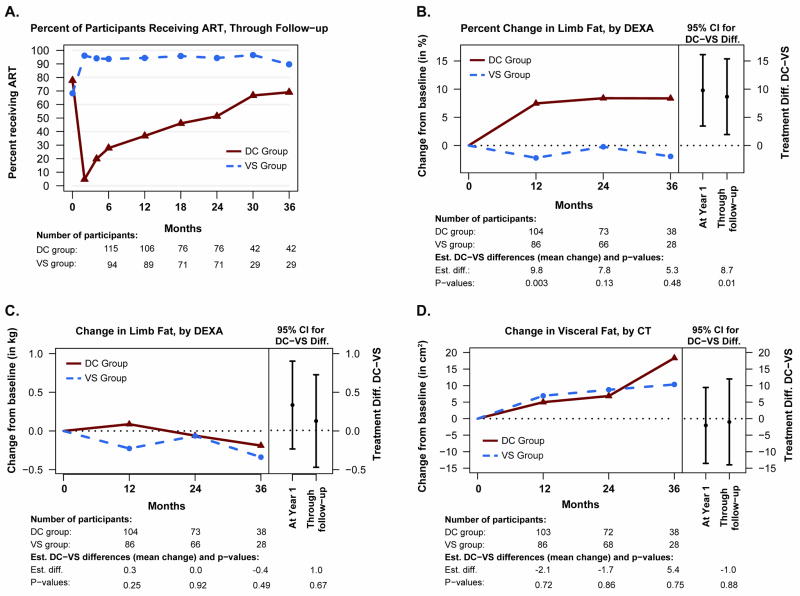
Participants were followed for a median of 24 months. In the DC group, the median time to the commencement of ART was 13 months. By the time of the year-1 scan dates, the DC group had received ART for 2.7 of 12.3 months (22%) of follow-up time. Overall, the DC and the VS groups received ART for 37% and 94% of the total follow-up time, respectively. Figure 1A shows the proportion of participants receiving ART, at selected time points through follow-up.
Figure 1.
Percentage of participants who were taking ART in the DC and VS groups, through follow-up (panel A); changes in limb fat by DEXA from baseline through follow-up (percent relative to baseline, panel B; absolute change from baseline, panel C) and changes in visceral adipose tissue by CT (panel D). Right-hand panels of B–D show estimated treatment differences with 95% confidence intervals for change from baseline to month 12, and through follow-up. Treatment differences, confidence intervals and p-values were estimated using regression models (at each visit) and longitudinal models (through month 36). All models included race, sex and their interaction as covariates.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CT, computed tomography; DC, Drug Conservation (intermittent ART) group; DXA, dual x-ray absorptiometry; VAT, visceral adipose tissue; VS, Viral Suppression (continuous ART) group
Changes in Body Composition
Treatment comparisons for changes in fat and lean mass are presented in Table 2. Of the 275 participants, 192 had follow-up scans and were included in the primary analysis (106 in the DC and 86 in the VS group); of the remaining 83, 82 (99%) had no follow-up scans due to the early closing of the study, and one for other reasons. At month 36, scans were available for 68 participants (35% of the 192); 2 participants had died (DC group), 4 missed their visits (VS group), and the remaining 118 had no scans due to the early closing of the study. Treatment differences were largest in the first year. At 12 months, limb fat increased by 9.0% in the DC group and remained stable (−0.8%) in the VS group (estimated difference 9.8%, 95% CI 3.5 to 16.1, P=0.003). From baseline through 36 months, the DC group gained 8.7% more limb fat than the VS group (95% CI 2.0 to 15.4, P=0.01) (Figure 1B). The treatment difference was not statistically significant, however, when measuring absolute change in limb fat (Figure 1C). Results were similar when restricting the analysis to the cohort with at least 2 years of follow-up.
Table 2.
Treatment Differences in Body Composition Measures Between the Drug Conservation (DC) and Viral Suppression (VS) Groups
| Outcome | Mean Change from Baseline to Month 12A |
Mean Change from Baseline through Month 36B |
||||||
|---|---|---|---|---|---|---|---|---|
| DC | VS | DC-VS | (95% CI) | P-Value | DC-VS | (95% CI) | P-Value | |
| Limb fat, absolute change (kg) | 0.20 | −0.13 | 0.34 | (−0.23, 0.90) | 0.25 | 0.13 | (−0.47, 0.73) | 0.67 |
| Limb fat, percent change (%)C | 9.0 | −0.8 | 9.8 | (3.5, 16.1) | 0.003 | 8.7 | (2.0, 15.4) | 0.01 |
| Lean limb mass (kg) | −0.29 | −0.18 | −0.11 | (−0.51, 0.29) | 0.60 | −0.03 | (−0.44, 0.39) | 0.90 |
| Total body fat (kg) | 0.45 | −0.23 | 0.68 | (−0.59, 1.95) | 0.29 | 0.17 | (−1.18, 1.52) | 0.81 |
| Lean body mass (kg) | −0.48 | −0.08 | −0.40 | (−1.11, 0.31) | 0.27 | −0.25 | (−0.99, 0.50) | 0.52 |
| VAT (cm2) | 3.5 | 5.5 | −2.1 | (−13.5, 9.4) | 0.72 | −1.0 | (−14.0, 12.0) | 0.88 |
| SAT (cm2) | 19.3 | 5.0 | 14.3 | (−0.1, 28.7) | 0.05 | 10.3 | (−4.6, 25.3) | 0.17 |
| Patient satisfaction with body imageD | −0.3 | −0.0 | −0.3 | (−6.8, 6.3) | 0.94 | −0.8 | (−6.8, 5.2) | 0.80 |
Adjusted for sex, race, and the sex-by-race interaction.
Estimates and p-values obtained in longitudinal mixed models adjusted for sex, race, and the sex -by-race interaction.
Percent change = (limb fat mass at follow-up visit limb fat mass at baseline)/(limb fat mass at baseline) * 100%.
Using a visual analogue scale from 0–100, with 0 being the worst and 100 the best.
Abbreviations: CI, confidence interval; DC, Drug Conservation group (episodic ART); SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; VS, Viral Suppression group (continuous ART)
SAT increased in both groups by 12 months, with a borderline significant between-group difference of 14.3 cm2 (95% confidence interval −0.1 to 28.7; P=0.05; Table 2) in favor of the DC group. Both DC and VS groups gained VAT by 12 months, but the between-group difference was not significant at any time-point (Figure 1D). There were no significant differences in lean mass and patient satisfaction with body image.
Subgroup Analyses for Changes in Body Fat by 12 Months
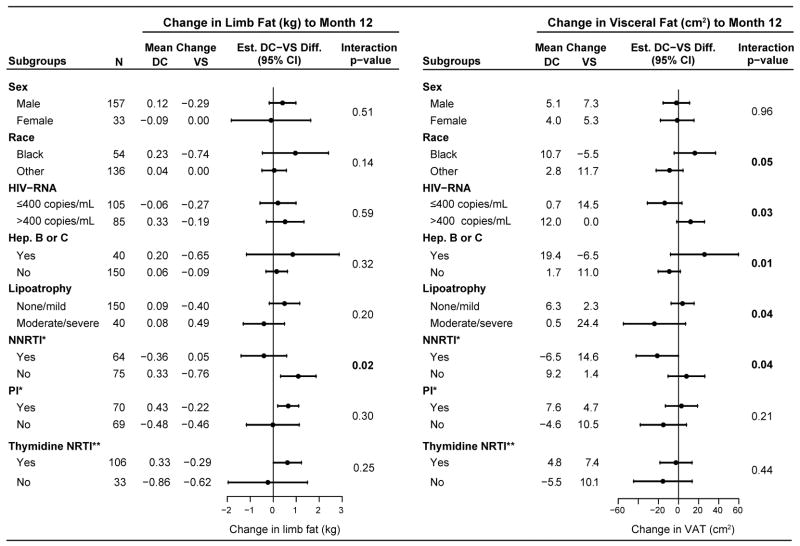
Figure 2 summarizes subgroup analyses for absolute change in limb fat and VAT from baseline to month 12. The analyses were not adjusted for race or sex because of the small sample size in some subgroups; however, results were similar after adjustment (data not shown).
Figure 2.
DC-VS group differences in change in limb fat and VAT from baseline to Month 12 within subgroups of participants, and tests for homogeneity of the treatment difference by subgroup factor.
* NNRTI or PI use, respectively, among those who were receiving ART at baseline.
** Among those who were receiving ART at baseline. In the DC and VS groups, 60 and 46 participants, respectively, used thymidine NRTIs at baseline. In the VS group, 35 (76% of 46) continued using thymidine NRTIs at year 1, and one more had switched to a thymidine NRTI. In the DC group, 24 participants had re-initiated ART by year 1 and 16 were taking thymidine NRTIs.
There was no evidence for differential treatment effect (interaction p-value > 0.05) by the following baseline factors: age, CD4+ count, nadir CD4+ count, prior AIDS, body mass index, smoking, use of ART, or duration of ART use.
The treatment difference in limb fat, absolute change from baseline, was larger among those using ART regiments without an NNRTI at baseline (n=75, DC-VS difference 1.1 kg, p=0.007) compared with those using NNRTIs (n=64, −0.4 kg, p=0.42), p=0.02 for differential treatment effect. The DC-VS treatment differences in VAT varied across several subgroups, including race, HIV-RNA level, hepatitis, lipoatrophy, or NNRTI use at baseline versus other ART regimens (Figure 2).
Additionally to the subgroups presented in Figure 2, we also analyzed subgroups by age, baseline CD4 cell count, nadir CD4 cell count, prior AIDS, BMI, smoking, and use of any ART at baseline. There was no evidence that the DC-VS treatment difference varied by any of these factors (p > 0.05 for interaction between subgroup factor and treatment group).
In subgroup analyses with respect to change in limb fat in percent of baseline limb fat, there was no evidence that the DC-VS difference differed by any of these factors (p>0.05 for interaction between treatment groups and subgroup factors). There was a trend towards a larger DC-VS difference among those using thymidine NRTIs (n=106, DC-VS difference 15.3%, p<0.001) compared with those using ART but no thymidine NRTIs (n=33, −1.5%, p=0.84), p=0.07 for interaction, and a trend for differential treatment effect by NNRTI use (p=0.08 for interaction); among those using an NNRTI, the estimated DC-VS difference was 4.0% (p=0.52) compared with 17.7% (p<0.001) among those using ART but no NNRTI.
Changes in Laboratory Parameters
Table 3 summarizes changes in metabolic parameters from baseline to month 4, and through 12 and 36 months. Substantial reductions in total, LDL and HDL-cholesterol, and triglycerides (analyzed on the log10 scale) were evident in the DC group within 4 months and were sustained through the 36 months of follow up whereas these lipid parameters were stable or increased in the VS group; treatment differences were significant (P≤0.001). Both total and HDL-cholesterol changed in the same direction, and the treatment effect on the total:HDL-cholesterol ratio was not statistically significant.
Table 3.
Treatment Differences in Metabolic Parameters Between the Drug Conservation (DC) Versus Viral Suppression (VS) Groups.
| Outcome | Mean Change from baseline to Month 4A |
Mean Change from baseline through Month 12A |
Mean Change from baseline through Month 36A |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ChangeB |
DC-VS | (95% CI) | P | DC-VS | (95% CI) | P | Mean ChangeB |
DC-VS | (95% CI) | P | |||
| DC | VS | DC | VS | ||||||||||
| Total cholesterol (mg/dl) | −23.6 | 18.0 | −41.7 | (−53.8, −29.6) | <0.001 | −29.5 | (−39.8, −19.3) | <0.001 | −21.6 | 6.4 | −27.8 | (−38.2, −17.5) | <0.001 |
| LDL-cholesterol (mg/dl) | −13.2 | 5.5 | −18.8 | (−27.6, −9.9) | <0.001 | −12.0 | (−19.3, −4.8) | 0.001 | −12.5 | −0.1 | −11.7 | (−18.8, −4.5) | 0.001 |
| HDL-cholesterol (mg/dl) | −3.4 | 3.2 | −6.6 | (−9.4, −3.8) | <0.001 | −6.6 | (−9.3, −4.0) | <0.001 | −3.9 | 1.8 | −6.0 | (−8.6, −3.4) | <0.001 |
| Triglycerides (mg/dl) | −29.0 | 44.7 | −73.7 | (−146, −1.2) | 0.05 | −63.6 | (−109, −18) | 0.01 | −38.4 | 17.9 | −57.2 | (−98.8, −15.5) | 0.01 |
| Log Triglycerides | −0.087 | 0.048 | −0.14 | (−0.20, −0.07) | <0.001 | −0.10 | (−0.16, −0.05) | <0.001 | −0.08 | 0.03 | −0.10 | (−0.15, −0.04) | <0.001 |
| Lactate (mmol/l) | −0.06 | −0.16 | 0.10 | (−0.14, 0.34) | 0.42 | 0.11 | (−0.14, 0.36) | 0.39 | −0.09 | −0.17 | 0.09 | (−0.13, 0.32) | 0.41 |
| C peptide (ng/ml) | 0.21 | −0.14 | 0.35 | (−0.11, 0.80) | 0.14 | 0.41 | (0.08, 0.75) | 0.02 | 0.41 | 0.08 | 0.37 | (0.04, 0.75) | 0.03 |
| Insulin (μU/mL) | 1.6 | −1.3 | 2.9 | (−1.8, 7.6) | 0.23 | 2.8 | (−1.1, 6.6) | 0.16 | 3.2 | 1.4 | 1.88 | (−1.6, 5.4) | 0.29 |
| Glucose (mg/dl) | 6.4 | −0.2 | 6.6 | (−3.9, 17.1) | 0.22 | 2.8 | (−3.9, 9.5) | 0.41 | 1.6 | −1.9 | 2.78 | (−3.4, 9.0) | 0.38 |
| HOMAC | 0.57 | −0.55 | 1.13 | (−0.32, 2.57) | 0.13 | 1.08 | (−0.3, 2.5) | 0.13 | 0.9 | 0.2 | 0.73 | (−0.5, 1.9) | 0.23 |
| Hemoglobin A1c (%)D | --- | --- | --- | --- | --- | 0.3 | (0.1, 0.5) | 0.003 | 0.5 | 0.1 | 0.3 | (0.1, 0.5) | 0.02 |
Estimates and p-values were obtained in regression models (month 4) or longitudinal models (through months 12 and 36) which also contained race, sex, and the race-by-sex interaction.
Estimated means, covariate-adjusted for race, sex, and the race-by-sex interaction.
HOMA(homeostasis model assessment of insulin resistance) = (fasting plasma glucose [mmol/l]) * (serum insulin [mU/l])/22.5
Hemoglobin A1c was not measured at 4 months or 8 months. Through month 12, hemoglobin A1c increased by 0.3% in the DC group and had no change in the VS group.
Abbreviations: CI, confidence interval; DC, Drug Conservation group (episodic ART); HDL, high-density lipoprotein; HOMA, Homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; VS, Viral Suppression group (continuous ART);
To convert: mg/dL of cholesterol to mmol/L divide by 39; mg/dL of triglycerides to mmol/L divide by 89; mmol of lactate to mg/dL divide by 9; ng/mL of C peptide to nmol/L divide by 3; μU/mL of insulin to μg/L divide by 24; mg/dL of glucose to mmol/L divide by 18.
Treatment differences in glucose, insulin, HOMA, and lactate were not significant. However, hemoglobin A1C levels at 12 months had increased in the DC group (+0.3%) but had remained stable in the VS group (estimated difference 0.3%, 95% CI 0.1 to 0.5, P=0.003). Treatment differences in hemoglobin A1C remained significant through 36 months (P=0.02). After removing patients receiving therapy for diabetes at baseline (n=13, DC group, and n=14, VS group), the variability of hemoglobin A1C decreased but the significant treatment differences persisted at 12 months (P=0.001) and through 36 months (P<0.001). Changes in hemoglobin A1C were not explained by changes in total hemoglobin (data not shown).
Discussion
In the SMART Body Composition sub-study, the strategy of intermittent ART in HIV-infected patients had significant effects on body fat and plasma lipids. For most endpoints, the treatment difference was fully evident at 12 months and did not increase with longer follow-up. This pattern is consistent with the increase over time in the proportion of participants receiving ART in the DC group.
Limb fat increased (as percent of baseline fat) in the DC group and remained stable in the VS group; SAT increased in both groups, but substantially more in the DC group. There was no evidence for a treatment difference in VAT, and no evidence for a difference in limb fat when measuring absolute change (kg) rather than percent change.
We presented results for limb fat both as percent change from baseline and as absolute change, because (1) the model assumptions for the longitudinal analyses of limb fat were better met when using the percent scale to measure change from baseline, suggesting more reliable estimates and p-values, while (2) the study protocol specified absolute change. Percent fat changes are not often reported, possibly because they appear less intuitive.
In our study, the strategy of intermittent ART resulted in increased limb fat compared with standard, continuous ART, at least in the short-term. This finding confirms those from previous studies (19-22), and is also in accordance with a recently reported, randomized clinical trial in which intermittent thymidine-sparing ART lead to significantly greater increases in limb fat at 24 months compared with continuous thymidine NRTI-sparing ART in HIV-infected patients with lipoatrophy (28).
The absolute change in limb fat in the DC group at 12 months (+200 g) was lower than that reported in studies switching from thymidine NRTI-containing to thymidine NRTI-sparing ART after a similar period of time (from +400 to +800 g) (16–18). Several reasons may explain this smaller increase. Firstly, only 74% of the participants were receiving ART at enrollment, and only 52% were taking a thymidine NRTI. In fact, there was a trend towards larger increments in percent limb fat with the intermittent ART strategy compared with continuous ART in patients on a thymidine NRTI at study than among those not. Second, lipoatrophy was an entry criteria required for the switching studies but not for the SMART Body Composition sub-study. In fact, the median limb fat in the participants (predominantly males) of the SMART Body Composition sub-study was 6.6 kg, similar to that recently reported in predominantly male, HIV-infected patients starting ART (15) and slightly lower than that reported for HIV-uninfected men (29, 30), whereas the median limb fat in the switching studies was 3–3.5 kg (16–18). Among participants with below-median limb fat (< 6.6 kg), limb fat increased by 480 g at 12 months in the DC group, and decreased slightly (−40 g) in the VS group. Finally, by 12 months, 38% of participants in the DC group had re-initiated ART, thus decreasing the difference in exposure to ART between the treatment groups.
There was no significant between-group difference in VAT. Potential reasons to explain this finding might be that visceral fat is less influenced by ART than subcutaneous fat, that factors other than ART may have a differential impact on visceral versus limb fat or, less likely, that visceral fat is relatively resistant to change in this population. Also, the treatment difference in VAT varied across several subgroups, (p-value <0.05 for treatment differential effect), suggesting that the DC and VS strategies may have different effects depending on HIV-RNA level, hepatitis B or C status, lipoatrophy or NNRTI use. There was also no evidence for a between-group difference with respect to change in lean mass. Prolonged uncontrolled HIV replication may lead to wasting syndrome (31), an AIDS-defining condition characterized by decreased lean mass. Wasting is typically observed at low CD4+ count levels, however, while participants in the DC group tended to re-initiate ART well above those levels.
All plasma lipids decreased significantly in the DC group as compared with the VS group. The reduction in lipid values was evident at 4 months. Antiretroviral-induced lipid changes have been usually reported as occurring within a few weeks after the introduction of ART (32). Our study showed rapid decrease in lipids after ART discontinuation, which mirrored the increase in lipids after starting ART reported in other studies (19–22). The decrease in plasma lipids associated with ART interruption was initially considered to be a positive effect (33). However, low HDL-cholesterol would be expected to increase the risk of cardiovascular disease even if LDL cholesterol is low (34). HIV infection is a condition associated with a relative decrease in HDL-cholesterol (32). HIV infection increases plasma triglycerides and decreases cholesterol and its lipoprotein fractions, but the reduction in HDL-cholesterol is relatively higher than that in total and LDL cholesterol. ART irrespective of the drugs used increases all cholesterol fractions in a kind of “return-to-normality” effect, but the raise in HDL-cholesterol may be proportionately less than that for total and LDL cholesterol (35). The difference in total:HDL-cholesterol ratio in this sub-study was not significant, but it was in favor of VS in the main SMART study (36), which had more power. Besides lipid changes, increases in proinflammatory cytokines and immune activation (parameters not assessed in the SMART Body Composition sub-study) have been observed under intermittent ART in the SMART and other studies (37–39).
Although there were no differences in glucose, insulin, C-peptide, HOMA, or lactate between both strategies, hemoglobin A1C increased in the DC group while it remained stable in the VS group. This difference was maintained throughout the study and was not explained by fasting glycemia, estimated insulin resistance, or total hemoglobin levels. Hemoglobin A1C may be falsely decreased with reduced hemoglobin, hemoglobinopathies, increased turnover of red blood cells, or uremia, and falsely elevated with alcoholism, lead poisoning, opiate addiction, excessive use of salicylates, or pregnancy (40). We did not systematically assess most of those conditions in our study. Due to the randomized design of the study, however, no substantial between-group differences in these characteristics would be expected. Although there was a race and sex imbalance in our substudy, we are not aware that hemoglobin A1C levels were affected by these factors. Inappropriately low hemoglobin A1C levels have been reported in HIV-infected patients receiving ART containing NRTIs (41–43) that were associated to subclinical hemolysis in one study (42) and to increased medium corpuscular volume of erythrocytes in another study (43). Consistent with previous findings (41–43), patients in the Body Composition sub-study had increases in hemoglobin A1C under intermittent ART while hemoglobin A1C levels remained stable with continuous ART. Altogether these data indicate that hemoglobin A1C may not be appropriate for reliably monitoring diabetes mellitus in HIV-infected patients receiving ART.
Potential limitations of our study include: (1) the premature closing of the SMART study limiting the power for treatment comparisons and subgroup analyses; (2) antiretroviral therapy was heterogeneous, limiting the abiltiy to assess specific antiretroviral drugs or drug classes; (3) analyses were not adjusted for multiple comparisons; in particular, we compared treatment effects across many subgroups, and some of the subgroup findings may be false positives; and (4) only few participants had lipoatrophy at study entry, limiting the power of analyses for this subgroup.
In conclusion, intermittent ART in the SMART Body Composition sub-study increased subcutaneous fat, had no effect on VAT, decreased plasma lipids, and increased hemoglobin A1C compared with continuous ART.
Acknowledgments
Supported in part by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, grants U01AI042170, U01AI46362 and U01AI068641.
We gratefully acknowledge the work of Chris Mullin and Lisa Thackeray as protocol managers for the Body Composition sub-study of SMART, and thank the patients participating in the SMART Body Composition sub-study. We thank reviewers for the INSIGHT network and for the AIDS journal for their helpful comments.
The following sites and investigators enrolled participants in the SMART Body Composition sub-study (number of participants in parentheses); similar information for the main study has been published (25):
Houston AIDS Research Team, Houston, Texas (n=94): Estela A. Acosta, Roberto C. Arduino, MD, Jorge Darcourt Riso-Patron, Carmen Machado, Pablo C. Okhuysen, MD, Maria C. Rodriguez-Barradas, MD, Maria Tadea Insignares, A. Clinton White, MD
The Research & Education Group, Portland, Oregon (n=56): Diana Antoniskis, MD, Doug Beers, MD, David Gilbert, MD, Joel Godbey, MD, Gordon Johnson, MD, Todd Korthuis, MD, James Leggett, MD, Michael McVeigh, MD, Melinda Mueller, MD, Mary O’Hearn, MD, James Sampson, MD
Australia Coordinating Center, Sydney, Australia (n=50): Jonathan Anderson, Kathy Barnes, Alison Cain, David Cooper, Beng Eu, Martyn French, Jennifer Hoy, Nic Medland, Richard Moore, Sally Price, Norm Roth, Jega Sarangapany, Don Smith, Bak Kiem Tee
Harlem AIDS Treatment Group, New York, New York (n=39): Susan Caras, MS, Rosetta Contreras, RN, John Corser, MD, Livette Johnson, MD, Subha Raghavan, PhD, Helen May Seedhom, RN
Spain Coordinating Center, Barcelona, Spain (n=13): Javier Fernandez, RN, José M. Gatell, MD, Maria Larrousse MD, Merce Poal, RN, Ana Rodriguez, RN, Sergi Vidal MD
Richmond AIDS Consortium, Richmond, Virginia (n=11): Robert Brennan, MD, Vivian Bruzzese, MD, Anne Chiang, MD, Clarence Childress, MD, Carol Clark, RN, BSN, Patricia Dodson, RN, BSN, CCRC, Kathleen Genther, RN, BSN, CCRC, Robert Higginson, PAC, Jane Kaatz, RN, ANP, Johanna McKee, RN, CCRC, Daniel Nixon, DO, PhD, Jane Settle, RN, ANP, Vicky Watson, RN, Joy Zeh, RN, FNP
University of North Texas Health Science Center, Fort Worth, Texas (n=10): Isabel Vecino, MD, Stephen E. Weis, DO
Louisiana Community AIDS Research Program, New Orleans, Louisiana (n=2): Suzanne L. Adams, RN, BSN, Sr. Sue Pabolvich, RN, MPH, C-FNP, Connie Z. Scott, RN, BSN, ACRN, Janice Y. Walker, RN, NP, MN, CCRC
Footnotes
Clinical Trials.gov identifier: NCT00027352.
Conflicts of interest statement
Fraser Drummond, Simon Edwards, Wafaa El-Sadr, Cynthia Gibert, Birgit Grund, Judith Shlay, Fehmida Visnegarwala, Avis Thomas report no conflicts. Andrew Carr has received research funding, consultancy fees, or lecture sponsorships from, or served on advisory boards for Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Roche and Tibotec. Esteban Martínez has received research funding, consultancy fees, or lecture sponsorships from, or served on advisory boards for Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and Tibotec. Daniel Pearce has received research funding, consultancy fees, or lecture sponsorships from, or served on advisory boards for GlaxoSmithKline, Bristol-Myers Squibb, Boehringer Ingelheim, Ortho Biotech, Serono, Roche, Pfizer, Gilead, Abbott, Merck Sharp & Dohme, Pharmacia Upjohn, Astra Zeneca, Unimed, Solvay, Watson, and Tibotec. Peter Reiss has received research funding, consultancy fees, or lecture sponsorships from, or served on advisory boards for Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Science, GlaxoSmithKline, Roche, Merck Sharp & Dohme, Pfizer, Theratechnologies, and Tibotec.
References
- 1.Heath KV, Hogg RS, Chan KJ, Harris M, Montessori V, O’Shaughnessy MV, et al. Lipodystrophy-associated morphological, cholesterol and triglyceride abnormalities in a population-based HIV/AIDS treatment database. AIDS. 2001;15:231–239. doi: 10.1097/00002030-200101260-00013. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, Jr, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Miller J, Carr A, Emery S, Law M, Mallal S, Baker D, et al. HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med. 2003;4:293–301. doi: 10.1046/j.1468-1293.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernasconi E, Boubaker K, Junghans C, Flepp M, Furrer HJ, Haensel A, et al. Abnormalities of body fat distribution in HIV-infected persons treated with antiretroviral drugs: The Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2002;31:50–55. doi: 10.1097/00126334-200209010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Blanch J, Rousaud A, Martinez E, De Lazzari E, Peri JM, Milinkovic A, et al. Impact of lipodystrophy on the quality of life of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2002;31:404–407. doi: 10.1097/00126334-200212010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Blanch J, Rousaud A, Martínez E, De Lazzari E, Milinkovic A, Peri JM, et al. Factors associated with severe impact of lipodystrophy on the quality of life of patients infected with HIV-1. Clin Infect Dis. 2004;38:1464–1470. doi: 10.1086/383573. [DOI] [PubMed] [Google Scholar]
- 7.Ammassari A, Antinori A, Cozzi-Lepri A, Trotta MP, Nasti G, Ridolfo AL, et al. Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr. 2002;31 (Suppl 3):S140–S144. doi: 10.1097/00126334-200212153-00011. [DOI] [PubMed] [Google Scholar]
- 8.Guaraldi G, Luzi K, Murri R, Granata A, De Paola M, Orlando G, et al. Sexual dysfunction in HIV-infected men: role of antiretroviral therapy, hypogonadism and lipodystrophy. Antivir Ther. 2007;12:1059–1065. doi: 10.1177/135965350701200713. [DOI] [PubMed] [Google Scholar]
- 9.Falutz J. Therapy insight: Body-shape changes and metabolic complications associated with HIV and highly active antiretroviral therapy. Nat Clin Pract Endocrinol Metab. 2007;3:651–661. doi: 10.1038/ncpendmet0587. [DOI] [PubMed] [Google Scholar]
- 10.Martinez E, Mocroft A, García-Viejo MA, Pérez-Cuevas JB, Blanco JL, Mallolas J, et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet. 2001;357:592–598. doi: 10.1016/S0140-6736(00)04056-3. [DOI] [PubMed] [Google Scholar]
- 11.Dubé MP, Komarow L, Mulligan K, Grinspoon SK, Parker RA, Robbins GK, et al. Long-term body fat outcomes in antiretroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides: dual X-ray absorptiometry results from A5005s, a substudy of Adult Clinical Trials Group 384. J Acquir Immune Defic Syndr. 2007;45:508–514. doi: 10.1097/QAI.0b013e3181142d26. [DOI] [PubMed] [Google Scholar]
- 12.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 13.Podzamczer D, Ferrer E, Sánchez P, Gatell JM, Crespo M, Fisac C, et al. Less lipoatrophy and better lipid profile with abacavir as compared to stavudine: 96-week results of a randomized study. J Acquir Immune Defic Syndr. 2007;44:139–147. doi: 10.1097/QAI.0b013e31802bf122. [DOI] [PubMed] [Google Scholar]
- 14.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 15.Haubrich RH, Riddler S, DiRienzo G, Komarow L, Powderly W, Klingman K, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin A, Smith DE, Carr A, Ringland C, Amin J, Emery S, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. AIDS. 2004;18:1029–1036. doi: 10.1097/00002030-200404300-00011. [DOI] [PubMed] [Google Scholar]
- 17.Moyle GJ, Sabin CA, Cartledge J, Johnson M, Wilkins E, Churchill D, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20:2043–2050. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
- 18.Murphy R, Zhang J, Hafner R, Yarasheski K, Tashima K, Berzins B, et al. Peripheral and visceral fat changes following a treatment switch to a nonthymidine analogue or nucleoside-sparing regimen in patients with peripheral lipoatrophy: 48-week final results of ACTG A5110, a prospective, randomized multicenter clinical trial. 13th Conference on Retroviruses and Opportunistic Diseases; 2006; Denver, CO. abstract 755. [Google Scholar]
- 19.Hatano H, Miller KD, Yoder CP, Yanovski JA, Sebring NG, Jones EC, Davey RT., Jr Metabolic and anthropometric consequences of interruption of highly active antiretroviral therapy. AIDS. 2000;14:1935–1942. doi: 10.1097/00002030-200009080-00008. [DOI] [PubMed] [Google Scholar]
- 20.Milinkovic A, Martinez E, Vidal S, Del Rio A, Pérez-Cuevas JB, Conget I, et al. The effect of structured therapy interruptions on the evolution of lipid abnormalities and body fat in patients with primary HIV-1 infection. Antivir Ther. 2001;6(Suppl 4):62. (abstract no. 91) [Google Scholar]
- 21.Milinkovic A, Martinez E, Vidal S, Blanco JL, Lonca M, Garcia F, et al. Impact of structured therapy interruption on body composition of chronically HIV-infected patients: preliminary 1-year results. Antivir Ther. 2003;8:L61. (abstract 88) [Google Scholar]
- 22.Arjona MM, Pérez-Cano R, García-Juárez R, Martín-Aspas A, del Alamo CF, Girón-González JA. Structured intermittent interruption of chronic HIV infection treatment with highly active antiretroviral therapy: effects on leptin and TNF-alpha. AIDS Res Hum Retroviruses. 2006;22:307–314. doi: 10.1089/aid.2006.22.307. [DOI] [PubMed] [Google Scholar]
- 23.Ananworanich J, Gayet-Ageron A, Le Braz M, Prasithsirikul W, Chetchotisakd P, Kiertuburanakul S, et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet. 2006;368:459–465. doi: 10.1016/S0140-6736(06)69153-8. [DOI] [PubMed] [Google Scholar]
- 24.Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 25.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 26.Ellis KJ, Grund B, Visnegarwala F, Thackeray L, Miller CG, Chesson CE, et al. Visceral and subcutaneous adiposity measurements in adults: influence of measurement site. Obesity. 2007;15:1441–1447. doi: 10.1038/oby.2007.172. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Martinez E, Milinkovic A, Garcia F, Larrousse M, Vidal S, Leon A, et al. Greater limb fat increase with intermittent (relative to continuous) thymidine-sparing antiretroviral therapy in HIV-infected patients with lipoatrophy. Antivir Ther. 2008;13 (Suppl 4):A9. [Google Scholar]
- 29.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Engelson ES, Kotler DP, Tan YX, et al. Fat distribution in HIV-infected patients reporting truncal enlargement quatified by whole-body magnetic resonance imaging. Am J Clin Nutr. 1999;69:1162–1169. doi: 10.1093/ajcn/69.6.1162. [DOI] [PubMed] [Google Scholar]
- 31.Manqili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42:836–842. doi: 10.1086/500398. [DOI] [PubMed] [Google Scholar]
- 32.Martinez E, Leyes P, Ros E. Effectiveness of lipid-lowering therapy in HIV patients. Curr Opin HIV AIDS. 2008;3:240–246. doi: 10.1097/COH.0b013e3282fb7bb9. [DOI] [PubMed] [Google Scholar]
- 33.Dybul M, Chun TW, Yoder C, Hidalgo B, Belson M, Hertogs K, et al. Short-cycle structured intermittent treatment of chronic HIV infection with highly active antiretroviral therapy: effects on virologic, immunologic, and toxicity parameters. PNAS. 2001;26:15161–15166. doi: 10.1073/pnas.261568398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J. 1985;110:1100–1107. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- 35.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 36.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 37.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calmy A, Gayet-Ageron A, Montecucco F, Nguyen A, Mach F, Burger F, et al. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS. 2009;23:929–939. doi: 10.1097/qad.0b013e32832995fa. [DOI] [PubMed] [Google Scholar]
- 39.Tebas P, Henry WK, Matining R, Weng-Cherng D, Schmitz J, Valdez H, et al. Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: implications for cardiovascular risk. PLoS ONE. 2008;3:e2021. doi: 10.1371/journal.pone.0002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran HA, Silva D, Petrovsky N. Case study: pitfalls of using hemoglobin A1c as the sole measure of glycemic control. Clin Diabetes. 2004;22:141–143. [Google Scholar]
- 41.Polgreen PM, Putz D, Stapleton JT. Inaccurate glycosilated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis. 2003:e53–e56. doi: 10.1086/376633. [DOI] [PubMed] [Google Scholar]
- 42.Diop ME, Bastard JP, Meunier N, Thevenet S, Maachi M, Capeau J, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retrovir. 2006;12:1242–1247. doi: 10.1089/aid.2006.22.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim PS, Woods C, Georgoff P, Crum D, Rosenberg A, Smith M, Hadigan C. Hemoglobin A1c underestimates glycemia in HIV infection. Diabetes Care. 2009;32:1591–1593. doi: 10.2337/dc09-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]