Abstract
The CHOIR trial in anemic patients with chronic kidney disease compared epoetin-alfa treatment with low (11.3 g/l) and high (13.5 g/l) hemoglobin targets on the composite end point of death, hospitalization for heart failure, stroke, and myocardial infarction. However, other anemia management trials in patients with chronic kidney disease found there was increased risk when hemoglobin is targeted above 13 g/dl. In this secondary analysis of the CHOIR trial, we compared outcomes among the subgroups of patients with diabetes and heart failure to describe the comparative relationship of treatment to these two different hemoglobin goals. By Cox regression analysis, there was no increased risk associated with the higher hemoglobin target among patients with heart failure. In patients without heart failure, however, the hazard ratio (1.86) associated with the higher target was significant. Comparing survival curves in an unadjusted model, patients with diabetes did not have a greater hazard associated with the higher target. Subjects without diabetes had a significantly greater hazard in the high as compared to the low target, but the interaction between diabetes and the target was not significant. We suggest that the increased risks associated with higher hemoglobin targets are not clinically apparent among subgroups with greater mortality risk. These differential outcomes underscore the need for dedicated trials in these subpopulations.
Keywords: anemia, diabetes mellitus, heart failure, kidney
The presence of anemia is associated with worse outcomes among many subgroups of patients including those with chronic kidney disease (CKD), end stage renal disease, and heart failure (HF).1–3 Paradoxically, however, trials comparing the effects of the treatment of anemia to two different hemoglobin goals in patients with CKD and end stage renal disease demonstrated harm 4,5 in the subjects randomized to the higher hemoglobin goals. This has led to hypotheses regarding the potential for a direct relationship between the erythrocyte-stimulating agent (ESA) dose and greater risk6 as mechanisms for these worse outcomes.
Patients with HF who have either advanced disease or recent hospitalization have a prevalence of anemia of 20% or greater.7–11 As among patients with CKD, the presence of anemia in those with HF is associated with an increased risk of poorer outcomes.11 Because many patients with HF have concurrent kidney disease related to the same underlying comorbidities, some of the causes of anemia in these two groups of patients (that is, CKD and HF) overlap. The term ‘cardiorenal syndrome’ has further been coined to identify the overlap within patients for these two comorbidities as a ‘state in which therapy to relieve congestive heart failure symptoms is limited by further worsening kidney function’.12 Although the large trials comparing outcomes between treatment strategies for anemia in CKD and end stage renal disease have been relatively consistent, trials comparing outcomes among patients with HF and anemia have been conflicting in terms of the effect of anemia management using ESA on exercise tolerance and symptoms.13–15
To further refine knowledge on the impact of anemia management among patients with comorbidities such as HF and DM, two large trials (RED-HF and TREAT) are ongoing to compare treatment with ESA to achieve hemoglobin goals of greater than 13 g/dl to placebo. These trials will significantly advance our understanding of how anemia treatment with ESAs affects outcomes in these two populations. Given the use of the placebo arm, however, they will not be able to shed light on the hemoglobin target that will best maximize a benefit to therapy. CHOIR was a randomized trial comparing the effect of treatment with epoetin-alfa to one of two hemoglobin targets (11.3 vs 13.5 g/dl) on the composite end point of death, hospitalization for congestive heart failure, stroke, and myocardial infarction in CKD patients with anemia. This secondary analysis of the CHOIR trial was undertaken to compare outcomes among the subgroups of patients with DM and HF to describe the comparative relationship with treatment to these two different hemoglobin goals.
RESULTS
Description of subgroups based on HF and DM
As previously reported,5,6 1432 subjects were randomized in CHOIR. Of these, 375 subjects had a previous history of HF, whereas 967 had no previous history (Table 1) and 90 subjects had missing previous history. In general, subjects with HF were older and were more likely to have concurrent comorbidities such as DM, cerebrovascular disease, coronary artery disease, peripheral vascular disease, and atrial dysrhythmias as compared with those without HF. Within subgroups based on the presence or absence of HF, there were few differences between the two randomized treatment groups. Of the entire cohort, 894 subjects had DM, 488 did not, and 50 had missing information on the presence of DM. Similar to the comparisons above, subjects with DM were more likely to have concurrent comorbidities such as HF, cerebrovascular disease, coronary artery disease, and peripheral vascular disease as compared with those without DM (Table 2). And within subgroups based on the presence or absence of DM, there were few differences between the two randomized treatment groups.
Table 1.
Demographics and previous medical history for subjects grouped by presence or absence of HF at baseline
| Subjects with HF at baseline (N=375) |
Subjects without HF at baseline (N=967) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hb 13.5 group (N=192) | Hb 11.3 group (N=183) | P-value | Total | Hb 13.5 group (N=477) | Hb 11.3 group (N=490) | P-value | Total | |
| Age (years) | 70.2 (11.71) | 69.5 (11.26) | 0.529 | 69.9 (11.49) | 64.5 (14.98) | 65.4 (13.98) | 0.313 | 64.9 (14.48) |
| Male gender | 89/192 (46.4%) | 103/183 (56.3%) | 0.054 | 192 (51.2%) | 198/477 (41.5%) | 212/490 (43.3%) | 0.581 | 410 (42.4%) |
| Race: black (vs non-white/black) | 56/191 (29.3%) | 50/183 (27.3%) | 0.668 | 106/374 (28.3%) | 131/477 (27.5%) | 146/490 (29.8%) | 0.422 | 277/967 (28.6%) |
| Hispanic ethnicity | 18/191 (9.4%) | 22/183 (12.0%) | 0.416 | 40/374 (10.7%) | 66/476 (13.9%) | 68/490 (13.9%) | 0.996 | 134/966 (13.9%) |
| Diabetes mellitus | 138/192 (71.9%) | 143/183 (78.1%) | 0.162 | 281/375 (74.9%) | 281/476 (59.0%) | 289/488 (59.2%) | 0.953 | 570/964 (59.1%) |
| Previous CVA or TIA | 31/192 (16.1%) | 38/183 (20.8%) | 0.249 | 69/375 (18.4%) | 58/477 (12.2%) | 60/488 (12.3%) | 0.949 | 118/965 (12.2%) |
| Previous coronary artery disease | 118/192 (61.5%) | 116/183 (63.4%) | 0.700 | 234/375 (62.4%) | 115/477 (24.1%) | 97/490 (19.8%) | 0.105 | 212/967 (21.9%) |
| Previous peripheral vascular disease | 51/192 (26.6%) | 44/183 (24.0%) | 0.575 | 95/375 (25.3%) | 65/477 (13.6%) | 70/488 (14.3%) | 0.748 | 135/965 (14.0%) |
| Previous atrial fibrillation/flutter | 43/192 (22.4%) | 34/183 (18.6%) | 0.360 | 77/375 (20.5%) | 20/477 (4.2%) | 24/490 (4.9%) | 0.599 | 44/967 (4.6%) |
| History of solid organ malignancy | 25/191 (13.1%) | 24/183 (13.1%) | 0.994 | 49/374 (13.1%) | 65/472 (13.8%) | 69/487 (14.2%) | 0.859 | 134/959 (14.0%) |
| Inflammation/malnutrition (albumin ≤3.6 g/dl or ferritin >600 ng/ml) | 83/192 (43.2%) | 68/179 (38.0%) | 0.305 | 151/371 (40.7%) | 180/475 (37.9%) | 173/489 (35.4%) | 0.417 | 353/964 (36.6%) |
| Baseline albumin (g/dl) | 3.7 (0.55) | 3.7 (0.48) | 0.118 | 3.7 (0.52) | 3.8 (0.50) | 3.8 (0.46) | 0.463 | 3.8 (0.48) |
| Baseline ferritin (ng/ml) | 159.5 (142.08) | 193.5 (186.05) | 0.050 | 175.9 (165.41) | 170.7 (163.08) | 177.8 (169.36) | 0.510 | 174.3 (166.25) |
| Baseline eGFR (ml/min) | 26.9 (8.94) | 26.0 (8.35) | 0.291 | 26.5 (8.66) | 27.0 (8.57) | 28.0 (9.29) | 0.103 | 27.5 (8.95) |
| Baseline cholesterol | 172.5 (50.08) | 178.5 (51.52) | 0.254 | 175.4 (50.80) | 189.8 (53.45) | 186.5 (49.24) | 0.318 | 188.1 (51.35) |
| Baseline TSAT (%) | 22.1 (9.84) | 24.1 (9.47) | 0.043 | 23.1 (9.70) | 26.4 (12.48) | 25.1 (10.55) | 0.069 | 25.7 (11.55) |
| Baseline hemoglobin | 10.0 (0.96) | 10.0 (0.96) | 0.847 | 10.0 (0.96) | 10.1 (0.83) | 10.2 (0.85) | 0.431 | 10.1 (0.84) |
For age and baseline laboratory assessments, values presented are Mean (s.d.) and t-test P-values. Otherwise, n/N (%) and Pearson χ2 P-values are provided.
CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; HF, heart failure; TIA, transient ischemic attack; TSAT, transferrin saturation.
Table 2.
Summary of baseline characteristics among groups based on the presence or absence of DM at baseline
| Subjects with DM at baseline (N=894) |
Subjects without DM at baseline (N=488) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hb 13.5 group (N=436) | Hb 11.3 group (N=458) | P-value | Total | Hb 13.5 group (N=249) | Hb 11.3 group (N=239) | P-value | Total | |
| Age (years) | 65.6 (12.37) | 65.9 (11.42) | 0.624 | 65.8 (11.89) | 66.9 (17.10) | 67.5 (16.32) | 0.698 | 67.2 (16.70) |
| Male gender | 193/436 (44.3%) | 224/458 (48.9%) | 0.164 | 417 (46.6%) | 107/249 (43.0%) | 97/239 (40.6%) | 0.593 | 204 (41.8%) |
| Race: black (vs non-white/black) | 136/435 (31.3%) | 141/458 (30.8%) | 0.877 | 277/893 (31.0%) | 58/249 (23.3%) | 64/239 (26.8%) | 0.374 | 122/488 (25.0%) |
| Hispanic ethnicity | 64/434 (14.7%) | 75/457 (16.4%) | 0.494 | 139/891 (15.6%) | 24/249 (9.6%) | 19/239 (7.9%) | 0.511 | 43/488 (8.8%) |
| Previous HF composite | 138/419 (32.9%) | 143/432 (33.1%) | 0.959 | 281/851 (33.0%) | 54/249 (21.7%) | 40/239 (16.7%) | 0.166 | 94/488 (19.3%) |
| Previous CVA or TIA | 60/420 (14.3%) | 67/430 (15.6%) | 0.596 | 127/850 (14.9%) | 29/249 (11.6%) | 31/239 (13.0%) | 0.656 | 60/488 (12.3%) |
| Previous coronary artery disease | 163/420 (38.8%) | 153/432 (35.4%) | 0.305 | 316/852 (37.1%) | 70/249 (28.1%) | 60/239 (25.1%) | 0.452 | 130/488 (26.6%) |
| Previous peripheral vascular disease | 88/419 (21.0%) | 88/431 (20.4%) | 0.833 | 176/850 (20.7%) | 28/249 (11.2%) | 26/238 (10.9%) | 0.910 | 54/487 (11.1%) |
| Previous atrial fibrillation/flutter | 32/419 (7.6%) | 34/432 (7.9%) | 0.899 | 66/851 (7.8%) | 31/249 (12.4%) | 24/239 (10.0%) | 0.400 | 55/488 (11.3%) |
| History of solid organ malignancy | 45/414 (10.9%) | 52/431 (12.1%) | 0.586 | 97/845 (11.5%) | 45/249 (18.1%) | 42/238 (17.6%) | 0.903 | 87/487 (17.9%) |
| Inflammation/malnutrition (albumin ≤3.6 g/dl or ferritin >600 ng/ml) | 190/436 (43.6%) | 190/453 (41.9%) | 0.622 | 380/889 (42.7%) | 85/247 (34.4%) | 66/239 (27.6%) | 0.105 | 151/486 (31.1%) |
| Baseline albumin (g/dl) | 3.7 (0.53) | 3.7 (0.46) | 0.270 | 3.7 (0.49) | 3.8 (0.50) | 3.8 (0.47) | 0.154 | 3.8 (0.48) |
| Baseline ferritin (ng/ml) | 169.9 (154.62) | 178.0 (167.38) | 0.453 | 174.0 (161.21) | 162.4 (164.58) | 185.1 (182.28) | 0.151 | 173.6 (173.70) |
| Baseline eGFR (ml/min) | 27.0 (8.81) | 27.5 (8.84) | 0.434 | 27.2 (8.82) | 27.0 (8.48) | 26.9 (9.53) | 0.953 | 26.9 (9.00) |
| Baseline cholesterol (mg/dl) | 183.9 (54.57) | 183.3 (49.95) | 0.860 | 183.6 (52.23) | 187.0 (49.07) | 186.4 (48.88) | 0.899 | 186.7 (48.93) |
| Baseline TSAT (%) | 23.9 (10.17) | 24.3 (9.61) | 0.524 | 24.1 (9.88) | 27.2 (14.13) | 25.6 (11.14) | 0.142 | 26.4 (12.76) |
| Baseline hemoglobin (g/dl) | 10.0 (0.87) | 10.1 (0.88) | 0.018 | 10.0 (0.88) | 10.3 (0.88) | 10.2 (0.86) | 0.177 | 10.2 (0.87) |
For age and baseline laboratory assessments, values presented are Mean (s.d.) and t-test P-values. Otherwise, n/N (%) and Pearson χ2 P-values are provided.
CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; HF, heart failure; TIA, transient ischemic attack; TSAT, transferrin saturation.
Outcomes for subjects with and without HF
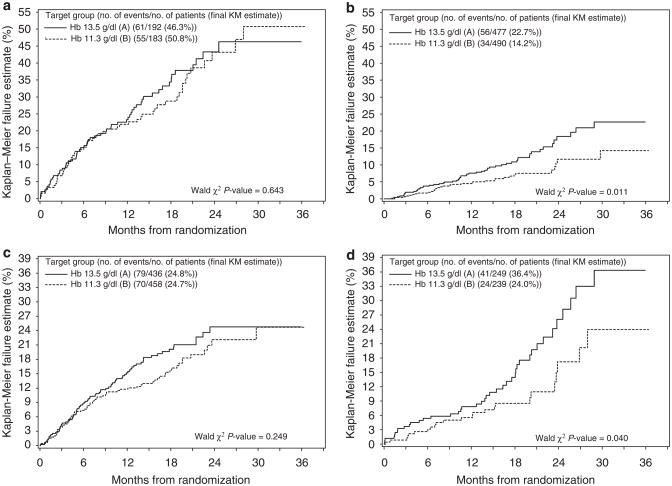
Subjects with HF had a greater hazard of experiencing a primary end point than subjects without HF in a univariable model (P<0.001) (Table 3a). Among subjects with HF, Kaplan–Meier estimates of the 3-year failure rate for the primary end point was 46.3% of those randomized to the higher hemoglobin arm as compared with 50.8% of those randomized to the lower hemoglobin arm (Figure 1a). These differences were not significant (P =0.643 based on Wald’s χ2-test from the unadjusted Cox model with interaction, Table 3b). Of subjects without previous HF at baseline, Kaplan–Meier estimates of the 3-year failure rate for the primary end point was 22.7% of those randomized to the higher hemoglobin arm as compared with only 14.2% of those randomized to the lower hemoglobin arm (Figure 1b) (P =0.011 based on Wald’s χ2 test from the unadjusted Cox model with interaction, Table 3b).
Table 3.
Univariate and multivariable Cox proportional hazard models of the primary composite event among subjects with and without HF
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| (a) Univariable model (n=1342, 90 excluded for missing information on HF) | ||
| Previous HF composite (HF, Cardiomyopathy, LVD, or RVD) vs no previous HF | 4.08 (3.09, 5.37) | <0.001 |
| (b) Unadjusted model with interaction (n=1342, 90 excluded for missing information on HF) | ||
| Hb 13.5 g/dl (vs 11.3 g/dl) | 1.73 (1.13, 2.65) | 0.011 |
| Previous HF composite (HF, Cardiomyopathy, LVD, or RVD) | 5.29 (3.45, 8.12) | <0.001 |
| Interaction of Hb 13.5 g/dl group and previous HF composite | 0.63 (0.36, 1.10) | 0.105 |
| Contrasts | ||
| No previous HF Patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 1.73 (1.13, 2.65) | 0.011 |
| Prior HF Patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 1.09 (0.76, 1.57) | 0.643 |
| (c) Adjusted multivariable model (n=1342, without missing HF with imputations for missing data in other fields) | ||
| Hb 13.5 g/dl (vs 11.3 g/dl) | 1.86 (1.21, 2.85) | 0.004 |
| Baseline albumin (g/dl) | 0.53 (0.40, 0.71) | <0.001 |
| Baseline cholesterol linear splines (per 10 mg/dl) | ||
| <240 mg/dl | 0.95 (0.92, 0.99) | 0.015 |
| ≥240 mg/dl | 1.09 (1.03, 1.16) | 0.003 |
| Age linear splines (per 5 years) | ||
| <55 years | 1.55 (1.12, 2.15) | 0.008 |
| ≥55 to <75 years | 0.97 (0.85, 1.10) | 0.591 |
| ≥75 years | 1.41 (1.17, 1.70) | <0.001 |
| Previous HF composite (HF, cardiomyopathy, LVD, or RVD) | 4.47 (2.89, 6.92) | <0.001 |
| Previous CVA or TIA | 1.42 (1.01, 2.00) | 0.042 |
| Previous DVT | 2.07 (1.20, 3.56) | 0.009 |
| History of solid organ malignancy | 0.64 (0.41, 0.99) | 0.046 |
| Previous atrial fibrillation/flutter | 1.77 (1.23, 2.54) | 0.002 |
| Interaction of Hb 13.5 g/dl group and previous HF composite | 0.53 (0.30, 0.94) | 0.028 |
| Contrasts | ||
| No previous HF patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 1.86 (1.21, 2.85) | 0.004 |
| Previous HF patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 0.99 (0.68, 1.43) | >0.999 |
For adjusted multivariable model, t-test P-values based on multiple imputation are provided. Otherwise, Wald χ2 P-values are reported.
DVT, deep vein thrombosis; LVD, left ventricular dysfunction; RVD, right ventricular dysfunction.
Figure 1. Kaplan–Meier plot of the primary end point.
(a) Subjects with a previous history of HF. (b) Subjects without a previous history of HF. (c) Subjects with diabetes mellitus. (d) Subjects without diabetes mellitus.
The presence or absence of HF at baseline interacted significantly with the hemoglobin target (P =0.028) (adjusted model, Table 3c). Other predictors for the occurrence of the primary end point included a previous history of HF (P<0.001), lower serum albumin (P<0.001), increasing age (Table 3c for spline P-values), previous cerebrovascular disease (P =0.042), previous deep venous thrombosis (P =0.009), previous atrial fibrillation or flutter (P =0.002) and previous malignancy (P =0.046). The association between cholesterol level and outcomes was not linear with a decreasing risk as serum cholesterol rose to 240 mg/dl (P =0.015) followed by an increasing risk associated with higher levels above that threshold (P =0.003). The presence of DM was not a significant predictor in this model.
In the adjusted model stratified on the presence or absence of HF at baseline (Table 3c), there was no increased risk associated with the higher target hemoglobin among subjects with baseline HF (HR =0.99; 95% CI 0.68, 1.43; P≥0.99). Among subjects without HF at baseline, the increased risk associated with the higher target hemoglobin was highly significant (HR =1.86; 95% CI 1.21, 2.85; P =0.004).
Outcomes for subjects with and without DM
Subjects with DM had a greater hazard of experiencing a primary end point than subjects without DM (P =0.067, Table 4a). Among subjects with DM, Kaplan–Meier estimates of the 3-year failure rate for the primary end point were 24.8% of those randomized to the higher hemoglobin arm as compared with 24.7% of those randomized to the lower hemoglobin arm (Figure 1c). These differences were not significant (HR =1.21, 95% CI 0.88, 1.67, P =0.249 in the unadjusted Cox model with interaction, Table 4a). Of subjects without previous DM at baseline, Kaplan–Meier estimates of the 3-year failure rate for the primary end point were 36.4% of those randomized to the higher hemoglobin arm as compared with only 24.0% of those randomized to the lower hemoglobin arm (HR =1.70, 95% CI 1.03, 2.81, P =0.040 in the unadjusted Cox model with interaction) (Figure 1d, Table 4b). The presence or absence of DM at baseline did not interact significantly with hemoglobin target in either the unadjusted or adjusted models (Table 4b and c, P =0.265 and 0.559, respectively).
Table 4.
Univariate and multivariable Cox proportional hazard models of the primary composite event among subjects with and without DM
| Hazard ratio 95% CI | P-value | |
|---|---|---|
| (a) Univariate models (n=1382, 50 excluded for missing information on DM) | ||
| Previous DM (history or etiology) vs no DM | 1.31 (0.98, 1.76) | 0.067 |
| (b) Unadjusted model with interaction (n=1382, 50 excluded for missing information on DM) | ||
| Hb 13.5 g/dl (vs 11.3 g/dl) | 1.70 (1.03, 2.81) | 0.040 |
| Previous DM (history or etiology) | 1.61 (1.01, 2.56) | 0.044 |
| Interaction of Hb 13.5 g/dl group*previous DM (history or etiology) | 0.71 (0.39, 1.29) | 0.265 |
| Contrasts | ||
| No previous DM patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 1.70 (1.03, 2.81) | 0.040 |
| Previous DM patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 1.21 (0.88, 1.67) | 0.249 |
| (c) Adjusted multivariate model (n=1382, 50 excluded for missing information on DM) | ||
| Hb 13.5 g/dl (vs 11.3 g/dl) | 1.47 (0.88, 2.45) | 0.143 |
| Baseline albumin (g/dl) | 0.55 (0.41, 0.72) | <0.001 |
| Baseline cholesterol linear splines (per 10 mg/dl) | ||
| <240 mg/dl | 0.96 (0.93, 1.00) | 0.053 |
| ≥240 mg/dl | 1.09 (1.02, 1.15) | 0.006 |
| Age linear splines (per 5 years) | ||
| <55 years | 1.50 (1.09, 2.05) | 0.012 |
| ≥55 to <75 years | 0.96 (0.85, 1.09) | 0.555 |
| ≥75 years | 1.44 (1.19, 1.74) | <0.001 |
| Previous CHF composite (CHF, cardiomyopathy, LVD, or RVD) | 2.97 (2.22, 3.98) | <0.001 |
| Previous CVA or TIA | 1.48 (1.05, 2.10) | 0.028 |
| Previous DVT | 1.99 (1.15, 3.44) | 0.014 |
| History of solid organ malignancy | 0.65 (0.42, 1.02) | 0.059 |
| Previous atrial fibrillation/flutter | 1.77 (1.22, 2.58) | 0.003 |
| Previous DM (history or etiology) | 1.37 (0.85, 2.21) | 0.198 |
| Interaction of Hb 13.5 g/dl group*previous DM (history or etiology) | 0.83 (0.45, 1.53) | 0.559 |
| Contrasts | ||
| No previous DM patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 1.47 (0.88, 2.45) | 0.143 |
| Previous DM patients—Hb 13.5 g/dl (vs Hb 11.3 g/dl) | 1.22 (0.88, 1.69) | 0.225 |
| (d) Adjusted multivariate model (n=1339, 93 subjects excluded for missing information on HF or DM) | ||
| Hb 13.5 g/dl (vs 11.3 g/dl) | 1.96 (1.09, 3.52) | 0.024 |
| Baseline albumin (g/dl) | 0.53 (0.40, 0.71) | <0.001 |
| Baseline cholesterol linear splines (per 10 mg/dl) | ||
| <240 mg/dl | 0.96 (0.92, 0.99) | 0.017 |
| ≥240 mg/dl | 1.10 (1.03, 1.16) | 0.003 |
| Age linear splines (per 5 years) | ||
| <55 years | 1.53 (1.10, 2.12) | 0.011 |
| ≥55 to <75 years | 0.97 (0.85, 1.11) | 0.692 |
| ≥75 years | 1.42 (1.17, 1.72) | <0.001 |
| Previous CHF composite (CHF, cardiomyopathy, LVD, or RVD) | 4.30 (2.76, 6.69) | <0.001 |
| Previous CVA or TIA | 1.42 (1.01, 2.00) | 0.042 |
| Previous DVT | 2.10 (1.22, 3.61) | 0.005 |
| History of solid organ malignancy | 0.65 (0.42, 1.02) | 0.060 |
| Previous atrial fibrillation/flutter | 1.79 (1.24, 2.60) | 0.002 |
| Previous DM (history or etiology) | 1.25 (0.77, 2.03) | 0.367 |
| Interaction of Hb 13.5 g/dl group*previous CHF composite | 0.56 (0.31, 0.98) | 0.043 |
| Interaction of Hb 13.5 g/dl group*previous DM (history or etiology) | 0.90 (0.48, 1.66) | 0.724 |
| Contrasts | ||
| Hb 13.5 g/dl (vs Hb 11.3 g/dl) within the following patient subgroups: | ||
| No previous CHF and no previous DM patients | 1.96 (1.09, 3.52) | 0.024 |
| No previous CHF and previous DM patients | 1.75 (1.08, 2.84) | 0.023 |
| Previous CHF and no previous DM patients | 1.09 (0.61, 1.95) | >0.999 |
| Previous CHF and previous DM patients | 0.97 (0.65, 1.46) | 0.895 |
Because HF is more common in subjects with DM, the potential for confounding of treatment effect within DM subgroups by previous HF was investigated. The model with all possible two-way and three-way interactions of treatment failed to demonstrate a significant interaction among HF, DM, and hemoglobin target (P =0.674, model not shown). Within four subgroups based on the presence or absence of DM or HF the adjusted HR (95% CI; P-value) for hemoglobin target were: (1) no previous CHF and no previous DM: 1.96 (1.09, 3.52; P =0.024), (2) no previous CHF and previous DM: 1.75 (1.08,2.84; P =0.023), (3) previous CHF and no previous DM: 1.09 (0.61, 1.95; P =0.999), and (4) previous CHF and previous DM: 0.97 (0.65, 1.46; P =0.895). This indicates that for subjects without previous HF, treatment effects (HR =1.96 and 1.75) are similar and significant for both patients without and with DM. For subjects with HF, the treatment effects for patients without and with DM again are similar, but not significant.
DISCUSSION
CHOIR was a randomized trial that tested the effect of two different hemoglobin targets among 1423 subjects with CKD and anemia on cardiovascular outcomes. This secondary analysis of the CHOIR trial examines this treatment effect among subjects with and without diabetes mellitus and heart failure. Although an increased risk of death, congestive heart failure hospitalization, myocardial infarction and stroke was shown in the group randomized to the higher arm in the primary analysis, this secondary analysis suggests that the risk was not homogeneous among these subgroups. In unadjusted analyses, no differences in outcomes were seen between high and low hemoglobin target groups for those subjects with either of these two comorbidities. Conversely, the demonstration of increased risk in the higher hemoglobin target group was entirely within those groups without diabetes or HF. In adjusted multivariable analyses, these relationships were maintained among subjects based on the presence or absence of HF. Among those subjects with HF, hemoglobin goal did not appear to affect outcomes.
Although CHOIR and the Normalization of Hematocrit Trial showed either harm or a strong trend toward harm,4,5 the mechanism by which targeting a higher hemoglobin confers this risk is still not clear. A secondary analysis of the CHOIR trial previously published suggested that higher doses of epoetin-alfa are associated with a direct risk supported by the fact that subjects targeted to a higher hemoglobin required more epoetin-alfa.6 This relationship could not be assessed among subgroups defined by DM or HF because of the limited power within each related to sample size. If the relationship between dose and risk were to be consistent among all subgroups, one could hypothesize that the failure to show a risk based on targeting a higher hemoglobin may be related to the balance between competing risks and how that balance differs in the presence of a comorbidity. Clearly, the overall rate of experiencing an end point was greater among subjects with either DM or HF than without. The possibility that the risk imposed by targeting a higher hemoglobin or receiving high doses of epoetin-alfa is not clinically apparent in the setting of that higher baseline risk should be considered and could be the mechanism for the differential associations presented here.
The comparison of study design between CHOIR and these two important trials of anemia correction in patients with either HF (RED-HF) or DM (TREAT) must be considered carefully before generalizing these results. With respect to enrollment criteria, many trials assessing anemia correction in HF require symptomatic heart failure with an ejection fraction below a threshold (for example, 40%) and test either a fixed dose or weight-based dose of ESA to placebo. Although most do not have a target hemoglobin and are phase II trials looking at intermediate outcomes,13–15 the largest of the trials which is ongoing (RED-HF) has a goal hemoglobin of at least 13.0 g/dl in the arm-receiving active therapy and will be assessing morbidity and mortality.16 In this secondary analysis of the CHOIR trial, it should be recognized that the subgroup of subjects with HF were identified based on past medical history questions rather than objective echocardiographic criteria. Furthermore, the clinical question being tested is subtly different between trials. RED-HF is designed to compare active therapy to placebo. In CHOIR, there was no placebo arm with all patients receiving active therapy having been randomized to one of two hemoglobin goals (11.3 vs 13.5 g/dl), and the trials ask and answer two subtly different questions. If RED-HF shows a benefit to ESA treatment as compared with placebo, the results of the analysis presented here would be relevant to designing trials that might test hemoglobin targets.
With respect to DM trials, the comparison of study designs is similarly relevant. TREAT-randomized subjects who are similar in many ways to the subjects enrolled in CHOIR.17 The key design difference is that TREAT enrolled only patients with type II DM. The analysis presented here has the ability to identify patients based on a history of diabetes supporting the comparison. However, in terms of treatment arms, TREAT-randomized patients to receive darbepoetin alfa to achieve and maintain target hemoglobin of 13 g/dl or to the control arm-receiving placebo for hemoglobin levels ≥9 g/dl with rescue therapy with darbepoetin alfa for hemoglobin levels <9 g/dl. If TREAT shows a benefit to the arm-receiving active therapy, combined with the results presented here, this suggests the hypothesis that a goal of 11.3 is either equivalent to or a potentially lower risk than the higher goal tested. At the time of this analysis, however, preliminary results of the ITT analysis of TREAT showed no statistically significant effect of treatment18 qualitatively similar to the univariate analysis presented here.
The major limitations of this analysis include the consideration of the validity of post hoc subgroup analyses as well as the limited power that exists within subgroups in the cohort. The limitations of subgroup analyses particularly with respect to chance findings have been widely discussed.19–21 These limitations are relevant considerations in the interpretation of the subgroup analyses presented here. Although chance findings may result in a significant result among subgroups where no treatment effect was seen in the overall cohort, the subgroups without HF or DM had a treatment effect similar in direction to the overall cohort. The relevant consideration here should therefore be focused on the lack of treatment effect among those patients with HF or DM. The interpretation of these findings must be considered in the context of the decreased power afforded within each subgroup. The potential for a type II error because of the decreased power within these subgroups particularly among the analyses based on the presence or absence of DM must be considered. In addition, given that subjects were identified as having HF based on their self-reported history or documentation in their medical record, the potential for misclassification is present. Although misclassification generally biases against finding a difference between groups, this limitation should be considered.
This secondary analysis of the CHOIR trial looks at the effect of treating anemia in CKD to two different treatment goals based on clinical subgroups defined by HF and DM. These results suggest that the presence of these comorbidities attenuates the risk seen in the group randomized to the higher hemoglobin goal. These results will either serve to validate the important findings of RED-HF and TREAT or will serve as an example of the need for careful interpretation of subgroup analyses of completed trials. Although the mechanism for this differential treatment effect might be due to the stronger effect of the comorbidity on outcome (that is, competing risk), this is not clear. However, it does allow a continued focus as to the subgroups of patients in whom the risk associated with higher treatment goal and potentially higher ESA dose is potentially concentrated or conversely not statistically or clinically apparent. Although these results do not support the correction of hemoglobin to 13 gm/dl in these subgroups, they allow us to clinically hone in on strategies to safely maximize the exercise tolerance of those with comorbidities and minimize the potential detrimental effects on those without comorbidities.
MATERIALS AND METHODS
Description of data set
CHOIR was a randomized trial comparing the effect of treatment with epoetin-alfa to one of two hemoglobin targets on the composite end point of death, hospitalization for congestive heart failure, stroke, and myocardial infarction in CKD patients with anemia. Methods, baseline characteristics, and results of CHOIR have been reported.5 Inclusion criteria were hemoglobin <11.0 g/dl and MDRD glomerular filtration rate (GFR) of 15–50 ml/min per 1.73 m2.
Definition of variables
A past medical history of the following diseases or conditions was defined as the presence of one or more of the variables in the case report form. Coronary artery disease was defined as the presence of past myocardial infarction, angina at baseline, or previous coronary artery bypass grafting or percutaneous coronary intervention. Heart failure (HF) was defined as a history of congestive heart failure, cardiomyopathy, left ventricular dysfunction, or right ventricular dysfunction reported by the subject or in the medical record. Diabetes mellitus (DM) was defined as either the presence of a history of or etiology of renal failure by type 1 or 2 DM. Cerebrovascular disease was defined as a previous history of either a stroke or transient ischemic attack. Thromboembolic disease was defined as a previous history of pulmonary embolism, arterial or deep venous thrombosis, or a hypercoaguable state. Peripheral vascular disease was defined as the presence of either peripheral vascular disease or lower extremity amputation. A single composite variable reflecting the presence of either malnutrition or inflammation was defined as a baseline serum albumin≤3.6 g/dl or ferritin >600 ng/ml.
Analyses
The primary goal of this analysis was to identify differences in the primary composite outcome between randomization arms within clinically relevant subgroups defined by the presence or absence of heart failure (HF) and diabetes mellitus (DM). Descriptive statistics were examined based on Kaplan–Meier survival curves for subjects randomized to each treatment arm within the subgroups of interest and the unadjusted treatment effect was obtained based on the Wald test from Cox proportional hazard models containing the main effects of treatment and HF (or DM) and the interaction of HF (or DM) with treatment.
To estimate the adjusted treatment effects within the subgroups, Cox proportional hazards regressions with the backward selection process at a stay level of 0.05 was used to select baseline variables for adjustment. The bootstrap method was used in combination with the backwards Cox proportional hazard regression analysis to select the final set of baseline variables included in the adjusted model. In the bootstrap procedure, 200 samples of 80% of the 1432 patients were sampled at random with replacement. A Cox proportional hazards regression with the stepwise selection process at an entry level of 0.10 and a stay level of 0.05 was applied to every bootstrap sample. If the variable occurred in at least 50% of the bootstrap models, the variable was judged to be reliable and was included in the adjusted model.22 Candidate variables for the model included baseline laboratory measurements (eGFR, albumin, total cholesterol, ferritin, transferrin saturation (TSAT), hemoglobin, protein/creatinine ratio, and the composite indicating the presence of inflammation or malnutrition defined as albumin ≤3.6 or ferritin>600) and demographic and clinical measurements (age, gender, race, ethnicity, coronary artery disease, HF, DM, cerebrovascular disease, thromboembolic disease, hypertension, peripheral vascular disease, any malignancy, cigarette smoking and previous atrial fibrillation). The treatment variable was always included in the model selection process. For continuous variables whose effect is not linear, piecewise linear splines with cut points based on minimizing the −2 log likelihood were used. Multiple imputation method was used to impute all missing data so that the model development was based on all original 1432 patients. Specifically, missing data were imputed via Markov Chain Monte Carlo method that draws simulation from a Bayesian predictive distribution based on the demographic, medical history, previous medication and baseline lab data. For binary variables where the randomly drawn values are ≥0.5, the imputed values were set to 1 and 0 otherwise. For continuous variables, in which the randomly drawn values are less than (or greater than) the minimum (or maximum) of the observed values for the variables, the imputed values were set to the minimum (or maximum) of the observed values. Five imputed data sets were generated using SAS procedure MI. The s.e. for the parameter estimates were obtained by using the SAS procedure MIANALYZE to account for uncertainty because of imputation. The variables selected in this process are used for the adjustment described below.
The selected variables in the final model were used to obtain the adjusted treatment effect within subgroups defined by the absence or presence of HF or DM based on models including the interaction of HF with treatment or the interaction of DM with treatment. Specifically, to evaluate the treatment effect within subgroups defined by HF, subjects without missing baseline HF were used for the models. The unadjusted model includes the main effects of HF and treatment and the interaction of HF and treatment. The adjusted model contains these factors as well as the main effects of all selected variables for adjustment. The subgroup comparisons were carried out by forming contrasts in the models to estimate the treatment effect within the subgroups of presence or absence of HF. Similarly, the treatment effect within subgroups defined by the absence or presence of DM was investigated based on subjects without missing baseline DM in an unadjusted model with the main effects of DM and treatment and the interaction of DM and treatment as well as an adjusted model containing these factors plus the selected variables. Because HF is more common in subjects with DM, the potential for confounding of treatment effects by HF within DM subgroups was investigated. The treatment effects within four subgroups: no prior HF and no prior DM, no prior HF and prior DM, prior HF and no prior DM, prior HF and prior DM were examined through all possible two-way and three-way interactions of treatment, HF and DM in the fully adjusted model. Multiple imputation method described above was used to deal with missing data in the selected variables for adjustment. Analyses were also performed on data sets without imputation providing similar results (data not shown). All statistical analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC, USA). A P-value ≤0.05 was considered as statistically significant.
Acknowledgments
The original CHOIR trial was supported by Ortho Biotech Clinical Affairs and Johnson & Johnson Pharmaceutical Research and Development, both subsidiaries of Johnson & Johnson.
This analysis was supported by a grant from the National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK080094-01A1).
Footnotes
DISCLOSURE
Dr Szczech reports receiving consulting fees from Ortho Biotech Clinical Affairs, Nabi Pharmaceuticals, Gilead, Fresenius Medical Care, Kureha, Affymax, and Acologix; lecture fees from Nabi Biopharmaceuticals, Fresenius Medical Care, GlaxoSmithKline, Gilead, Genzyme, Abbott, Amgen, and Ortho Biotech; and grant support from GlaxoSmithKline, Pfizer, and Genzyme. Dr Barnhart reports receiving consulting fees and grant support from Ortho Biotech Clinical Affairs. Dr Inrig reports support by NIH Grant 1KL2 RR024127. She has also received investigator initiatied research support from Genzyme. Dr Reddan reports receiving consulting fees from Ortho Biotech Clinical Affairs and Shire Pharmaceuticals; lecture fees from Amgen, Novartis, Pfizer, AstraZeneca, and General Electric; and grant support from Ortho Biotech Clinical Affairs, Amgen, and Novartis. Ms Sapp has no disclosures. Dr Patel has received research/grant support from Abbott Laboratories and Merck & Co. Dr Singh reports receiving consulting fees and lecture fees from Johnson & Johnson and Watson; and receiving grant support from Ortho Biotech Clinical Affairs, Johnson & Johnson, Amgen, and Watson. Dr Hernandez reports receiving research grants from GlaxoSmithKline, Johnson & Johnson (Scios), Medtronic, Merck, Roche Diagnostics, and serving on the speaker’s bureau or receiving honoraria within the past 5 years from AstraZeneca, Medtronic, Novartis, Sanofi-Aventis, and Thoratec Corporation. Dr Hernandez has made available online detailed listings of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp). Dr Felker reports receiving research grants from the NHLBI, Amgen, Cytokinetics, Corthera, and Roche Diagnostics. He also reports consulting for Corthera, Amgen, XDX, and Cytokinetics. Dr Califf reports receiving research support from Novartis and Schering Plough. He receives lecture support from the HEART.ORG, Novartis, and Kowa Research. He is a consultant for Annenberg, Heart.org, Kowa Research Institute, NITROX LLC, Novartis Pharmaceutical, and Schering Plough and holds equity interest in NITROX LLC.
References
- 1.Ma JZ, Ebben J, Xia H, et al. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol. 1999;10:610–619. doi: 10.1681/ASN.V103610. [DOI] [PubMed] [Google Scholar]
- 2.Xia H, Ebben J, Ma JZ, et al. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol. 1999;10:1309–1316. doi: 10.1681/ASN.V1061309. [DOI] [PubMed] [Google Scholar]
- 3.McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510. doi: 10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 4.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. New Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 5.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 6.Szczech LA, Barnhart HX, Inrig JK, et al. Epoetin-alfa dose and achieved hemoglobin are associated with outcomes among patients with anemia and chronic kidney disease: A secondary analysis of the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) Trial. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associatedwith worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk In Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 9.Szachniewicz J, Petruk-Kowalczyk J, Majda J, et al. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol. 2003;90:303–308. doi: 10.1016/s0167-5273(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 10.Arant CB, Wessel TR, Olson MB, et al. Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: results from the National Heart, Lung, and Blood Institute Women’s Ischemia Syndrome Evaluation Study. J Am Coll Cardiol. 2004;43:2009–2014. doi: 10.1016/j.jacc.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Tang YD, Katz SD. The prevalence of anemia in chronic heart failure and its impact on the clinical outcomes. Heart Fail Rev. 2008;13:387–392. doi: 10.1007/s10741-008-9089-7. [DOI] [PubMed] [Google Scholar]
- 12.NHLBI Working Group. [Accessed March 30, 2009];Cardio-Renal Connections in Heart Failure and Cardiovascular Disease. 2004 August 20; Available at: http://www.nhlbi.nih.gov/meetings/workshops/cardiorenal-hf-hd.htm.
- 13.Ghali JK, Anand IS, Abraham WT, et al. on behalf of the Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group Randomized Double-Blind Trial of Darbepoetin Alfa in Patients With Symptomatic Heart Failure and Anemia. Circulation. 2008;117:526–535. doi: 10.1161/CIRCULATIONAHA.107.698514. [DOI] [PubMed] [Google Scholar]
- 14.Veldhuisen DJ, Dickstein K, Cohen-Solal A, et al. Randomized, double-blind, placebo-controlled study to evaluate the effect of two dosing regimens of darbepoetin alfa in patients with heart failure and anaemia. Euro Heart J. 2007;28:2208–2216. doi: 10.1093/eurheartj/ehm328. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Anker SD, Szachniewicz J, et al. Effect of Darbepoetin Alfa on Exercise Tolerance in Anemic Patients With Symptomatic Chronic Heart Failure: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am College Cardiol. 49:753–762. doi: 10.1016/j.jacc.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed on April 20, 2009];Clinical Trials identifier NCT00358215. http://www.clinicaltrials.gov/ct2/show/NCT00358215?term=red-hf&rank=2.
- 17.Mix TC, Brenner RM, Cooper ME, et al. Rationale—Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): Evolving the management of cardiovascular risk in patients with chronic kidney disease. Am Heart J. 2005;149:408–413. doi: 10.1016/j.ahj.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 18.http://wwwext.amgen.com/media/media_pr_detail.jsp?year=2009&releaseID=1324296 (accessed September 10, 2009).
- 19.Assman SF, Pocock SJ, Enos LE, et al. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 20.Pocock SJ, Hughes MD, Lee RJ. Statistical problems in the reporting of clinical trials: a survey of three medical journals. New Eng J Med. 1987;317:426–432. doi: 10.1056/NEJM198708133170706. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Wittes J, Probstfield J, et al. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991;266:93–98. [PubMed] [Google Scholar]
- 22.Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med. 1992;11:2093–2109. doi: 10.1002/sim.4780111607. [DOI] [PubMed] [Google Scholar]