Abstract
The aim of the present study was to investigate the potential of a phospholipid-based microemulsion formulation for parenteral delivery of anticancer drug, etoposide. The microemulsion area was identified by constructing pseudoternary phase diagrams. The prepared microemulsions were subjected to different thermodynamic stability tests. The microemulsion formulations that passed thermodynamic stability tests were characterized for optical birefringence, droplet size, viscosity measurement, and pH measurements. To assess the safety of the formulations for parenteral delivery, the formulation was subjected to compatibility studies with various intravenous infusions and in vitro erythrocyte toxicity study. The developed formulation was found to be robust and safe for parenteral delivery.
Key words: etoposide, microemulsion, parenteral, phospholipid
INTRODUCTION
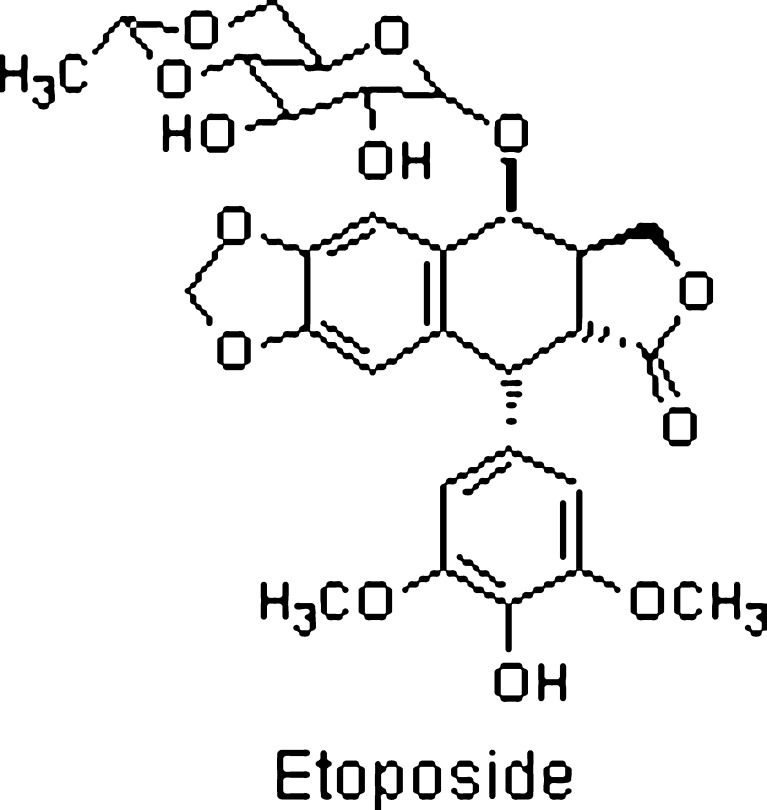
Etoposide (Fig. 1), an epipodophyllotoxin, is an anticancer drug useful for the treatment of small cell lung cancer and testicular carcinoma. Prior to administration, the drug has to be diluted in the infusion fluid; its low aqueous solubility thus acts as a constraint in the formulation of its parenteral dosage form. This attribute results in drug precipitation in the infusion fluid thereby proving detrimental to the health owing to the possibility of capillary blockade. The currently marketed formulation contains 20 mg/ml etoposide in nonaqueous vehicle including 2 mg citric acid, 30 mg benzyl alcohol, 80 mg polysorbate 80, 650 mg polyethylene glycol 300, and 30.5% (v/v) alcohol (1). The components of this formulation have been associated with serious side effects such as pain, inflammation, tissue damage, necrosis at the site of injection, and substantial hemolysis (2). Furthermore, the risk of precipitation as microcrystals encountered on the dilution of the organic solvents phase with consequent venous sequelae and too rapid an infusion of etoposide precipitates hypotension of the patient (3). Hence, an etoposide preparation compatible with the infusion fluids would serve as an attractive alternative to the marketed system (Fig. 1).
Fig. 1.
Chemical structure of etoposide
Recently, much attention has been paid to the application of microemulsions as drug delivery systems, since microemulsions are thermodynamically stable and are formed spontaneously by simple mixing of the various components (4–9).
Thus, the focus of the present investigation was to improve the solubility of etoposide, a poorly water-soluble drug without chemical modification and by using microemulsion drug delivery system that is suitable for parenteral administration and stable after dilution with commonly used infusion fluids.
MATERIALS AND METHODS
Materials
Etoposide was a gift from Cadila Pharmaceuticals, India, Epikuron®135 F (fluid phosphatidyl choline enriched lecithin) was a kind gift from Degussa, Capmul® MCM was a kind gift from Abitec Corp. U.S.A., Tween® 80 and Polyethylene Glycol 400 (PEG 400) were purchased from s.d. Fine chemicals. All other chemicals were of AR-grade and purchased from Merck.
Solubility Studies
The fixed amount of surfactant/oil was weighed accurately and transferred to a small test tube. In this test tube, the drug was added to assess the equilibrium solubility of the drug at ambient condition of 25 to 27°C. Thereafter, the amount of surfactant/oil required to solubilize the drug was determined. Table I depicts the solubility data compounded for etoposide in various oils, surfactants, and solubilizers.
Table I.
Solubility Data of Etoposide in Oils/Surfactants/Solubilizers
| Oils/surfactants/solubilizers | Solubility (mg/mL) |
|---|---|
| Miglyol-812 | 4 |
| Soyabean oil | 1.5 |
| Capmul MCM | 10 |
| Epikuron 135 F | 5.5 |
| PEG-400 | 110 |
| Tween-80 | 35 |
| Tween-20 | 30 |
| Cremophor EL | 23 |
Preparation of Microemulsion
Formulation Methodology
Etoposide was solubilized in PEG 400 by vortexing. Thereafter, oil was mixed, followed by surfactant and phospholipid. The resultant solution was mixed uniformly by vortexing. Then, the fixed amount of water was added and gently mixed to obtain a microemulsion. The resultant microemulsion was sterilized by autoclaving at 121°C and 15 psi for 15 min.
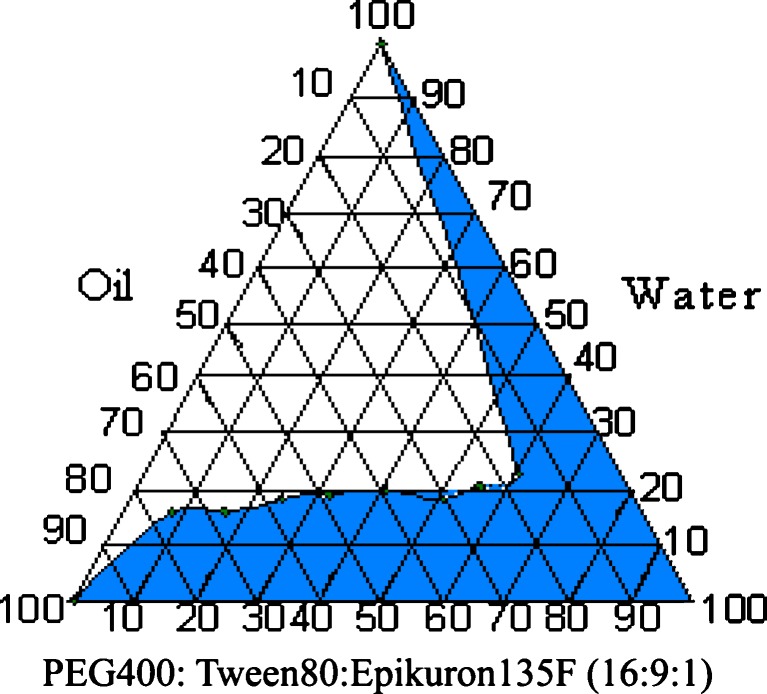
Preparation of Pseudoternary Phase Diagram
The pseudo-ternary phase diagrams of oil, surfactant:cosurfactant [PEG 400: Tween®80: Epikuron®135F (16:9:1)], and water were constructed prior to optimization of the formulation. Surfactant was mixed with cosurfactant in fixed weight ratios. Aliquots of each surfactant–cosurfactant mixture were then mixed with oil and finally with aqueous phase. Mixtures were gently shaken or mixed by vortexing and kept at ambient temperature (25°C) to attain equilibrium. The equilibrated samples were assessed visually for their appearance, i.e., it was noted as being clear and transparent microemulsions, or crude emulsions or gels.
Characterization of Microemulsion
Optical Birefringence
The microemulsion was placed between two polarizing plates and then observed for light transmittance. After this, one of the plates was rotated relative to the other through 90° (crossed polarizer) and then examined.
Determination of Particle Size
Photon correlation spectroscopy using laser light scattering is frequently used to determine particle size of emulsions. A Beckman N4 Plus submicron Particle Size Analyzer was employed to monitor particle size of the microemulsion. The instrument calculated the mean particle size and polydispersity from intensity, assuming spherical particles. Polydispersity is a measure of particle homogeneity and it varies from 0.0 to 1.0. The closer to zero the polydispersity value, the more homogeneous are the particles. Prior to analysis, samples were diluted fivefold using 0.22 µ filtered double distilled water. Light scattering was monitored at 90° scattering angle and temperature of 25°C or 37°C. All measurements were performed in triplicate.
Accelerated Stability Testing
Centrifugation
Centrifugal methods have been employed to subject the system to assess accelerated stability. The microemulsion was subjected to centrifugation at 5,175×g for 30 min and observed for any separation, i.e., incompatibility (8).
Freeze–Thaw Cycling
A 5-ml microemulsion was subjected for six heating/cooling cycles between 40°C and −4°C with storage at each temperature for not less than 24 h and assessed for their physical instability like phase separation and precipitation (8).
Viscosity Measurements
The viscosity of the formulation was ascertained by an Oswald-type viscometer (Techniko 841, D). The viscometer tube was filled with the exact amount of the formulation. The meniscus of the liquid in capillary tube was adjusted to the level of the top graduation mark with the aid of vacuum. The time in seconds was recorded for liquid to flow from the upper mark to the lower mark in the capillary tube. The time required for water was also recorded. The procedure was repeated three times and the average was taken for calculation.
pH Measurement
The acceptable range is pH 2–12 for intravenous and intramuscular administration whereas for subcutaneous administration, the range is reduced to pH 2.7–9.0 as the rate of in vivo dilution is significantly reduced resulting in more potential for irritation at the injection site.
The pH of formulation was measured using by Systronic Digital pH meter 335, standardized using pH 4.0 and 7.0 standard buffers use.
Compatibility Assessment with Different Injectable Diluents
The dilutability and compatibility of developed formulations with the 0.9% sodium chloride injection or 5% dextrose injection was assessed by diluting the developed microemulsion formulation in different concentration ranges (0.2–1 mg/ml) with 0.9% sodium chloride injection and 5% dextrose injection and kept them for visual inspection with respect to phase separation or precipitation for a period of 3 h.
In Vitro Erythrocyte Toxicity Study
The erythrocyte toxicity assay was conducted as described by Bock et al. (10). Fresh blood was collected in the vial containing EDTA (anticoagulant). Red blood cells (RBCs) were isolated by centrifugation (5,000 rpm for 5 min) and the RBCs were washed three times with isotonic phosphate buffer pH 7.4 before diluting with buffer to prepare erythrocyte stock dispersion (three parts of centrifuged erythrocytes plus 11 parts buffer). The buffer consisted of Na2PO4·10H2O (7.95 g), KH2PO4 (0.76 g), NaCl (7.2 g), and distilled water (add 1,000 ml). The washing step was repeated in order to remove debris and serum protein. A 100-μl aliquot stock dispersion was added per milliliter of test sample. The resulting solution was incubated at 37°C for a period of 1 h. After incubation under shaking, debris and intact erythrocytes were removed by centrifugation. One hundred milliliters of resulting supernatant was added to 2 ml of an ethanol/HCl mixture [(39 parts ethanol (99% v/v) + 1 part of HCl (37% w/v)]. This mixture dissolved all components and avoided the precipitation of hemoglobin. The absorbance of the mixture was determined at 398 nm by spectrometer monitoring against a blank sample. Control sample of 100% lysis (in TritonX 100) was employed in the experiment. The percentage of hemolysis caused by the test sample was calculated by following equation:
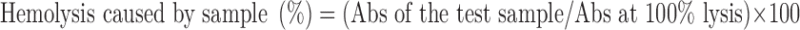 |
Test for Sterility
Test for sterility was performed by using nutrient broth medium and incubated at 37°C for 7 days. For the study, groups including developed microemulsion, negative control medium, and positive control medium incubated with Bacillus subtilis were evaluated.
RESULTS AND DISCUSSION
Solubility Studies
Parenteral administration of microemulsion requires the component such oil, surfactant(s) and co surfactant(s) to be biocompatible and safe. In this regards, nonionic or zwitterionic surfactants have found to favorable for pharmaceutical applications since they are less toxic and less affected by changes in pH and ionic strength (2).
Moreover, for a lipophilic drug, the principle objective is to achieve a formulation where the drug is dissolved in the liquid vehicle. By selecting the optimum liquid vehicle composition, it is possible to minimize or eliminate precipitation of the drug. As shown in Table I, among the limited choice of excipients, Capmul® MCM was selected as the oil component. However, as the dose of the drug was 20 mg, the oil component was insufficient to solubilize the drug, hence Tween®80 and PEG 400 was selected as surfactant and solubilizer components, respectively. In the process of formulation, it was observed that drug precipitated in most of the microemulsion formed by varying the combinations of aforementioned excipients. In order to form a stable microemulsion, complex interfacial film of mixture of surfactants is favorable and therefore an amphiphilic surfactant, Epikuron®135 F (a transparent, liquid fraction of soybean lecithin and soybean oil with enriched phosphatidylcholine content), having miscibility with oil, was chosen.
Preparation of Pseudoternary Phase Diagram
Phase studies were done to investigate the effect of surfactant to cosurfactant ratio on the extent of stable o/w microemulsion region. The microemulsions in the present study formed spontaneously at ambient temperature when their components were brought into contact. The areas of microemulsion and isotropic regions increased with increasing ratio of surfactant: cosurfactant (Fig. 2). It indicates that the maximum proportions of oil incorporated in microemulsions increased significantly with increasing ratio of surfactant and cosurfactant to oil. It is recommended that etoposide be administered by slow intravenous infusion over a period of 30 to 60 min to prevent hypotension. Hence, using a constructed phase diagram, the optimum ratios of the components for microemulsion which would remain stable and prevent drug precipitation over infinite dilution was selected (Table II).
Fig. 2.
Pseudoternary phase diagram
Table II.
Composition of Parenteral Microemulsion of Etoposide
| Component | mg/mL | % w/v |
|---|---|---|
| Drug | 20 | 2 |
| PEG-400 | 400 | 40 |
| Capmul MCM | 50 | 5 |
| Epikuron 135 F | 25 | 2.5 |
| Tween-80 | 225 | 22.5 |
| Water for injection | q. s. to 1 ml | 33 |
Characterization of Microemulsion
Optical Birefringence
Birefringence (6) is a light-scattering phenomenon. It is also called as double refraction and is found in liquid crystals and anisotropic systems. In birefringence, the light passing through a material is divided into two components with different velocities and hence different refractive indices. Thus, when a liquid crystal is observed between crossed polarizer, intense bands of colors are seen which is known as birefringence. In contrast, microemulsion appears completely black.
The developed microemulsion appeared completely dark when observed between cross-polarizing plates validating that the formulation was an isotropic, colloidal dispersion.
Determination of Particle Size
It is known that the particle size is distribution one of the most important characteristics emulsion for the evaluation of its stability and also in vivo fate of emulsion. Furthermore, it is also well documented that size plays a pivotal role in the circulation time of the particulate carriers. Some literature states, particulate carriers with smaller size can evade recognition by MPS; consequently have longer circulation time in the bloodstream. In addition, smaller size eludes capillary blockade resulting in attenuation of adverse effects often associated with intravenous administration of particulate carriers. Therefore, the particle size of microemulsion was assessed in triplicates. Table III depicts the particle size before and after autoclaving. There was marginal increase in particle size of the microemulsion globule; however, it was lower than 100 nm.
Table III.
Globule Size and Polydispersity Index of Developed Formulation
| Samples | Particle size (nm) | Polydispersity index (PI) |
|---|---|---|
| Formulation before autoclaving | 58.2 | 0.912 |
| Formulation after autoclaving | 68.0 | 0.978 |
Accelerated Stability Testing
Centrifugation
Centrifugal methods (8) have long been employed by emulsion technologists to induce and accelerate instability by gravitational means. It is commonly accepted that shelf life under normal storage conditions can be predicted rapidly by observing the separation of disperse phase when the microemulsion is subjected to centrifugation.
The technique to determine behavior of small particles in the gravitational force, i.e., their separation rates, is quite simple and inexpensive providing a rapid fool-proof identification of the systems as microemulsions. Brownian movement is associated with particles smaller than 0.5 µm. The microparticles in this size range are small enough to absorb kinetic energy from bombardment by the molecules of dispersion medium. It has been calculated that it causes such a particle to change direction 1024 times per second. This keeps the dispersed droplets in a state of violent motion preventing their settling under gravitational field. So long as they do not coalesce, it is Brownian movement that keeps the droplets of microemulsion droplets from settling or creaming. The reason that microemulsion droplets do not coalesce is due to surface free energy of a microemulsion system. Just as soon as two droplets coalesce to form a single droplet of larger size, the interfacial tension of the new droplet becomes negative, i.e., the system has negative free-surface energy. The larger droplet now spontaneously increases its curvature to effect zero interfacial tension again and two droplets of the original size result. This process appears continuously as does the bombardment of droplets by molecules of dispersion medium. It is this dynamic equilibrium that keeps the microemulsion systems stable.
At the end of 30 min, the developed microemulsion showed absence of phase separation and drug precipitation after centrifugation at 3,000 rpm, verifying the stability of the formulation.
Freeze–Thaw Cycling
This test induces stress in the microemulsion (8). At temperature below freezing, the formation of ice crystals in an o/w type microemulsion may cause oil particles to elongate and flatten. In addition, the lipophilic portion of the emulsifier molecule will lose their mobility while the hydrophilic portions are simultaneously “dehydrated” due to the freezing action of water. As the sample is thawed, water is released and travels rapidly through the microemulsion. If the system can “heal” itself before coalescence occurs, then the microemulsion survives the test. However, if the rate of redissolution of the ingredients is slow, instability may occur in case of microemulsion which is not related to normal temperature processes.
The developed formulation did not show any evidence of instability, the physical integrity of the formulation was maintained at the end of the cycle.
Viscosity Measurements
The stability of the microemulsion is often governed by its viscosity, i.e., is an expression of the resistance to flow. The viscosity defines the tendency of the system to agglomerate. Moreover, for the developed microemulsion, the viscosity measurement was of utmost importance, since it had to be diluted using infusion fluids prior to administration. It is well known that the viscosity of parenteral formulation may also affect the syringeability. Using the following equation, the viscosity of the developed formulated was calculated to be 106.92 cP. The viscosity was calculated from the equation:
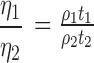 |
where η1 and η2 are the viscosities of the test and the standard liquids, ρ1 and ρ2 are the densities of the liquids, and t1 and t2 are the respective flow times in seconds.
The low viscosity of the developed microemulsion ensures ease of syringeability as well as ease of mixing with intravenous fluids with minimum mechanical agitation.
pH Measurement
The pH of formulation measured regularly over a period of 10 days showed that the pH of the formulation was in an acceptable range for intravenous administration. For etoposide, pH plays a pivotal role in the drug stability. The pH for stability of etoposide was found to be pH 5.4 which is considered optimum to prevent the degradation of drug (11). Due to constant pH, the drug content (Table IV) was found to be in acceptable limits over a period of 3 months.
Table IV.
Drug Content Stability Data
| Conditions | % Drug content | ||||
|---|---|---|---|---|---|
| Initial | 7 days | 1 month | 2 months | 3 months | |
| RT | 102.05 ± 0.66 | 99.93 ± 0.32 | 97.60 ± 0.73 | 99.31 ± 0.84 | 97.20 ± 0.91 |
| 30°C/60%RH | – | 99.04 ± 0.41 | 98.70 ± 0.33 | 99.37 ± 0.96 | 100.88 ± 1.34 |
| 40°C/75%RH | – | 103.74 ± 0.29 | 101.34 ± 0.51 | 98.32 ± 0.88 | 102.86 ± 0.73 |
RT room temperature, RH relative humidity
Compatibility Assessment with Different Injectable Diluents
As prescribed, etoposide for injection needs to be diluted for administration by intravenous infusion in either 5% dextrose injection or 0.9% sodium chloride injection to produce a solution containing 200 to 400 µg (0.2 to 0.4 mg) of etoposide per milliliter. This is essential to prevent hypotension, a side effect of the drug. As shown in Tables V and VI, the diluted solution was stable for sufficient time enabling slow intravenous infusion of etoposide in concentration range up to 1 mg/ml for 1.5 and 2 h in 0.9% sodium chloride injection and 5% dextrose injection, respectively. However, it should be noted that at the recommended concentration of 0.4 mg/ ml, there was absence of drug precipitation for 3 h in 0.9% sodium chloride injection and 5% dextrose injection, respectively. This clearly reflects the superiority of the developed formulation over the existing etoposide injection which reports drug precipitation as its limitations.
Table V.
Compatibility of Microemulsion with 0.9% Sodium Chloride Injection
| Conc. of drug mg/mL | Time in hours | |||||
|---|---|---|---|---|---|---|
| 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | |
| 0.2 | C | C | C | C | C | C |
| 0.4 | C | C | C | C | C | C |
| 0.6 | C | C | C | C | C | C |
| 0.8 | C | C | C | C | C | P |
| 1 | C | C | C | P | – | – |
C clear, P precipitation
Table VI.
Compatibility of Microemulsion with 5% Dextrose Injection
| Conc. of drug mg/mL | Time in hours | |||||
|---|---|---|---|---|---|---|
| 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | |
| 0.2 | C | C | C | C | C | C |
| 0.4 | C | C | C | C | C | C |
| 0.6 | C | C | C | C | C | C |
| 0.8 | C | C | C | C | C | P |
| 1 | C | C | C | C | P | P |
C clear, P precipitation
In Vitro Erythrocyte Toxicity Study
Colloidal drug carrier systems serve to minimize the side effects of drugs used for parenteral applications. Side effects often result from the destruction of corpuscles of blood or tissue cells at the site of injection and these may be reduced by incorporating the drug in the colloidal carriers (e.g., microemulsions). To corroborate this statement, the hemolytic activity was done for estimating the membrane damage caused by formulation in vivo. Since phospholipid and PEG-400 are known for their relative non-toxic nature, they were not considered for this study (2). However, the samples tested for erythrocyte toxicity; developed microemulsion, Capmul MCM andTween-80 showed considerably less hemolytic activity (Table VII). This study indicated the safety of the developed microemulsion for parenteral administration.
Table VII.
Comparative Hemolysis after 1 Hour Incubation Period
| Component | % Hemolysis after 1 hour of incubation |
|---|---|
| Capmul MCM | 0.16 |
| Tween 80 | 0.05 |
| Microemulsion | 0.15 |
| TritonX 100 | 6.69 |
Test for Sterility
Sterility is one of the prerequisites for the parenteral preparations. However, at extreme temperatures, phase separation may occur but the microemulsions spontaneously return to their initial state when brought back to normal temperature and on adequate mixing. As it is established, microemulsions can be sterilized by autoclaving (12); the developed microemulsion was sterilized by autoclaving at 121°C and 15 psi for 15 min. Although there was phase separation after autoclaving, after shaking it gave a homogeneous microemulsion. The sterility testing of this sterile microemulsion showed absence of microbial growth indicating the effectiveness of autoclaving. Furthermore, this was attested by the stability of the developed microemulsion over a period of 3 months (Table IV).
CONCLUSION
The parenteral phospholipid-based microemulsion was successfully developed with particle size less than 100 nm. The developed formulation was amenable to sterilization by autoclaving and was found to be robust to dilution with intravenous fluids. The in vitro erythrocyte toxicity study demonstrated the safety and acceptability of the formulation for parenteral administration.
Acknowledgments
The authors are grateful to the University Grant Commission (New Delhi, India) for providing financial support for this project. The authors are also thankful to Degussa and Abitec Corp. USA for providing the gift samples of oils, surfactants, and cosurfactants.
References
- 1.(2000) Physicians desk reference, 54th edition. Medical Economics Company, Inc. Montvale, New Jersey, pp. 888–889
- 2.Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201–230. doi: 10.1023/B:PHAM.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 3.Darwish IA, Florence AT, Saleh AM. Effects of hydrotropic agents on the solubility, precipitation, and protein binding of etoposide. J Pharm Sci. 1989;78:577–581. doi: 10.1002/jps.2600780714. [DOI] [PubMed] [Google Scholar]
- 4.Date AA, Nagarsenker MS. Parenteral microemulsions: an overview. Int J Pharm. 2008;355:19–30. doi: 10.1016/j.ijpharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Rowe RC, Sheskey PJ, Weller PJ (eds) (2003) Handbook of pharmaceutical excipients, 4th edition. Pharmaceutical Press, London/American Pharmaceutical Association, Washington
- 6.Panaggio A, Rhodes CT, Worthen LR. The possible use of autoclaving microemulsion for sterilization. Drug Dev Ind Pharm. 1979;5:169–173. doi: 10.3109/03639047909055670. [DOI] [Google Scholar]
- 7.Prince LM. In: Microemulsions: theory and practice. Prince LM, editor. London: Academic; 1977. pp. 1–20. [Google Scholar]
- 8.Attwood D. Microemulsions. In: Kreuter J, editor. Colloidal drug delivery systems, vol. 66. New York: Marcel Dekker; 1994. pp. 31–71. [Google Scholar]
- 9.Block LH. Pharmaceutical emulsions and microemulsions. In: Lieberman HA, Rieger MM, editors. Pharmaceutical dosage forms: disperse systems, vol. 2. New York: Marcel Dekker; 1996. pp. 47–109. [Google Scholar]
- 10.Bock TK, Muller BW. A novel assay to determine the hemolytic activity of drugs incorporated in colloidal carriers systems. Pharm Res. 1994;11:589–591. doi: 10.1023/A:1018987120738. [DOI] [PubMed] [Google Scholar]
- 11.Tian L, He H, Tang X. Stability and degradation kinetics of etoposide-loaded parenteral lipid emulsion. J Pharm Sci. 2007;96(7):1719–1728. doi: 10.1002/jps.20830. [DOI] [PubMed] [Google Scholar]
- 12.Panaggio A, Rhodes CT, Worthen LR. The possible use of autoclaving microemulsion for sterilization. Drug Dev Ind Pharm. 1979;5:169–173. doi: 10.3109/03639047909055670. [DOI] [Google Scholar]