Abstract
Lactate esters are widely used as food additives, perfume materials, medicine additives, and personal care products. The objective of this work was to investigate the effect of a series of lactate esters as penetration enhancers on the in vitro skin permeation of four drugs with different physicochemical properties, including ibuprofen, salicylic acid, dexamethasone and 5-fluorouracil. The saturated donor solutions of the evaluated drugs in propylene glycol were used in order to keep a constant driving force with maximum thermodynamic activity. The permeability coefficient (Kp), skin concentration of drugs (SC), and lag time (T), as well as the enhancement ratios for Kp and SC were recorded. All results indicated that lactate esters can exert a significant influence on the transdermal delivery of the model drugs and there is a structure-activity relationship between the tested lactate esters and their enhancement effects. The results also suggested that the lactate esters with the chain length of fatty alcohol moieties of 10–12 are more effective enhancers. Furthermore, the enhancement effect of lactate esters increases with a decrease of the drug lipophilicity, which suggests that they may be more efficient at enhancing the penetration of hydrophilic drugs than lipophilic drugs. The influence of the concentration of lactate esters was evaluated and the optimal concentration is in the range of 5∼10 wt.%. In sum, lactate esters as a penetration enhancer for some drugs are of interest for transdermal administration when the safety of penetration enhancers is a prime consideration.
Key words: lactate esters, penetration enhancer, percutaneous permeation, rat skin, transdermal drug delivery system
INTRODUCTION
Skin is the largest organ in the body and an obvious route for both local and systemic drug delivery. Transdermal drug delivery system (TDDS) possesses many advantages over the conventional oral route, such as circumvention of the first-pass effect, provision of sustained plasma levels, attainment of controlled release of therapeutic agents for longer periods, and hence improvement in patient compliance (1,2). However, excellent impervious nature of skin is the greatest challenge for successfully delivering drug molecules to the systemic circulation by TDDS. The stratum corneum, the outermost layer of the skin, is the primary barrier to transdermal permeation, which is comprised of keratin-rich cells embedded in multiple lipid bilayers (3). Only potent drugs with appropriate physicochemical properties (low molecular weight, adequate solubility in aqueous and non-aqueous solvents, appropriate melting point, etc.) are suitable candidates for transdermal delivery (4). As a result, penetration enhancement technology is a challenging development that would increase significantly the number of drugs available for transdermal administration. Many different approaches have been used to facilitate drug permeation through the skin, including chemical penetration enhancers and physical enhancement strategies, such as iontophoresis, phonophoresis, electroporation, and microneedles (5,6). One of the most extensively studied techniques is the use of penetration enhancers, which can provide advantages including design flexibility with formulation chemistry, possibility of patch application over a large area (>10 cm2) and ability to work without external physical delivery mechanisms (7). These penetration enhancers are chemical compounds which can reversibly alter the barrier function of the skin and allow an increased rate of percutaneous permeation of administered drugs. Many chemicals have been identified as penetration enhancers that can increase skin permeability, such as azone derivatives, fatty acids, alcohols, esters, sulphoxides, pyrrolidones, glycols, surfactants, and terpenes (8). However, with the safety of permeation enhancers being of a prime consideration, the search continues for an ideal enhancer that is pharmacologically inert, odorless, colorless, nontoxic, nonirritating, nonallergenic, and compatible with most drugs and excipients (9).
Fatty acid–alcohol esters are commonly used as adjuvants for cosmetics and pharmaceuticals. Moreover, a large number of studies have suggested that aliphatic esters displayed excellent enhancement activities for a wide variety of drugs (10–19). Chukwumerije et al. measured the in vitro enhancement effects of a series of methyl esters of n-alkyl fatty acids using various skin types and revealed that the medium chain n-alkyl fatty acid esters appeared to be better enhancers of a variety of drugs than Azone (20). In addition, Karande et al. (21) investigated the enhancement ratio (ER) and irritation potential (IP) of about 300 chemical penetration enhancers in silico and in vitro and found the stearyl methacrylate yielded the highest ER/IP among the commercially available substance of structures which indicated stearyl methacrylate should be greatly safe and competent to enhance percutaneous penetration. In addition, stearyl methacrylate also significantly enhanced skin permeability of a macromolecule, insulin. Especially, due to excellent biocompatibility and particular physicochemical properties, as well as the absence of irritating and toxic properties, lactic acid and its derivatives of fatty esters have been approved by the FDA (USA) and are widely used as food additives, perfume materials, medicine materials and medicine additives, personal care products, and pharmaceuticals (22,23). Moreover, owing to their high safety and non-irritancy to skin, lactate esters are suitable not only for foods but also for medication and cosmetics as exfoliants, moisturizers, and emollients (24). Lactate esters have a structure which can be considered to be a chemical combination of lactic acid and fatty alcohols, both of which are known to be potent percutaneous absorption enhancers. Moreover, the lactate esters harbor a hydrophilic hydroxy head group and a lipophilic fatty alcohol chain in their structure, and belong to the family of non-ionic surfactants with well-documented activity (25). Furthermore, it was speculated that the structures of fatty acid esters enabled them to interact with the components of the stratum corneum and, consequently, to enhance permeation of drugs through the skin (26). Given these mechanisms of action, they could be effective in increasing the permeation of drugs across the skin. A series of studies have shown that lactate esters are an effective enhancer for some drugs (e.g., indomethacin, oxybutynin, alprazolam, testosterone, estradiol, and ibuprofen lysine) in TDDS (23–26). However, lactate esters have been largely ignored as potential penetration enhancer, though these esters are readily available, inexpensive, and safe. Moreover, systematic work for investigating their percutaneous penetration enhancement activity has been rarely performed.
In this study, a series of lactate esters with different alkyl chain length were investigated as penetration enhancers. Four drugs with different lipophilicities, including ibuprofen, salicylic acid (SA), dexamethasone (DEX), and 5-fluorouracil (5-FU) were chosen as the model drugs and hairless rat skin was used in vitro penetration experiment. Saturated solution of the model drugs in propylene glycol (PG) were used as the donor solution in order to ensure equal thermodynamic activity of drugs in the donor compartment.
MATERIALS AND METHODS
Materials
Ibuprofen (IBU) and SA were supplied by Pharmaceutical Factory of Juhua Company (China). 5-FU was obtained from Changzhou Jianhu Chemical Co., Ltd. (China). DEX was obtained from Tianjin Jinyao Group Co., Ltd. (China). (The physicochemical parameters of these model drugs are presented in Table I). Ethyl lactate (LE-2), butyl lactate (LE-4), hexyl lactate (LE-6), octyl lactate (LE-8), lauryl lactate (LE-12), myristic lactate (LE-14), and cetyl lactate (LE-16) were all obtained from Wujiang Ciyun Flavor And Fragrance Co., Ltd. (China). Decyl lactate (LE-10) and octadecyl lactate (LE-18) were obtained from Shanghai Julong Perfumery Co., Ltd. (China) (The physicochemical parameters of these enhancers are obtained from the Merck Index and supplied in Table II). Methanol and acetonitrile (Merck, Germany) were high-performance liquid chromatography (HPLC) grade and the other chemicals were analytical grade and used as received.
Table I.
Physicochemical Properties of Model Drugs
| Properties | Drugs | |||
|---|---|---|---|---|
| IBU | SA | DEX | 5-FU | |
| Molecular weight (g/mol) | 206.27 | 138.12 | 392.46 | 130.08 |
| Solubility in water (mg/ml) | 0.098 ± 0.007 | 2.24 ± 0.16 | 0.11 ± 0.02 | 11.05 ± 0.93 |
| Solubility in PG (mg/ml) | 453.1 ± 37.6 | 201.6 ± 17.9 | 11.5 ± 1.4 | 6.1 ± 0.4 |
| Solubility parameters ((J/cm3)1/2)a | 20.91 | 31.33 | 28.58 | 62.72 |
| Log K o/w | 3.94 b | 2.26b | 0.72c | −0.86b |
| Melting point (°C) | 74 | 157-159 | 256-260 | 283 |
Table II.
Physicochemical Properties of Penetration Enhancers
| Penetration enhancer | Abbreviation | log K o/w | Solubility parametersa ((J/cm3)1/2) | Melting point (°C) | Molecular weight (g/mol) |
|---|---|---|---|---|---|
| Propylene glycol | PG | −0.91 | 62.05 | −59 | 76.09 |
| Ethyl lactate | LE-2 | 0.06 | 46.22 | −26 | 118.13 |
| Butyl lactate | LE-4 | 1.10 | 44.13 | −43 | 146.18 |
| Hexyl lactate | LE-6 | 1.94 | 42.42 | −22 | 174.23 |
| Octyl lactate | LE-8 | 3.17 | 41.74 | −8 | 202.34 |
| Decyl lactate | LE-10 | 4.21 | 41.03 | −5 | 230.34 |
| Lauryl lactate | LE-12 | 4.83 | 40.44 | −2 to 8 | 258.51 |
| Myristic lactate | LE-14 | 5.64 | 39.98 | 29-34 | 286.58 |
| Cetyl lactate | LE-16 | 6.44 | 39.61 | 23-41 | 314.66 |
| Octadecyl lactate | LE-18 | 7.25 | 39.27 | 45-49 | 342.74 |
aCalculated using the method of Fedors (27)
Adult male Kunming rats, weight 180-220 g, were obtained from Tianjin Institute of Pharmaceutical Research. The rats were housed in plexiglas cages with free access to water and food. The cages were placed in a room under a controlled environmental condition (temperature 25°C, humidity 40–60%). All animal experiments were in compliance with local law in accordance with the Ethical Principles for the Use of Animals for Scientific Purposes of Tianjin Institute of Pharmaceutical Research, Tianjin, China.
Determination of Drug Solubility in Water and in PG
To determine the saturation solubility of the drugs in water and in PG, excess drug was added to a known volume of vehicle at room temperature and equilibrated at 37°C for 24 h while agitating. Finally, the suspensions were centrifuged at 8,000 rpm for 15 min. The saturated solution was then filtered through 0.45 µm membrane filters and the concentration of drug was measured by HPLC after appropriate dilution. The experiments were performed in quadruplicate and the results were shown in Table I.
Preparation of Donor Solution
Donor solutions of the different drugs, including IBU, SA, DEX, and 5-FU were obtained by equilibration of excess amounts of solute in PG, with and without selected concentration enhancers. The mixture was allowed to stand at room temperature under agitation for 24 h and the resulting suspension after filtration was used for the experiments. Without special indication, 5% was selected as a suitable concentration of each enhancer. However, it is worth pointing out that the donor solution with LE-16 or LE-18 are prepared by dissolving the CPE in propylene glycol at 50°C, because the solubility of LE-16 and LE-18 is found to be about 3 wt.% at room temperature.
In vitro Skin Permeation Studies
The skin samples were obtained from the abdominal area of Kunming rats. After careful removal of the hair on the abdominal area of the sacrificed rat using an electric clipper, the skin was cut and the adherent fat and other debris were carefully removed. Finally, the full-thickness skin was obtained and examined microscopically to ensure the integrity of the skin (20,26).
The permeability of drug through hairless rat skin was measured in vitro with a Franz diffusion cell system (1.65 cm2 in area and 17 ml in receptor cell volume, Shishin Technology Co. LTD, China) at 37 ± 0.5°C through the use of a circulating water bath. The treated skin pieces were mounted over diffusion cells with the dermal side in contact with the receptor phase. The receptor compartments were filled with phosphate buffered saline (PBS, pH 7.4) containing 0.02% w/v of sodium azide to retard microbial growth. The receptor phase was stirred at 600 rpm with a small magnetic bar to mix the concentrations uniformly. Skins were allowed to equilibrate for 1 h before experimentation. The test solution (2.5 ml) was placed on the donor side. Saturation solubilities of the model drugs in PG were determined and presented in Table I. All donors were then occluded with Parafilm®. Samples (2 ml) were withdrawn from the receiver solution at predetermined time intervals (1, 2, 4, 8, 12, 16, and 24 h) and frozen at −20°C until analyzed using HPLC. The cells were replenished to their marked volumes with fresh buffer solution. Addition of solution to the receiver compartment was performed with great care to avoid trapping air beneath the dermis samples. All experiments were performed in quadruplicate.
At the end of the in vitro permeation experiment (24 h), the amount of drug retained in the skin was determined according to a method reported previously (30). The skin was removed from the Franz cell and the effective permeation area of skins (1.65 cm2) was taken and was carefully washed five times using a cotton cloth immersed in methanol to remove drug remaining on the surface. Subsequently, the obtained skin was patted dry with a lint-free wipe. Following room temperature drying, each treated skin was weighed and then placed in 10 ml of methanol, and homogenized using a tissue homogenizer (PRO400, PRO Scientific Inc, Beijing). The homogenate was centrifuged for 5 min at 3,000 rpm. Following centrifugation, 1 ml of the supernatant was removed and stored at −20°C until analyzed using HPLC.
Drug Analysis
The amounts of drug in the receptor solution were measured by HPLC (Agillent1100, USA) using Krcmafsis (250 × 4 mm, 5 μm) C18 column. IBU was detected at 225 nm with a mobile phase of methanol, acetonitrile, and 0.05 mol/L KH2PO4 aqueous solution (adjusted to pH 4.0 with phosphoric acid; ratio 175:44:81, v/v/v) at a flow rate of 1.0 ml/min (31). SA was detected with flow rate at 1.5 ml/min at 295 nm at 25°C. The mobile phase for SA assay was prepared by adding 15 ml acetic acid and 0.25 g ammonium acetate to 300 ml acetonitrile. The volume was completed to 1,000 ml using distilled water according to our previous research (32). DEX was detected at 242 nm with a mobile phase of 0.01 mol/L phosphate buffer (pH 3.0) and acetonitrile (ratio 25:75, v/v) at a flow rate of 1.0 ml/min according to reference (33). The mobile phase for 5-FU consisted of acetonitrile and 0.0364 mol/L monobasic potassium phosphate in distilled water (1:99, v/v), the wavelength was set at 265 nm (34).
Data Analysis
The cumulative amounts of drugs (μg/cm2) at each collection time was plotted against time (h) and linear regression of the steady state portion of the curve was used to estimate drug flux (J, μg/cm2/h) through rat skin. Permeability coefficient (Kp, cm/h) was calculated from the ratio of flux to drug concentration in the donor chamber. The lag time (T, h) was determined by extrapolating the linear portion of the curve to the abscissa. In addition, permeation profiles were also analyzed by the skin concentration of drugs (μg/g). The ER were calculated from the Kp and skin concentration of drugs with enhancer divided by the same parameter without enhancer (control). All percutaneous permeation data are mean ± S.D. Statistical significance was checked by student’s t test and considered to be granted at P < 0.05.
RESULTS AND DISCUSSION
The skin permeation enhancement of penetration enhancers is greatly dependent upon their structure, the characteristics of the permeants, and the skin model (35). In this work, in order to analyze the effect of a series of lactate esters on skin permeability, the in vitro percutaneous penetration of compounds with different lipophilicities (log KO/W from −0.86 to 3.94) in saturated donor solution of PG were investigated.
Drug Solubilities in PG and in Water
The solubility of a drug, which depends on the physicochemical properties of the drug and of the vehicle, is important in determining the rate of delivery into the skin. As can be seen from Table I, 5-FU has the highest solubility in water at 11.05 ± 0.93 mg/ml. SA has moderately solubility in water at 2.24 ± 0.16 mg/ml. IBU and DEX have very low solubilities in water at 0.098 ± 0.007 mg/ml and 0.11 ± 0.02 mg/ml, respectively. Contrarily, IBU and SA possess good solubilities in PG at higher than 200 mg/ml. DEX and 5-FU have low solubilities in PG at 11.5 ± 1.4 mg/ml and 6.1 ± 0.4 mg/ml, respectively.
Penetration Enhancement of Lactate Esters on Percutaneous Permeation of IBU In vitro
The effect of the lactate esters on percutaneous permeation of IBU through hairless rat skin is summarized in Table III. All results are expressed as means ± standard deviations. A control experiment was carried out without enhancer but using PG as the vehicle. The control flux and permeability coefficient of IBU is 127.36 ± 10.85 μg/cm2/h and 28.11 ± 2.20 × 10−5 cm/h, respectively. The control value for the skin concentration of drugs and lag time is 478.37 ± 33.25 μg/g and 1.52 ± 0.41 h. Compared with the control experiments, the percutaneous permeation of IBU increased when lactate esters were used as enhancers except LE-4, and significant increase in percutaneous permeation was observed with LE-12, LE-14, and LE-16. Moreover, it can be clearly seen that the most effective enhancer of the lactate series is LE-14, which has a permeability coefficient of 83.98 ± 3.58 × 10−5 cm/h (ER(Kp) = 2.99). The results obtained are consistent with the previous studies which LE-12, LE-14, and LE-16 in vivo can have a significant effect on the flux of indometacin with a log Ko/w of 3.80 that is very close to the properties of IBU with a log Ko/w of 3.94 (23,36). Lee et al. reported that LE-12 can effectively enhanced the permeation of a highly lipophilic drug oxybutynin (log Ko/w = 4.9) (26). In addition, it can be observed that there is a relationship between the flux values of IBU and the carbon chain length of fatty alcohol moieties of lactate esters. The permeations of ibuprofen firstly increase with the increase of the chain length of fatty alcohol moieties from two to 14 (except with LE-4) and then decrease when the chain length of fatty alcohol moieties increases from 14 to 18. However, The enhancing activity of lactate esters for IBU is low (ER(Kp) < 3), which may be attributed to the fact that PG is also an excellent enhancer for IBUand IBU saturated solutions possess the properties of self-permeation enhancement due to IBU-skin interaction (31,37). As far as skin concentration of drugs is concerned, the drug skin content in the presence of LE-12, LE-16 and LE-18 is higher than 1,000 μg/g and presents a significant increase. All of them cause enhancement of skin concentration of drugs about threefold compared to the control. However, except lactate esters with a small number of carbon atoms, almost all the evaluated enhancers do not significantly shorten the lag time of IBU. Considering that lag time is inversely related to drug diffusivity according to the Fick’s law and the high IBU content in skin, we thought that the lactate esters may retain the drug in the skin and their promotion in the permeation of IBU can be attributed chiefly to the increase in the drug partition rather than drug diffusion into skin. Some studies have demonstrated that the aliphatic esters may influence partitioning between vehicle and skin by solubilization effects (12).
Table III.
Effect of the Lactate Esters (5 wt.%) on Percutaneous Permeation Parameters of Ibuprofen Through Hairless Rat Skin
| Enhancer | J (μg/cm2/h) | K p ×105 (cm/h) | ER(Kp) | SC (μg/g) | ER(SC) | T (h) |
|---|---|---|---|---|---|---|
| PG | 127.36 ± 10.85 | 28.11 ± 2.20 | 1.00 | 478.37 ± 33.25 | 1.00 | 1.52 ± 0.41 |
| LE-2 | 132.45 ± 7.03 | 29.23 ± 2.31 | 1.04 | 905.11 ± 24.21a | 1.89 | 1.17 ± 0.45a |
| LE-4 | 111.82 ± 7.96 | 24.68 ± 1.74a | 0.88 | 624.56 ± 20.94 | 1.31 | 1.46 ± 0.51 |
| LE-6 | 151.55 ± 9.19 | 33.30 ± 2.03a | 1.19 | 785.09 ± 42.93 | 1.64 | 1.13 ± 0.37 |
| LE-8 | 173.21 ± 10.11 | 38.22 ± 3.09 | 1.36 | 1,050.15 ± 93.21 | 2.20 | 1.38 ± 0.65 |
| LE-10 | 268.73 ± 13.70 | 59.32 ± 3.02a | 2.11 | 1,103.21 ± 79.52 | 2.31 | 1.47 ± 0.49 |
| LE-12 | 375.71 ± 13.17 | 82.92 ± 2.90 | 2.95 | 1,329.28 ± 82.59 | 2.78 | 1.42 ± 0.51a |
| LE-14 | 380.43 ± 16.23 | 83.98 ± 3.58 | 2.99 | 987.84 ± 66.17 | 2.07 | 1.55 ± 0.34a |
| LE-16 | 284.02 ± 15.92 | 62.68 ± 4.26 | 2.23 | 1,462.38 ± 123.14a | 3.06 | 1.69 ± 0.73 |
| LE-18 | 257.27 ± 12.12 | 56.78 ± 2.67 | 2.02 | 1,350.15 ± 93.21a | 2.82 | 2.01 ± 0.65a |
J drug flux through rat skin, K p permeability coefficient, ER (Kp) the enhancement ratio of permeability coefficient, SC the skin concentration of drugs, ER (sc) the enhancement ratio of the skin concentration of drugs, T the lag time
Values are the mean ± SD (n = 4)
aNo significant differences vs. control (P > 0.05)
Penetration Enhancement of Lactate Esters on Percutaneous Permeation of SA In vitro
The skin permeation parameters for SA are shown in Table IV. The values for the percutaneous permeation parameters of SA control group are as follows: 204.11 ± 20.12 μg/cm2/h for the flux, 10.12 ± 1.04 × 10−4 cm/h for Kp, 273.64 ± 19.95 μg/g for SC, and 1.83 ± 0.42 h for lag time. These expected high permeability coefficients might be a result of the very high concentrations. SA was proposed to be a keratolytic molecule that can alter the barrier properties of the skin (38). Furthermore, some studies reported there is a parabolic dependence between skin permeation and the log Ko/w with an optimum value of log Ko/w≈2 (39,40). Therefore, SA with optimal log Ko/w = 2.26 may be able to permeate through the skin readily due to the appropriate lipophilicity. Interestingly, hydrophobic SA possesses a considerable solubility in water at 2.24 ± 0.16 mg/ml, which may probably facilitate SA partition into hydrophilic hypoderma of the rat full skin. It can be seen from Table IV that LE-2 and LE-4 do not show any significant enhancement, whereas LE-8, LE-10 and LE-12 show great enhancement in the percutaneous permeation of SA. LE-14, LE-16, and LE-18 exhibit a similar enhancement effect on the percutaneous permeation of SA and they do not show significant n-alkyl group chain length effect upon enhancer potency for SA. Moreover, it can also be clearly seen that the most effective enhancer of the lactate esters is LE-10 which increases the SA in Kp by 4.70-fold. Han et al. found that N-adamantyl n-alkanamide has the strongest transdermal penetration-enhancing activity on the penetration of SA when the length of the alkyl chain of fatty acid moieties is ten (41). However, the permeation enhancers tested do not showed a same rule in SC. LE-12 and LE-14 provide the best enhancement activity in SC (P < 0.05), and both of them cause increase of skin concentration of drugs over 3.5-fold. Furthermore, similar to IBU, nearly all the evaluated enhancers do not present any significant decrease in the lag time of SA.
Table IV.
Effect of the Lactate Esters (5 wt.%) on Percutaneous Permeation Parameters of Salicylic Acid Through Hairless Rat Skin
| Enhancer | J (μg/cm2/h) | K p ×104 (cm/h) | ER(Kp) | SC (μg/g) | ER(SC) | T (h) |
|---|---|---|---|---|---|---|
| PG | 204.11 ± 20.12 | 10.12 ± 1.04 | 1.00 | 273.64 ± 19.95 | 1.00 | 1.83 ± 0.42 |
| LE-2 | 191.92 ± 25.04 | 9.51 ± 1.31 | 0.94 | 244.37 ± 16.39 | 1.12 | 1.76 ± 0.19 |
| LE-4 | 223.08 ± 41.47 | 11.05 ± 1.77a | 1.09 | 482.13 ± 20.73 | 1.77 | 1.31 ± 0.36 |
| LE-6 | 419.07 ± 45.70 | 20.79 ± 3.53 | 2.05 | 882.13 ± 10.73a | 3.23 | 1.21 ± 0.23a |
| LE-8 | 487.82 ± 50.46 | 24.18 ± 3.18 | 2.39 | 307.36 ± 13.15 | 1.13 | 1.44 ± 0.31a |
| LE-10 | 959.4 ± 82.58 | 47.59 ± 5.24 | 4.70 | 944.66 ± 28.69a | 3.45 | 1.77 ± 0.28 |
| LE-12 | 861.6 ± 77.14 | 42.74 ± 5.52 | 4.22 | 868.88 ± 24.05 | 3.17 | 1.55 ± 0.34a |
| LE-14 | 613.98 ± 68.3 | 30.46 ± 4.12 | 3.01 | 1005.05 ± 32.54 | 3.67 | 1.82 ± 0.27a |
| LE-16 | 520.74 ± 66.64 | 25.83 ± 3.28 | 2.55 | 831.90 ± 35.69a | 3.04 | 2.09 ± 0.33 |
| LE-18 | 574.36 ± 5.16 | 28.49 ± 2.54a | 2.82 | 581.75 ± 29.31 | 2.13 | 1.88 ± 0.45a |
J drug flux through rat skin, K p permeability coefficient, ER (Kp) the enhancement ratio of permeability coefficient, SC the skin concentration of drugs, ER (sc) the enhancement ratio of the skin concentration of drugs, T the lag time
Values are the mean ± SD (n = 4)
aNo significant differences vs. control (P > 0.05)
Penetration Enhancement of Lactate Esters on Percutaneous Permeation of DEX In vitro
The effect of the lactate esters on percutaneous permeation of DEX is summarized in Table V. The values for the percutaneous permeation parameters of DEX control group are as follows: 0.28 ± 0.03 μg/cm2/h for the flux, 2.43 ± 0.15 × 10−5 cm/h for Kp, 1.14 ± 0.09 μg/g for SC, and 3.64 ± 0.95 h for lag time. It can clearly be observed from Table V that most of the enhancers showed significant influences on the percutaneous permeation of DEX. Moreover, it can also be seen that the most competent enhancers for DEX are LE-10 and LE-12. Both of them can provide an increases in Kp of DEX about tenfold. Furthermore, the highest increase in SC is also provided by LE-10 and LE-12 around 20-fold. It is worth pointing out that there is a significant increase in flux and SC of DEX in the presence of LE-2. With regard to the lag time, except LE-14, LE-16, and LE-18, lactate esters with a small number of carbon atoms distinctly shorten the lag time of DEX. These results indicate that the short chain esters are able to increase the diffusivity of DEX in the stratum corneum. It has been reported that some simple alkyl esters, such as methyl acetate, ethyl acetate, and butyl acetate, are relatively polar, hydrogen-bonding compounds that may enhance permeation by penetrating into the stratum corneum and increasing the lipid fluidity by disruption of lipid packing (10,11).
Table V.
Effect of the Lactate Esters (5 wt.%) on Percutaneous Permeation Parameters of Dexamethasone Through Hairless Rat Skin
| Enhancer | J (μg/cm2/h) | K p ×105 (cm/h) | ER(Kp) | SC (μg/g) | ER(SC) | T (h) |
|---|---|---|---|---|---|---|
| PG | 0.28 ± 0.03 | 2.43 ± 0.15 | 1.00 | 1.14 ± 0.09 | 1.00 | 3.64 ± 0.95 |
| LE-2 | 2.00 ± 0.16 | 17.35 ± 1.34a | 7.14 | 11.47 ± 0.07 | 10.06 | 1.37 ± 0.69 |
| LE-4 | 0.96 ± 0.09 | 8.35 ± 0.29 | 3.36 | 3.10 ± 0.44a | 2.71 | 1.81 ± 0.26a |
| LE-6 | 1.31 ± 0.11 | 11.40 ± 1.34 | 4.69 | 4.13 ± 0.13 | 3.62 | 1.31 ± 0.36 |
| LE-8 | 1.40 ± 0.07 | 12.18 ± 0.46a | 5.08 | 9.09 ± 0.61 | 7.97 | 1.56 ± 0.31a |
| LE-10 | 2.78 ± 0.26 | 24.15 ± 1.52 | 9.76 | 19.91 ± 0.17a | 17.47 | 1.77 ± 0.19 |
| LE-12 | 3.01 ± 0.19 | 26.17 ± 2.36 | 10.77 | 21.74 ± 0.93 | 19.07 | 1.55 ± 0.14 |
| LE-14 | 1.19 ± 0.17 | 10.30 ± 1.18a | 4.24 | 7.28 ± 0.09 | 6.39 | 4.05 ± 0.27 |
| LE-16 | 0.74 ± 0.11 | 6.44 ± 0.31 | 2.65 | 4.37 ± 0.18 | 3.83 | 3.09 ± 0.33a |
| LE-18 | 0.57 ± 0.09 | 4.91 ± 0.45a | 2.02 | 7.80 ± 0.53 | 2.89 | 3.77 ± 0.35 |
J drug flux through rat skin, K p permeability coefficient, ER (Kp) the enhancement ratio of permeability coefficient, SC the skin concentration of drugs, ER (sc) the enhancement ratio of the skin concentration of drugs, T the lag time
Values are the mean ± SD (n = 4)
aNo significant differences vs. control (P > 0.05)
Penetration Enhancement of Lactate Esters on Percutaneous Permeation of 5-FU In vitro
The influence of the lactate esters on percutaneous permeation of 5-FU through hairless rat skin is summarized and shown in Table VI. The control experiment was carried out without enhancers. The control flux, permeability coefficient and the skin concentration of 5-FU is 2.43 ± 0.31 μg/cm2/h, 3.98 ± 0.51 × 10−5 cm/h and 9.67 ± 1.19 μg/g, respectively. The control lag time of 5-FU is 8.51 ± 1.14 h, which is the longest lag time among the four model drugs. As can be seen, all of the evaluated enhancers have significant effects on the percutaneous permeation of 5-FU. The highest increase in Kp is observed with LE-12, which increases the Kp by 22.09-fold, followed by LE-10 (18.77-fold) and LE-2 (11.69-fold). Similar to the Kp of 5-FU, LE-12 provides the highest increase in SC (74.33 ± 9.19 μg/g), followed by LE-2 (72.59 ± 10.17 μg/g). Moreover, almost all the evaluated enhancers have a shorter lag time relative to the control. These results suggest that lactate esters, besides leading to 5-FU partitioning across the potential barriers more easily, also result in an increase in the diffusion rate of 5-FU. A series of investigations have indicated that lactate esters chiefly act on stratum corneum and increase the fluidity of the lipid layer of the stratum corneum (23,36). Considering that the stratum corneum is the primary barrier of hydrophilic drug, therefore, it is of no wonder that the lactate esters possess effective enhancement activity on permeation of 5-FU. Furthermore, compared with other hydrophobic model drugs, lactate esters show more significant enhancement activity on percutaneous permeation of 5-FU, which can be attributed to several reason. First, lactate esters can exert a hydration effect on stratum corneum which will lead to a reduction in the stratum corneum-viable epidermis partition coefficient of the penetrant (because the two tissue phases now appear more similar). This effect should facilitate the kinetics of transfer of polar molecule from stratum corneum to the viable epidermis. Secondly, it has been reported that PG at high concentration withdraws water from skin due to its distinct hygroscopicity, and the dehydration effect of PG on stratum corneum can affect the polar region in the stratum corneum and as a consequence reduce the apparent diffusivity of hydrophilic drug (42,43). Conversely, lactate esters being solubilized in PG, can exert a hydration effect, which may neutralize the dehydration effect of high concentration PG on stratum corneum and allow hydrophilic molecule to permeate through horny stratum corneum more easily. Last, a large number of studies have demonstrated that the flux and Kp are minimized when solubility parameter of the penetrant is close to that of the vehicle (38,44,45). 5-FU with a solubility parameter of 62.72 (J/cm3)1/2 is very close to PG (62.05 (J/cm3)1/2). The solubility parameter of PG is so adjacent to 5-FU that the distance of solubility parameters between them will be remarkably altered by adding the lactate esters. It is expected that such a small alteration caused by enhancers would produce a significant increase in 5-FU permeation. In a word, lactate esters may be a trail of outstanding penetration enhancer for hydrophilic drug, such as 5-FU. These results are in agreement with the previous study by Lopes et al., who demonstrated that the transdermal delivery of the hydrophilic drug, but not of the lipophilic one, was significantly enhanced by medium-chain glycerides (15,19).
Table VI.
Effect of the Lactate Esters (5 wt.%) on Percutaneous Permeation Parameters of 5-Fluorouracil Through Hairless Rat Skin
| Enhancer | J×10 (μg/cm2/h) | K p ×105 (cm/h) | ER(Kp) | SC (μg/g) | ER(SC) | T (h) |
|---|---|---|---|---|---|---|
| PG | 2.43 ± 0.31 | 3.98 ± 0.51 | 31.00 | 9.67 ± 1.19 | 1.00 | 8.51 ± 1.14 |
| LE-2 | 28.41 ± 3.17 | 46.57 ± 6.98 | 11.69 | 72.59 ± 10.17 | 7.55 | 4.79 ± 0.59a |
| LE-4 | 20.61 ± 4.06 | 33.78 ± 5.41a | 35.48 | 53.61 ± 12.51 | 5.54 | 6.88 ± 0.37 |
| LE-6 | 9.84 ± 0.57 | 16.12 ± 1.28a | 4.05 | 66.13 ± 9.73a | 6.84 | 3.31 ± 0.36 |
| LE-8 | 19.17 ± 1.42 | 31.42 ± 2.43 | 7.89 | 55.32 ± 14.24a | 5.72 | 2.15 ± 0.18a |
| LE-10 | 45.65 ± 3.35 | 74.84 ± 5.36 | 18.77 | 39.78 ± 11.12 | 4.11 | 3.47 ± 0.23 |
| LE-12 | 53.71 ± 4.23 | 88.05 ± 8.13 | 22.09 | 74.33 ± 9.19 | 7.69 | 2.03 ± 0.40 |
| LE-14 | 23.72 ± 1.70 | 38.84 ± 4.89 | 9.76 | 40.12 ± 4.35 | 4.15 | 4.52 ± 1.45 |
| LE-16 | 7.78 ± 1.58 | 12.76 ± 1.32a | 3.20 | 50.33 ± 17.58a | 5.20 | 4.18 ± 0.52 |
| LE-18 | 9.07 ± 0.68 | 11.58 ± 1.05 | 2.91 | 27.99 ± 6.29 | 2.89 | 6.96 ± 0.44a |
J drug flux through rat skin, K p permeability coefficient, ER (Kp) the enhancement ratio of permeability coefficient, SC the skin concentration of drugs, ER (sc) the enhancement ratio of the skin concentration of drugs, T the lag time
Values are the mean ± SD (n = 4)
aNo significant differences vs. control (P > 0.05)
Correlation Between Enhancement Efficacy of Lactate Esters and Carbon Chain Length of Lactate Esters
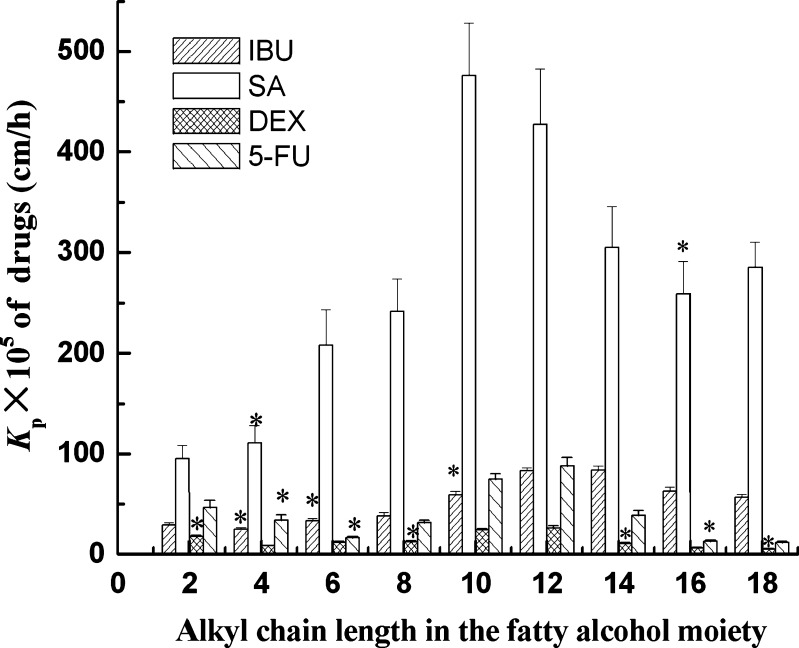
The effect of alkyl chain length from two to 18 in the fatty alcohol moiety of lactate esters on drug permeation through hairless rat skin is presented in Fig. 1. It can be clearly seen that the permeation of drug is related to the carbon chain length of the fatty alcohol moiety. An increase in the permeation of IBU is observed when the carbon chain length of the fatty alcohol moiety increases from two to 14 carbons. However, the permeation of IBU decreased when the chain length was increased beyond 14 carbons. It can also be observed that the Kp of SA has a parabolic relationship with the carbon chain length of the carbon chain length of the fatty alcohol moiety, and LE-10 shows the strongest enhancing effect on the permeation of SA. The permeation of DEX and 5-FU is also found to have a parabolic relationship with the carbon chain length, and LE-10 and LE-12 are the most effective. All results suggest that there is a parabolic relationship between the carbon chain length of the fatty alcohol moiety of lactate esters and enhancement activity for permeation of all four drugs with different properties. This kind of parabolic relationship has been observed by others with different enhancers such as alcohols (46), fatty acids (47), n-alkyl piperidones (48), and n-alkanes (49). In addition, maximal enhancement is generally attained for enhancers with a carbon chain length in the range of 10-14 (50). This optimal chain length may be interpreted as follows: adequate length of alkyl chain is required for best incorporation of the enhancer into the stratum corneum lipid layers. The most effective carbon chain lengths correspond to the chain length of the steroid nucleus of cholesterol, suggesting that these may act by disrupting ceramide-cholesterol or cholesterol-cholesterol interaction and increase penetration of drug (51). Furthermore, the enhancers have the optimum alkyl chain for enhancement activity, which could be attributed to the physicochemical properties of compounds. Table II shows the physicochemical parameters of lactate esters including molecular weight, log Ko/w, melting point and solubility parameters. As the carbon chain length of the fatty alcohol moieties increases, their molecular weights, log Ko/w and melting points also increase linearly. The lower permeation enhancement effect of lactate esters with over 12 carbon atoms may be attributed to the decreased mobility of lactate esters within the skin layers due to the combined effect of their higher molecular weight, log Ko/w and melting point. The melting point of LE-16 and LE-18 is higher than room temperature. According to ideal solubility theory, the higher the melting point of a substance, the lower its solubility in a given solvent, including skin lipids (52). Moreover, LE-10 and LE-12 may have an optimal balance of partition coefficient and affinity to lipids in the stratum corneum. Short chain lactate esters have insufficient lipophilicity to penetrate the skin, and long-chain fatty acids have too much affinity to the lipids in stratum corneum and actually retard the penetration of drugs. Therefore, the solubility parameters have been considered very important, when the characteristics of a compound are discussed in the study of percutaneous penetration enhancers (44,45). An ideal enhancer should have solubility parameter similar to that of the skin (53). It can be seen from Table II that the solubility parameters of lactate esters decrease linearly as the carbon chain length of the fatty alcohol moieties increases. LE-10 with a solubility parameter of 41.03 (J/cm3)1/2 and LE-12 with a solubility parameter of 40.44 (J/cm3)1/2 are very close to skin, since it has been suggested that the skin may have a solubility parameter of about 41 (J/cm3)1/2 (54). Therefore, it might be hypothesized that such enhancers should mix freely with the stratum corneum lipids and have maximal enhancement properties.
Fig. 1.
Influences of alkyl chain length in the fatty alcohol moiety of lactate esters on drug permeation through hairless rat skin; 5 wt.% lactate esters were dissolved in PG; asterisk means no significant differences vs. control (P > 0.05). IBU ibuprofen, SA salicylic acid, DEX dexamethasone, 5-FU 5-fluorouracil, PG propylene glycol
Correlation Between Enhancement Efficacy of Lactate Esters and Log Ko/w of Drugs
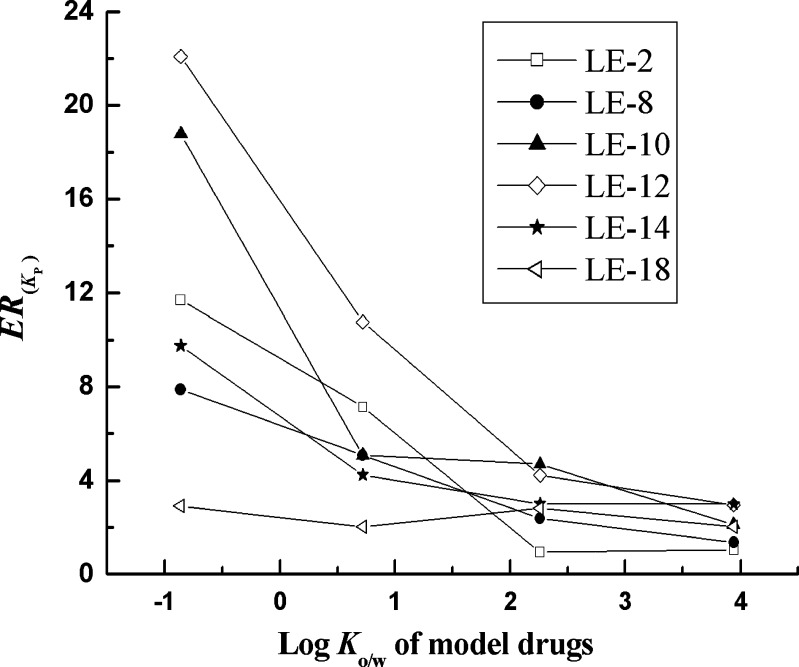
The physicochemical properties of the permeants are thought to play an important role in determining the promoting activity of penetration enhancers on the permeation of the drug across the skin (8). Drugs are considered to penetrate through the skin by one of three pathways: the polar, non-polar, or polar/non-polar route depending on their physicochemical properties, in which the molecular weight and log Ko/w of drugs are thought to be the key factor (55,56). In this study, the molecular weight of the drugs, however, may have no significant effect on the penetration rate of drugs due to the relatively narrow range of molecular weight (200-500 g/mol; 57). The log Ko/w value, which is a measure of how well a substance partitions between a lipid and water, determines the route of drug penetration through the skin. Consequently, the log Ko/w value of drug molecule has a most direct and important effect on the enhancement efficacy of penetration enhancers. Figure 2 exhibits the relationships between log Ko/w values of the model drugs and their ER(Kp) with several lactate esters as penetration enhancers. It can be seen that the lipophilicities of model drugs have a significant impact on the promoting activity of lactate esters and the increase in the log Ko/w values of the model drugs leads to a decrease in the enhancement efficacy of penetration enhancers. The enhancers show the highest activity for the most hydrophilic drug, i.e., 5-FU, and the lowest activity of the enhancers was recorded with the most lipophilic compound, i.e., IBU. In order to explain these differences, it is necessary to consider the different molecular mechanisms involved in the diffusion through the stratum corneum of hydrophilic and lipophilic molecules and also the main mechanisms of action of percutaneous enhancers (53). Polar and non-polar substances diffuse through the stratum corneum by different molecular mechanisms. The stratum corneum is the main barrier for “hydrophilic” drugs, whereas, in the case of “lipophilic” drugs, the limiting step for absorption is the lipid-to-aqueous (viable tissue) partitioning step. As mentioned above, the lactate esters can exert a skin hydration effect on stratum corneum. Generally, increased tissue hydration appears to increase transdermal delivery of both hydrophilic and low lipophilic compounds due to an increase in partition into the skin of drugs (8). It is proposed that the hydration effect of them on the stratum corneum could make the penetration of hydrophilic drugs easier. In a similar study, Barry and Bennett speculated that polar molecules, because of their low partition coefficient and high hydrogen-bonding potential, would show a dramatic increase in permeation with appropriate enhancers (58). However, for the high lipophilic compounds (log Ko/w > 2), partition into the “hydrated” stratum corneum are made difficult, consequently, which results in a reduction in their permeation capacity through the skin.
Fig. 2.
Relationship between enhancement ratios and the Log K o/w value of model drugs using 5 wt.% LE-2, LE-8, LE-12, LE-14 and LE-18 as penetration enhancers in PG. LE-2 ethyl lactate, LE-8 octyl lactate, LE-10 decyl lactate, LE-12 Lauryl lactate, LE-14 Myristic lactate, LE-18 Octadecyl lactate, PG propylene glycol
Correlation Between Enhancement Efficacy of Lactate Esters and the Concentration of Penetration Enhancers
The effects of lactate esters at various concentrations in PG on the in vitro percutaneous permeation of lipophilic drug (IBU) and hydrophilic drug (5-FU) from the saturated solution through hairless rat skin were investigated. LE-2 as the representative of lactate esters with short hydrocarbon chains and LE-12 as the representative of long-chain lactate esters at concentrations of 1, 3, 5, 8, 10, 15, and 20 wt.% were employed. As can be seen in Fig. 3a, the concentration of LE-2 does not show a significant influence on the transdermal flux of IBU, because, as know from experiments, LE-2 is not an effective enhancer for permeation of IBU. However, a parabolic relationship was observed between the transdermal flux of IBU and the LE-12 concentration. The flux of IBU firstly increases with the increase of concentration of LE-12 concentration, but the flux of IBU obviously decreases when the enhancer concentration was beyond 10 wt.%, which was in agreement with the research results of Taskovich et al. that 12 wt.% of LE-12 provides the greatest enhancement on lipophilic drug (24). The high concentration of LE-12 results in a reduction of the enhancer effect. This is because LE-12 exerts an effect on the hydration of the stratum corneum and the water content in skin increases as the concentration of LE-12 increases. This condition makes the penetration of hydrophilic drugs easier, whereas it would make the partition into the hydrated stratum corneum for the most lipophilic compounds difficult and, consequently, the penetration is reduced. As far as 5-FU was concerned, the transdermal flux of 5-FU shows obvious increase with the increase of LE-2 and LE-12 concentration. The increase in flux of 5-FU with the increase of the concentrations of LE-2 may be attributed to the fact that increasing LE-2 concentration would promote skin penetration capability of LE-2 itself and result in enhancing the permeation of drug. Friend et al. has reported that some simple alkyl esters, such as methyl acetate and ethyl acetate, can penetrate into skin and increase skin concentration of drugs (10). The increase in the enhancement activity on hydrophilic 5-FU attained by increasing the concentration of LE-12 can be attributed to the water content increase in stratum corneum which allows the polar molecule to partition across the potential barriers more easily. Consequently, it can be deduced from these results that the influence of the lactate enhancer concentration depends on the lipophilicity of the tested model drugs. However, taking into account that the enhancement no longer increases proportionally when the concentration of lactate esters above 10 wt.%, the enhancer concentration in the range of 5∼10 wt.% should be an appropriate selection.
Fig. 3.
Influences of lactate esters concentration on the transdermal flux of IBU (a) and 5-FU (b). Values are the mean ± SD of four determinations. LE-2 ethyl lactate, LE-12 Lauryl lactate, IBU ibuprofen, 5-FU 5-fluorouracil
CONCLUSION
This study has found lactate esters to be effective penetration enhancers for drugs with different physicochemical properties, such as IBU, SA, DEX, and 5-FU. Furthermore, the optimal enhancement is obtained for lactate esters with a carbon chain length in the range of 10-12. Moreover, the enhancement effect increases with a decrease in the drug lipophilicity. These results suggest that the lactate esters are more effective for enhancing the penetration of hydrophilic drugs than lipophilic drugs. In a word, with the characters like safety, non-irritancy, and non-toxicity to skin, the lactate esters, especially decyl lactate and lauryl lactate, have the potential to be a moderately efficient, facile penetration enhancer in TDDS. However, further study is needed to determine the effectiveness of PG on the penetration enhancing effects of lactate esters tested here. It is likely that PG has distinct effects on the efficacy of lactate esters. In addition, it is well known that human skin is much different from hairless rat skin, and lactate esters may have different behavior in different skin models. Therefore, further studies are also needed in order to assess the performance of lactate esters in a more authentic model such as human skin.
ACKNOWLEDGMENTS
This project was supported by the National Natural Science Foundation of China (Number 30672554).
REFERENCES
- 1.Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta. 2009;1788:2362–2373. doi: 10.1016/j.bbamem.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Hadgraft J, Lane ME. Historical perspectives skin permeation: the years of enlightenment. Int J Pharm. 2005;305:2–12. doi: 10.1016/j.ijpharm.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Darlenski R, Sassning S, Tsankov N, Fluhr JW. Non-invasive in vivo methods for investigation of the skin barrier physical properties. Eur J Pharm Biopharm. 2009;72:295–303. doi: 10.1016/j.ejpb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Kanikkannan N, Kandimalla K, Lamba SS, Singh M. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr Med Chem. 2000;7:593–608. doi: 10.2174/0929867003374840. [DOI] [PubMed] [Google Scholar]
- 5.Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14:101–114. doi: 10.1016/S0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 6.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nature Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 7.Finnin BC, Morgan TM. Transdermal penetration enhancers: applications, limitations, and potential. J Pharm Sci. 1999;88:955–958. doi: 10.1021/js990154g. [DOI] [PubMed] [Google Scholar]
- 8.Williams AC, Barry BW. Penetration enhancers. Adv Drug Dev Rev. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Pfister WR, Dean S, Hsieh ST. Permeation enhancers compatible with transdermal drug delivery systems. I. Selection and formulation considerations. Pharm Tech. 1990;8:132–140. [PubMed] [Google Scholar]
- 10.Friend DR, Catz P, Heller J. Simple alkyl esters as skin permeation enhancers. J Control Release. 1989;9:33–41. doi: 10.1016/0168-3659(89)90031-X. [DOI] [Google Scholar]
- 11.Catz P, Friend DR. Alkyl esters as skin permeation enhancers for indomethacin. Int J Pharm. 1989;55:17–23. doi: 10.1016/0378-5173(89)90271-8. [DOI] [Google Scholar]
- 12.Sato K, Sugibayashi K, Morimoto Y. Effect and mode of action of aliphatic esters on the in vitro skin permeation of nicorandil. Int J Pharm. 1988;43:31–40. doi: 10.1016/0378-5173(88)90055-5. [DOI] [Google Scholar]
- 13.Ozawa Y, Yamahira T, Sunada H, Nadai T. Influence of fatty acid-alcohol esters on percutaneous absorption of hydrocortisone butyrate propionate. Chem Pharm Bull. 1988;36:2145–2151. doi: 10.1248/cpb.36.2145. [DOI] [PubMed] [Google Scholar]
- 14.Panigrahi L, Pattnaik S, Ghosal SK. The effect of ph and organic ester penetration enhancers on skin permeation kinetics of terbutaline sulfate from pseudolatex-typentransdermal delivery systems through mouse and human cadaver skins. AAPS PharmSciTech. 2005;6:167–173. doi: 10.1208/pt060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes LB, Murphy N, Nornoo A. Enhancement of transdermal delivery of progesterone using medium-chain mono and diglycerides as skin penetration enhancers. Pharm Dev Techol. 2009;14:524–529. doi: 10.1080/10837450902814180. [DOI] [PubMed] [Google Scholar]
- 16.Hosmer J, Reed R, Bentley MVLB, Nornoo A, Lopes LB. Microemulsions containing medium-chain glycerides as transdermal delivery systems for hydrophilic and hydrophobic drugs. AAPS PharmSciTech. 2009;10:589–596. doi: 10.1208/s12249-009-9251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chukwumerije O, Nash RA, Matias JR, Orentreich N. Studies on the efficacy of methyl esters of n-alkyl fatty acids as penetration enhancers. J Invest Dermatol. 1989;93:349–352. doi: 10.1111/1523-1747.ep12280256. [DOI] [PubMed] [Google Scholar]
- 18.Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. PNAS. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta R, Henry M. Lactic acid: recent advances in products, processes and technologies—a review. J Chem Technol Biotechnol. 2006;81:1119–1129. doi: 10.1002/jctb.1486. [DOI] [Google Scholar]
- 20.Clary JJ, Feron VJ, van Velthuijsen JA. Safety assessment of lactate esters. Regul Toxicol Pharmacol. 1998;27:88–97. doi: 10.1006/rtph.1997.1175. [DOI] [PubMed] [Google Scholar]
- 21.Tung RC, Bergfeld WK, Vidimos AT, Remzi BK. Alpha-hydroxy acid-based cosmetic procedures. Guidelines for patient management. Am J Clin Dermatol. 2000;1:81–88. doi: 10.2165/00128071-200001020-00002. [DOI] [PubMed] [Google Scholar]
- 22.Roenne TH, Xu XB, Tan TW. Lipase-catalyzed esterification of lactic acid with straight-chain alcohols. JAOCS. 2005;82:881–885. doi: 10.1007/s11746-005-1159-1. [DOI] [Google Scholar]
- 23.Kaiho F, Koike R, Nomura H, Hara H, Maruoka K, Dohi M, Kato Y. Enhancing effect of cetyl lactate on the percutaneous absorption of indomethacin in rats. Chem Pharm Bull. 1989;37:1114–1116. doi: 10.1248/cpb.37.1114. [DOI] [PubMed] [Google Scholar]
- 24.Taskovich LT, Yum SI, Crisologo NM. Monoglyceride/lactate ester permeation enhancer for codelivery of steroids. US Patent 5,686,097, Nov 11, 1997.
- 25.Taskovich LT, Yum SI, Lee ES, Crisologo NM. Monoglyceride/lactate ester permeation enhancer. US Patent 5,750,137, May 12, 1998.
- 26.Lee ES, Watanabe T, Gale RM, Burkoth TL. Monoglyceride/lactate ester permeation enhancer for oxybutynin. US Patent 5,747,065, May 5, 1998.
- 27.Fedors RF. A method for estimating both the solubility parameters and molar volumes of liquids. Polym Eng Sci. 1974;14:147–154. doi: 10.1002/pen.760140211. [DOI] [Google Scholar]
- 28.Hatanaka T, Inuma M, Sugibayashi K, Morimoto Y. Prediction of skin permeability of drugs I: comparison with artificial membrane. Chem Pharm Bull. 1990;38:3452–3459. doi: 10.1248/cpb.38.3452. [DOI] [PubMed] [Google Scholar]
- 29.Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Lennernäs H, Hussain AS, Junginger HE, Stavchansky SA, Midha KK, Shah VP, Amidon GL. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol Pharm. 2004;1:85–96. doi: 10.1021/mp034006h. [DOI] [PubMed] [Google Scholar]
- 30.Godwin DA, Player MR, Sowell JW, Michniak BB. Synthesis and investigation of urea compounds as transdermal penetration enhancers. Int J Pharm. 1998;167:165–175. doi: 10.1016/S0378-5173(98)00060-X. [DOI] [Google Scholar]
- 31.Al-Saidan SM. Transdermal self-permeation enhancement of ibuprofen. J Control Release. 2004;100:199–209. doi: 10.1016/j.jconrel.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JH, Liu ZP, Du H, Zeng Y, Deng LD, Xing JF, Dong AJ. A novel hydrophilic adhesive matrix with self-enhancement for transdermal drug delivery. Pharm Res. 2009;26:1398–1406. doi: 10.1007/s11095-009-9850-1. [DOI] [PubMed] [Google Scholar]
- 33.Gallego JML, Arroyo JP. Simultaneous determination of dexamethasone and trimethoprim by liquid chromatography. J Pharm Biomed Anal. 2002;30:1255–1261. doi: 10.1016/S0731-7085(02)00468-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhao LG, Fang L, Xu YN, Liu S, He ZG, Zhao YY. Transdermal delivery of penetrants with differing lipophilicities using O-acylmenthol derivatives as penetration enhancers. Eur J Pharm Biopharm. 2008;69:199–213. doi: 10.1016/j.ejpb.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Bach M, Lippold BC. Percutaneous penetration enhancement and its quantification. Eur J Pharm Biopharm. 1998;46:1–13. doi: 10.1016/S0939-6411(97)00149-5. [DOI] [PubMed] [Google Scholar]
- 36.Dohi M, Kaiho F, Suzuki A, Sekiguchi N, Nakajima N, Nomura H, Kato Y. Enhancing effects of myristryl lactate and lauryl lactate on percutaneous absorption of indomethacin in rats. Chem Pharm Bull. 1990;38:2877–2879. doi: 10.1248/cpb.38.2877. [DOI] [PubMed] [Google Scholar]
- 37.Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Effect of propylene glycol on ibuprofen absorption into human skin in vivo. J Pharm Sci. 2008;97:185–197. doi: 10.1002/jps.20829. [DOI] [PubMed] [Google Scholar]
- 38.Dias M, Hadgraft J, Lane ME. Influence of membrane-solvent-solute interactions on solute permeation in skin. Int J Pharm. 2007;340:65–70. doi: 10.1016/j.ijpharm.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Hadgraft J. Dermal and transdermal drug design. Int J Pharm Med. 1999;13:155–158. [Google Scholar]
- 40.Yano T, Noda K. Skin permeability of various non-steroids anti-inflammatory drugs in man. Life Sci. 1986;39:1043–1050. doi: 10.1016/0024-3205(86)90195-5. [DOI] [PubMed] [Google Scholar]
- 41.Han SK, Park YH, Kim CK. Preparation of N-adamantyl n-alkanamides and evaluation of their transdermal penetration in the rabbit. Int J Pharm. 1995;126:35–40. doi: 10.1016/0378-5173(95)04072-2. [DOI] [Google Scholar]
- 42.Megrab NA, Williams AC, Barry BW. Oestradiol permeation through human skin and silastic membrane: effects of propylene glycol and supersaturation. J Control Release. 1995;36:277–294. doi: 10.1016/0168-3659(95)00062-D. [DOI] [Google Scholar]
- 43.Arellano A, Santoyo S, Martin C, Ygartua P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur J Pharm Sci. 1999;7:129–135. doi: 10.1016/S0928-0987(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 44.Sloan KB, Koch SAM, Siver KG, Flowers FP. Use of solubility parameters of drug and vehicle to predict flux through skin. J Invest Dermatol. 1986;87:244–252. doi: 10.1111/1523-1747.ep12696635. [DOI] [PubMed] [Google Scholar]
- 45.Sherertz EF, Sloan KB, McTiernan RG. Use of theoretical partition coefficients determined from solubility parameters to predict permeability coefficients for 5-fluorouracil. J Invest Dermatol. 1987;89:147–151. doi: 10.1111/1523-1747.ep12470550. [DOI] [PubMed] [Google Scholar]
- 46.Aungst BJ, Rogers NJ, Shefter E. Enhancement of naloxone penetration through human skin in vitro using fatty acids, fatty alcohols, surfactants, sulfoxides, and amides. Int J Pharm. 1986;33:225–234. doi: 10.1016/0378-5173(86)90057-8. [DOI] [Google Scholar]
- 47.Cooper ER, Merritt EW, Smith RL. Effect of fatty acids and alcohols on the penetration of acyclovir across human skin in vitro. J Pharm Sci. 1985;74:688–689. doi: 10.1002/jps.2600740623. [DOI] [PubMed] [Google Scholar]
- 48.Quan D, Higuchi RI, Takayama K, Higashiyama T, Nagai T. Enhancement effect of piperidone derivatives on the percutaneous absorption of indomethacin. Drug Des Deliv. 1990;6:61–71. [PubMed] [Google Scholar]
- 49.Hori M, Satoh S, Maibach HI, Guy RH. Enhancement of proparanol hydrochloride and diazepam skin absorption in vitro: effect of enhancer lipophilicity. J Pharm Sci. 1991;80:32–35. doi: 10.1002/jps.2600800109. [DOI] [PubMed] [Google Scholar]
- 50.Li GL, van der Geest R, Chanet L, van Zanten E, Danhof M, Bouwstra JA. In vitro iontophoresis of R-apomorphine across human stratum corneum: structure-transport relationship of penetration enhancement. J Control Release. 2002;84:49–57. doi: 10.1016/S0168-3659(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 51.Brain KR, Walters KA. Molecular modeling of skin permeation enhancement by chemical agents. In: Walters KA, Hadgraft J, editors. Pharmaceutical skin penetration enhancement. New York: Marcel Dekker; 1993. pp. 389–416. [Google Scholar]
- 52.Mackay KMB, Williams AC, Barry BW. Effect of melting point of chiral terpenes on human stratum corneum uptake. Int J Pharm. 2001;228:89–97. doi: 10.1016/S0378-5173(01)00808-0. [DOI] [PubMed] [Google Scholar]
- 53.Barry BW. In: Skin pharmacokinetics. Barry BW, Shroot B, Schaefer H, editors. Basel: Karger; 1987. pp. 121–137. [Google Scholar]
- 54.Liron Z, Cohen S. Percutaneous absorption of alkanoic acids II: application of regular solution theory. J Pharm Sci. 1984;73:538–542. doi: 10.1002/jps.2600730426. [DOI] [PubMed] [Google Scholar]
- 55.Nanayakkara GR, Bartlett A, Forbes B, Marriott C, Whitfield PJ, Brown MB. The effect of unsaturated fatty acids in benzyl alcohol on the percutaneous permeation of three model penetrants. Int J Pharm. 2005;301:129–139. doi: 10.1016/j.ijpharm.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 56.Moser K, Kriwet K, Naik A, Kalia YN, Guy RH. Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm. 2001;52:103–112. doi: 10.1016/S0939-6411(01)00166-7. [DOI] [PubMed] [Google Scholar]
- 57.Guy RH, Hadgraft J. Transdermal drug delivery: a simplified pharmacokinetic approach. Int J Pharm. 1985;24:267–274. doi: 10.1016/0378-5173(85)90026-2. [DOI] [Google Scholar]
- 58.Barry BW, Bennett SL. Effect of penetration enhancers on the permeation of mannitol, hydrocortisone and progesterone through human skin. J Pharm Pharmacol. 1987;39:535–546. doi: 10.1111/j.2042-7158.1987.tb03173.x. [DOI] [PubMed] [Google Scholar]