Abstract
Polymeric coating materials have been widely used to modify release rate of drug. We compared physical properties and release-controlling efficiency of polymeric coating materials using matrix-type casted film and diffusion-controlled coated tablet. Hydroxypropylmethyl cellulose (HPMC) with low or high viscosity grade, ethylcellulose (EC) and Eudragit® RS100 as pH-independent polymers and Eudragit S100 for enteric coatings were chosen to prepare the casted film and coated tablet. Tensile strength and contact angle of matrix-type casted film were invariably in the decreasing order: EC> Eudragit S100> HPMC 100000> Eudragit RS100>HPMC 4000. There was a strong linear correlation between tensile strength and contact angle of the casted films. In contrast, weight loss (film solubility) of the matrix-type casted films in three release media (gastric, intestinal fluid and water) was invariably in the increasing order: EC < HPMC 100000 < Eudragit RS100 < HPMC 4000 with an exception of Eudragit S100. The order of release rate of matrix-type casted films was EC > HPMC 100000 > Eudragit RS100 > HPMC 4000 > Eudragit S100. Interestingly, diffusion-controlled coated tablet also followed this rank order except Eudragit S100 although release profiles and lag time were highly dependent on the coating levels and type of polymeric coating materials. EC and Eudragit RS100 produced sustained release while HPMC and Eudragit S100 produced pulsed release. No molecular interactions occurred between drug and coating materials using 1H-NMR analysis. The current information on release-controlling power of five different coating materials as matrix carrier or diffusion-controlled film could be applicable in designing oral sustained drug delivery.
Key words: diffusion-controlled coated tablet, drug release rate, matrix-type casted film, polymeric coating materials, release-controlling power
INTRODUCTION
Numerous oral modified-release dosage forms are widely used for many therapeutic reasons. Polymeric coating materials have been widely used for achieving modified release of various dosage forms to provide sustained or pulsed drug release (1–6). Pharmaceutically available polymers such as polymethacrylates (Eudragit RS100, and Eudragit S100), hydroxypropylmethyl cellulose (HPMC), and ethylcellulose (EC) in either a single or mixed composition are widely applicable as matrix carriers and coating materials of pharmaceutical dosage forms to obtain modified releases.
There are numerous reports to investigate the effect of coating formulations (coating levels, solvent, particle size, type of polymer and plasticizer, etc.) and processing parameters (air pressure and temperature, etc.) on physicochemical properties of the coated films and drug release using a single polymer or blends of various polymers (2,7–9). The physicochemical properties of the coated film in a single or binary blend of coating materials are related with drug release profiles from coated dosage forms. Among them, tensile strength, contact angle (wettability), and weight loss (solubility) of coating materials in release media are the most key factors which influence drug release (2,8,10,11).
However, pharmaceutical product formulation and processing are very complex tasks and it is often difficult to optimize the drug release patterns. The release-controlling power of coating materials is very variable and scattered even though the coating formulation and processing conditions are fixed. Recently, neurofuzzy logic, neural networks, and decision trees were successfully developed as predictive models for tensile strength and drug release profiles of immediate release tablet formulation to overcome the complex non-linear relationship between formulation composition, process conditions, and product properties (12,13). Therefore, establishment of physical properties and release-controlling efficiencies of the various coating materials are needed to produce the intended controlled-release profiles as well as to select proper coating materials in dosage form design. So far, no comparison of release-controlling power of different polymeric coating materials has been investigated despite the wide applications of coating materials for controlled drug delivery in pharmaceutical sciences.
The aims of this work were to compare physical properties and release-controlling efficiency of polymeric coating materials using matrix-type film and diffusion-controlled coated tablet. Five pharmaceutically available coating materials were chosen for comparison. HPMC 4000, HPMC 100000, EC and Eudragit® RS100 are pH-independent polymers while Eudragit® S100 is a pH-dependent enteric polymer. Acetaminophen (APAP) was chosen as a model drug. Release profiles of casted films and coated tablets were evaluated in enzyme-free simulated gastric fluid (SGF) for 2 h and subsequently continued in simulated intestinal fluid (SIF). Physical properties such as tensile strength, contact angle, weight loss (film solubility) in media (SGF, SIF, and water) and surface morphology of the matrix-type casted films were characterized. Release rate of matrix-type casted film and diffusional-controlled coated tablet were then investigated. Molecular interactions between drug and coating materials were also investigated using 1H NMR analysis.
MATERIALS AND METHODS
Materials
APAP and cross-linked carboxymethyl cellulose sodium (Ac-Di-Sol®) were obtained from Korea United Pharm. Inc. (Seoul, Korea). Eudragit S100 and RS100 were provided as a courtesy of Degussa (Seoul, Korea). Two different kinds of HPMC (Methocel grade K4MP and K100MP) with the same particle size (90SH type) were provided by Richwood (Seoul, Korea). Ethyl cellulose (ethoxy content, 48.2%) and dibutyl sebacic acid (DBS) were purchased from Sigma (St. Louis, MO). Talc, a purified and hydrated magnesium silicate, was purchased from Showa (Tokyo, Japan). Magnesium stearate was purchased from Katayama (Osaka, Japan). All other chemicals were reagent grade and used without further purification.
Methods
Preparation of Polymeric Coating Solutions
The formulation compositions of the polymeric coating solutions are shown in Table I. Eudragit S100 and Eudragit RS100 were dissolved in organic solvents. HPMC was dissolved in a mixture of ethanol and deionized water. The talc and DBS (20% based on solid polymer content) were added to the coating solutions and then stirred for 24 h to ensure sufficient plasticization of the polymer. DBS was used as a plasticizer. The concentration of the polymer in the solvent was 10% by weight, except for EC, which was 5%.
Table I.
Formulation Compositions for the Preparation of Polymeric Coating Solution (g)
| Polymera | DBS | Talc | Solvents | |||
|---|---|---|---|---|---|---|
| Acetone | Isopropyl- alcohol | Ethanol | Water | |||
| Eudragit S100 | 2 | 0.5 | 30 | 100 | – | – |
| Eudragit RS100 | 2 | 0.5 | 30 | 100 | – | – |
| HPMC 4000 | 2 | 0.5 | – | – | 150 | 30 |
| HPMC 100000 | 2 | 0.5 | – | – | 150 | 30 |
| EC | 2 | 0.5 | 140 | – | 60 | – |
aSolid contents of polymeric solution used was invariably 10%
Preparation of Matrix-type Casted Films
The solvent-based matrix-type casted films were prepared from polymeric coating solutions as described previously (8). The polymeric coating solution in an ethanol:deionized water (9:1) mixture was casted onto a glass plate that was placed on a level, flat surface and then slowly evaporated at ambient conditions for about 72 h. Film thickness (approximately 300 μm) was measured in triplicate using a dial thickness gauge (Mitutoyo, #7326, Tokyo, Japan) with a micrometer unit. Measurements were taken at three positions on each specimen for determining average tensile strength. The films were cut into bow-shaped sections. The rectangular matrix-type casted film with 10 cm length, 2 cm ends and 300 μm thickness was used for tensile strength determinations, while film with 2.5 cm length, 2 cm ends and 300 μm thickness was used for the weight loss tests and surface morphology using SEM.
The matrix-type casted films containing drug were also prepared by adding 5 g APAP to five coating solutions and then the process was repeated as described above.
Weight Loss of Matrix-type Casted Film in Release Media
The weight loss (film solubility) of matrix-type casted films was determined by gravimetric analysis. Casted films (approximately 500 mg) were weighed (W1), immersed in 900 mL of media (SGF, SIF, and deionized water), and maintained at 37 ± 0.5°C. The dissolution apparatus 1 (basket; Fine Scientific DST600A, Seoul, Korea) was rotated for 3 h at a rate of 100 rpm. After 3 h, film residues were gently removed from the vessels and completely dried at 40°C using a vacuum drying oven (Misung Engineering Co., Seoul, Korea) until a constant mass was reached (W2). Each experiment was performed in triplicate. The weight loss of polymeric film was then calculated according to the following equation:
 |
1 |
Tensile Strength of Matrix-type Casted Film
The matrix-type casted films were preconditioned for at least 48 h in a constant temperature humidity chamber set at 23°C and 50% relative humidity before testing. Tensile strength and percent elongation at break of rectangular film samples were examined using a Universal Testing Machine (Model-LR10K, TIRA Mechinehban GmbH, Germany) with an extension speed of 1.0 mm/min. The test procedure was based on the ASTM D638 method. The stress–strain curve was recorded for each film sample, and the mechanical parameters (tensile strength and elongation) were determined in triplicate.
Contact Angle of Matrix-type Casted Film
Polymeric coating materials were completely dissolved in solvents (see Table I) and filtered through a PTFE syringe filter with a pore size of 0.2 μm. The clear solution was then spin-coated onto a silicon wafer at a speed of 3,000 rpm for 30 s using a Head-Way PM101DT-R485 spinner (Shinu M.S.T Co. Ltd) and then dried in air to form a thin film. Contact angles were measured by the sessile drop technique using a contact angle goniometer (DSA 100, KRUSS GmbH, Germany). In each experiment, a drop of deionized water was placed on the surface of the polymeric thin film at room temperature. The measurements of contact angles were taken five times by direct reading of different zones of the polymeric thin films.
Surface Morphology of Matrix-type Casted Film Using SEM
Surface morphology of matrix-type casted film was determined using scanning electron microscopy (SEM) to evaluate the coating integrity. The casted films were coated with gold under an argon atmosphere using a Jeol JFC-1100 sputter coater (Jeol, Japan) at the Korean Basic Science Institute (Chuncheon, Korea) for about 2 min to obtain a 200 Å coating thickness. Micrographs were taken with a Cambridge Stereo Scan 200 (London, UK) at an accelerating voltage of 15 kV.
Preparation of Core Tablets
Powders containing drug, Avicel®, superdisintegrant (Ac-Di-Sol), magnesium stearate, and talc (66.7:30.7:1.3:0.65:0.65 w/w%) were thoroughly blended together using a mortar and pestle. The resulting powder mixtures were directly compressed into tablets using a rotary tablet machine (Korea Machine, Seoul, Korea) equipped with a capsule-shaped punches (small outside diameter, 8.6 mm and large outside diameter, 16 mm). The hardness of tablets was in triplicate measured using the Erweka® hardness tester (Model SVM-12, Heusenstamm, Germany). The mean weight and hardness of 30 core tablets were 450.0 ± 24.0 mg and 100.0 ± 20.0 N, respectively.
Preparation of Coated Tablets
The core tablets were manually coated with various polymeric coating solutions by dipping. The core tablet was completely dipped into the polymeric suspension and was then dried with a hair dryer. The dipping steps were repeated until desired coating levels, defined as percent of weight gain compared to uncoated core tablets, were obtained.
Release Studies
Release studies were performed in triplicate using the KP VIII dissolution paddle method (Fine Scientific DST600A, Seoul, Korea) at 37 ± 0.5°C with a stirring speed of 50 rpm. The enzyme-free SGF and SIF were prepared according to the method previously reported (6). APAP solubility was not affected by pH condition. It was previously reported that APAP solubility in water (approximately 15 mg/ml) is almost constant over a physiological range of pH 1–7 (14). Therefore, it is unnecessary to perform the release test in the sink condition. Matrix-type casted films or diffusion-controlled coated tablet was placed in 900 ml of the SGF (pH 1.2 ± 0.1) for 2 h and then switched to 900 ml of the SIF (pH 6.8 ± 0.1) for 24 h. The grilles were used to prevent floating of the casted films. Five milliliters of release media were collected at given time intervals and replaced with an equal volume of temperature-equilibrated media. The collected media was filtered through a 0.45-μm membrane (regenerated cellulose), and immediately diluted with the mobile phase for prevention of drug precipitation. The concentration of dissolved drug was measured using a UV–Vis spectrophotometer at a wavelength of 254 nm.
The time required for the percentage of the dose to be released was designated as T%. For example, the time required for 100% of the 300 mg dose to be released was designated as T100%. It indicates that the higher T%, the lower drug release rate. T% was directly obtained from the release-time curves. The lag time (Tlag time) was also determined from the x-intercept of the release profiles.
1H-NMR Analysis
To study molecular interactions between the drug, DBS, and polymeric coating materials, 1H-NMR spectra were measured. For 1H-NMR studies, the lipophilic polymers, Eudragit RS100, Eudragit S100, and EC, and their mixtures with DBS or APAP were dissolved in DMSO. D2O was used in the case of hydrophilic HPMC. The 1H-NMR for each specimen was measured at 24°C using a 600 MHz Brucker Avance spectrometer (JEOL, Germany). 1H-NMR spectra of APAP and DBS were also compared as references.
Data Analysis
All data were expressed as average ± standard deviations. Linear regression modeling between tensile strength and contact angle among five polymeric coating materials was used for comparison using Sigmaplot 2004, Systat Software, Inc. (San Jose, CA, USA). Data were analyzed using SAS program (SAS Institute, Inc., version 9.1) for statistical differences by a one-way ANOVA, applying Duncan’s multiple comparison test. A p ≤ 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Surface Morphologies of the Matrix-type Casted Film
Film surface morphology of the casted films was further characterized by SEM (Fig. 1). The surface of the films was homogenous and smooth. No phase separation or aggregation was observed. Non-uniform, rough, and small pores were observed in the case of casted EC films. However, these phenomena were not related to drug release rates because EC had high tensile strength and longer drug release compared with other coating polymers as described below.
Fig. 1.
Surface morphology of the matrix-type casted films using SEM
Physical Properties of Matrix-type Casted Film and Their Correlations
To compare the release-modulating power of five polymeric coating materials, the casted films with identical composition of tablet coatings were prepared and their physical properties, such as tensile strength, elongation, contact angle, and weight loss (solubility) in release media were characterized. The physical properties of the matrix-type casted films are then compared in Table II. Incorporation of different coating polymers into the coated films significantly changed the physical properties by the ANOVA test, applying multiple comparison (p < 0.05). However, the contact angle and weight loss between Eudragit S100 and EC had no statistical significance. Tensile strength is defined as the maximum stress sustained by the material (15). Tensile strength test is a general approach used to evaluate the mechanical properties of coated films in pharmaceutical coating. Film tensile strength revealed the following rank order: EC ≥ Eudragit S100 > HPMC 100000 > Eudragit RS100 > HPMC 4000, indicating that EC was strong and flexible. Tensile strength of HPMC 4000 and Eudragit RS100 film exhibited the lowest mechanical strength (about 1,300 N/m2)
Table II.
Comparison of Physical Properties of the Matrix-type Casted Films
| Parameters | Eudragit S100 | Eudragit RS100 | HPMC 4000 | HPMC 100000 | EC |
|---|---|---|---|---|---|
| Tensile strength (N/m2)a | 2,040 ± 62 | 1,418 ± 18 | 1,265 ± 71 | 1,614 ± 88 | 2,213 ± 57 |
| Elongation (%)a | 14 ± 0.33 | 27 ± 0.25 | 31 ± 0.41 | 22 ± 0.29 | 12 ± 0.28 |
| Contact angle (o)b | 65 ± 2.91 | 31 ± 0.47 | 13 ± 1.03 | 43 ± 1.91 | 68 ± 0.79 |
| Weight loss in SGF (%)b | 0.45 ± 0.12 | 4.79 ± 0.79 | 9.32 ± 0.11 | 2.05 ± 0.12 | 0.24 ± 0.13 |
| Weight loss in water (%)a | 1.43 ± 0.25 | 4.13 ± 0.74 | 9.24 ± 0.38 | 2.48 ± 0.22 | 0.41 ± 0.41 |
| Weight loss in SIF (%)a | 18.95 ± 0.81 | 4.33 ± 0.07 | 10.02 ± 0.60 | 2.49 ± 0.28 | 0.25 ± 0.13 |
Each value represents the mean ± SD (n = 3)
aSignificantly different among matrix-type casted films by ANOVA test, applying multiple comparisons (p < 0.05)
bSignificantly different among matrix-type casted films by multiple comparisons except Eudragit S100 and EC
To confirm the wettability of the polymeric coating films in release media, the contact angles of water on the hydrophilic and hydrophobic surfaces of polymer films were determined. The order of the contact angles was EC > Eudragit S100 > HPMC 100000 > Eudragit RS100 > HPMC 4000. A contact angle is an indication of degree of wetting, and the higher the less degree of wetting (15). Generally, a contact angle above 90° indicates poor wettability, and a reduced contact angle improves wetting and drug release (11). EC had a high contact angle (68°) compared with the other coating materials due to their hydrophobic properties. Interestingly, a linear correlation of tensile strength and contact angle of the matrix-type casted films was observed (Fig. 2). There was a positively strong correlation between tensile strength and contact angle of the five casted films. The higher the tensile strength, the higher the contact angle was obtained, giving poor wettability of the coating films in release media.
Fig. 2.
Linear correlation of tensile strength and contact angle of the matrix-type casted films
In addition to the tensile strength and contact angle, film weight loss (solubility) was investigated in three different media to index drug release patterns. Film weight loss indicates the extent of surface erosion and polymer degradation from coated tablets via subsequent water uptake. Water uptake, erosion, and drug release occur simultaneously during the release of drug from coated films (2,7,8). The rate of weight loss of the coated films is also affected by the testing media due to their dependence on film solubility.
The order of weight loss was invariably HPMC 4000 > Eudragit RS100 > HPMC 100000 > Eudragit S100 > EC in deionized water and SGF and these results are in accordance with the tensile strength results. However, this order was Eudragit S100 > HPMC 4000 > Eudragit RS100 > HPMC 100000 > EC in SIF. Eudragit S100, an enteric polymer, showed the highest weight loss (18.95 ± 0.81%) due to its high solubility in high pH conditions. In contrast, the repeating carboxyl groups of the Eudragit S100 is un-ionized in SGF produces a closely packed structure, leading to reduced release.
Release Rates of Matrix-type Casted Film and Diffusion-controlled Coated Tablets
Release rate of drug from matrix-type casted films are shown in Fig. 3. Release profiles and lag time were also highly dependent on the type of polymeric coating materials. In the case of Eudragit S100, lag time was observed at 2 h due to its resistance to SGF as an enteric coating polymer. In contrast, release profiles of other matrix-type casted films showed sustained release with no distinct lag time. The order of T100% of matrix-type casted films was the decreasing order: EC > HPMC 100000 > Eudragit RS100 > HPMC 4000 > Eudragit S100.
Fig. 3.
Release rate profiles from matrix-type casted films
The effect of types and levels of polymeric coating materials on drug release rate from coated tablets is also shown in Fig. 4. The uncoated core tablet showed immediate release within 1 h. Release rate decreased as invocating levels increased. In the case of Eudragit S100, no lag time was observed at 5% coating level due to the high solubility of APAP, even though Eudragit S100 is an enteric coating polymer. As the film thickness increased, lag time accordingly increased in a pulsed manner. About 2.75 h lag time was obtained at 10% coating levels. In case of Eudragit RS100, release rate gradually decreased in a sustained fashion with no distinct lag time as the coating level increased, even at the 10% coating level. Eudragit RS100 was not efficient to modulate the lag time for pulsed release. In contrast, the lag time of tablet coated with EC was also significantly increased (0.75 h to 4 h) as the coating levels increased. After a determined lag time, there was sustained release of drug from the coated tablets. Release profiles of tablets coated with different viscosity grades of hydrophilic HPMC at different coating levels were also compared. About 14% APAP slowly released in a near-zero-order fashion for 4 or 8 h when 10% or 15% coatings with high-viscosity-grade HPMC 100000 was applied, respectively. Thereafter, drug was gradually released in a pulsed manner. We reported that high-viscosity-grade HPMC100000 could produce pulsed release after coating of the core tablet (3). In contrast, low-viscosity-grade HPMC 4000 gave much faster release and a 1.5 h lag time. High-viscosity HPMC retarded release rate more significantly and gave a longer lag time for pulsed release due to its swelling and gelling capacity. Drug release from tablets coated with HPMC is highly dependent on the viscosity grade of the HPMC and its coating levels (3,4).
Fig. 4.
Effect of types and levels of coating materials on release rate from diffusion-controlled coated tablets
Release T10–100% (time to release % drug) increased as the coating levels increased in all formulations tested. Release profiles and lag time were highly dependent on the coating levels and type of coating materials. Pulsed release with a lag time was obtained when core tablets were coated with HPMC, EC, or Eudragit S100 in the following lag time order: HPMC 100000 > Eudragit S100 > HPMC 4000 > EC > Eudragit RS100, whereas the T100% was EC > HPMC 100000 > (Eudragit S100) > Eudragit RS100 > HPMC 4000. The EC and Eudragit RS100 produced sustained release while HPMC and Eudragit S100 produced pulsed release. These results are in accordance with release rate of matrix-type casted film with an exception of pH-dependent Eudragit S100. Thus, matrix-type casted films among coating materials could be also used as an index to expect release rate of diffusion-controlled coated tablet.
Drug release rate tended to decrease as the tensile strength increased, and EC, with the highest tensile strength, showed the slowest release rate. A harder film must have a high tensile strength (stress) and a large extension before breaking. As the contact angle of the film increased, T100% of the drug took longer. It was reported that a negative correlation between contact angle of the triglyceride film and the amount of drug release (or positive correlation with T%) (11). It was known that the extent of weight loss in release media was correlated with drug release rate from coated dosage forms (2,7). In addition to thickness (coating levels), weight loss in release media was crucial to the drug release rate because the high weight loss (solubility) of the film increased the drug release rate (2).
1H-NMR spectra for Molecular Interaction of Coating Materials
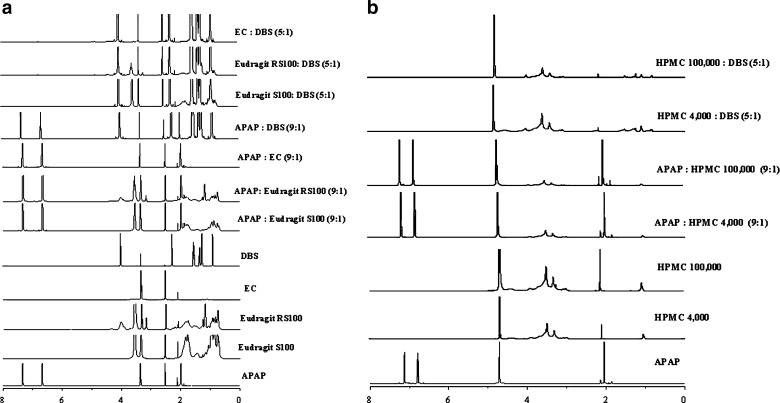
We also used 1H-NMR to investigate the molecular interactions between coating materials. Figure 5 shows the 1H-NMR spectra of APAP, DBS, and polymeric coating materials and their mixtures in DMSO or D2O. Specific APAP peaks appeared at 1.97, 6.66, and 7.33 ppm, respectively, whereas DMSO and D2O solvent peaks were at 2.5, 3.34 (Fig. 5a), and 4.72 ppm (Fig. 5b), respectively. The peaks of binary mixtures of APAP/polymer, APAP/DBS, and polymers/DBS were unchanged and appeared at the same position as peaks of the single component, although peak intensity decreased due to the decreased mixture ratio. Thus, no chemical degradation or complexation of the coating materials occurred during the coating process. In addition, the molecular interaction between drug and polymeric coating materials was not a factor to modulate release rates of coated tablets.
Fig. 5.
1H-NMR spectra of APAP, Eudragit S100, Eudragit RS100, HPMC4000, HPMC 100000, EC, DBS, and their mixtures in DMSO (a) or D2O solution (b)
CONCLUSIONS
Release-controlling efficiencies of polymeric coating materials using matrix-type film and diffusion-controlled coated tablet were compared. The release rate of drug from coated tablet was rather faster as compared to matrix-type film. The order of release rate of matrix-type casted films and diffusion-controlled coated tablet was invariably followed: EC > HPMC 100000 > Eudragit RS100 > HPMC 4000 with an exception of Eudragit S100. Interestingly, release-controlling efficiency of coating materials using matrix-type casted films was well matched with diffusion-controlled coated tablet in rank order of release rate. The physical parameters among coating materials such as tensile strength, contact angle and weight loss (film solubility) in media showed this rank of order. A strong linear correlation between tensile strength and contact angle was observed. Thus, the current information can be also useful for understanding release-modulating power and selection guidelines of different coating materials in preparing oral sustained drug delivery.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF- R01-2008-000-11777-0), and by a grant of the 2009 Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Korea. The authors are grateful to Mr. Kyu-Yeol Nam for his partial contributions to the experiments. We also thank the Central Research Laboratory for the use of the 1H-NMR and the Research Institute of Pharmaceutical Sciences, Kangwon National University for allowing the use of their UV–Vis spectrometer.
References
- 1.Lee B-J, Ryu S-G, Cui J-H. Controlled release of dual drug-loaded hydroxypropyl methyl cellulose matrix tablet using drug-containing polymeric coatings. Int J Pharm. 1999;188:71–80. doi: 10.1016/S0378-5173(99)00204-5. [DOI] [PubMed] [Google Scholar]
- 2.Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Blends of enteric and GIT-insoluble polymers used for film coating: physicochemical characterization and drug release patterns. J Contr Rel. 2003;89:457–471. doi: 10.1016/S0168-3659(03)00155-X. [DOI] [PubMed] [Google Scholar]
- 3.Cao Q-R, Choi H-G, Kim D-C, Lee B-J. Release behavior and photo-image of nifedipine tablet coated with high viscosity grade hydroxypropylmethylcellulose: effect of coating conditions. Int J Pharm. 2004;274:107–117. doi: 10.1016/j.ijpharm.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Sangalli ME, Maroni A, Foppoli A, Zema L, Giordano F, Gazzaniga A. Different HPMC viscosity grades as coating agents for an oral time and /or site-controlled delivery system: a study on process parameters and in vitro performances. Eur J Pharm Sci. 2004;22:469–476. doi: 10.1016/j.ejps.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Cao Q-R, Lee E-S, Choi Y-J, Lee B-J. Rumen bypass and biodistribution of l-carnitine from dual-layered coated pellets in cows, in vitro and in vivo. Int J Pharm. 2008;359:87–93. doi: 10.1016/j.ijpharm.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Piao Z-Z, Lee M-K, Lee B-J. Colonic release and reduced intestinal tissue damage of coated tablets containing naproxen inclusion complex. Int J Pharm. 2008;350(1–2):205–211. doi: 10.1016/j.ijpharm.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 7.Siepmann F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Blends of aqueous polymer dispersions used for pellet coating: importance of the particle size. J Contr Rel. 2005;105:226–239. doi: 10.1016/j.jconrel.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Bando H, McGinity JW. Physicochemical properties of enteric films prepared from aqueous dispersions and organic solutions. Int J Pharm. 2006;313:43–48. doi: 10.1016/j.ijpharm.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Kim T-W, Ji C-W, Shim S-Y, Lee B-J. Modified release of coated sugar spheres using drug-containing polymeric suspensions. Arch Pharm Res. 2007;30(1):124–130. doi: 10.1007/BF02977788. [DOI] [PubMed] [Google Scholar]
- 10.Heng PWS, Wan LSC, Tan YTF. Relationship between aggregation of HPMC coated spheroids and tackiness/viscosity/additives of the coating formulations. Int J Pharm. 1996;138:57–66. doi: 10.1016/0378-5173(96)04529-2. [DOI] [Google Scholar]
- 11.Koennings S, Berié A, Tessmar J, Blunk T, Goepferich A. Influence of wettability and surface activity on release behavior of hydrophilic substances from lipid matrices. J Contr Rel. 2007;119:173–181. doi: 10.1016/j.jconrel.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Shao Q, Rowe RC, York P. Comparison of neurofuzzy logic and decision trees in discovering knowledge from experimental data of an immediate release tablet formulation. Eur J Pharm Sci. 2007;31:129–136. doi: 10.1016/j.ejps.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Shao Q, Rowe RC, York P. Comparison of neurofuzzy logic and neural networks in modelling experimental data of an immediate release tablet formulation. Eur J Pharm Sci. 2006;28(5):394–404. doi: 10.1016/j.ejps.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Sako K, Mizumoto T, Kajiyama A, Ohmura Y. Influence of physical factors in gastrointestinal tract on acetaminophen release from controlled-release tablets in fasted dogs. Int J Pharm. 1996;137:225–232. doi: 10.1016/0378-5173(96)04524-3. [DOI] [Google Scholar]
- 15.Yoo JW, Dharmala K, Lee CH. The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int J Pharm. 2006;309:139–145. doi: 10.1016/j.ijpharm.2005.11.020. [DOI] [PubMed] [Google Scholar]