Abstract
The purpose of this study was to improve dissolution behavior of poorly water-soluble drugs by application of cyclodextrin in extrusion processes, which were melt extrusion process and wet extrusion process. Indomethacin (IM) was employed as a model drug. Extrudates containing IM and 2-hydroxypropyl-β-cyclodextrin (HP-β-CyD) in 1:1 w/w ratio were manufactured by both melt extrusion process and wet extrusion process. In vitro drug release properties of IM from extrudates and physiochemical properties of extrudates were investigated. The dissolution rates of IM from extrudates manufactured by melt extrusion and wet extrusion with HP-β-CyD were significantly higher than that of the physical mixture of IM and HP-β-CyD. In extrudate manufactured by melt extrusion, γ-form of IM changed to amorphous completely during melt extrusion due to heating above melting point of IM. On the other hand, in extrudate manufactured by wet extrusion, γ-form of IM changed to amorphous partially due to interaction between IM and HP-β-CyD and mechanical agitating force during process. Application of HP-β-CyD in extrusion process is useful for the enhancement of dissolution rate for poorly water-soluble drugs.
KEY WORDS: 2-hydroxypropyl-β-cyclodextrin, dissolution, extrusion, indomethacin, poor water-soluble drug
INTRODUCTION
Poorly water-soluble drugs often show low bioavailability when administered orally, since the absorption of the drugs in the gastrointestinal tract can usually be a rate-limiting step. Therefore, it is important for such kind of drugs to enhance their dissolution rate. Some of the dissolution-enhancing methods have been applied for the production of pharmaceutical preparations. Various studies have been reported in attempt to improve solubilities of poorly water-soluble drugs. Increasing the available surface area for dissolution via particle size reduction is one of the oldest methods for improving the dissolution rates of poorly water-soluble drugs (1). Solid dispersion systems have been widely studied and repeatedly shown to improve the dissolution properties of poorly water-soluble drugs (2,3).
Cyclodextrins (CyDs) comprise a family of cyclic oligosaccharides, and several members of this family are used industrially in pharmaceutical and allied applications. CyDs are potential candidates because of their ability to alter physical, chemical, and biological properties of guest molecules through the formation of inclusion complexes (4,5). Recently, various kinds of CyD derivatives have been prepared so as to extend the physicochemical properties and inclusion capacity of natural CyDs as novel drug carriers (6–9). 2-Hydroxypropyl-β-cyclodextrin (HP-β-CyD) has superior properties (a highly soluble, amorphous powder with no detectable oral toxicity) as pharmaceutical additive. HP-β-CyD can modify the release rate of poorly water-soluble drugs, which can be used for the enhancement of drug absorption across biological barriers, serving a potent drug carrier in the immediate release formulations (10–12) and be useful for inhibition of polymorphic transition and crystallization rates of poorly water-soluble drugs during storage, which can consequently maintain the higher dissolution characteristics and oral bioavailability of the drugs (13–15).
Extrusion process is one of the processes of applying pressure to mass until it flows through an orifice or a defined opening (16). An extruder is a mixer that operates continuously and involves processes such as kneading, shearing, heating, melting, and cooling. The extruder has been used for polymer processing and in the plastic sand food industries (17,18). Melt extrusion may be applied to disperse drugs in a given matrix down to the molecular level, e.g., to form a true solution. It is the convenience of the technology that gives new hope to the glass or solid solution approach as a delivery system for poorly soluble drugs. The use of melts in order to obtain solid molecular dispersions, e.g., glass or solid solutions, is well known, and the essential advantage of a melt process in this domain is its solvent-free formation of such dispersions (19). The melt extrusion process is capable of handling active agents of different particle sizes as well as amorphous solids or other polymorphic forms leading to the same product. Wet granulation is considered one of the most important processes in the manufacturing of solid dosage forms. Production of solid dosage forms using granules has several advantages such as enhanced flowability, improved compactability, reduced segregation, and less dust. In the pharmaceutical industry, the use of a twin-screw extruder for wet extrusion was introduced by Gamlen and Eardley (20). Kleinebudde and Lindner (21) performed preliminary studies with an instrumented, co-rotating twin-screw extruder. Schroeder and Steffens (22) used rotary screw extrusion for continuous wet extrusion. Lindberg et al. (23–25) were the first to report on the possibility of using a twin-screw extruder for the continuous granulation of an effervescent paracetamol preparation.
Recently, there were some reports about application of CyDs in extrusion process to improve stability and dissolution behavior of poorly water-soluble drugs. Rambali et al. (26) used a melt extrusion process with HP-β-CyD to improve the poor solubility of itraconazole. In other studies, Fukuda et al. (27) investigated the influence of sulfobutyl ether-β-CyD on the dissolution properties of ketoprofen from extrudates prepared by melt extrusion at a processing temperature close to the melting point of ketoprofen, but below the melting point of sulfobutyl ether-β-CyD, the dissolution rate of ketoprofen from extrudate was significantly higher than both the physical mixture and melt extrudate prepared with parent β-CyD.
However, no literature was presented so far on the comparison of dissolution behavior and physicochemical properties of extrudate between melt extrusion and wet extrusion with application of CyDs in extrusion process.
In this study, indomethacin (IM), which is a model for poorly water-soluble drugs, and HP-β-CyD were used. The improvement of dissolution behavior of IM by application of HP-β-CyD in extrusion process was investigated, and the mechanism of enhancing effect for dissolution properties in both melt extrusion process and wet extrusion process was evaluated.
MATERIALS AND METHOD
Materials
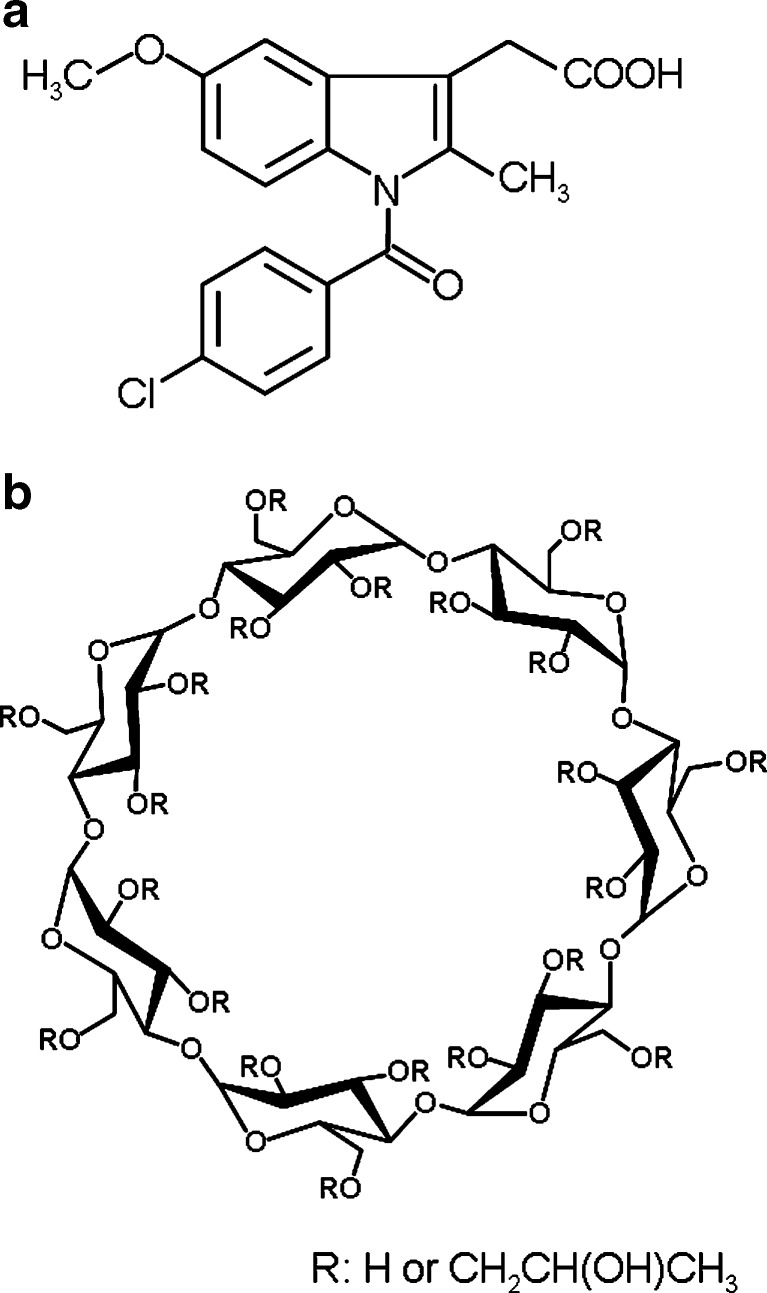
IM was purchased from Sogo Pharmaceutical Co., LTD. (Tokyo, Japan). HP-β-CyD, with the degree of substitution of 4.8, was purchased from Nihon Shokuhin Kako Co., LTD. (Tokyo, Japan). The chemical structures of IM and HP-β-CyD are shown in Fig. 1. Lactose monohydrate (GranuLac® 200) was given as a gift by Molkerei Meggle Wasserburg GmbH & Co. KG (Wasserburg, Germany). Dicalcium phosphate (Dicafos® C92-22) was given as a gift by Chemische Fabrik Budenheim KG (Budenheim, Germany). Microcrystalline cellulose (MCC Sanaq® 101) was given as a gift by Pharmatrans Sanaq AG (Basel, Switzerland). All other chemicals and solvents were of analytical reagent grade, and deionized water was used throughout study.
Fig. 1.
Chemical structures of IM (a) and HP-β-CyD (b)
Methods
Sample Preparation
Melt Extrusion
IM and HP-β-CyD were weighed in a 1:1 w/w ratio (500 g/batch) and mixed in a high-shear mixer (Mini-MGT, Huluerscheidt, Germany) at 300 rpm for 3 min. The powder mixture was gravimetrically fed by a dosing device (KT20K-Tron Soder, Lenzhard, Switzerland) into the barrel of a co-rotating twin-screw extruder (Mikro 27GL-28D, Leistritz, Nürnberg, Germany). The die head contained one hole exhibiting 2 mm diameter. Since the melting range of IM is reported to be 155–162°C in Japanese Pharmacopeia XV (JP XV), the processing temperatures were set at 140°C (zone 1), 173°C (zone 2), 173°C (zone 3), 173°C (zone 4), 160°C (zone 5), 140°C (zone 6), and 130°C (zone 7) in order to melt IM in the heated barrel of the extruder and to obtain extrudates. A constant screw speed was kept at 150 rpm with a fixed powder feeding rate of 40 g/min. Following extrusion, the extrudates were gently ground into a fine powder using a mortar and pestle and then passed through a 180-μm screen for further studies.
Wet Extrusion
IM and HP-β-CyD were weighed in a 1:1 w/w ratio (500 g/batch) and mixed in a high-shear mixer (Mini-MGT, Huluerscheidt, Germany) at 300 rpm for 3 min. The powder mixture was gravimetrically fed by a dosing device (KT20K-Tron Soder, Lenzhard, Switzerland) into the barrel of a co-rotating twin-screw extruder (Mikro 27GL-28D, Leistritz, Nürnberg, Germany). The die plate contained 23 holes exhibiting 1 mm diameter, 2.5 mm thickness. The temperature of the extruder barrel was set at 25°C. A constant screw speed was kept at 150 rpm with a fixed powder feeding rate of 50 g/min and a fixed liquid (deionized water) feeding rate of 6 g/min. Following extrusion process, the wet extrudates were dried at 40°C below 100 hPa for 12 h. Following drying process, the extrudates were gently ground into a fine powder using a mortar and pestle and then passed through a 180-μm screen for further studies.
Physical Mixture
IM and HP-β-CyD were weighed in a 1:1 w/w ratio (5 g/batch) and mixed by using a mortar and pestle for 5 min. Following mixing, the sample was passed through a 180-μm screen.
Heat-Treated Sample
IM and other excipients (HP-β-CyD or dicalcium phosphate) were weighed in a 1:1 w/w ratio (5 g/batch) and mixed by using a mortar and pestle for 5 min. The powder mixture was placed in an oven and heated at 170°C for 5 min. Following heating, the sample was gently ground into a fine powder using a mortar and pestle and then passed through a 180-μm screen.
Kneaded Sample
IM and other excipients (HP-β-CyD, lactose monohydrate, or dicalcium phosphate) were weighed in a 1:1 w/w ratio (5 g/batch) and mixed by using a mortar and pestle for 5 min. The powder mixture and deionized water (10% w/w) were kneaded for 5 min and dried at 40°C overnight below 100 hPa for 12 h. Following drying, the sample was gently ground into a fine powder using a mortar and pestle and then passed through a 180-μm screen.
Amorphous IM
Amorphous IM was prepared to estimate its solid characteristics by melt quenching method (28); γ-form of IM (2 g) was heated at 170°C for 5 min. After melting IM, the melted IM was poured in liquid nitrogen quickly. After quenching, the material was milled softly by using mortar and pestle. Melt-quenched sample was stored at −20°C.
Differential Scanning Calorimetry
Thermograms were recorded using a differential scanning calorimetry (DSC) 821e calorimeter (Mettler-Toledo, Gießen, Germany). Hermetically sealed aluminum pans contained weighed samples of approximately 5 mg. Experiments were conducted twice with a heating rate of 10°C/min within a temperature range of 20–250°C.
X-ray Powder Diffraction
Diffractograms were recorded with X-ray powder diffractometer (D8 Advance powder-diffractometer, Bruker AXS GmbH, Karlsruhe, Germany). The X-ray source was Cu Kα1 radiation under 40 kV and 40 mA. The scanning range (2θ) was from 5–50°, and the scan step and the measuring time were 0.0198° and 1 s per step, respectively.
Dissolution Study
The dissolution test of samples (equivalent to 50 mg IM) was carried out in a medium consisting 900 mL phosphate buffer (pH 6.8) using the paddle method. The stirring speed was kept constant at 100 rpm and the temperature was 37 ± 0.5°C during dissolution testing. The samples used for dissolution studies were prepared by mixing with microcrystalline cellulose at a weight ratio of 1:1 to prevent agglomeration of material at initial point of test. The drug concentration in solution was determined at 318 nm with a UV-Vis spectrometer (Lambda 40, Perkin-Elmer, Rodgau-Juegesheim, Germany). Each experiment was conducted in triplicate. Relative area under the curve (AUC) ratio was calculated to investigate dissolution enhancing effect based on dissolution profile using following equation.
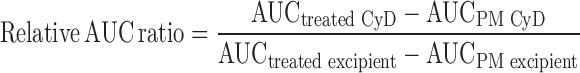 |
1 |
where AUCtreated CyD is area under the curve of dissolution profile for heat-treated or kneaded sample containing IM and HP-β-CyD, AUCPM CyD is area under the curve of dissolution profile for physical mixture containing IM and HP-β-CyD, AUCtreated excipient is area under the curve of dissolution profile for heat-treated or kneaded sample containing IM and dicalcium phosphate or lactose monohydrate, and AUCPM excipient is area under the curve of dissolution profile for physical mixture containing IM and dicalcium phosphate or lactose monohydrate. There is no interaction between IM and HP-β-CyD when relative AUC ratio is 1. On the other hand, relative AUC ratio is larger than 1; the interaction between IM and HP-β-CyD contributes to increasing dissolution rate of IM.
Near Infrared Chemical Imaging
In this study, near infrared (NIR) Chemical Imaging System (NIR-CI 2450; Malvern Instruments Ltd, UK) was used to image extrudates. The spectral range used in this study was 1,200–2,400 nm, and the magnification was selected to achieve 38.7-μm/pixel resolution. The data collected were processed using ISys® chemical imaging software (Malvern Instruments Ltd, UK). The data were first pre-processed in order to remove physical effects and to enhance chemical contrast. Each data cube was background and dark corrected, converted to absorbance units, and then normalized. Partial least squares model was created from a pure component library and was applied to each data cube in order to determine spectral contribution from each component at every pixel. The library was constructed from images acquired of the pure components (γ-form of IM, amorphous IM, and HP-β-CyD). Each pixel is scored between “0” and “1” relative to pure components. A score value of “0” indicated that the component was not present at that pixel, and “1” indicated that there was 100% of that pure component. Often, the values fall between 0 and 1, indicating more than one component present at that pixel. The scored image provides a visual representation of the spatial distribution of the material in the sample. In visualization of scored image, Red-Green-Blue (RGB) image technique was used (red: amorphous IM, blue: γ-form of IM)
Interaction between IM and HP-β-CyD
The interaction of IM with HP-β-CyD was studied by the solubility method. The solubility method was conducted according to the method of Higuchi and Connors (29). The phase solubility diagrams, i.e., plots of solubility of guest as a function of cyclodextrin concentration, are generally classified as either type A (a soluble complex is formed) or type B (a complex with definite solubility is formed). Type A can be further classified as subtypes AL, AP, and AN, where the guest solubility of the first type increases linearly with cyclodextrin concentration while those of the second and third types deviate positively and negatively, respectively, from the straight line. The complex formation with a 1:1 stoichiometry gives the AL type diagram.
An excess amount of IM (about 100 mg) was added in a glass bottle containing HP-β-CyD solutions at various concentrations (0–50 mM) in phosphate buffer (10 mL), and the mixture was shaken at 25°C for about 7 days. After the equilibrium was attained, an aliquot was taken by a syringe with filter, diluted appropriately with water, and analyzed for IM by UV spectroscopy (Spekol 1200; Carl Zeiss Technology, Jena, Germany) at 318 nm. The stability constant (K1:1) of inclusion complexes was calculated by the following equation using slopes and intercepts of the initial straight line portion of the phase solubility diagrams.
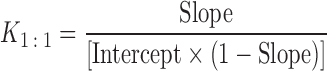 |
2 |
Storage
The samples were placed in glass bottle and were stored in desiccator with silica gel for 1 month at 40°C.
RESULTS AND DISCUSSION
Effect of Extrusion Processing on IM Release
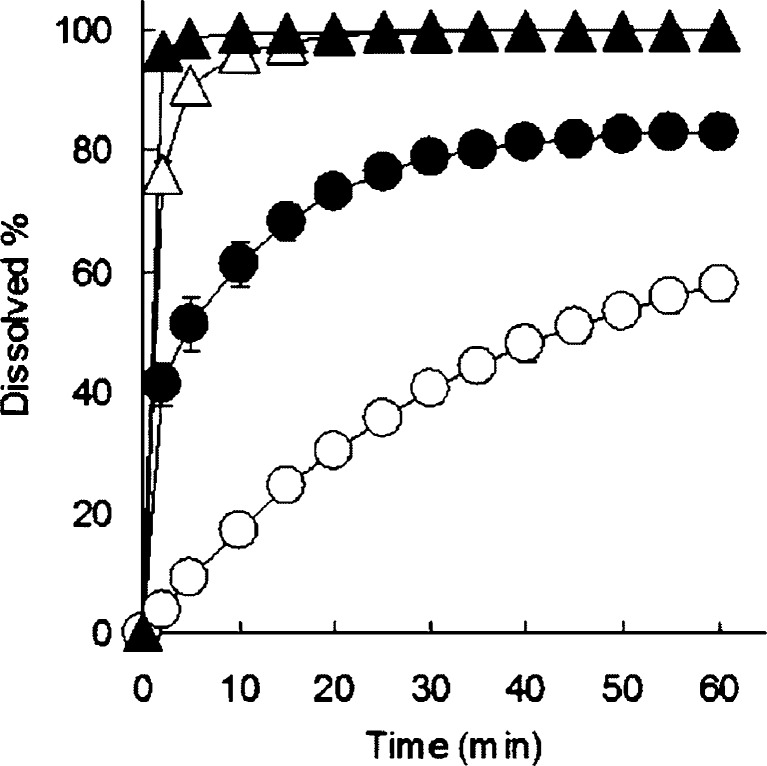
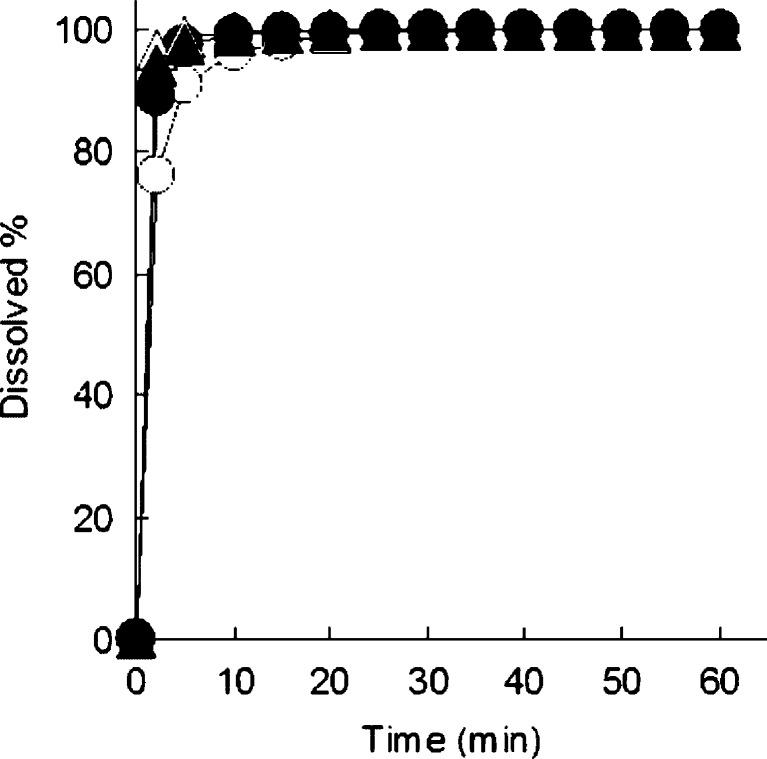
Figure 2 shows the dissolution profiles of IM alone, physical mixture of IM, and HP-β-CyD and extrudates containing IM and HP-β-CyD manufactured by melt extrusion and wet extrusion in pH 6.8 phosphate buffer. The amount of IM released after 15 min from IM alone and physical mixture of IM and HP-β-CyD was 24% and 68%, respectively. On the other hands, IM release from extrudates containing IM and HP-β-CyD manufactured by melt extrusion and wet extrusion exhibited rapid dissolution rate of drug release with 96% and 100% after 15 min, respectively. These results suggest that dissolution rates of IM from extrudates manufactured by both melt extrusion and wet extrusion with application of HP-β-CyD were significantly higher than that of the physical mixture of IM and HP-β-CyD. Surprisingly, dissolution rate of IM from wet extrudates was also significantly higher than that of the physical mixture of IM and HP-β-CyD, nevertheless γ-form of IM did not change to amorphous IM completely in wet extrusion process.
Fig. 2.
Dissolution profiles of IM from melt extrusion system and wet extrusion system of IM and HP-β-CyD (equivalent to 50 mg of IM) in pH 6.8 phosphate buffer at 37°C. Unfilled circles IM alone, filled circles physical mixture, unfilled triangles extrudate containing IM and HP-β-CyD manufactured by melt extrusion, unfilled triangles extrudate containing IM and HP-β-CyD manufactured by wet extrusion. Each point represents the mean ± SD of three experiments
Solid Characteristics of Extrudates Containing IM and HP-β-CyD
Thermal Characteristics of Extrudates
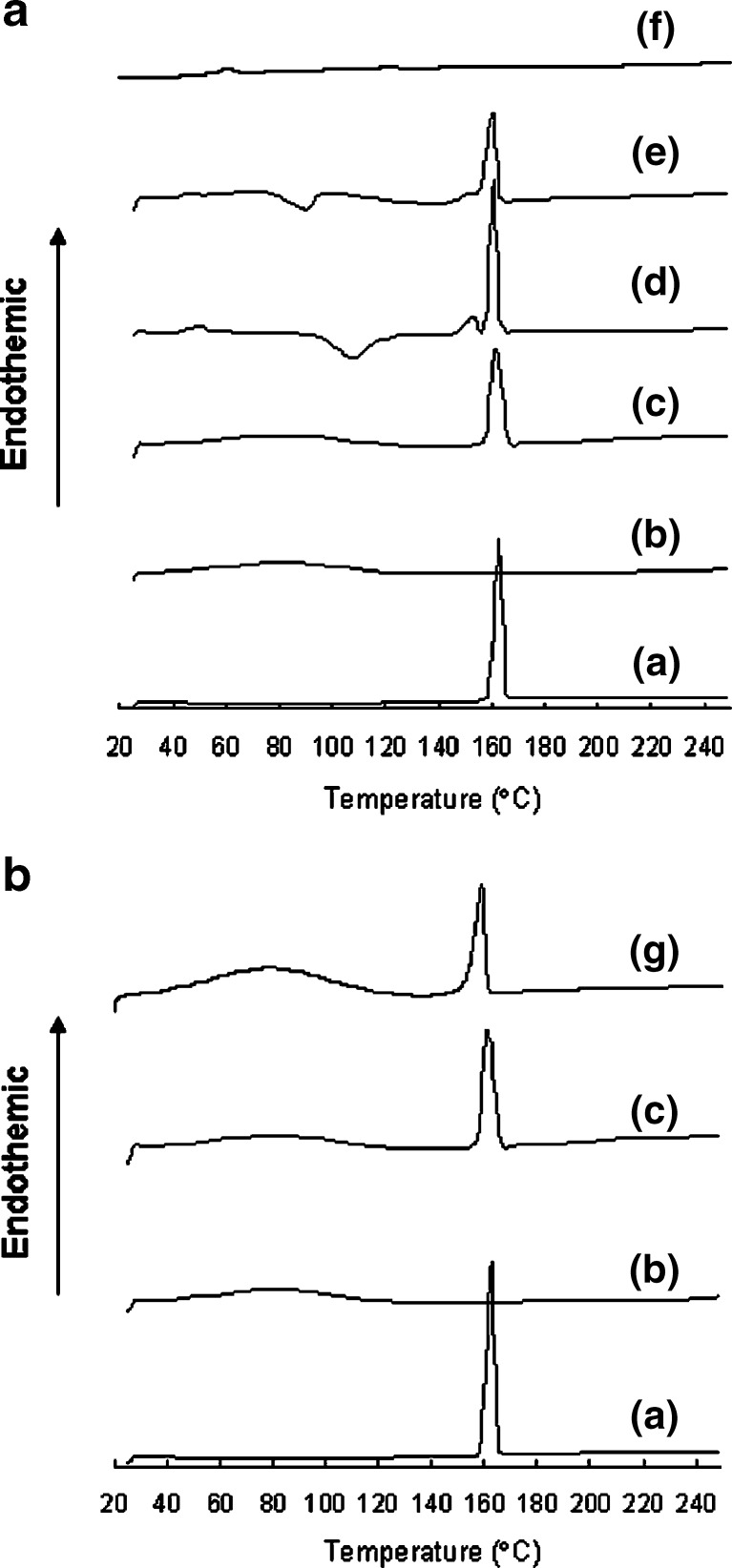
Figure 3 shows the DSC thermograms of γ-form of IM, amorphous IM, physical mixture of γ-form of IM and HP-β-CyD, physical mixture of amorphous IM and HP-β-CyD, extrudates manufactured by melt extrusion and wet extrusion. DSC thermogram for γ-form of IM exhibited endothermic peaks at 161°C, which correspond to its respective melting point. DSC thermogram for HP-β-CyD did not exhibit peaks since HP-β-CyD is amorphous compound. For melt-quenched sample, an exothermic peak at ca. 50°C, an exothermic peak at 100–140°C, and an endothermic peak at 160°C appeared which correspond to its respective glass transition, recrystallization of amorphous to crystalline of IM, and melting point of IM, respectively. For physical mixture and extrudate manufactured by wet extrusion, the endothermic melting peak of IM occurred at slightly lower temperature due to the incorporation of HP-β-CyD (Fig. 3b). On the other hand, on DSC thermogram for extrudate manufactured by melt extrusion, the endothermic peak of melting point of IM disappeared (Fig. 3a).
Fig. 3.
DSC curves for IM and HP-β-CyD system. a IM and HP-β-CyD system in melt extrusion, b IM and HP-β-CyD system in wet extrusion. a γ-form of IM, b HP-β-CyD, c physical mixture of γ-form of IM and HP-β-CyD, d amorphous IM, e physical mixture of amorphous IM and HP-β-CyD, f extrudate containing IM and HP-β-CyD manufactured by melt extrusion, g extrudate containing IM and HP-β-CyD manufactured by wet extrusion
Evaluation for Crystalline State of IM in Extrudates with XRPD and NIR Imaging
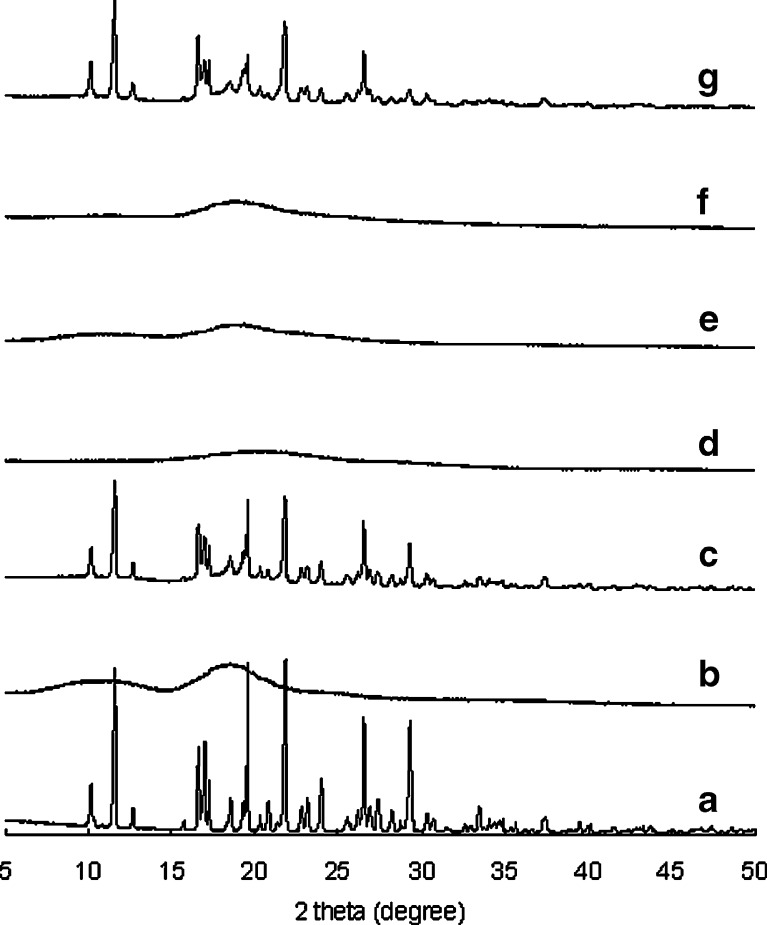
The crystalline states of IM in extrudates were investigated with X-ray powder diffraction (XRPD) and NIR imaging. Figure 4 shows the XRPD of γ-form of IM, amorphous IM, physical mixture of γ-form of IM, and HP-β-CyD and amorphous IM and HP-β-CyD, extrudate manufactured by melt extrusion, and wet extrusion. In the XRPD pattern of extrudate manufactured by melt extrusion, crystalline peaks of IM were not observed. This was due to amorphization of IM by thermal processing at a temperature above the melting point of IM. On the other hand, in the XRPD pattern of extrudate manufactured by wet extrusion, crystalline peaks of native IM (γ-form) were observed (2θ = 11.6°, 16.6°, 19.6°, 21.8°, and 26.6°). This indicated that γ-form of IM was present in extrudate manufactured by wet extrusion process.
Fig. 4.
XRPD for IM and HP-β-CyD system. a γ-form of IM, b HP-β-CyD, c physical mixture of γ-form of IM and HP-β-CyD, d amorphous IM, e physical mixture of amorphous IM and HP-β-CyD, f extrudate containing IM and HP-β-CyD manufactured by melt extrusion, g extrudate containing IM and HP-β-CyD manufactured by wet extrusion
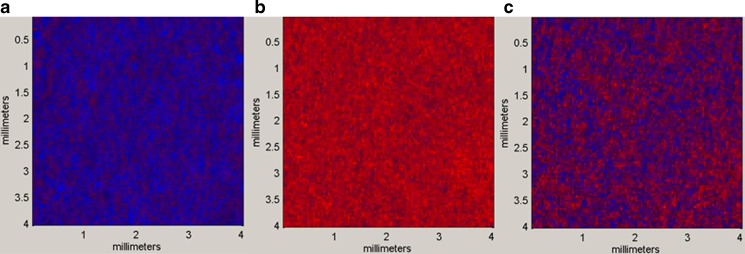
Figure 5 shows NIR images of physical mixture of γ-form of IM and HP-β-CyD and extrudates manufactured by melt extrusion and wet extrusion. In RGB image, red and blue represent amorphous IM and γ-form of IM, respectively. In the image of physical mixture of γ-form of IM and HP-β-CyD (Fig. 5a), blue color was shown in all area of image due to existing γ-form of IM in powder of physical mixture. In the image of extrudate containing IM and HP-β-CyD manufactured by melt extrusion (Fig. 5b), red color was shown in all area of image due to existing amorphous IM in extrudate. On the other hand, red color and blue color were shown in the area of image for extrudate manufactured by wet extrusion (Fig. 5c), which means both γ-form of IM and amorphous IM exist in that extrudate.
Fig. 5.
RGB images for physical mixture (a), melt extrusion system (b), and wet extrusion system (c) of IM and HP-β-CyD. Red amorphous IM; blue γ-form of IM
In melt extrusion process, manufacturing condition for part of IM melting was set at 173°C since IM had a melting point at 161°C. Fukuda et al. (27) reported successful improvement for dissolution properties of ketoprofen by setting the temperature near the melting point of the ketoprofen in melt extrusion process by using sulfobutyl ether-β-CyD as same manufacturing condition of this study. In this study, the crystalline state of IM was changed during melt extrusion process, i.e., γ-form of IM in mixture of IM and HP-β-CyD was completely changed to amorphous form during melt extrusion process. On the other hand, γ-form of IM in mixture of IM and HP-β-CyD was partially changed to amorphous form during wet extrusion process. The composition of IM and HP-β-CyD was selected 1:1 weight ratio, which corresponds to approximately 4:1 molar ratio, from the standpoint of manufacturability in this study. Therefore, approximately 20% of IM in sample can theoretically form 1:1 inclusion complex with HP-β-CyD during wet extrusion process.
Effect of Application of HP-β-CyD, Heat-Treating, and Kneading with Water on IM Release
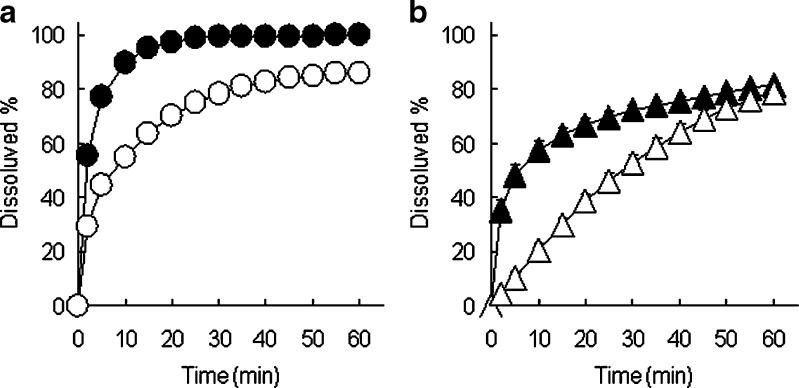
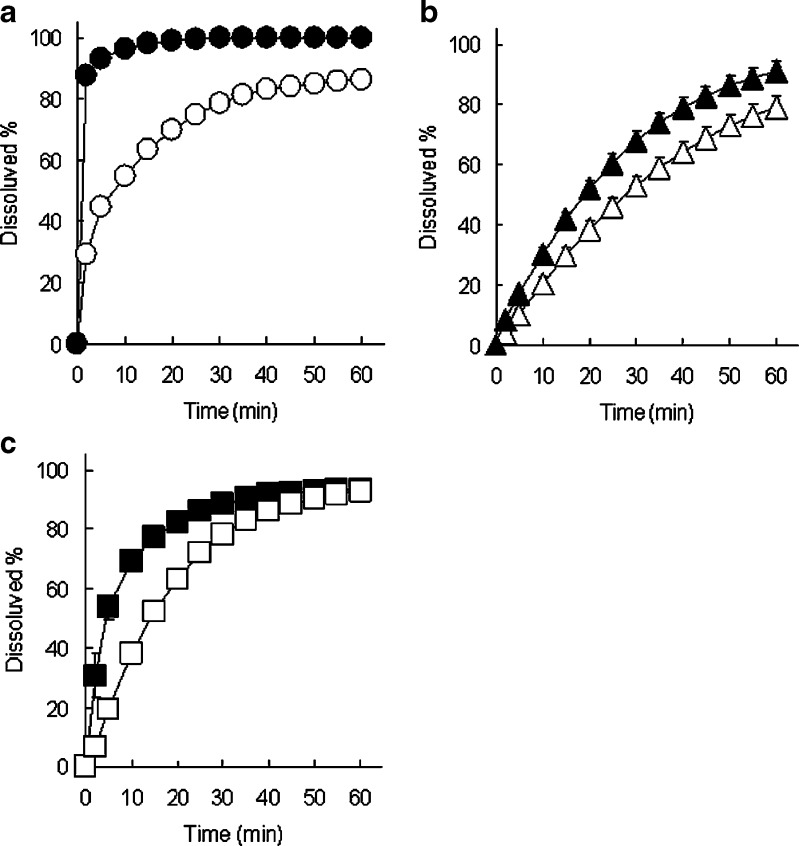
As shown in Fig. 2, dissolution rates of IM from extrudates manufactured by melt extrusion and wet extrusion with HP-β-CyD were significantly higher than that of the physical mixture of IM and HP-β-CyD. From solid characteristics of extrudates manufactured by melt extrusion and wet extrusion (Figs. 3, 4, and 5), the crystalline state of IM was changed to amorphous form during melt extrusion process, and γ-form of IM in mixture of IM and HP-β-CyD was partially changed to amorphous form during wet extrusion process. Therefore, the effects of application of HP-β-CyD, heat-treating, and kneading with water during extrusion process on IM release were investigated. Dicalcium phosphate and lactose monohydrate which are insoluble and soluble excipients, respectively, were used as control for calculation of relative AUC ratio. Figure 6 shows the dissolution profiles of heat-treated samples for IM for IM-HP-β-CyD system (Fig. 6a) and IM-dicalcium phosphate system (Fig. 6b) in pH 6.8 phosphate buffer. The dissolution rates of IM from heat-treated samples for both IM-HP-β-CyD system and IM-dicalcium phosphate system were higher than that for each physical mixture. Moreover, the dissolution rate of IM from heat-treated sample in IM-HP-β-CyD system was highest in IM-HP-β-CyD system and IM-dicalcium phosphate system. From these results, the combination of application of HP-β-CyD and heat-treating enhanced dissolution rate of IM. Figure 7 shows the dissolution profiles of kneaded samples for IM-HP-β-CyD system (Fig. 7a), IM-dicalcium phosphate system (Fig. 7b), and IM-lactose monohydrate system (Fig. 7c) in pH 6.8 phosphate buffer. The dissolution rates of IM from kneaded samples were significantly higher than those from each physical mixture in IM-HP-β-CyD system, IM-dicalcium phosphate system, and IM-lactose monohydrate system. Moreover, the dissolution rate of IM from kneaded sample in IM-HP-β-CyD system was highest in IM-HP-β-CyD system, IM-dicalcium phosphate system and IM-lactose monohydrate system. From these results, the combination of application of HP-β-CyD and kneading with water-enhanced dissolution rate of IM same as heat-treated sample.
Fig. 6.
Effect of heating on dissolution profiles of IM from IM-HP-β-CyD system (a) and IM-dicalcium phosphate system (b) (equivalent to 50 mg of IM) in pH 6.8 phosphate buffer at 37°C. Unfilled circles physical mixture of IM and HP-β-CyD, filled circles heated sample for mixture of IM and HP-β-CyD, unfilled triangles physical mixture of IM and dicalcium phosphate, filled triangles heated sample for mixture of IM and dicalcium phosphate. Each point represents the mean ± S.D. of three experiments
Fig. 7.
Effect of kneading with water on dissolution profiles of IM from IM-HP-β-CyD system (a), IM-dicalcium phosphate system (b), IM-lactose monohydrate system (c) (equivalent to 50 mg of IM) in pH 6.8 phosphate buffer at 37°C. Unfilled circles physical mixture of IM and HP-β-CyD, filled circles kneaded sample for mixture of IM and HP-β-CyD, unfilled triangles physical mixture of IM and dicalcium phosphate, filled triangles kneaded sample for mixture of IM and dicalcium phosphate, unfilled squares physical mixture of IM and lactose monohydrate, filled squares kneaded sample of IM and lactose monohydrate. Each point represents the mean ± SD of three experiments
Relative AUC ratios were calculated by using Eq. 1 based on the data of Figs. 6 and 7 to investigate the enhancing effect of application of HP-β-CyD, heat-treating itself and kneading with water on dissolution rate. Relative AUC ratios for each system were summarized in Table I. Relative AUC ratio for IM-HP-β-CyD system vs IM-dicalcium phosphate system with heat-treating was shown to be 1.1. These results suggest that the heat-treating contributed enhancing dissolution of IM, and enhancing effect in IM-HP-β-CyD system on IM dissolution was the same as that in IM-dicalcium phosphate system. On DSC thermogram of physical mixture of IM and HP-β-CyD, the endothermic melting peak of IM occurred at slightly lower temperature due to the incorporation of HP-β-CyD (Fig. 3). However, that interaction between IM and HP-β-CyD during heat-treating was not greatly impacted on enhancement of IM dissolution since relative AUC ratio in IM-HP-β-CyD system vs IM-dicalcium phosphate system was 1.1. Therefore, the main mechanism of improvement for dissolution behavior of IM from extrudate containing IM and HP-β-CyD manufactured by melt extrusion process was changing crystalline state of IM, i.e., amorphization of IM during melt extrusion process.
Table I.
Effect of Application of HP-β-CyD, Heat-Treating, and Kneading with Water on Dissolution Behavior for IM
| System | Relative AUC ratio |
|---|---|
| Heat-treating | |
| HP-β-CyD system vs dicalcium phosphate system | 1.1 |
| Kneading with water | |
| HP-β-CyD system vs dicalcium phosphate system | 2.0 |
| HP-β-CyD system vs lactose monohydrate system | 1.9 |
On the other hand, relative AUC ratios for IM-HP-β-CyD system vs IM-dicalcium phosphate system and IM-HP-β-CyD system vs IM-lactose monohydrate system with kneading with water were shown to be about 2.0. These results suggest that the kneading with water could improve dissolution rate of IM, and moreover, dissolution enhancing effect by kneading with water in IM-HP-β-CyD system was greater than those in IM-dicalcium phosphate system and IM-lactose monohydrate system. Furthermore, combination of application of HP-β-CyD and kneading with water had a great potential for dissolution enhancing effect since relative AUC ratios for IM-HP-β-CyD system vs IM-dicalcium phosphate system and IM-HP-β-CyD system vs IM-lactose monohydrate system with kneading with water were approximately 2, not around 1. Wet extrusion process can be considered one of the wet granulation processes. When powder mixture of IM and HP-β-CyD was kneaded in the presence of water, particles of IM and HP-β-CyD were consolidated by mechanical agitating force during wet extrusion process and extrudate containing IM and HP-β-CyD was manufactured as finished product. It was estimated that the improvement of wettability of IM caused by application hydrophilic cyclodextrin, HP-β-CyD, in wet extrusion process was one of the reasons for enhancement of dissolution rate of IM. Therefore, the mechanism of enhancement for dissolution rate of IM from extrudate containing IM and HP-β-CyD manufactured by wet extrusion process was that interaction between IM and HP-β-CyD and improvement of wettability of IM due to consolidation of IM and HP-β-CyD by mechanical agitating force during process.
Interaction between IM and HP-β-CyD
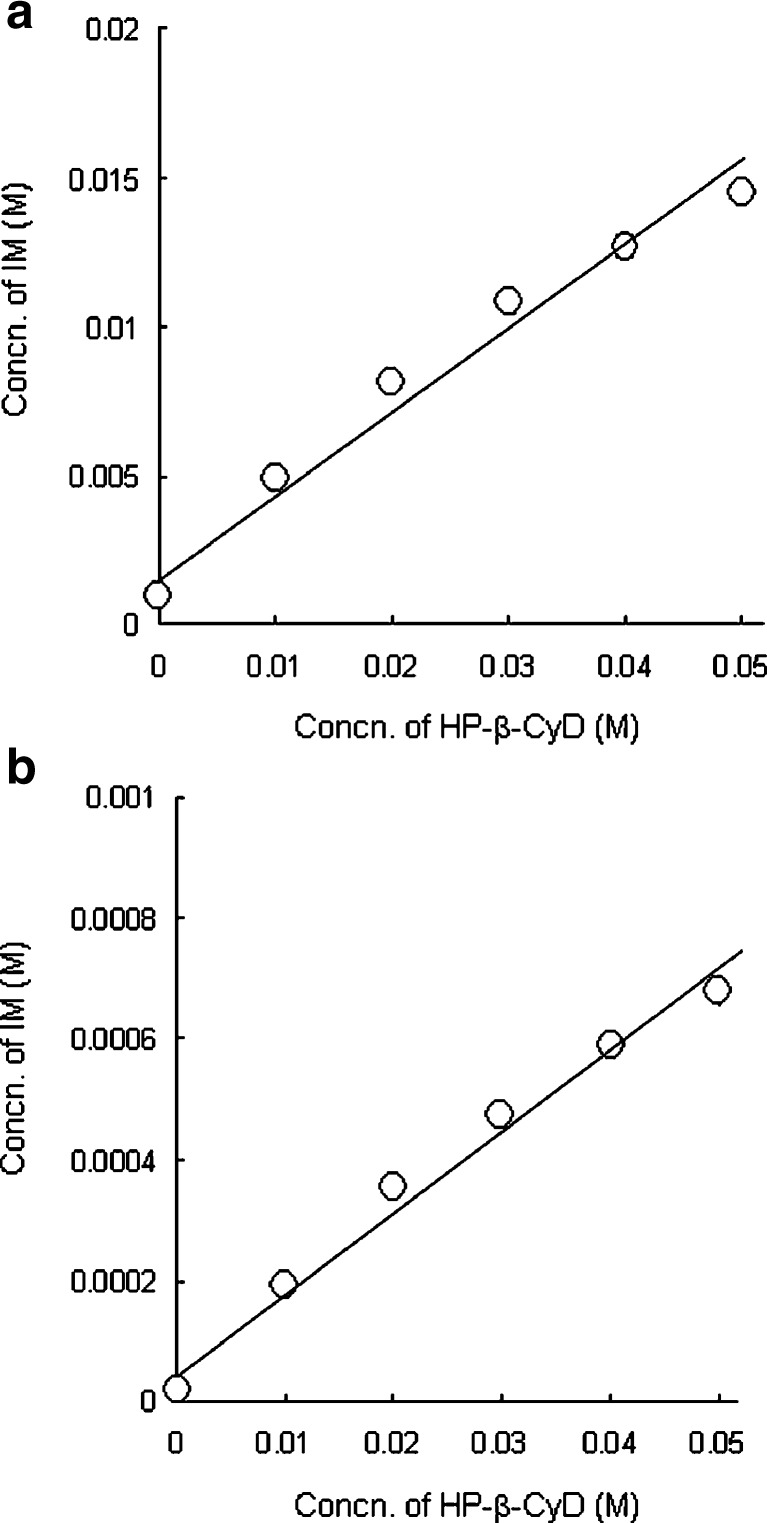
As shown in Fig. 7, kneaded sample in IM-HP-β-CyD system for IM and HP-β-CyD have a property for great enhancing effect for dissolution rate of IM. Therefore, interaction between IM and HP-β-CyD was investigated by solubility method. Figure 8 shows the phase solubility diagrams for IM and HP-β-CyD system in pH 6.8 and 5.0 phosphate buffer at 25°C. The solubility of IM increased linearly with increasing HP-β-CyD concentrations showing typical AL-type phase solubility diagrams, indicating the presence of only one species of 1:1 complex. Therefore the stability constant (K1:1) of 1:1 IM/HP-β-CyD complexes was calculated by analyzing data of initial straight line portion of diagrams according to Eq. 2. The K1:1 value for IM and HP-β-CyD in pH 6.8 and 5.0 phosphate buffer were 450 ± 4 and 751 ± 20 M−1, respectively. Hoshino et al. (30) reported that CyDs interacted with IM and could be made inclusion complex with IM, molar ratio of IM and CyDs was 1:1 based on phase solubility diagram and some spectroscopic studies and suggested that the chlorobenzen ring moiety of IM molecule was predominantly included in the cavity of CyDs. In addition, Backensfeld et al. (31) reported that HP-β-CyD interacted with IM in aqueous solution by using phase solubility diagram in different pH. These results, coupled with those of Hoshino et al. and Backensfeld et al., suggest that IM could interact with HP-β-CyD in presence of water during wet extrusion process.
Fig. 8.
Phase solubility diagrams of IM-HP-β-CyD systems in pH 6.8 (a) and pH 5.0 (b) phosphate buffer at 25°C. Each point represents the mean ± SD of three experiments
Stability Study for IM Dissolution Rate
Dissolution profiles of IM from extrudates before and after storage for 1 month at 40°C were shown in Fig. 9. There was no significant change of IM dissolution rates from extrudates containing IM and HP-β-CyD manufactured by both melt extrusion and wet extrusion during stability study. Therefore dissolution properties of extrudates containing IM and HP-β-CyD manufactured by both melt extrusion and wet extrusion maybe stable during storage.
Fig. 9.
Dissolution profiles of IM from melt extrusion system and wet extrusion system of IM and HP-β-CyD (equivalent to 50 mg of IM) in pH 6.8 phosphate buffer at 37°C before and after storage for 1 month at 40°C. Unfilled circles extrudate containing IM and HP-β-CyD manufactured by melt extrusion (initial), filled circles extrudate containing IM and HP-β-CyD manufactured by melt extrusion (stored for 1 M), unfilled triangles extrudate containing IM and HP-β-CyD manufactured by wet extrusion (initial), filled triangles extrudate containing IM and HP-β-CyD manufactured by wet extrusion (stored for 1 M). Each point represents the mean ± SD of three experiments
CONCLUSIONS
The purpose of this study was to achieve improvement of dissolution behavior of poorly water-soluble drug with application with HP-β-CyD in extrusion process.
The dissolution rates of IM from melt extrudates were significantly higher than that of the physical mixture of IM and HP-β-CyD. In melt extrudate containing IM and HP-β-CyD, γ-form (stable form) of IM changed to amorphous completely during melt extrusion due to heating above melting point of IM. The application of HP-β-CyD in melt extrusion process could improve dissolution rate of IM. However, interaction between IM and HP-β-CyD during melt extrusion was not greatly impacted on enhancement of IM dissolution. The main mechanism of improvement for dissolution behavior of IM from melt extrudate was changing crystalline state of IM, i.e., amorphization of IM during process.
On the other hand, dissolution behavior of IM also improved significantly by application of HP-β-CyD in wet extrusion process, nevertheless γ-form of IM did not change to amorphous IM completely. The application of HP-β-CyD in wet extrusion has a great potential for dissolution enhancing effect due to interaction between IM and HP-β-CyD and improvement of wettability with consolidation during process.
In conclusion, application of HP-β-CyD in extrusion process is useful for the enhancement of dissolution rate for poorly water-soluble drugs.
ACKNOWLEDGEMENT
The authors are grateful to Ms. Karin Matthée of Institute of Pharmaceutics and Biopharmaceutics, Heinrich-Heine-University for helpful measurement of DSC. Hideki Yano expresses his appreciation to Daiichi Sankyo Co., LTD. for granting him a 1-year leave of absence to pursue these studies.
References
- 1.Thanos CG, Liu Z, Goddard M, Reineke J, Bailey N, Cross M, Burrill R, Mathiowitz E. Enhancing the oral bioavailability of the poorly soluble drug dicumarol with a bioadhesive polymer. J. Pharm. Sci. 2003;92:1677–1689. doi: 10.1002/jps.10446. [DOI] [PubMed] [Google Scholar]
- 2.Serajuddin ATM. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 1999;88:1058–1066. doi: 10.1021/js980403l. [DOI] [PubMed] [Google Scholar]
- 3.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 4.Szejtli J. Cyclodextrin technology. Dordrecht: Kluwer; 1988. [Google Scholar]
- 5.Duchêne D. New trends in cyclodextrins and derivatives. Paris: Editions de Santé; 1991. [Google Scholar]
- 6.Uekama K. Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. 2004;52(8):900–915. doi: 10.1248/cpb.52.900. [DOI] [PubMed] [Google Scholar]
- 7.Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem. Rev. 1998;98:2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 8.Uekama K, Hirayama F, Arima H. Recent aspect of cyclodextrin-based drug delivery system. J. Incl. Phenom. Macrocycl. Chem. 2006;56:3–8. doi: 10.1007/s10847-006-9052-y. [DOI] [Google Scholar]
- 9.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past present and future. Nat. Rev. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 10.Pitha J, Milecki J, Fales H, Pannell L, Uekama K. Hydroxypropyl- β-cyclodextrin: preparation and characterization; effects on solubility of drugs. Int. J. Pharm. 1986;29:73–82. doi: 10.1016/0378-5173(86)90201-2. [DOI] [Google Scholar]
- 11.Yoshida A, Yamamoto M, Itoh T, Irie T, Hirayama F, Uekama K. Utility of 2-hydroxypropyl-beta-cyclodextrin in an intramuscular injectable preparation of nimodipine. Chem. Pharm. Bull. 1990;38(1):176–179. doi: 10.1248/cpb.38.176. [DOI] [PubMed] [Google Scholar]
- 12.Loftsson T, Ólafsdóttir BJ. The effect of 2-hydroxypropyl-β-cyclodextrin on the simultaneous dissolution and degradation of chlorambucil. Int. J. Pharm. 1990;66:289–292. doi: 10.1016/0378-5173(90)90410-6. [DOI] [Google Scholar]
- 13.Hirayama F, Wang Z, Uekama K. Effect of 2-hydroxypropyl-β-cyclodextrin on crystallization and polymorphic transition of nifedipine in solid state. Pharm. Res. 1994;11:1766–1770. doi: 10.1023/A:1018971501909. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama F, Usami M, Kimura K, Uekama K. Crystallization and polymorphic transition behavior of chloramphenicol palmitate in 2-hydroxypropyl-β-cyclodextrin matrix. Eur. J. Pharm. Sci. 1997;5:23–30. doi: 10.1016/S0928-0987(96)00250-3. [DOI] [Google Scholar]
- 15.Kimura K, Hirayama F, Arima H, Uekama K. Solid-state 13C nuclear magnetic resonance spectroscopic study on amorphous solid complexes of tolbutamide with 2-hydroxypropyl-α- and -β-cyclodextrins. Pharm. Res. 1999;16:1729–1734. doi: 10.1023/A:1018958116349. [DOI] [PubMed] [Google Scholar]
- 16.Hicks DC, Freese HL. Extrusion and spheronizing equipment. In: Ghebre-Sellassie I, editor. Pharmaceutical pelletization technology. New York: Marcel Dekker; 1989. pp. 71–100. [Google Scholar]
- 17.Faubion JM, Hoseney RC, Seib PA. Functionality of grain components in extrusion. Cereal Foods World. 1982;27:212–216. [Google Scholar]
- 18.Sokhey AS, Kollengode AN, Hanna MA. Screw configuration effects on corn starch expansion during extrusion. J. Food. Sci. 1994;59:895–898. doi: 10.1111/j.1365-2621.1994.tb08152.x. [DOI] [Google Scholar]
- 19.Lefebvre C, Brazier M, Robert H, Guyot-Hermann AM. Solid dispersions why and how? Industrial aspect. STP Pharma. 1985;4:300–322. [Google Scholar]
- 20.Gamlen MJ, Eardley C. Continuous extrusion using a Baker Perkins MP50 (multipurpose) extruder. Drug Dev. Ind. Pharm. 1986;12:1701–1713. doi: 10.3109/03639048609042604. [DOI] [Google Scholar]
- 21.Kleinebudde P, Lindner H. Experiments with an instrumented twin-screw extruder using a single-step granulation/extrusion process. Int. J. Pharm. 1993;94:49–58. doi: 10.1016/0378-5173(93)90008-4. [DOI] [Google Scholar]
- 22.Schroeder R, Steffens KJ. A new system for continuous wet granulation. Pharm. Ind. 2002;64:283–288. [Google Scholar]
- 23.Lindberg NO, Tufvesson C, Olbjer L. Extrusion of an effervescent granulation with twin screw extruder, Baker Perkins MPF 50 D. Drug Dev. Ind. Pharm. 1987;13:1891–1913. doi: 10.3109/03639048709068698. [DOI] [Google Scholar]
- 24.Lindberg NO. Some experiences of continuous wet granulation. Acta Pharm. Suec. 1988;25:239–246. [Google Scholar]
- 25.Lindberg NO, Tufvesson C, Holm P, Olbjer L. Extrusion of an effervescent granulation with twin screw extruder, Baker Perkins MPF 50 D. Influence on intragranular porosity and liquid saturation. Drug Dev. Ind. Pharm. 1988;14:1791–1798. doi: 10.3109/03639048809151987. [DOI] [Google Scholar]
- 26.Rambali B, Verreck G, Baert L, Massart DL. Itraconazole formulation studies of the melt-extrusion process with mixture design. Drug Dev. Ind. Pharm. 2003;29:641–652. doi: 10.1081/DDC-120021313. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda M, Miller DA, Peppas NA, McGinity JW. Influence of sulfobutyl ether β-cyclodextrin (Captisol®) on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion. Int. J. Pharm. 2008;350:188–196. doi: 10.1016/j.ijpharm.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Otsuka M, Kato F, Matsuda Y. Comparative evaluation of the degree of indomethacin crystallinity by chemoinfometrical fourie-transformed near-infrared spectroscopy and conventional powder X-ray diffractiometry phase solubility techniques. AAPS PharmSci. 2000;2(1):9. doi: 10.1208/ps020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higuchi T, Connors KA. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965;4:117–122. [Google Scholar]
- 30.Hoshino T, Tagawa Y, Hirayama F, Otagiri M, Uekama K. Inclusion complexation of indomethacin and its related compounds with cyclodextrin in aqueous solution. Yakugaku Zasshi. 1982;102:1184–1190. [Google Scholar]
- 31.Backensfeld T, Müller BW, Kolter K. Interaction of NSA with cyclodextrins and hydroxypropyl cyclodextrin derivatives. Int. J. Pharm. 1991;74:85–93. doi: 10.1016/0378-5173(91)90225-D. [DOI] [Google Scholar]