Abstract
Preventive and therapeutic efficacies of resveratrol on several lower gastrointestinal (GI) diseases (e.g., colorectal cancer, colitis) are well documented. To overcome the problems due to its rapid absorption and metabolism at the upper GI tract, a delayed release formulation of resveratrol was designed to treat these lower GI diseases. The current study aimed to develop a delayed release formulation of resveratrol as multiparticulate pectinate beads by varying different formulation parameters. Zinc-pectinate (Zn-pectinate) beads exhibited better delayed drug release pattern than calcium-pectinate (Ca-pectinate) beads. The effects of the formulation parameters were investigated on shape, size, Zn content, moisture content, drug encapsulation efficiency, swelling–erosion, and resveratrol retention pattern of the formulated beads. Upon optimization of the formulation parameters in relative to the drug release profiles, the optimized beads were further subjected to morphological, chemical interaction, enzymatic degradation, and stability studies. Almost all prepared beads were spherical with ∼1 mm diameter and efficiently encapsulated resveratrol. The formulation parameters revealed great influence on resveratrol retention and swelling–erosion behavior. In most of the cases, the drug release data more appropriately fitted with zero-order equation. This study demonstrates that the optimized Zn-pectinate beads can encapsulate very high amount of resveratrol and can be used as delayed release formulation of resveratrol.
Key words: bead, delayed release, formulation parameters, resveratrol, Zn-pectinate
INTRODUCTION
Trans-3,5,4′-trihydroxystilbene, commonly known as resveratrol, displays various pharmacological activities and is widely known for its cardioprotective, chemopreventive, antioxidant, anti-inflammatory, analgesic, neuroprotective, and anti-aging activities (1,2). Although in vitro pharmacological studies in several disease models exhibited positive effects (3), the very short half-life (∼8–14 min; 1,4) and extremely low oral bioavailability of resveratrol have raised concerns regarding its systemic action (1,5). At the same time, researchers have demonstrated the efficacy of resveratrol in lower gastrointestinal (GI) diseases such as colorectal cancer and colitis (6–9). Our hypothesis for the current study was that the development of a delayed release drug delivery system that prevents drug release in the upper GI tract and allows its release at the colonic region could provide a potential approach to treat these lower GI diseases.
There are various colon-specific drug delivery systems (time, pressure, pH, and colonic bacteria controlled; 10,11). The system that is stable in contact with upper GI bacterial enzymes but specifically degraded in the presence of colonic bacterial enzymes is suggested as a more realistic approach due to their less interindividual variability than the other three approaches (10,12). Several biopolymers (pectin, chitosan, guar gum, etc.) have shown this peculiar property (11).
Because of the wide variety of types and flexibility in use, pectins appeared to be of great practical interest as carriers for colon-specific drug delivery. Pectins are heterogeneous polysaccharide (partially methoxylated poly α (1–4)-D-galacturonic acids with some 1,2-linked L-rhamnose groups), present in the cell wall of most plants which are consumed as part of the human diet and used as a food additive. It is resistant to enzymes present in the stomach and intestine but can be almost completely degraded by the colonic bacterial enzymes (11). Unfortunately, their solubility and swellability in aqueous fluid cause premature drug release before the delivery system reaching to the colon. However, this can be controlled through the choice of pectin type or the presence of additives. Usually, low methoxy pectins (LM pectins) that have more free carboxylic group can be cross-linked with divalent cations (e.g., calcium, zinc) to produce a more water insoluble pectinate gel which has the potential to be an effective vehicle for drug delivery (12). Furthermore, amidated pectins are more prone to form a rigid gel structure with divalent cations than nonamidated pectin (13,14). Therefore, amidated LM pectins allow the formation of a more compact Ca- or Zn-pectinate network than nonamidated and/or high methoxy (HM) pectins.
Additionally, multiparticulate systems proved to be better than single unit dosage forms due to their reproducible and predictable GI transit time, more reliable drug release profile, and less local irritation (15). Further, multiple unit dosage forms of enzyme specificity quickly spread out upon their arrival to the colon, and due to an enhanced surface area being exposed to the enzymes, rapid drug release occurs at the colon (16).
Various reports suggest that multiparticulate Ca- or Zn-pectinate bead system may be a viable carrier for site-specific drug delivery to the colon (15,17–21). Indeed, we have recently exploited Ca-pectinate beads as colon-specific carrier of resveratrol (22), and to our knowledge, our study was the only one that explored the delivery of resveratrol specifically to the colonic region. However, it has been observed that zinc is a better cross-linking agent for pectin chains than calcium and it forms stronger pectinate network (19–21,23). Hence, in the present study, we attempted to compare the resveratrol release pattern from the beads prepared with both cross-linking agents (i.e., calcium and zinc). Our present study indicated Zn-pectinate beads as better formulation than Ca-pectinate beads, in terms of superior delayed drug release pattern. Subsequently, we aimed to optimize the formulation parameters (cross-linking solution pH, cross-linking agent concentration, cross-linking time, drying condition, pectin concentration, and pectin to resveratrol ratio) of resveratrol-loaded Zn-pectinate beads after studying their effect on different physicochemical properties and resveratrol retention pattern of the formulated beads.
MATERIALS AND METHODS
Chemicals
GENU® pectin LM-104 AS-FS (degree of esterification = 28% and degree of amidation = 20%) was a generous gift from CPKelco (Denmark). Resveratrol (fine crystalline powder with 99.12% purity) and calcium chloride dihydrate were purchased from Shaanxi Sciphar Biotechnology Co., Ltd. (Xi’an, China) and Merck (Darmstadt, Germany), respectively. Sodium hydroxide, sodium phosphate monobasic, zinc acetate dehydrate, and Pectinex® Ultra SP-L (pectinase/pectinolytic enzyme from Aspergillus aculeatus, activity > 9,500 PG ml−1) were obtained from Sigma (St. Louis, Missouri, USA). HPLC grade methanol and Fourier transform infra-red (FTIR) grade potassium bromide were bought from Tedia Company, Fairfield, Ohio, USA and Aldrich, Germany, respectively. MiliQ water (18.2 MΩ·cm at 25°C from Millipore Direct-Q® ultra-pure water system (Billerica, Massachusetts, USA)) was used throughout the study. Disodium hydrogen phosphate anhydrous and monobasic potassium phosphate were purchased from Fluka (Steinheim, Germany). All materials were used as received.
Formulation of Resveratrol-Loaded Pectinate Beads
Ionotropic gelation method was used to prepare the beads (15,22,24,25). Briefly, resveratrol was homogeneously dispersed in the aqueous solution of pectin with the help of a homogenizer. The dispersion was then sonicated on a bath sonicator to remove the air bubbles. The pectin–resveratrol mixture (6 ml) was added dropwise (1 ml min−1) through a 25G needle (blunt end) from 5 cm above into gently agitated cross-linking solution (100 ml) at room temperature (RT). Upon contact with cross-linking solution, the pectin drops instantly formed pectinate beads. Mild agitation of the solution was continued for further cross-linking of the beads. The beads were isolated from the solution after different time intervals, subsequently washed with distilled water, and then dried under various conditions. The different formulation parameters used to prepare the beads are listed in Table I. All formulations were prepared in triplicate.
Table I.
Formulation Design
| Variables | Values | Constants | |||||
|---|---|---|---|---|---|---|---|
| Zinc acetate concentration (% w/v) | Cross-linking solution pH | Cross-linking time (h) | Drying | Pectin concentration (% w/v) | Pectin–Resveratrol | ||
| Cross-linking agent | Zn | 5 | 1.5 | 0.5 | RT | 5 | 3:1 |
| Ca | 5a | 1.5 | 0.5 | RT | 5 | 3:1 | |
| Zinc acetate concentration (% w/v) | 1 | – | 1.5 | 0.5 | RT | 5 | 3:1 |
| 2.5 | – | 1.5 | 0.5 | RT | 5 | 3:1 | |
| 5 | – | 1.5 | 0.5 | RT | 5 | 3:1 | |
| 10 | – | 1.5 | 0.5 | RT | 5 | 3:1 | |
| Cross-linking pH | 1.5 | 5 | – | 0.5 | RT | 5 | 3:1 |
| 6.3 | 5 | – | 0.5 | RT | 5 | 3:1 | |
| Cross-linking time (h) | 0.25 | 5 | 1.5 | – | RT | 5 | 3:1 |
| 0.5 | 5 | 1.5 | – | RT | 5 | 3:1 | |
| 2 | 5 | 1.5 | – | RT | 5 | 3:1 | |
| 24 | 5 | 1.5 | – | RT | 5 | 3:1 | |
| Drying | RT | 5 | 1.5 | 0.5 | – | 5 | 3:1 |
| 37°C | 5 | 1.5 | 0.5 | – | 5 | 3:1 | |
| 50°C | 5 | 1.5 | 0.5 | – | 5 | 3:1 | |
| FD | 5 | 1.5 | 0.5 | – | 5 | 3:1 | |
| Pectin concentration (% w/v) | 3 | 5 | 1.5 | 0.5 | RT | – | 3:1 |
| 4 | 5 | 1.5 | 0.5 | RT | – | 3:1 | |
| 5 | 5 | 1.5 | 0.5 | RT | – | 3:1 | |
| 6 | 5 | 1.5 | 0.5 | RT | – | 3:1 | |
| Pectin–Resveratrol | 1:1 | 5 | 1.5 | 0.5 | RT | 5 | – |
| 2:1 | 5 | 1.5 | 0.5 | RT | 5 | – | |
| 3:1 | 5 | 1.5 | 0.5 | RT | 5 | – | |
| 4:1 | 5 | 1.5 | 0.5 | RT | 5 | – | |
| Blank | – | 5 | 1.5 | 0.5 | RT | 5 | – |
aCalcium chloride was used instead of zinc acetate
Morphology
JEOL scanning electron microscopy (JSM-5200, Japan) was used for the morphological examination (bead surface) of the resveratrol-free and resveratrol-loaded beads. The excitation voltage was set at 20 kV. Pectinate beads were fixed on an aluminum stub and coated with platinum (30 s) to a thickness of 2 nm under vacuum with the JEOL auto fine coater (JFC-1600, Japan). The micrographs were recorded at different magnifications.
Shape and Size
The lengths and breadths of randomly selected 50 beads from each batch were measured by precalibrated image analysis program (LAS EZ, version 1.4.0) after images were taken through a digital camera (LEICA EC3, Switzerland) connected with an optical microscope (LEICA DM IL, Switzerland). The shape of the beads was represented by elongation ratio (ER), which is the quotient of length to breadth of the beads (24). ER = 1 means perfect spherical shape, while ER > 1 indicates more deviation from spherical shape. The size of each bead was calculated as the average of length and breadth (24).
FTIR Spectroscopy
Infra-red (IR) spectra of pure resveratrol, resveratrol-free pectin bead, and resveratrol-loaded pectin bead were compared to examine chemical interactions between resveratrol and bead components. Before FTIR study, pectin beads were crushed and dried in a vacuum desiccator for 3 days to remove the moisture completely. Each sample (∼2 mg) was mixed with ∼198 mg FTIR grade anhydrous potassium bromide and ground into a fine powder using mortar and pestle. The mixture was then compressed into a disc. IR spectrum of each disc was recorded over a wave number region of 400–4,000 cm−1 at a resolution of 4 cm−1 using the FTIR spectrometer (PerkinElmer Spectrum 100 Series, Norwalk, Connecticut, USA).
Zinc Content
Zinc content in the beads was determined to confirm the safety aspect of the prepared beads. The amount of zinc retained in the beads was determined as follows. The beads (∼20 mg) were dissolved in 19 ml of 50 mM sodium phosphate buffer (pH 7.4) containing 1% ethylenediaminetetraacetic acid. To acidify the solution, 1 ml of concentrated hydrochloric acid was added to it and mixed well. The solution obtained was then centrifuged at 10,000 × g for 10 min. The amount of zinc present in the supernatants was determined by atomic absorption spectroscopy (AAS, PerkinElmer AAnalyst 100 model, Norwalk, Connecticut, USA).
Moisture Content
The moisture content (MC) was determined by gravimetric method: 50 dry and fully dry beads were randomly selected from each batch and weighed on an analytical balance with readability of 0.00001 g (Metler Toledo, Switzerland; 22). The mean MC was estimated by the following equation:
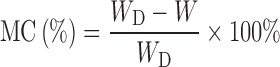 |
1 |
where, WD is the weight of the dry beads and W is the weight of fully dry beads (dried at 60°C until no further weight change was observed).
Resveratrol Encapsulation Efficiency
During formulation of the resveratrol-loaded pectinate beads (as described in the above section), resveratrol content in the beads was determined using a spectrophotometer (UV–Visible Spectrophotometer UV-1601, Shimadzu, Japan) at a wavelength of 320 nm (22). Encapsulation efficiency (EE) was determined by analyzing spectrophotometrically the total amounts of resveratrol lost to the cross-linking and washing solutions during formulation. EE of the beads were then calculated by the following equation:
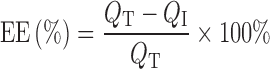 |
2 |
where, QT is the amount of resveratrol initially added in 6 ml pectin gel mixture, and QI is the total amount of resveratrol lost in the cross-linking and washing solutions.
In Vitro Drug Release Study
The in vitro release study of the resveratrol-loaded pectinate beads was carried out according to the procedures developed in our previous study (22). Because of the marginal solubility of resveratrol in simulated intestinal fluid (SIF) and simulated gastric fluid (SGF), the release of resveratrol (in SIF/SGF) is not observed even in the presence of a high amount of surfactant (SIF with 0.2% Tween® 80 exhibited resveratrol solubility of 0.72 ± 0.13 μg ml−1). Thus, the standard dissolution methods are not feasible in this case as the drug is released in the dissolution media as particulate form rather than dissolved form due to the poor aqueous solubility of resveratrol, which impede proper quantification of the released drug.
Hence, we devised an alternative method in which we estimated the amount of resveratrol remaining in the intact beads at each time point instead of measuring the drug released in the media (SIF/SGF). This method precludes measurement of drug released (either in dissolved or particulate form) in the release media. Briefly, resveratrol-loaded beads (∼25 mg) were suspended in 10 ml releasing medium (preheated at 37 ± 0.2°C) in screw cap glass test tubes. Separate tubes were used for each time point. The tubes were kept on a shaking water bath (100 rpm) at 37 ± 0.2°C. Intact beads were separated from the specified tubes at predetermined time intervals. The isolated intact beads were then dissolved (pectinate matrix)/dispersed (resveratrol particles) in 5 ml of 50 mM PBS (pH 7.4) and fully dissolved (resveratrol) by the addition of 10 ml methanol. The mixture was mixed well on a magnetic stirrer and centrifuged at 10,000 × g for 10 min. The supernatant was collected and diluted with methanol–water (1:1) to the calibration range (0.1–10 µg ml−1). The resveratrol content in the supernatant (i.e., resveratrol remaining in the beads) was determined by a UV–Visible Spectrophotometer (UV-1601, Shimadzu) at 320 nm. Resveratrol-free beads were used as a control.
SIF (pH 6.8) was used as release media throughout the bead optimization process. Further, release study of the optimized beads was performed in SIF (pH 6.8) containing 300 PG Pectinex® Ultra SP-L (simulated colonic fluid) and SGF (pH 1.2) to investigate the effect of colonic and gastric fluids on resveratrol release from the bead.
Analysis of the Drug Release Kinetics
The drug release kinetics from the beads were investigated by fitting the drug release data into zero order and square root of time equations. The zero order equation can be obtained by plotting the percent drug released versus time as follows:
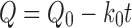 |
3 |
The square root of time equation can be obtained by plotting the percent drug released versus square root of time as follows:
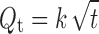 |
4 |
where, Q is the percentage of drug remaining at time t (h), Q0 is the percentage of drug at t = 0 h, k0 is the zero order release rate constant, Qt is the percentage of drug released at time t (h), and k is the Higuchi’s release rate constant.
The drug release data obtained from the release studies were fitted into the above models, and the appropriate model was selected after linear regression analysis. R2 > 0.95 was set for linearity. The release rate constants were calculated from the slope of each straight line of best fit model. Times corresponding to 25%, 50%, and 75% resveratrol retention in the beads (T25, T50, and T75, respectively) during release study were determined.
Swelling–Erosion Behavior
The experimental conditions for swelling–erosion study were similar to the release study. The beads were removed at the predetermined time point from the specified tubes, blotted with filter paper to remove the excess water, and weighed. Swelling–erosion behavior of the optimized beads were also checked in SGF (pH 1.2) and in SIF (pH 6.8) containing 300 PG Pectinex® Ultra SP-L. The swelling–erosion ratio (SER) was calculated using the following equation:
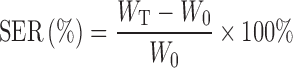 |
5 |
where, WT and W0 represent the weight of the beads at the given time point and the initial weight of the dry beads, respectively. Positive SER and upward trend denote overall swelling, while negative SER and downward trend demonstrate the erosion and/or drug release of beads. SER = −100% denotes complete dissolution of beads.
Enzymatic Degradation
The beads prepared with the optimized formulation parameters were incubated in SIF (pH 6.8) with or without 300 PG pectinase enzyme at 37 ± 0.2°C. After 3 h incubation, the beads were separated from the media and lyophilized. The appearance of the enzyme-treated and nonenzyme-treated beads were compared by scanning electron microscopy (JSM-5200) using the same parameters to those of the morphological study.
Stability
About 100 mg of the beads prepared with the optimized formulation parameters were stored at three different conditions: cold (4°C), RT, and accelerated temperature (40°C). Resveratrol content of the samples was analyzed at predetermined time intervals (0, 3, 7, 15, 30, 90, and 180 days).
Statistical Analysis
Graph-Pad Prism Version 2.00 (San Diego, California, USA) was used for statistical analysis. All experiments were performed in triplicate and data were expressed as mean ± standard deviation (SD). One-way ANOVA with the post hoc Tukey test and two-tail unpaired t test were performed for all statistical analysis. Statistical significance was set at p < 0.05.
RESULTS AND DISCUSSIONS
Formulation
When the pectin droplets come in contact with divalent cation (e.g., Ca2+, Zn2+), ionic interaction between the negatively charged carboxylic groups of the pectin chains and the positively charged divalent calcium or zinc ions leads to intermolecular cross-linking (known as ‘egg-box’ conformation (26)) and forms gelled spheres. This process is known as ionotropic gelation method. In addition, this cross-linking reduces the solubility and induces noncovalent associations of the carbohydrate chains in pectin. These interactions in amidated LM pectin allowed the formation of a compact pectinate network, in which insoluble resveratrol particles were entrapped. Nevertheless, the beads were easily prepared without any sophisticated instruments.
Morphology
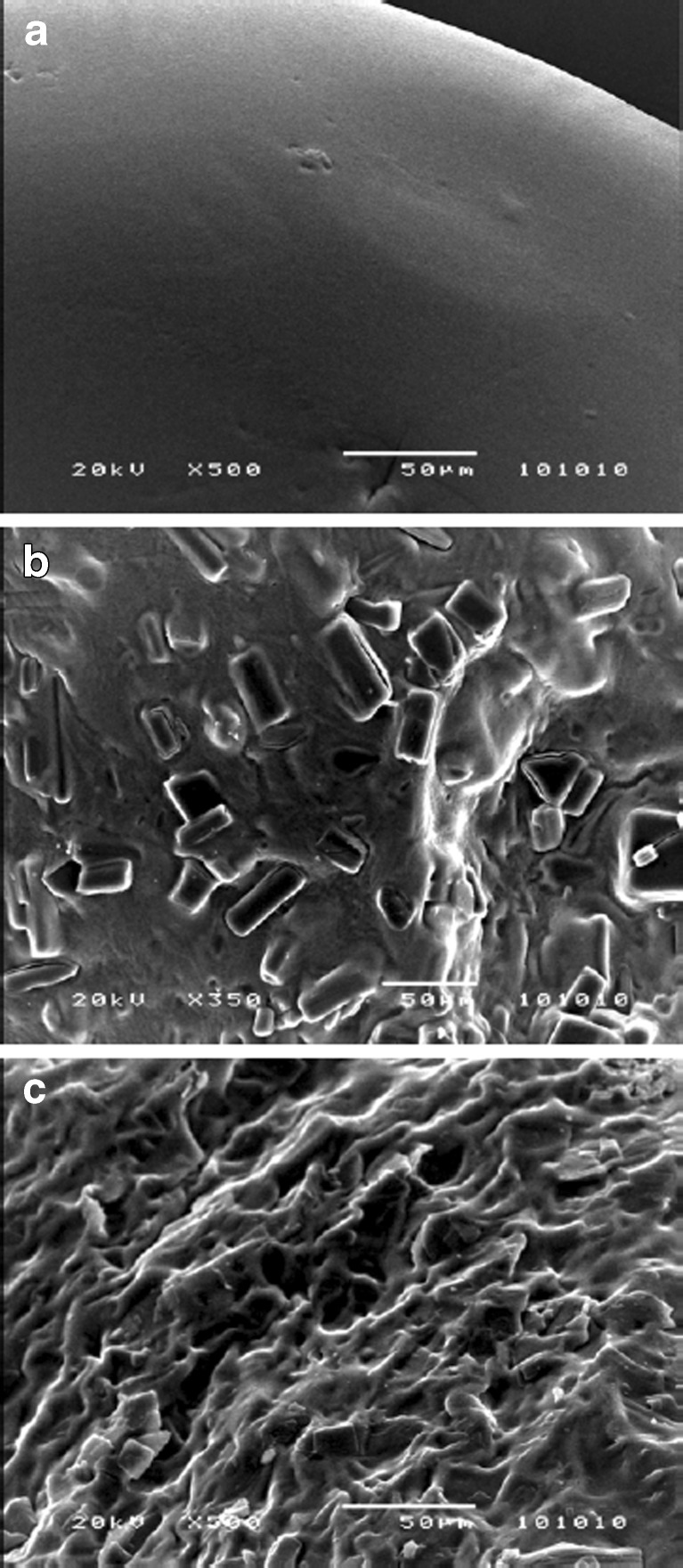
All beads were spherical in shape (ER < 1.15) with ∼1 mm size (Table II). Scanning electron micrographs of the beads are presented in Fig. 1. SEM image of the resveratrol-free bead shows a smooth surface topography (Fig. 1a). Contrarily, resveratrol-loaded bead shows rough and rugged surface which indicates that the drug crystals are embedded in the bead matrix (Fig. 1b). The surface of the Zn-pectinate beads (globular; Fig. 1b) are found to be different from Ca-pectinate bead surface (rough and thread-like structure; Fig. 1c). Similar finding was reported in earlier studies (19,21).
Table II.
Size, Shape, Weight, Zn Content, WL, MC, EE, and L of the Beads (Data Represents Mean ± SD (N = 3))
| Variables | Values | Shape (ER)v | Size (µm)v | Weight of 50 beads (mg)v | Zn content (µg mg−1 bead) | MC (%) | EE (%) |
|---|---|---|---|---|---|---|---|
| Cross-linking agent | Zn | 1.06 ± 0.03 | 896.44 ± 7.90 | 23.12 ± 0.03 | 54.12 ± 1.92 | 10.09 ± 0.43k | 97.99 ± 0.10k |
| Ca | 1.07 ± 0.05 | 1,077.77 ± 72.97 | 32.62 ± 0.77 | – | 16.16 ± 1.00 | 98.65 ± 0.21 | |
| Cross-linking pH | 1.5 | 1.06 ± 0.03 | 896.44 ± 7.90 | 23.12 ± 0.03 | 54.12 ± 1.92a | 10.09 ± 0.43 | 97.99 ± 0.10 |
| 6.3 | 1.06 ± 0.03 | 1,111.76 ± 40.61 | 24.49 ± 0.14 | 81.73 ± 0.94 | 11.05 ± 0.76 | 97.77 ± 0.68 | |
| Zinc acetate concentration (%) | 1 | 1.03 ± 0.01 | 881.09 ± 38.29 | 19.84 ± 0.08 | 33.55 ± 0.87b | 6.29 ± 0.95b | 98.29 ± 0.12q |
| 2.5 | 1.07 ± 0.02 | 886.96 ± 9.81 | 22.83 ± 0.11 | 50.44 ± 2.78 | 9.51 ± 0.66 | 98.01 ± 0.18 | |
| 5 | 1.06 ± 0.03 | 896.44 ± 7.90 | 23.12 ± 0.03 | 54.12 ± 1.92 | 10.09 ± 0.43 | 97.99 ± 0.10 | |
| 10 | 1.06 ± 0.02 | 1,035.16 ± 43.22 | 25.54 ± 0.46 | 88.80 ± 0.60c | 11.23 ± 0.79 | 97.68 ± 0.27 | |
| Cross-linking time (h) | 0.25 | 1.13 ± 0.07 | 894.28 ± 14.38 | 20.38 ± 0.12 | 42.35 ± 2.94d | 7.78 ± 0.19l | 98.38 ± 0.04r |
| 0.5 | 1.06 ± 0.03 | 896.44 ± 7.90 | 23.12 ± 0.03 | 54.12 ± 1.92 | 10.09 ± 0.43m | 97.99 ± 0.10s | |
| 2 | 1.07 ± 0.07 | 920.48 ± 14.47 | 23.24 ± 0.05 | 75.29 ± 0.96 | 12.25 ± 0.79 | 97.06 ± 0.85 | |
| 24 | 1.10 ± 0.08 | 943.97 ± 15.04 | 24.74 ± 0.24 | 84.00 ± 0.91 | 13.21 ± 0.12 | 96.29 ± 0.11 | |
| Drying | RT | 1.06 ± 0.03 | 896.44 ± 7.90 | 23.12 ± 0.03 | 54.12 ± 1.92e | 10.09 ± 0.43e | 97.99 ± 0.10 |
| 37°C | 1.06 ± 0.03 | 895.76 ± 11.13 | 22.03 ± 0.73 | 54.65 ± 0.65f | 9.56 ± 0.72f | 97.99 ± 0.10 | |
| 50°C | 1.06 ± 0.03 | 886.88 ± 7.820 | 20.95 ± 0.51 | 85.30 ± 0.42 | 1.45 ± 0.45 | 97.99 ± 0.10 | |
| FD | 1.06 ± 0.03 | 2,040.51 ± 46.66 | 19.59 ± 0.05 | 87.76 ± 0.61 | 0.66 ± 0.23 | 97.99 ± 0.10 | |
| Pectin concentration (%) | 3 | 1.09 ± 0.03 | 892.87 ± 13.27 | 23.38 ± 0.98 | 116.25 ± 1.18g | 10.70 ± 1.44 | 97.44 ± 0.08t |
| 4 | 1.07 ± 0.03 | 898.47 ± 16.83 | 22.06 ± 0.55 | 96.02 ± 0.86h | 11.01 ± 0.78 | 97.05 ± 0.09 | |
| 5 | 1.06 ± 0.03 | 896.44 ± 7.90 | 23.12 ± 0.03 | 54.12 ± 1.92 | 10.09 ± 0.43 | 97.99 ± 0.10 | |
| 6 | 1.08 ± 0.03 | 907.63 ± 20.25 | 23.81 ± 0.18 | 53.43 ± 2.84 | 10.24 ± 0.72 | 97.75 ± 0.05 | |
| Pectin–Resveratrol | 1:1 | 1.07 ± 0.03 | 1140.67 ± 80.12 | 40.16 ± 0.42 | 45.95 ± 2.05 | 15.07 ± 0.49n | 99.28 ± 0.10u |
| 2:1 | 1.07 ± 0.04 | 993.01 ± 48.82 | 31.57 ± 0.42 | 51.63 ± 2.10 | 12.43 ± 0.24o | 98.55 ± 0.02 | |
| 3:1 | 1.06 ± 0.03 | 896.44 ± 7.90 | 23.12 ± 0.03 | 54.12 ± 1.92 | 10.09 ± 0.43p | 97.99 ± 0.10 | |
| 4:1 | 1.05 ± 0.03 | 891.37 ± 11.06 | 21.81 ± 0.20 | 92.33 ± 0.24i | 9.09 ± 0.07 | 97.50 ± 0.03 | |
| Blank | – | 1.04 ± 0.01 | 866.62 ± 23.17 | 21.02 ± 0.09 | 111.58 ± 6.60j | 8.54 ± 0.17 | – |
a p < 0.05 between cross-linking pH 1.5 and 6.3
b p < 0.05 between zinc acetate concentration 1% with 2.5%, 5%, and 10%
c p < 0.05 between zinc acetate concentration 10% with 2.5% and 5%
d p < 0.05 among cross-linking time 0.25, 0.5, 2, and 24 h
e p < 0.05 between drying at RT with 50°C and FD
f p < 0.05 between drying at 37°C with 50°C and FD
g p < 0.05 between pectin concentration 3% with 4%, 5%, and 6%
h p < 0.05 between pectin concentration 4% with 5% and 6%
i p < 0.05 between pectin to resveratrol ratio 4:1 with 1:1, 2:1, and 3:1
j p < 0.05 between blank beads with pectin to resveratrol ratio 1:1, 2:1, 3:1, and 4:1
k p < 0.05 between cross-linking agent Ca and Zn
l p < 0.05 between cross-linking time 0.25 h with 0.5, 2, and 24 h
m p < 0.05 between cross-linking time 0.5 h with 2 and 24 h
n p < 0.05 between pectin to resveratrol ratio 1:1 with 2:1, 3:1, 4:1, and blank
o p < 0.05 between pectin to resveratrol ratio 2:1 with 3:1, 4:1, and blank
p p < 0.05 between pectin to resveratrol ratio 3:1 with 4:1 and blank
q p < 0.05 between zinc acetate concentration 1% and 10%
r p < 0.05 between cross-linking time 0.25 h with 2 and 24 h
s p < 0.05 between cross-linking time 0.5 and 24 h
t p < 0.05 among pectin concentration 3%, 4%, 5%, and 6%
u p < 0.05 among pectin to resveratrol ratio 1:1, 2:1, 3:1, and 4:1
vStatistical analysis is not shown
Fig. 1.
Scanning electron micrographs of the surface of a resveratrol-free Zn-pectinate bead (× 500), b resveratrol-loaded Zn-pectinate bead (× 350), and c resveratrol-loaded Ca-pectinate bead (× 500). Magnifications corresponding to each figure are presented in parentheses
FTIR
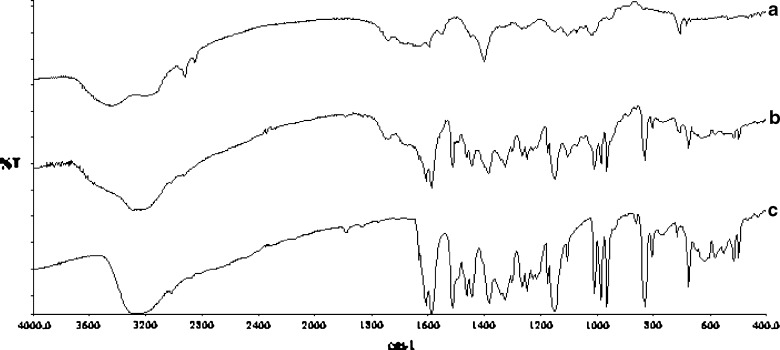
Comparison of the IR spectra of pure resveratrol, resveratrol-free and resveratrol-loaded Zn-pectinate beads indicates no chemical interaction of resveratrol with beads’ components when formulated into Zn-pectinate beads (Fig. 2). All characteristic peaks of resveratrol (olefinic band at 966 cm−1, C–O stretching at 1383, C–C olefinic stretching at 1585, and C–C aromatic double-bond stretching at 1607 cm−1) are present in the resveratrol-loaded Zn-pectinate bead.
Fig. 2.
FTIR spectra of a blank Zn-pectinate bead, b resveratrol-loaded Zn-pectinate bead, and c pure resveratrol
Zinc Content
The zinc contents per mg of the beads are depicted in Table II. The results suggest the potential impact of formulation parameters on the zinc retention in the beads. Zinc content increased with cross-linking solution pH, zinc acetate concentration, cross-linking time, and pectin to resveratrol ratio, while content decreased with pectin concentration. Lower amount of zinc is expected to bind with pectin chains due to suppression of ionization of the carboxylic group of the pectin chains at low cross-linking solution pH, which might repress the zinc entrapment. Augmentation of zinc content with increasing zinc acetate concentration and cross-linking time was due to availability of more zinc and more time for the cross-linking, respectively. One previous report evidenced that only small amount of calcium is required for Ca-pectinate network formation with the majority of calcium remaining as free calcium chloride in the Ca-pectinate beads (27). Similar to Ca-pectinate beads, we anticipate that majority of zinc remained as free zinc acetate in Zn-pectinate beads. At lower pectin to resveratrol ratio, less pectin is available for cross-linking which could probably explain lower zinc retention. During bead preparation, when dilute pectin droplets come in contact with zinc acetate solution, zinc acetate can easily penetrate into the pectin droplets. This might be the reasons for higher zinc content at lower pectin concentration. Zinc content was found in the order of RT < 37°C < 50°C < FD. The higher zinc content per mg of beads is rational for lower weighing beads as the weight of the beads decreased with drying temperature and was lowest in case of freeze dried beads (Table II). Similar trends were observed in case of calcium retention in the Ca-pectinate beads (22). Zinc is an essential micronutrient and 47 weeks oral administration of 25.5 mg.kg−1.day−1 zinc (zinc acetate in drinking water) displayed no toxic effect in rats (28). Thus, observed zinc contents in the beads are anticipated to be safe for practical use.
Effect of Formulation Parameters
The size, weight, MC, and EE corresponding to each type of formulations are given in Table II. Percent resveratrol retentions in the intact beads (in comparison to the initial resveratrol amount in the beads) are plotted against time to represent the drug retention profile (Figs. 3, 4, 5, 6, 7, 8, 9, and 10a), whereas percent SERs are plotted against time to depict the swelling–erosion behavior of the beads in the release media (Figs. 3–10b). Further, release parameters (T75, T50, and T25) are cited in Table III. The effects of different formulation parameters on the size, weight, MC, EE as well as resveratrol retention profiles, release parameters, and swelling–erosion behavior of the beads in the release media will be discussed in the following sections.
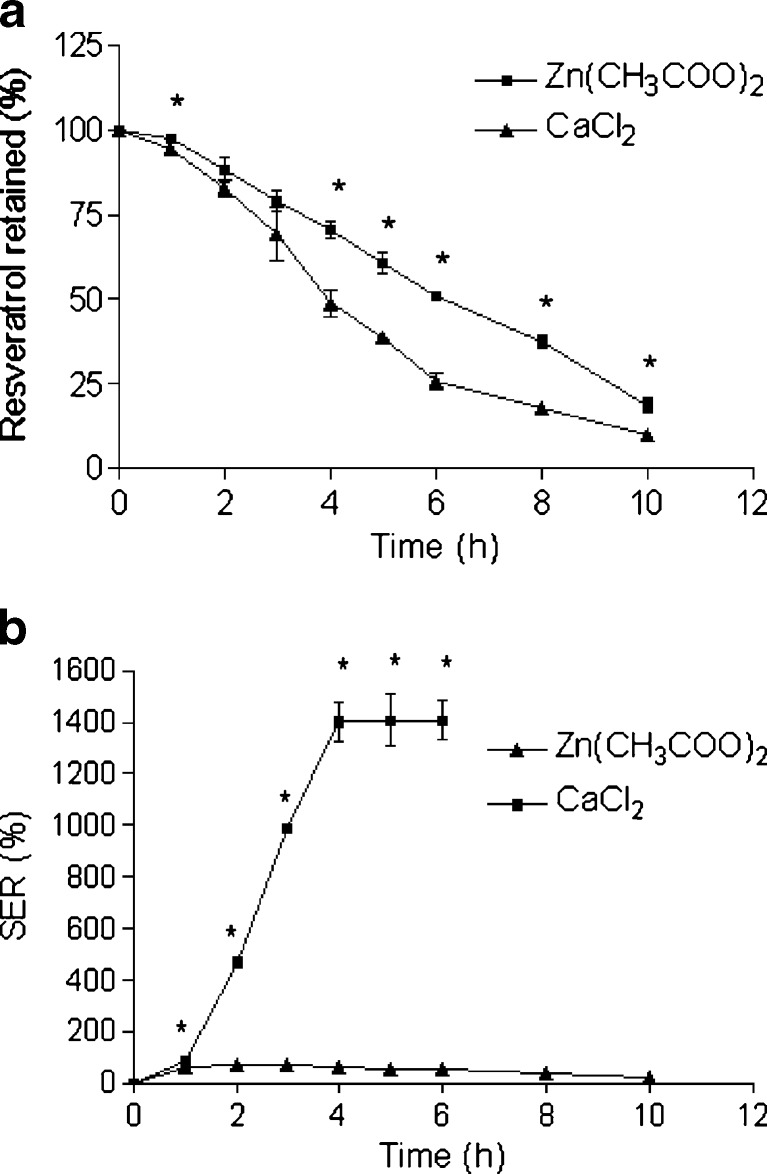
Fig. 3.
Effect of cross-linking agents (Zn(CH 3 COO) 2: zinc acetate, CaCl 2: calcium chloride) on a retention of resveratrol in the beads and b SER of the beads. The beads were incubated in SIF. Data represents mean ± SD. *p < 0.05
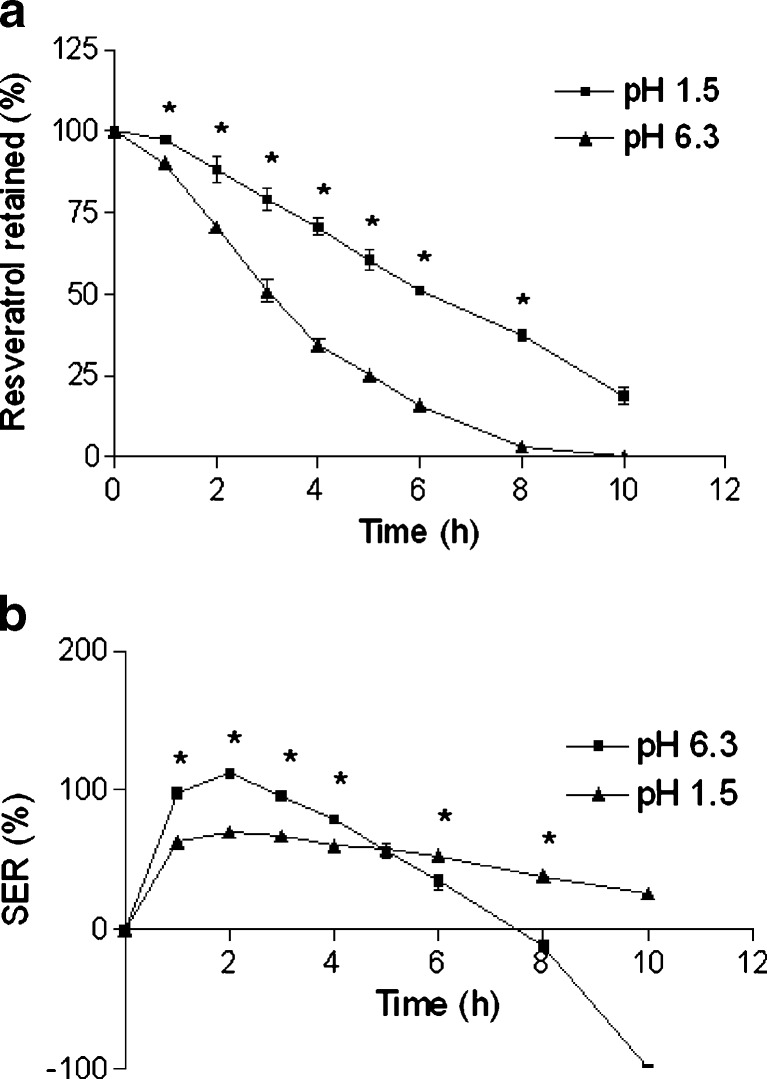
Fig. 4.
Effect of cross-linking solution pH on a retention of resveratrol in the beads and b SER of the beads. The beads were incubated in SIF. Data represents mean ± SD. *p < 0.05
Fig. 5.
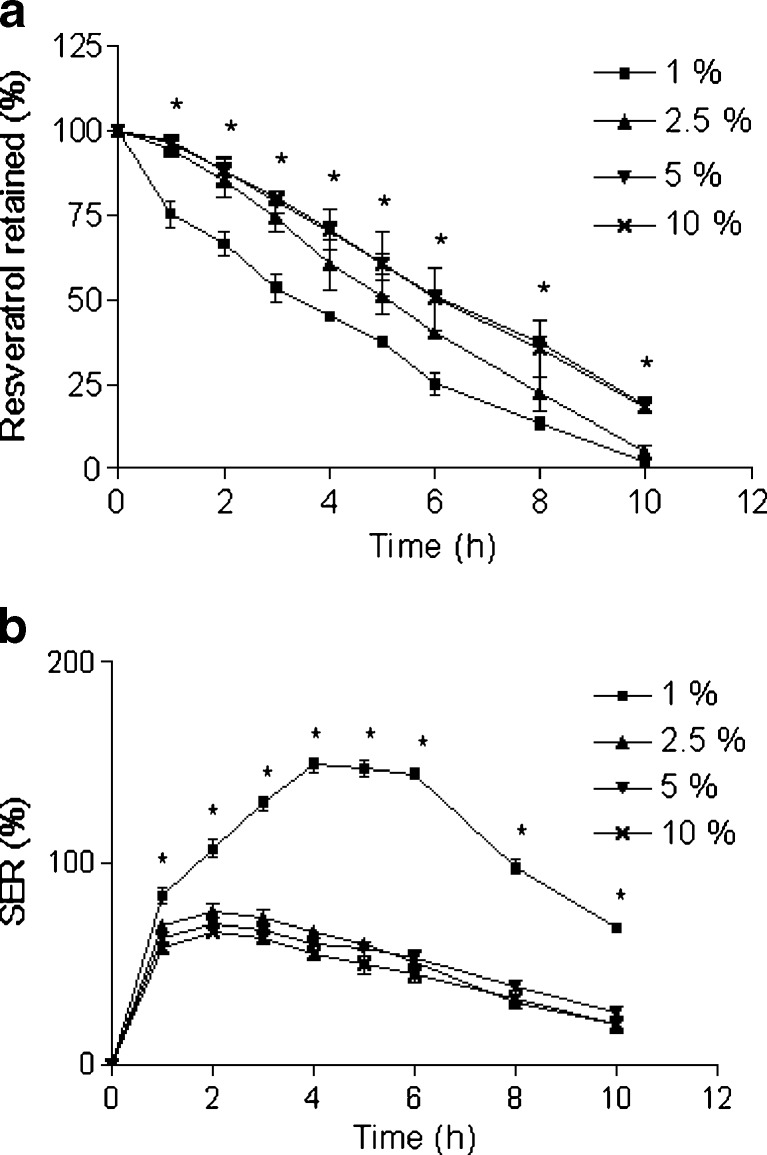
Effect of zinc acetate concentration on a retention of resveratrol in the beads and b SER of the beads. The beads were incubated in SIF. Data represents mean ± SD. *p < 0.05
Fig. 6.
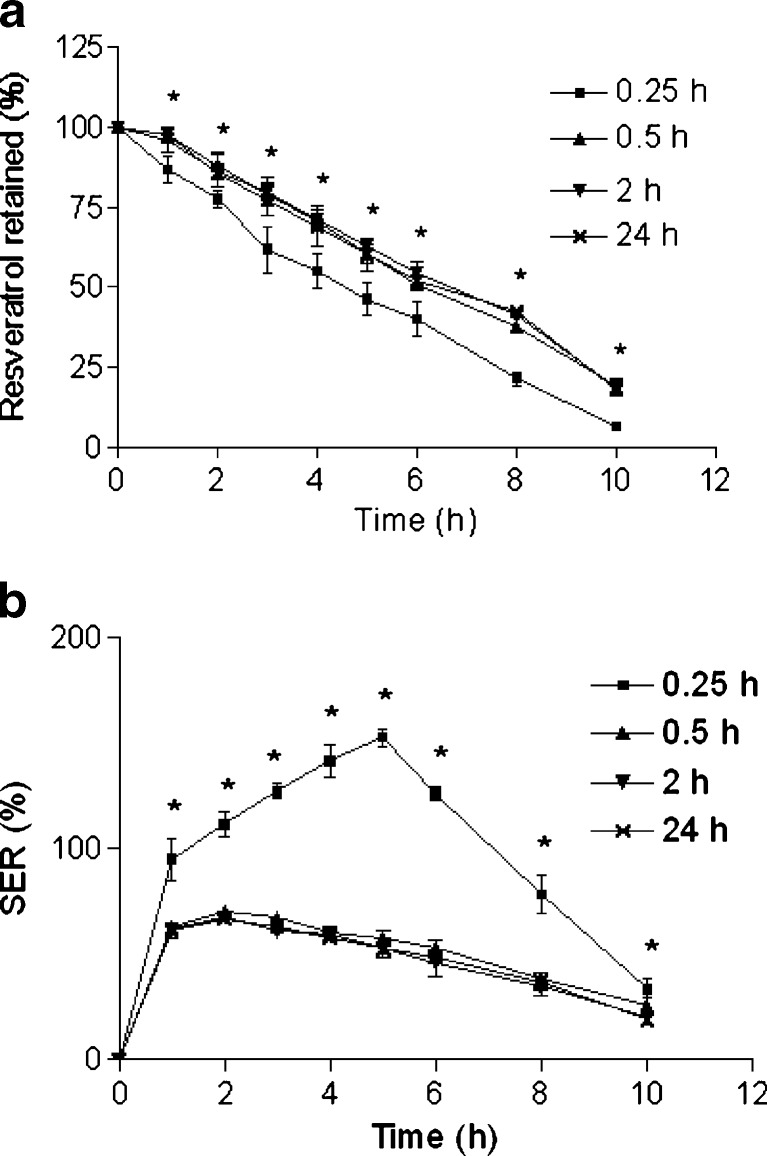
Effect of cross-linking time on a retention of resveratrol in the beads and b SER of the beads. The beads were incubated in SIF. Data represents mean ± SD. *p < 0.05
Fig. 7.
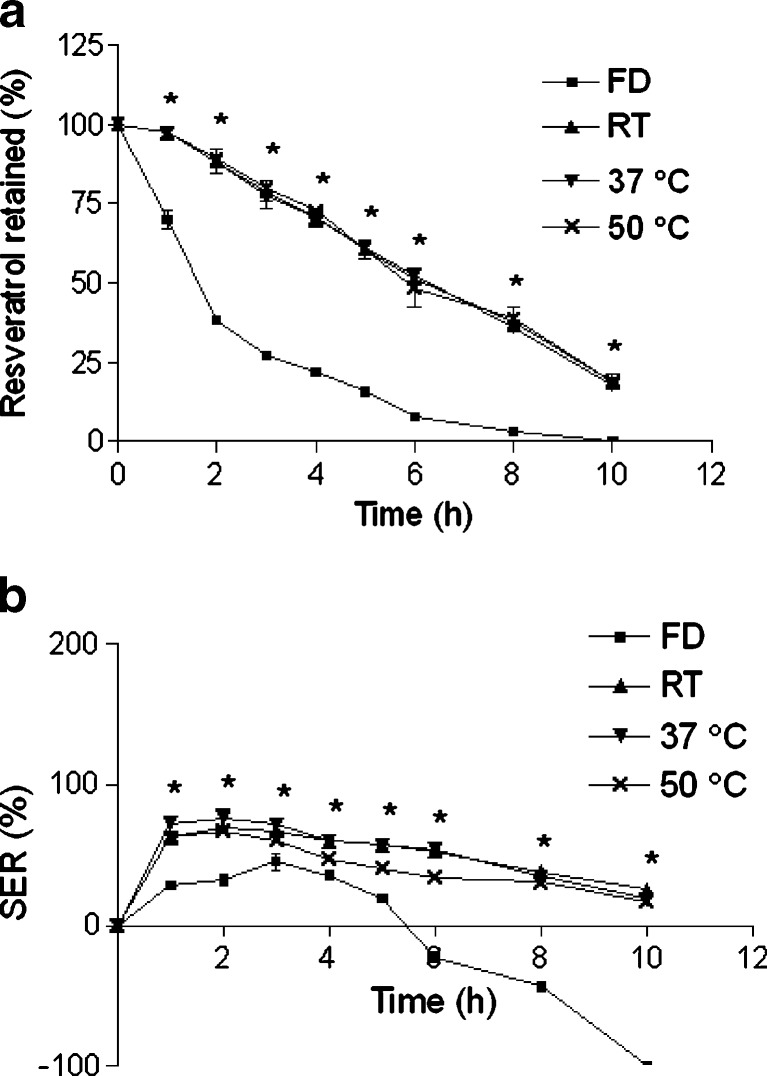
Effect of drying condition on a retention of resveratrol in the beads and b SER of the beads. The beads were incubated in SIF. Data represents mean ± SD. *p < 0.05
Fig. 8.
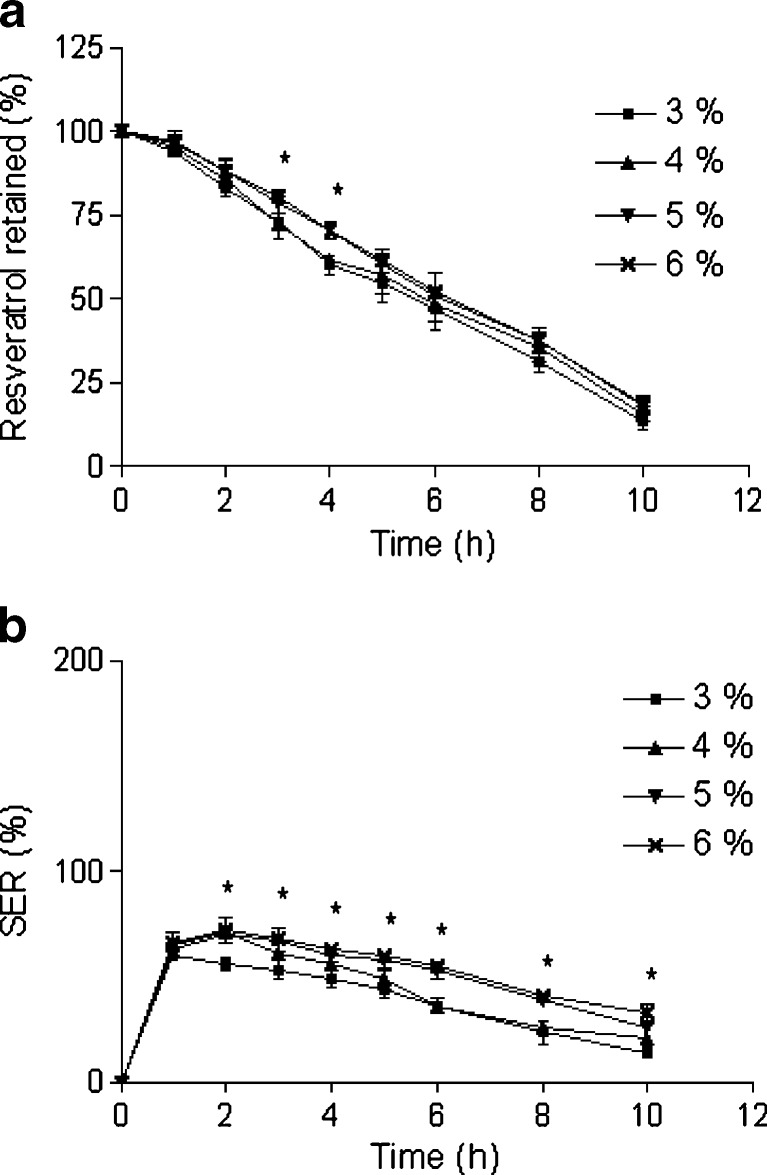
Effect of pectin concentration a retention of resveratrol in the beads and b SER of the beads. The beads were incubated in SIF. Data represents mean ± SD. *p < 0.05
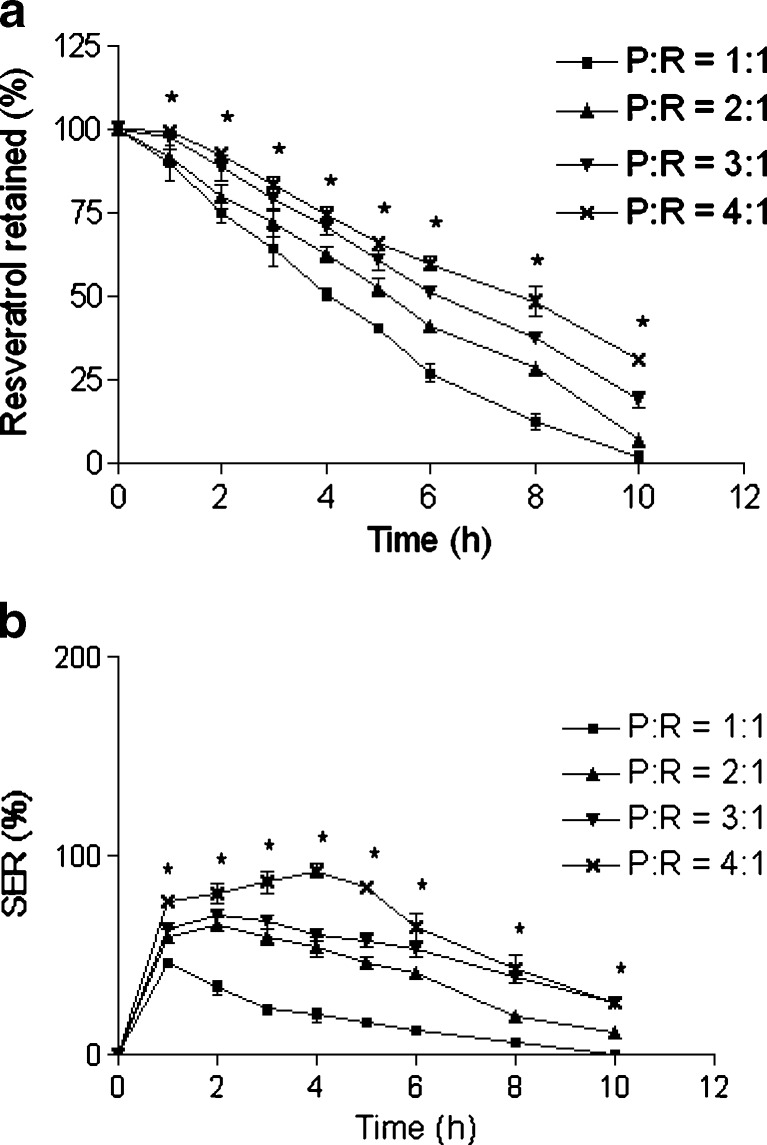
Fig. 9.
Effect of pectin to resveratrol ratio on a retention of resveratrol in the beads and b SER of the beads. The beads were incubated in SIF. Data represents mean ± SD. *p < 0.05
Fig. 10.
Effect of release media on a retention of resveratrol in the beads and b SER of the beads. Data represents mean ± SD. *p < 0.05
Table III.
Release Kinetics and Parameters of the Beads (Data Represents Mean ± SD (N = 3))
| Variables | Values | SQRT (R 2) | Zero order (R 2) | Time (h)i | ||
|---|---|---|---|---|---|---|
| T 75 | T 50 | T 25 | ||||
| Cross-linking agent | Zn | 0.980 | 0.997 | 3.48 ± 0.21a | 6.37 ± 0.20a | 9.26 ± 0.27a |
| Ca | 0.972j | 0.923 | 2.18 ± 0.17 | 4.27 ± 0.15 | 7.04 ± 0.08 | |
| Cross-linking pH | 1.5 | 0.980 | 0.997 | 3.48 ± 0.21b | 6.37 ± 0.20b | 9.26 ± 0.27b |
| 6.3 | 0.989j | 0.942 | 1.62 ± 0.02 | 3.16 ± 0.02 | 5.23 ± 0.03 | |
| Zinc acetate concentration (%) | 1 | 0.992j | 0.982 | 1.23 ± 0.23c | 3.30 ± 0.24c | 6.38 ± 0.18c |
| 2.5 | 0.986 | 0.995 | 2.82 ± 0.36 | 5.29 ± 0.34d | 7.75 ± 0.31d | |
| 5 | 0.980 | 0.997 | 3.48 ± 0.21 | 6.37 ± 0.20 | 9.26 ± 0.27 | |
| 10 | 0.976 | 0.998 | 3.46 ± 0.42 | 6.31 ± 0.47 | 9.16 ± 0.52 | |
| Cross-linking time (h) | 0.25 | 0.989 | 0.990 | 1.96 ± 0.41e | 4.80 ± 0.27e | 7.64 ± 0.18e |
| 0.5 | 0.980 | 0.997 | 3.48 ± 0.21 | 6.37 ± 0.20 | 9.26 ± 0.27 | |
| 2 | 0.964 | 0.995 | 3.51 ± 0.33 | 6.52 ± 0.22 | 9.53 ± 0.36 | |
| 24 | 0.974 | 0.988 | 3.41 ± 0.28 | 6.41 ± 0.23 | 9.41 ± 0.19 | |
| Drying | RT | 0.980 | 0.997 | 3.48 ± 0.21 | 6.37 ± 0.20 | 9.26 ± 0.27 |
| 37°C | 0.979 | 0.998 | 3.47 ± 0.13 | 6.33 ± 0.03 | 9.20 ± 0.18 | |
| 50°C | 0.975 | 0.992 | 3.53 ± 0.11 | 6.41 ± 0.19 | 9.28 ± 0.27 | |
| FD | 0.913k | 0.821k | – | – | – | |
| Pectin concentration (%) | 3 | 0.988 | 0.992 | 2.82 ± 0.42f | 5.70 ± 0.32 | 8.58 ± 0.22 |
| 4 | 0.986 | 0.987 | 3.00 ± 0.19 | 5.95 ± 0.35 | 8.90 ± 0.52 | |
| 5 | 0.980 | 0.997 | 3.48 ± 0.21 | 6.37 ± 0.20 | 9.26 ± 0.27 | |
| 6 | 0.971 | 0.998 | 3.51 ± 0.09 | 6.39 ± 0.18 | 9.27 ± 0.35 | |
| Pectin-Resveratrol | 1:1 | 0.994j | 0.972 | 1.95 ± 0.27g | 3.94 ± 0.27g | 6.63 ± 0.21g |
| 2:1 | 0.979 | 0.996 | 2.61 ± 0.08 | 5.33 ± 0.11 | 8.05 ± 0.16 | |
| 3:1 | 0.980 | 0.997 | 3.48 ± 0.21 | 6.37 ± 0.20 | 9.26 ± 0.27 | |
| 4:1 | 0.982 | 0.994 | 4.10 ± 0.14 | 7.43 ± 0.21 | 10.76 ± 0.29 | |
| Enzyme | – | 0.994j | 0.967 | 2.02 ± 0.23h | 4.20 ± 0.28h | 7.17 ± 0.31h |
a p < 0.05 between cross-linking agent Zn and Ca
b p < 0.05 between cross-linking pH 1.5 and 6.3
c p < 0.05 between zinc acetate concentration 1% with 2.5%, 5%, and 10%
d p < 0.05 between zinc acetate concentration 2.5% with 5% and 10%
e p < 0.05 between cross-linking time 0.25 h with 0.5, 2, and 24 h
f p < 0.05 between pectin concentration 3% and 6%
g p < 0.05 among pectin to resveratrol ratio 1:1, 2:1, 3:1, and 4:1
h p < 0.05 between enzyme and nonenzyme treatment
iData represents mean ± SD
jRelease data more appropriately fit with square root of time equation
kRelease data does not fit with both equations
Cross-linking Agent
Zn-pectinate beads were smaller than Ca-pectinate beads (Table II), indicating that Zn-pectinate beads are more compact than Ca-pectinate beads. Other researchers also noticed similar phenomena (19,21). Further, Zn-pectinate beads were lighter with less MC than Ca-pectinate beads. Instantaneous formation of pectinate network allowed easy encapsulation of resveratrol in the pectinate beads. Indeed, very high percent of resveratrol encapsulation (>95.9%) was observed in all cases (Table II). Poor aqueous solubility of resveratrol was responsible for its high encapsulation in the beads. However, Zn-pectinate beads revealed lower resveratrol encapsulation and loading than Ca-pectinate beads. The finding is similar to observation in other studies on pectinate beads (19,21). This is probably due to formation of stronger zinc-pectinate network during bead formation, which led to ejection of resveratrol towards the outer part of the beads and subsequent drug leakage in the cross-linking solution.
Figure 3a (resveratrol retention profile) and Table III (T75, T50, and T25) demonstrate that Zn-pectinate beads were able to retain higher amount of drug than Ca-pectinate beads in SIF. This observation is in the same line with others (19–21,23). Consequently, huge decrease of SER was observed for Zn-pectinate than Ca-pectinate beads (Fig. 3b). Ca-pectinate beads were hydrated and swelled rapidly in contact with SIF, leading to subsequent drug release due to their high molecular porosity. In contrast, Zn-pectinate beads swelled very little. Wellner et al. compared different divalent cations for gel forming ability with pectin and found zinc was a stronger cross-linker than calcium (29). It was also reported that zinc poses higher binding ability with a higher affinity than calcium (30), which can form pectinate bead with higher gel strength. Thus, we decided to use zinc acetate as the cross-linking agent for further optimization of the formulation parameters.
Cross-linking Solution pH
Diminution of pH of the cross-linking solution produced beads with smaller size (Table II). A probable reason is that at reduced pH, nonionic interaction occurs along with ionic interaction between pectin chains, which leads to small and compact beads. Weight of beads slightly increased with increasing cross-linking solution pH (Table II). Moreover, MC of the beads was also found to increase with increasing pH of cross-linking solution (Table II). Nevertheless, insignificant increase in EE was observed in the present study with decreased cross-linking solution pH (Table II).
Drug retention profile shows that resveratrol retention was significantly higher in case of beads prepared at cross-linking solution pH 1.5 (Fig. 4a). Table III shows that the release parameters (T75, T50, and T25) were significantly lower in case of beads prepared at cross-linking solution with unmodified pH (pH 6.3). Similar effect of cross-linking solution pH on pectinate beads was reported before (21). Our previous study with Ca-pectinate beads also evidenced similar effect of cross-linking solution pH (22). As shown in Fig. 4b, SER values were higher in case of beads prepared at pH 6.3 up to 4 h and then erosion dominated over swelling. This might be because of the formation of a stronger Zn-pectinate network at acidic cross-linking solution (31). In addition to the ionic interaction between carboxylic groups of pectin and zinc cation, several nonionic interactions (e.g., hydrophobic interaction, hydrogen bonding) are involve in amidated pectin at pH below 3 (lower than pKa value of pectin), leading to the formation of strong Zn-pectinate network at acidic pH (32–34). Similar to Ca-pectinate network, reduction in cross-linking solution pH may lead to conformational transition from the twofold to the more compact threefold helical conformation of the polygalacturonate chains of pectin in the Zn-pectinate network (22). These could account for the effects of adjusting the cross-linking solution pH on resveratrol retention and SER of Zn-pectinate beads. Subsequently, we set the cross-linking solution pH at 1.5, while optimizing the other formulation parameters of resveratrol-loaded Zn-pectinate beads.
Zinc Acetate Concentration
Our preliminary studies showed that zinc acetate concentration below 1% produced irregular-shaped beads and above 10% gave no improvement of drug retention (data not shown), concentration was then kept between 1% and 10% in the present study. Augmentation of the beads’ size and weight was observed with increasing zinc acetate concentration in the cross-linking medium (Table II). However, some other studies reported an inverse relationship between the size and concentration of cross-linking agent (17). Table II demonstrates that MC increased with increasing zinc acetate concentration. However, a little improvement in EE was noticed with decreasing zinc acetate concentration (Table II).
Figure 5a demonstrates that the retention of resveratrol in the beads depends on the zinc acetate concentration. The release of resveratrol was significantly faster at 1% and 2.5% zinc acetate than 5%, while 5% and 10% zinc acetate showed almost similar drug retention pattern. In addition, the release parameters (T75, T50, and T25) support the above phenomena (Table III). Our previous work with Ca-pectinate beads showed increased retention of drug with increasing calcium chloride concentration up to a certain value (22). Another earlier work also exhibited slower drug release at higher zinc acetate concentration (20). In general, SER decreased with increasing concentration of zinc acetate (Fig. 5b). Very high SERs were detected when beads were prepared at 1% zinc acetate. It demonstrates an early phase of swelling, followed by rapid erosion of the beads. These differences in drug retention and SER could be explained by difference in degree of cross-linking, which could effect the swelling rate and subsequent penetration of solvent into the beads. It was reported that the calcium cation leads to dimer formation with pectin chain at low calcium concentration, whereas at high calcium concentration, enhancement of the number and/or strength of cross-links between pectin and calcium ions as well as aggregation of initial dimmers take place (13,27). We anticipate similar phenomena with Zn-pectinate beads, which led to a higher degree of cross-linking with increasing concentration of zinc acetate. In summary, higher drug retention and lower SER were observed due to higher gel strength at higher zinc acetate concentrations. Based on this finding, 5% zinc acetate was chosen as the optimal concentration for subsequent optimization processes.
Cross-Linking Time
The longer cross-linking time resulted larger and heavier beads with higher MC and lower EE (Table II).
The release profiles of resveratrol from the beads prepared with various cross-linking times are shown in Fig. 6a. It is obvious from the figure that the cross-linking time has great influence on the release of resveratrol from the Zn-pectinate beads. Retention of resveratrol was higher when beads were prepared at longer cross-linking time (0.5–24 h); whereas, retention of resveratrol was the lowest at the shortest cross-linking time (0.25 h). However, insignificant differences in drug retention were observed among the beads prepared at cross-linking time of 0.5, 2, and 24 h, although more drug retention was noticed in all cases than beads prepared at cross-linking time of 0.25 h (Table III). El-Gibaly has also demonstrated reduced drug release with increasing cross-linking time (20). Lower drug release at higher cross-linking time was also evident in case of Ca-pectinate beads in our previous study (22). The SER profile is depicted in Fig. 6b. In our findings and as expected, 0.25 h cross-linking time showed the highest SER (swelling of the beads accompanied by erosion and drug release). However, almost similar SER profiles were noticed among 0.5, 2, and 24 h cross-linking time. Promotion of cross-linking between pectin chains and zinc ions with respect to time might be the possible explanation for the observed SER and resveratrol retention pattern. Additionally, the aggregation of primary dimmers probably takes place at higher cross-linking time, which improves the strength of Zn-pectinate network. In this study, the optimized cross-linking time was found to be 0.5 h.
Drying Condition
Beads’ size and weight decreased with increasing drying temperature (Table II). The size of the freeze-dried beads was almost similar to the wet beads. This might be because of moisture evaporation from the beads without affecting the bead structure during lyophilization. A clear correlation was found between MC of the beads with the drying method (Table II). Lower MC was observed with higher drying temperature. The lyophilized beads and those dried at 50°C exhibited minimum MC (FD < 50°C, MC < 1.5%).
The effect of drying condition on retention of resveratrol is illustrated in Fig. 7a and Table III. Lyophilized beads exhibited lowest drug retention profile among the all drying methods. Further, erosion overshadowed swelling of lyophilized beads (Fig. 7b). A significant slow release and low SER were observed in case of beads dried at RT, 37°C and 50°C, although there were almost no difference in drug retention and SER profiles among them. The rapid degradation and higher SER of lyophilized beads can be explained by the higher porosity created during drying. Therefore, lyophilized beads were unsuitable for our current purpose. RT was selected as optimum drying condition due to its convenience.
Pectin Concentration
Low pectin concentration has low consistency that leads to rapid shrinkage of beads during drying and lose their spherical shape. Our preliminary studies showed irregular-shaped bead formation when <3% pectin concentration was used. On the other hand, when a >6% pectin concentration was used, beads with a drop-like structure were formed due to very high viscosity of high pectin concentration (data not shown). Thus, pectin concentration between 3% and 6% was chosen for optimization. However, beads’ size, weight, MC, and EE were found to be independent of the pectin concentration (Table II).
As shown in Fig. 8a and b and Table III, both drug retention capability and SER of the beads slightly increased with increasing pectin concentration (3% to 5%). Higher pectin concentration forms a denser matrix, which probably improves drug retention behavior of beads. However, almost similar resveratrol retention and SER was observed between beads prepared with 5% and 6% pectin. These observations guided us to decide 5% as the optimum pectin concentration.
Pectin to Resveratrol Ratio
Reduction of pectin to resveratrol ratio produced heavier and bigger beads with higher MC (Table II). This is anticipated because of lower polymer to drug ratio. Further, blank beads were much smaller and lighter with lower MC than resveratrol-loaded beads. In general, enhancement of drug concentration results lower EE due to reduced polymer to drug ratio (35). However, the results revealed augmentation of EE with decreasing pectin to resveratrol ratio (Table II). This might be due to the poor solubility of resveratrol in the cross-linking solution. After a certain amount, resveratrol saturates the cross-linking solution at constant temperature. Thus, addition of high amount of resveratrol into the pectin solution led to the high EE in the beads as only limited amount of resveratrol can diffuse from the beads to the cross-linking solution.
Figure 9a demonstrated the influence of pectin to resveratrol ratio on retention of resveratrol in the beads. The retention of resveratrol decreased with decreasing pectin to resveratrol ratio. The release parameters (T75, T50, and T25) showed the following trend: 1:1 < 2:1 < 3:1 < 4:1 (Table III). Our earlier study with Ca-pectinate beads showed similar trend (22). SER decreased with decreasing pectin to resveratrol ratio (Fig. 9b). Since only little pectin would be available for drug retention and swelling at low pectin to resveratrol ratio, reduction of resveratrol retention and SER are expected in this case. The pectin to resveratrol ratio of 3:1 was selected for the formulation due to its high drug loading and sufficient delayed drug release pattern.
Effect of Release Media on Drug Release and SER
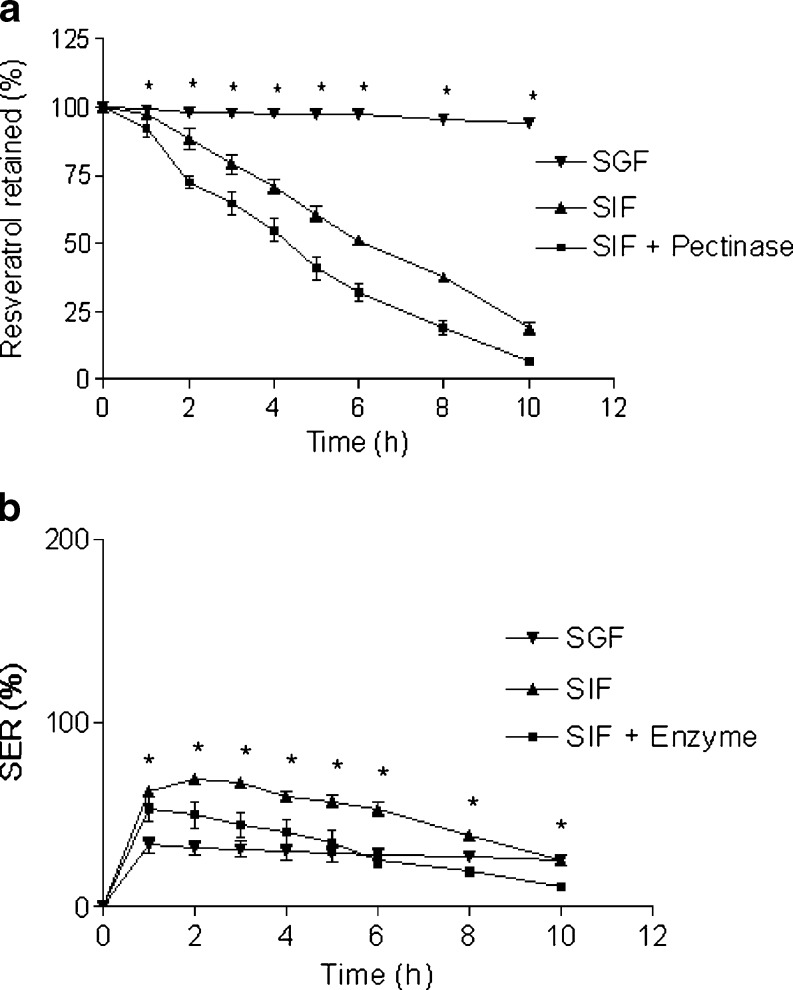
After careful and thorough observation of the effects of formulation parameters on the beads’ properties, the following parameters were optimized to prepare resveratrol-loaded pectin beads: zinc acetate as cross-linking agent, cross-linking solution pH at 1.5, zinc acetate concentration at 5%, cross-linking time of 0.5 h, drying at RT, pectin concentration at 5%, and pectin to resveratrol ratio of 3:1. Drug retention and SER profile of the optimized beads in SGF (pH 1.2) and in SIF containing 300 PG pectinase (simulating colonic fluid) were noted. Resveratrol retention (Fig. 10a) in the beads was very high (>94% after 10 h) and SER (Fig. 10b) was very low in SGF. This indicates the stability of Zn-pectinate beads in the gastric environment. Faster release of resveratrol from pectin beads was evident in SIF with pectinase than SIF alone. The release parameters (T75, T50, and T25) were lower in presence of pectinase than nonenzymatic degradation (Table III). Similar trend was observed with Ca-pectinate beads (22). Because of enzymatic degradation of the pectinate matrix, lower SER was observed in presence of enzyme.
Enzymatic Degradation
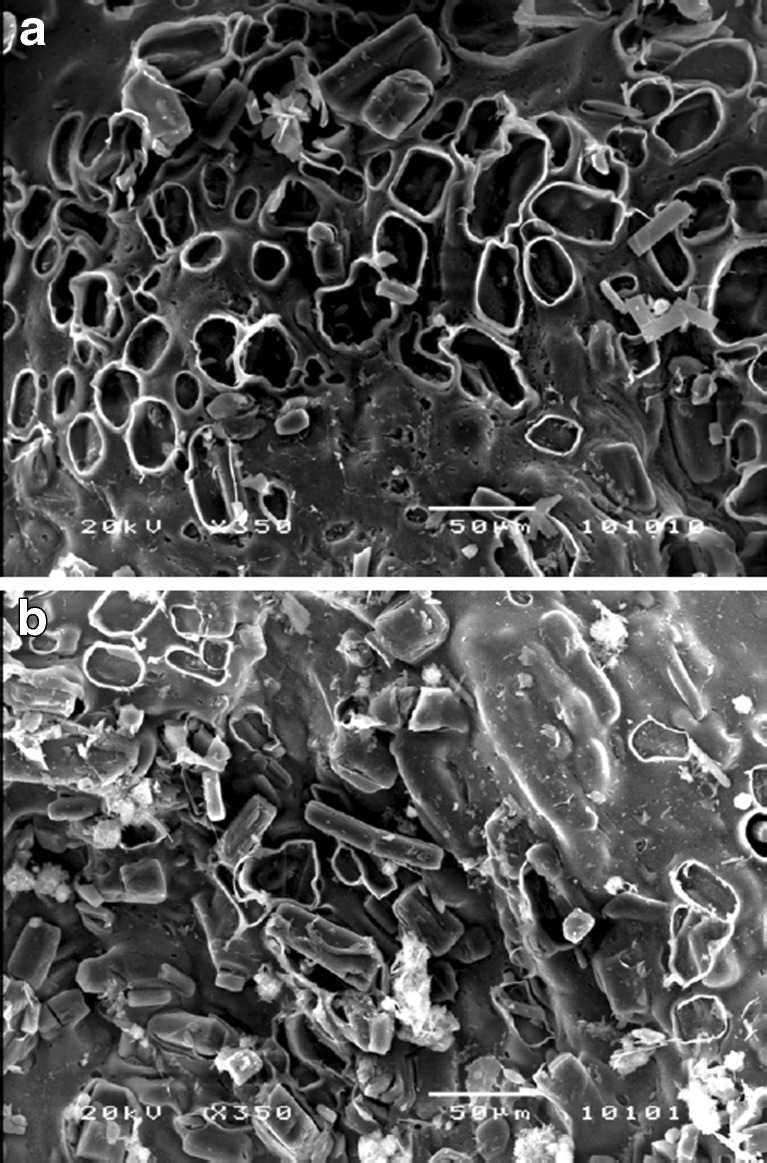
SEM images of the beads after 3 h incubation in SIF with and without enzyme exhibited many tiny pores throughout the beads (Fig. 11a and b). Much higher number of pores was evident with the beads incubated in SIF containing pectinase than SIF alone. Enzymatic degradation and subsequent erosion of the pectinate matrix might explain this observation. Resveratrol particles probably released through these pores during matrix degradation.
Fig. 11.
Scanning electron micrographs of a surface of resveratrol-loaded bead after 3 h incubation in SIF containing 300 PG pectinase (× 350) and b surface of resveratrol-loaded bead after 3 h incubation in SIF (× 350). Magnifications corresponding to each figure are presented in brackets
Release Kinetics
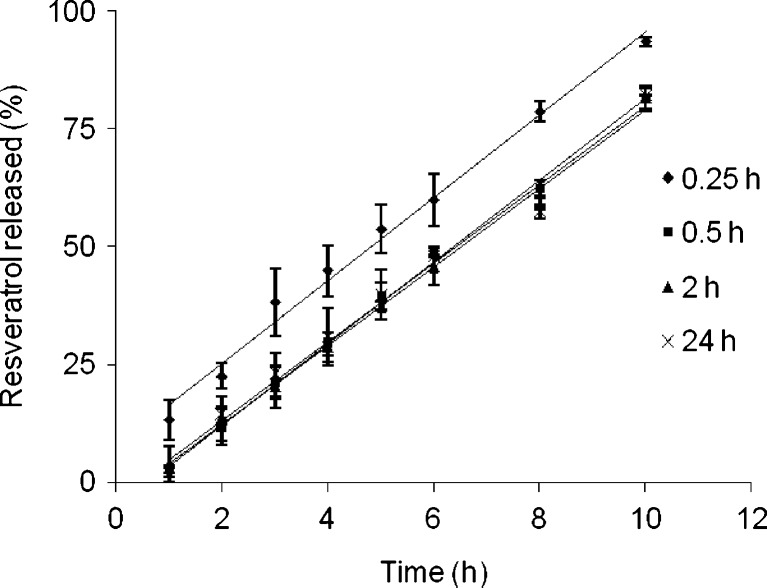
In the present study, we noticed that the drug release kinetics of Ca-pectinate beads as well as Zn-pectinate beads prepared with 1% zinc acetate, cross-linking solution pH of 6.3, and pectin to resveratrol ratio = 1:1 together with Zn-pectinate beads in presence of enzyme showed high correlation with the square root of time equation (Table III). The rest of the beads more appropriately fitted with the zero order equation (except lyophilized beads which did not fit both equations). However, these observations do not match with the release kinetics of the Ca-pectinate beads, where all release data more appropriately fitted with the square root of time equation (22). One example to show the linearity of plots for the cumulative percent resveratrol released versus time is presented in Fig. 12. Because of the marginal solubility of resveratrol in SIF (0.11 ± 0.01 μg ml−1; 4), most of the resveratrol particles should not be dissolved in the release media that penetrated into the bead matrix. Similar to the Ca-pectinate beads, the release of resveratrol from the Zn-pectinate beads probably follows the following three consecutive phenomena: (a) hydration of the matrix, (b) swelling and erosion of the matrix, and (c) the release of resveratrol from the matrix as undissolved particles rather than diffusion of the soluble resveratrol. Upon contact with SIF (basically a phosphate buffer), fluid penetrates into the Zn-pectinate network and ion exchange between Zn2+ and Na+/K+ ions (components of SIF) takes place. As a result, partially soluble pectin regions are formed due to displacement of the Zn2+ ions from Zn-pectinate network, which are more permeable (36). Pores are created in the matrix due to the release of undissolved resveratrol particles, which allow more fluid penetration in to the matrix and subsequent release of more resveratrol particles followed by erosion of the bead matrix. When the pectinate matrix is strong, the drug release from the beads is impeded by less fluid penetration and less erosion of the matrix.
Fig. 12.
The release of resveratrol from Zn-pectinate beads prepared at different cross-linking time, plotted as the cumulative percent resveratrol released versus time. Data represents mean ± SD
STABILITY
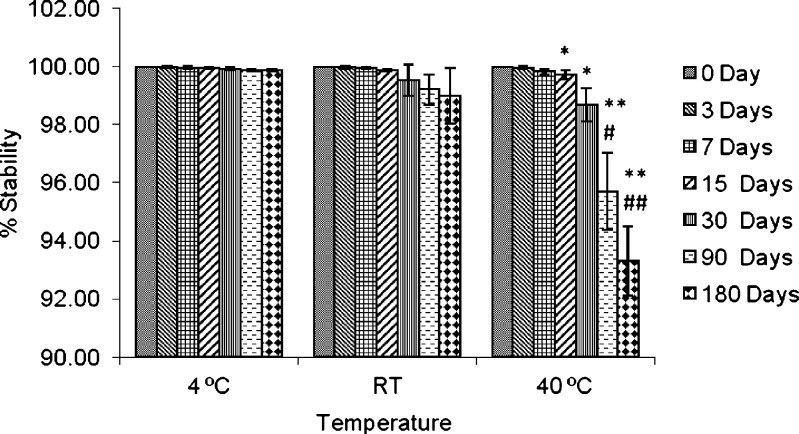
The stability profile of resveratrol in the optimized beads indicates that resveratrol was stable inside the beads at 4°C and RT, even after 6 months (Fig. 13). Stability was ∼99.87% and ∼98.99% in case of storage at 4°C and RT, respectively, whereas ∼93% when stored at accelerated condition (40°C). These results indicate desired stability of the formulation at 4°C and RT.
Fig. 13.
Stability of resveratrol in the optimized resveratrol-loaded Zn-pectinate beads. Data represents mean ± SD. # represents p < 0.05 for the difference between 90 days and 0, 3, 7, 15, and 30 days; ## represent p < 0.05 for the difference between 180 days and 0, 3, 7, 15, 30, and 90 days; *p < 0.05 for the difference between 40°C and 4°C; and **p < 0.05 for the difference between 40°C and 4°C and RT
CONCLUSIONS
Because resveratrol has shown preventive as well as therapeutic activity to the lower GI diseases (e.g., colorectal cancer, colitis) but quickly absorbed and rapidly metabolized at the upper GI tract, a delayed release or colon-specific drug delivery system is essential to treat these diseases. To fulfill this aim, we have attempted to develop a multiparticulate resveratrol-loaded pectinate bead. Our present study revealed that Zn-pectinate beads are better than Ca-pectinate beads as delayed release formulation of resveratrol. During optimization of the resveratrol-loaded Zn-pectinate formulation, formulation parameters appeared to have great influence on beads’ properties and drug release characteristics. The release mechanism of resveratrol from most of the Zn-pectinate beads followed zero order equation. Beads were able to encapsulate very high amount of resveratrol and the beads prepared with optimized formulation parameters exhibited delayed release of resveratrol. The beads were also stable at RT and 4°C storage. However, despite its delayed drug release pattern, additional modifications of the beads such as enteric coating, use of hardening agent, and complexation with other polymers are necessary to achieve colon-specific delivery of resveratrol. We are currently aiming to achieve this goal.
Acknowledgments
This work was partially supported through a National University of Singapore Academic Research Funds R148-050-068-101 and R148-050-068-133.
Abbreviations
- Ca
calcium
- CaCl2
calcium chloride
- Ca-pectinate
calcium-pectinate
- Ca2+
calcium cation
- EE
encapsulation efficiency
- ER
elongation ratio
- FD
freeze drying
- FTIR
Fourier transform infra-red
- GI tract
gastrointestinal tract
- MC
moisture content
- P–R
pectin–resveratrol
- RT
room temperature
- SER
swelling–erosion ratio
- T25
25% drug retention
- T50
50% drug retention
- T75
75% drug retention
- Zn(CH3COO)2
zinc acetate
- Zn
zinc
- Zn-pectinate
zinc-pectinate
- Zn2+
zinc cation
Contributor Information
Ka-Yun Ng, Phone: +65-651-62651, FAX: +65-677-91554.
Paul C. Ho, Phone: +65-651-62651, FAX: +65-677-91554
References
- 1.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 3.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7(4):423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 4.Das S, Lin HS, Ho PC, Ng KY. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm Res. 2008;25(11):2593–2600. doi: 10.1007/s11095-008-9677-1. [DOI] [PubMed] [Google Scholar]
- 5.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67(7):1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Martin AR, Villegas I, Sanchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147(8):873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis. 2000;21(8):1619–1622. doi: 10.1093/carcin/21.8.1619. [DOI] [PubMed] [Google Scholar]
- 9.Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39(1):102–107. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 10.Basit AW. Advances in colonic drug delivery. Drugs. 2005;65(14):1991–2007. doi: 10.2165/00003495-200565140-00006. [DOI] [PubMed] [Google Scholar]
- 11.Friend DR. New oral delivery systems for treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57(2):247–265. doi: 10.1016/j.addr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24(19):3333–3343. doi: 10.1016/S0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 13.Wakerly Z, Fell J, Attwood D, Parkins D. Studies on amidated pectins as potential carriers in colonic drug delivery. J Pharm Pharmacol. 1997;49(6):622–625. doi: 10.1111/j.2042-7158.1997.tb06856.x. [DOI] [PubMed] [Google Scholar]
- 14.Thakur BR, Singh RK, Handa AK. Chemistry and uses of pectin—a review. Crit Rev Food Sci Nutr. 1997;37(1):47–73. doi: 10.1080/10408399709527767. [DOI] [PubMed] [Google Scholar]
- 15.Maestrelli F, Cirri M, Corti G, Mennini N, Mura P. Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur J Pharm Biopharm. 2008;69(2):508–518. doi: 10.1016/j.ejpb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez M, Vila-Jato JL, Torres D. Design of a new multiparticulate system for potential site-specific and controlled drug delivery to the colonic region. J Control Release. 1998;55(1):67–77. doi: 10.1016/S0168-3659(98)00029-7. [DOI] [PubMed] [Google Scholar]
- 17.Sriamornsak P. Effect of calcium concentration, hardening agent and drying condition on release characteristics of oral proteins from calcium pectinate gel beads. Eur J Pharm Sci. 1999;8(3):221–227. doi: 10.1016/S0928-0987(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 18.Maestrelli F, Zerrouk N, Cirri M, Mennini N, Mura P. Microspheres for colonic delivery of ketoprofen-hydroxypropyl-beta-cyclodextrin complex. Eur J Pharm Sci. 2008;34(1):1–11. doi: 10.1016/j.ejps.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Dupuis G, Chambin O, Génelot C, Champion D, Pourcelot Y. Colonic drug delivery: influence of cross-linking agent on pectin beads properties and role of the shell capsule type. Drug Dev Ind Pharm. 2006;32(7):847–855. doi: 10.1080/03639040500536718. [DOI] [PubMed] [Google Scholar]
- 20.El-Gibaly I. Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int J Pharm. 2002;232(1–2):199–211. doi: 10.1016/S0378-5173(01)00903-6. [DOI] [PubMed] [Google Scholar]
- 21.Chambin O, Dupuis G, Champion D, Voilley A, Pourcelot Y. Colon-specific drug delivery: influence of solution reticulation properties upon pectin beads performance. Int J Pharm. 2006;321(1–2):86–93. doi: 10.1016/j.ijpharm.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Ng K-Y. Resveratrol-loaded calcium-pectinate beads: effects of formulation parameters on drug release and bead characteristics. J Pharm Sci. 2010;99(2):840–860. doi: 10.1002/jps.21880. [DOI] [PubMed] [Google Scholar]
- 23.Atyabi F, Majzoob S, Iman M, Salehi M, Dorkoosh F. In vitro evaluation and modification of pectinate gel beads containing trimethyl chitosan, as a multi-particulate system for delivery of water-soluble macromolecules to colon. Carbohydr Polym. 2005;61(1):39–51. doi: 10.1016/j.carbpol.2005.02.005. [DOI] [Google Scholar]
- 24.Wong TW, Nurjaya S. Drug release property of chitosan-pectinate beads and its changes under the influence of microwave. Eur J Pharm Biopharm. 2008;69(1):176–188. doi: 10.1016/j.ejpb.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Bourgeois S, Tsapis N, Honnas H, Andremont A, Shakweh M, Besnard M, et al. Colonic delivery of beta-lactamases does not affect amoxicillin pharmacokinetics in rats. J Pharm Sci. 2008;97(5):1853–1863. doi: 10.1002/jps.21115. [DOI] [PubMed] [Google Scholar]
- 26.Grant GT, Morris ER, Rees DA. Biological interactions between polysaccharides and divalent cations: the egg box model. FEBS Lett. 1973;32(1):195–198. doi: 10.1016/0014-5793(73)80770-7. [DOI] [Google Scholar]
- 27.Bourgeois S, Gernet M, Pradeau D, Andremont A, Fattal E. Evaluation of critical formulation parameters influencing the bioactivity of beta-lactamases entrapped in pectin beads. Int J Pharm. 2006;324(1):2–9. doi: 10.1016/j.ijpharm.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 28.Drinker KR, Thompson PK, Marsh M. An investigation of the effect upon rats of long continued ingestion of zinc compounds, with a special reference to the relation of zinc excretion to zinc intake. Am J Physiol. 1927;80:284–306. [Google Scholar]
- 29.Wellner N, Kacurakova M, Malovikova A, Wilson RH, Belton PS. FT-IR study of pectate and pectinate gels formed by divalent cations. Carbohydr Res. 1998;308(1–2):123–131. doi: 10.1016/S0008-6215(98)00065-2. [DOI] [Google Scholar]
- 30.Dronnet VM, Renard CMGC, Axelos MAV, Thibault JF. Characterisation and selectivity of divalent metal ions binding by citrus and sugar-beet pectins. Carbohydr Polym. 1996;30(4):253–263. doi: 10.1016/S0144-8617(96)00107-5. [DOI] [Google Scholar]
- 31.Pillay V, Fassihi R. In vitro release modulation from crosslinked pellets for site-specific drug delivery to the gastrointestinal tract. I. Comparison of pH-responsive drug release and associated kinetics. J Control Release. 1999;59(2):229–242. doi: 10.1016/S0168-3659(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 32.Cardoso SM, Coimbra MA, Lopes da Silva JA. JA. Temperature dependence of the formation and melting of pectin-Ca 2+ networks: A rheological study. Food Hydrocoll. 2003;17(6):801–807. doi: 10.1016/S0268-005X(03)00101-2. [DOI] [Google Scholar]
- 33.Gilsenan PM, Richardson RK, Morris ER. Thermally reversible acid-induced gelation of low-methoxy pectin. Carbohydr Polym. 2000;41(4):339–349. doi: 10.1016/S0144-8617(99)00119-8. [DOI] [Google Scholar]
- 34.Lootens D, Capel F, Durand D, Nicolai T, Boulenguer P, Langendorff V. Influence of pH, Ca concentration, temperature and amidation on the gelation of low methoxyl pectin. Food Hydrocoll. 2003;17(3):237–244. doi: 10.1016/S0268-005X(02)00056-5. [DOI] [Google Scholar]
- 35.Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and in vitro release studies. Int J Pharm. 1998;160(2):207–212. doi: 10.1016/S0378-5173(97)00310-4. [DOI] [Google Scholar]
- 36.Sriamornsak P. Investigation of pectin as a carrier for oral delivery of proteins using calcium pectinate gel beads. Int J Pharm. 1998;169(2):213–220. doi: 10.1016/S0378-5173(98)00129-X. [DOI] [Google Scholar]