Abstract
This paper deals with the synthesis of thermo-responsive microspheres with proteic structure exhibiting a transition temperature close to the body temperature. Temperature-sensitive hydrogels have attracted extensive interest due to their potential and promising applications in drug delivery field since they can undergo a rapid and reversible phase transition from a swollen to a shrunken state depending on environmental temperature. The hydrogels were synthesized by free-radical polymerization of hydrolyzed methacrylated gelatin (HGel-MA) and N,N′-methylenebisacrylamide as pro-hydrophilic multifunctional macromer and crosslinker, respectively, and N-isopropylacrylamide as thermo-responsive monomer. Thermal analyses showed negative thermo-responsive behavior for all compositions and, by increasing the content of the hydrophilic moieties in the network, the transition temperature raised to 36.9°C, close to the physiological values. In order to test the materials as drug carriers, diclofenac sodium salt was chosen as model drug. Drug release profiles, in phosphate buffer solution (pH 7.0, 10−3 M) at 25 and 40°C, depend on the hydrogel’s crosslinking degree and hydrophilic/hydrophobic balance in the polymeric network. For all formulations, in the shrunken state, the drug release percent values ranged from 80% to 100% after 24 h, and after 3 h, more than 60% of therapeutics was delivered. On the contrary, the swelling of the loaded microparticles produces, even after 30 h, a drug release percent of about 75%. By using semi-empirical equations, the release mechanism was extensively studied and the diffusional contribute was evaluated. The physico-chemical characteristics of thermo-responsive materials confirm the applicability of the microspheres as drug delivery device.
Key words: hydrogel, hydrolyzed gelatin, microspheres, radical polymerization, thermo-responsive release
INTRODUCTION
Hydrogels are polymeric networks that absorb large quantities of water while remaining insoluble in aqueous solutions due to chemical or physical crosslinking of individual polymer chains. In the swollen state, the hydrogels are soft and rubbery. Furthermore, some hydrogels, being similar to living tissue, possess excellent biocompatibility properties (1–4). Among polymers that can form hydrogels, natural polymers are often preferred to synthetic materials because of their non-toxicity, low cost, ready availability, and biocompatibility. Besides, they can be copolymerized with specific monomers to obtain copolymers with designed functionalities, such as thermo- or pH sensitivity. This kind of systems is of great interest as drug carrier devices, food additives, and so on (5–8).
Thermo-sensitive hydrogels have attracted extensive interest due to their potential and promising applications in many fields (9–12). Temperature is the most widely utilized triggering signal for a variety of triggered or pulsatile drug delivery systems. The use of temperature as a signal has been justified by the fact that the body temperature often deviates from the physiological value (37°C) in the presence of pathogens or pyrogens. This deviation sometimes can be a useful stimulus that activates the release of therapeutic agents from various temperature-responsive drug delivery systems for diseases accompanying fever. Among the family of the temperature-sensitive hydrogels, poly(N-isopropylacrylamide) (PNIPAAm) is one of the most widely studied. It exhibits a lower critical solution temperature (LCST) at 32°C in aqueous solution and shows an abrupt thermo-reversible change in volume as external temperature cycles around this critical temperature (13). The LCST can be adjusted to the human body temperature by copolymerization or interpenetration with other monomers (14). For this purpose, and to improve the mechanical properties of the resultant materials, much research has been done to associate biopolymers (such as protein and polysaccharides) with thermo-sensitive macromolecules.
Gelatin is a biopolymer with thermo-reversible properties (15), and at temperatures below 25°C, an aqueous gelatin solution solidifies due to the formation of triple helices and a rigid three-dimensional network. When the temperature is raised to approximately 30°C, the conformation changes from a helix to a more flexible coil, with a consequent formation of the liquid form of the gel (16). As the opposite thermal behavior is desired for biomedical applications, researchers have combined gelatin with other polymers showing thermal gelation close to body temperature (17). In addition to its good biological properties, such as non-toxicity and non-immunogenicity, gelatin has the advantage of allowing for easy modification on the amino acid level.
In literature, different methods to obtain gelatin thermo-sensitive hydrogels are reported, and these materials are of great interest in several fields such as drug delivery and tissue engineering. Chun et al. (18) proposed the synthesis of a hydrogel-dispersed composite membrane based on PNIPAAm and crosslinked gelatin, and its thermally actuated transport characteristics of 4-acetamidophen were investigated in a diffusion cell. Lee et al. (19) studied the effect of gelatin on the release profile of anionic, cationic, and neutral drugs from different organic hybrid gels, based on PNIPAAm and gelatin, crosslinked through a two-step process with genipin or glutaraldehyde.
This paper reports on the synthesis, by reverse phase suspension radical polymerization, of peptide-based thermo-responsive microspheres in which hydrolyzed methacrylated gelatin (HGel-MA) was employed as pro-hydrophilic monomer/crosslinker, NIPAAm as thermo-responsive co-monomer, and N,N′-methylenebisacrylamide (MBA) as crosslinking agent. Our objective was to design biodegradable thermo-responsive materials with a spherical shape and LCST values close to the body temperature in order to obtain pharmaceutical devices able to modulate the drug release profile depending on the temperature of the surrounding environment. The proposed materials should be usefully applied in the pharmaceutical field because they combine two essential features of drug delivery systems. They undergo reversible volume changes, a phase transition process, by swelling–shrinking in response to external stimuli such as temperature, and at the same time, their oligopeptide structure and spherical shape are suitable for a drug delivery device.
Three different polymeric networks have been synthesized by varying the HGel-MA and NIPAAm amount in the polymerization feed. Scanning electronic microscopy (SEM), Fourier transform infrared spectroscopy, and dimensional, calorimetric, and swelling analyses were performed to characterize the materials.
In order to verify the suitability of these materials as thermo-responsive devices for drug delivery, a commonly used anti-inflammatory drug, diclofenal sodium salt (DC), was loaded on the polymeric structures and release profiles at 25 and 40°C were evaluated. Furthermore, using semi-empirical equations, the diffusional contribute on the delivery of the DC was estimated. Finally, to test the thermo-responsive switching behavior, the pulsatile drug release experiments, by temperature cycling around the LCST value, were performed (20,21).
MATERIALS AND METHODS
Materials
Gelatin (Ph Eur, Bloom 160), methacrylic anhydride (MA), potassium hydroxide, N-isopropylacrylamide (NIPAAm), N,N′-methylenebisacrylamide (MBA), sorbitan trioleate (Span 85), polyoxyethylene sorbitan trioleate (Tween 85), N,N,N′,N′-tetramethylethylendiamine (TMEDA), ammonium persulfate, sodium hydrogen phospate, disodium hydrogen phosphate, ammonium acetate, and diclofenac sodium salt were provided by Sigma–Aldrich (Sigma Chemical Co., St. Louis, Missouri). Acetonitrile, methanol, and water were from Carlo Erba Reagents (Milan, Italy) and were all of high pressure liquid chromatography (HPLC) grade. 2-Propanol, ethanol, acetone, n-hexane, chloroform, glacial acetic acid, and diethyl ether were from Carlo Erba Reagents and were all of analytical grade. The n-hexane and chloroform were purified by standard procedures.
Synthesis of Methacrylated Gelatin Hydrolysate
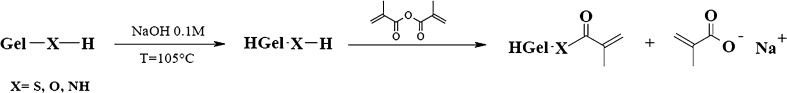
Hydrolyzed methacrylated gelatin (HGel-MA) was prepared according to the literature with some modifications (22). A reaction mixture containing 40 g of gelatin was taken up in 60 g of water, and after the addition of 1.6 g of sodium hydroxide, the solution was heated for 16 h at 130°C. Then, after cooling to room temperature, 3 ml of methacrylic anhydride was added to the reaction mixture. The pH value of the reaction mixture was kept at 10 by addition of dilute sodium hydroxide. After a reaction time of 5 h, the mixture was adjusted with dilute hydrochloridic acid to a pH value of 7 (Fig. 1). The obtained functional hydrolysate was precipitated by adding the polymeric solutions to an excess volume of acetone under agitation at room temperature. The suspensions were filtered by sintered glass filter funnel (Pyrex, Ø30 mm; porosity G3) and washed with diethyl ether, and the recovered gelatin hydrolysate was dried in a vacuum oven at 40°C.
Fig. 1.
Schematic representation of HGel-MA synthesis
Microspheres Preparation (Standard Procedure)
Microspheres based on HGel-MA, NIPAAm, and MBA were produced by radical copolymerization technique (23). Briefly, a mixture of n-hexane and chloroform was placed in a round-bottomed cylindrical glass reaction vessel fitted with an anchor-type stirrer and thermostated at 30°C, then treated, after 30 min of N2 bubbling, with an aqueous solution of HGel-MA, the co-monomer (NIPAAm), the crosslinker (MBA), and ammonium persulfate as radical initiator. The density of the organic phase was adjusted by the addition of chloroform or n-hexane so that the aqueous phase sank slowly when stirring stopped. Under stirring at 1,000 rpm, the mixture was treated with Span 85 and Tween 85, then after 10 min with TMEDA, and stirring was continued for another 60 min. Table I reports on the experimental conditions of each polymerization reactions. The microspheres were filtered, washed with 50-ml portions of 2-propanol, ethanol, acetone, and diethyl ether, and dried overnight under vacuum at 40°C.
Table I.
Polymerization Conditions for the Synthesis of Thermo-responsive Hydrogels
| Aqueous dispersed phase | Organic continuous phase | Hydrogel | |||
|---|---|---|---|---|---|
| HGel-MA (mg) | NIPAAm (mg/mmol) | MBA (mg/mmol) | CHCl3/n-hexane (ml/ml) | mg (conv.%) | Code |
| 200 | 300/3.0 | 100/0.6 | 19/22 | 450 (75%) | HG-1 |
| 300 | 300/3.0 | 120/0.8 | 19/23 | 400 (55%) | HG-2 |
| 300 | 400/4.0 | 120/0.8 | 18/22 | 425 (52%) | HG-3 |
For all polymerizations, the amount of aqueous phase is 2.5 ml; initiator system is (NH4)2S2O8/TMEDA (100 mg/150 µl); surfactants are Span 85/Tween 85 (150 µl/40 µl)
FT-IR Spectroscopy
Fourier transform infrared (FT-IR) spectra of HGel-MA, NIPAAm, MBA, HG-1, HG-2, and HG-3 were measured as pellets in KBr with a FT-IR spectrophotometer. (model Jasco FT-IR 4200) in the wavelength range of 4,000–400 cm−1. Signal averages were obtained for 100 scans at a resolution of 1 cm−1.
Shape and Surface Morphology
The shape and surface morphology of the microspheres were studied using SEM. The sample was prepared by lightly sprinkling the microspheres with powder on a double adhesive tape, which was stuck on an aluminum stub. The stubs were then coated with gold to a thickness of about 300 Ǻ using a sputter coater then viewed under a scanning electron microscope (Leo stereoscan 420) and shown in photomicrographs.
Dimensional Distribution
The particle size distribution was carried out using an image processing and analysis system, Leica DMRB, equipped with a Leica Wild 3D stereomicroscope. This image processor calculates the particle area and converts it to an equivalent circle diameter.
Water Content of HG Microspheres
The swelling characteristics were determined in order to test hydrophilic properties of the microspheres. Typically, aliquots (40–50 mg) of the microspheres dried to constant weight were placed in a tared 5-ml sintered glass filter (Ø10 mm; porosity G3), weighed, and left to swell by immersing the filter plus support in a beaker containing the swelling media [phosphate-buffered saline (PBS) solution 10−3 M, pH 7.0, at 25 and 40°C]. After 24 h, the excess water was removed by percolation at atmospheric pressure. Then, the filter was placed in a properly sized centrifuge test tube by fixing it with the help of a bored silicone stopper, then centrifuged at 3,500 rpm for 15 min and weighed. The filter tare was determined after centrifugation with only water. The weights recorded at the different times were averaged and used to give the water content percent (WR%) by the following Eq. (1):
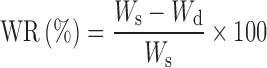 |
1 |
where Ws and Wd are the weights of swollen and dried microspheres, respectively. The WR (%) for all prepared materials are reported in Table II.
Table II.
Calorimetric Analyses, Water Uptake Experiments, Loading Efficiency (LE%), and Drug Loaded (DL%) Percentages of Thermo-responsive Microspheres
| Hydrogel | Calorimetric analysis | Swelling parameters | LE% | DL% | ||
|---|---|---|---|---|---|---|
| LCST (°C) | WR25 | WR40 | S r | |||
| HG-1 | 35.4 | 200 ± 5 | 129 ± 2 | 1.55 | 97 ± 2 | 9.8 ± 0.1 |
| HG-2 | 36.5 | 267 ± 3 | 128 ± 1 | 2.08 | 99 ± 1 | 9.9 ± 0.1 |
| HG-3 | 36.9 | 229 ± 7 | 128 ± 3 | 1.78 | 98 ± 1 | 9.8 ± 0.1 |
Thermo-behavior of HG Microspheres
The differential scanning calorimetry (DSC) thermograms of microspheres were recorded on a Netzsch DSC200 PC, and the LCST values for all polymers are reported in Table II. In a standard procedure, the sample was immersed in distilled water at room temperature for at least 2 days to reach a swollen state. About 10 mg of swollen samples was accurately weighed into a solid aluminum hermetic pan and then sealed tightly by a hermetic aluminum lid. The thermal analyses were performed from 25 to 55°C on the swollen hydrogel samples under constant purging of nitrogen at 25 ml/min and at a constant heating rate of 3°C min−1. All samples were run in duplicate.
Incorporation of Drug into Preformed Microspheres
Incorporation of diclofenac sodium salt into microspheres was performed as follows: 200 mg of preformed empty microspheres was wetted with 2.0 ml in a concentrated drug solution (10 mg/ml). After 3 days, under slow stirring at 37°C, the microspheres were filtered and dried at reduced pressure in presence of P2O5 to constant weight. The loading efficiency percent (LE%) of all samples are determined by HPLC analysis of filtered solvent according to Eq. (2) :
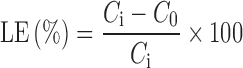 |
2 |
Here, Ci is the concentration of drug in solution before the loading study and C0 the concentration of drug in solution after the loading study. The values of calculated LE percent and the drug loaded percent (DL%) in each matrix are listed in Table II, according to Eq. 3:
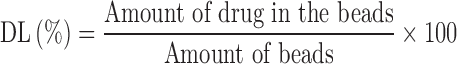 |
3 |
Drug Stability at pH 7.0 at 25 and 40°C
Drug stability was studied at pH 7.0 and at different temperatures (25 and 40°C). Aliquots of drug (10 mg) were incubated at 25 and 40°C in PBS solution 10−3 M at pH 7.0. At scheduled time intervals, corresponding to the condition of the drug release experiments, the samples were withdrawn and assayed by HPLC in order to determine the drug concentration. The HPLC conditions were a mixture of aqueous solution of ammonium acetate, methanol, and acetonitrile (40/30/30, v/v/v). The pH of the aqueous mobile phase portion of ammonium acetate buffer was adjusted with glacial acetic acid. The mobile phase was filtered, degassed, and pumped isocratically at a flow rate of 0.6 ml min−1; UV detection at 284 nm (24). The HPLC analyses were carried out using a Jasco PU-2080 liquid chromatography equipped with a Rheodyne 7725i injector (fitted with a 20-μl loop), a Jasco UV-2075 HPLC detector, and a Jasco-Borwin integrator. A reversed-phase C18 column (μBondapak, 10 μm of 150×4.6 mm internal diameter obtained from Waters) was used. Retention time was 4.2 min, limit of detection 0.7 μM, and limit of quantification 14 μM.
In Vitro Drug Release at 25 and 40°C from Microspheres
In vitro drug release profiles were obtained by HPLC analyses. Aliquots (10 mg) of drug-loaded microspheres were dispersed in flasks containing 10.0 ml of PBS solution (pH 7.0, 10−3 M), at 25.0 ± 0.1 and 40.0 ± 0.1°C, and sink conditions were maintained throughout the experiments. The samples, at suitable time intervals, were filtered and the solutions were analyzed by HPLC.
In Vitro Pulsatile Drug Release from 25 to 40°C
Oscillatory drug release profile of the loaded microspheres was investigated in depth by immersing the hydrogels in PBS solution at pH 7.0 (10.0 ml, 10−3 M) and alternating the temperature between 25 and 40°C at suitable time intervals. At each time, the samples were filtered and the solutions were analyzed by HPLC. The release period was extended over several cycles until no further drug was released. Two different experiments were performed, the first starting from 25°C and the second from 40°C, and the sink conditions were maintained throughout the experiments. The larger temperature difference was used to help increase the speed of collapse, since DC is a small molecule.
Statistical Analysis
All the experiments were done in triplicate and the results were in agreement within ±5% standard error. One-way analysis of variance was performed to assess the significance of the differences among data. Tukey–Kramer post test was used to compare the means of different treatment data. P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Synthesis of HG Microspheres
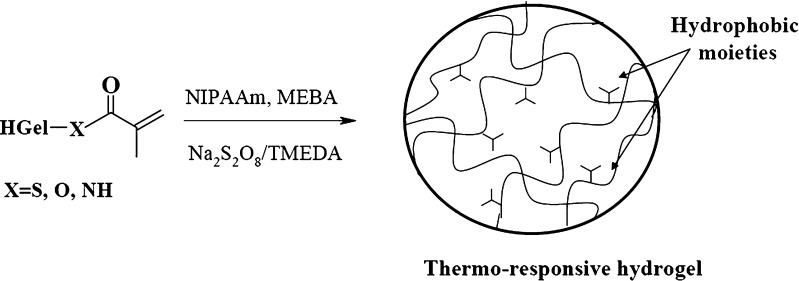
Chemical groups susceptible to radical polymerization were introduced onto hydrolyzed gelatin by acylation with methacrylic anhydride (MA) to synthesized polymerizable polypeptide chains (HGel-MA). Comparing to the native protein, the hydrolysates are characterized not only by enhanced water solubility, but also by a greater number of nucleophilic groups disposable for the reaction with the acylating agent. Together with the thiolic groups of cysteine, hydroxyl groups of serine and tyrosine, and with the amino groups in the side chain of lysine residues, the terminal amino groups, deriving from the alkaline hydrolysis of the peptide bonds, indeed represent reactive sites toward the derivatization with MA. Our goal was to obtain proteic moieties bearing polymerizable functionalities to be directly copolymerized with a stimuli-responsive monomer, producing a crosslinked structure in which the polypeptide chains are linked by hydrocarbon bridge and randomly interrupted by growing chains of the functional monomer. In order to synthesize useful spherical polymeric materials showing thermo-responsive behavior, biocompatibility characteristics, and hydrophilic properties, the HGel-MA was copolymerized with NIPAAm and MBA, acting as a temperature-sensitive and crosslinking agent, respectively (Fig. 2).
Fig. 2.
Radical polymerization of HGel-MA macromers with NIPAAm and MBA
On the other hand, the choice to obtain hydrogels characterized by spherical shape was dictated by the fact that this kind of materials are ideal vehicles for many controlled delivery applications due to their ability to encapsulate a variety of drugs, biocompatibility, high bioavailability, and sustained drug release characteristics (25). The microspheres were synthesized by free-radical suspension polymerization, in which TMEDA and ammonium persulfate were used as initiator system. The optimization of the polymerization method was performed, and it was observed that the hydrophilic/lipophilic balance (HLB) of surfactants represents an important parameter to produce a water-in-oil emulsion consisting of water drops uniformly dispersed in the organic phase (CHCl3/n-hexane) when stirring was stopped. Generally, water-in-oil emulsions are stabilized by surfactants in concentrations of 0.5–1.5% by weight into water and literature data report that the best results were obtained using surfactant mixtures with different values of HLB (26). In particular, the volume ratio of the surfactant mixture strictly depends on the dispersed phase. In our experiments, different tests were carried out, allowing to determine the correct ratio for Span 85 (HLB = 1.8) and Tween 85 (HLB = 11). A system with a total HLB = 3.7 was eventually found to be able to stabilize the aqueous phase dispersed in the organic one. Varying the amount of HGel-MA and the molar ratio NIPAAm/MBA in the polymerization feed, three different hydrogels were prepared, as reported in Table I. In particular, NIPAAm/MBA molar ratio was 5.0 for HG-1 and HG-3 and 3.7 for HG-2, while the amount of hydrophilic crosslinker (HGel-MA) was 33.3% (w/w) for HG-1 and was increased to 36.6% and 41.1% for HG-3 and HG-2, respectively. In the proposed polymerization protocol, we found that the change of both the crosslinking degree and the hydrophilic/hydrophobic balance of the polymeric networks seem to greatly influence the water-bead affinity and consequently the swelling/shrinking transition temperatures of the microspheres.
Characterization of Thermo-responsive Microspheres
The microspheres were characterized by FT-IR spectrophotometry, swelling behavior, and morphological and calorimetric analyses.
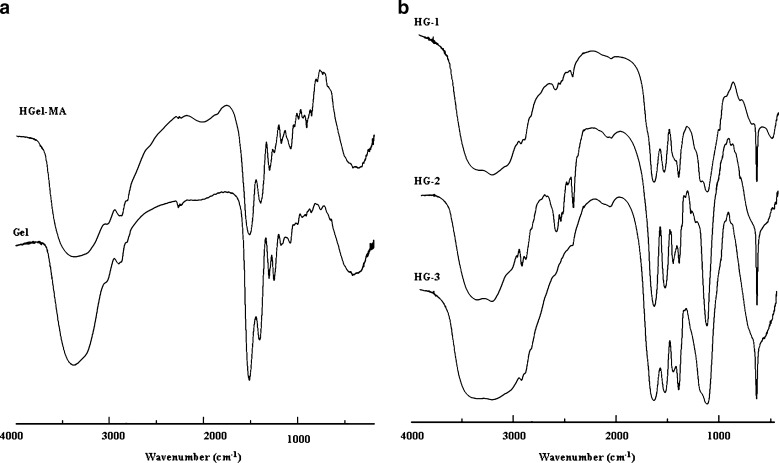
The FT-IR spectra of methacrylate hydrolyzed gelatin and native protein showed the appearance of a typical absorption band at 985 cm−1, ascribable to C=C double bond of methacrylic units inserted on the polypeptide chains after derivatization reaction (Fig. 3a). In addition, the FT-IR spectra of all samples showed the disappearance of the bands ascribable to C=C double bond of all the monomers in the polymerization feed and the appearance of the typical absorption bands of hydrolyzed gelatin and NIPAAm, confirming the insertion of both thermo-sensitive and hydrophilic co-monomers in the macromolecular network. In particular, the peaks at 3,442 and 3,417 cm−1 due to N–H stretching of secondary amide, C–H stretching at 2,921 and 2,855 cm−1, C=O stretching at 1,682 and 1,643 cm−1, N–H bending between 1,553 and 1,497 cm−1, and N–H out-of-plane wagging at 672 cm−1 are visible in the spectra of all the samples (Fig. 3b).
Fig. 3.
FT-IR spectra of HGel-MA and gel a and HG-1, HG-2, and HG-3 b
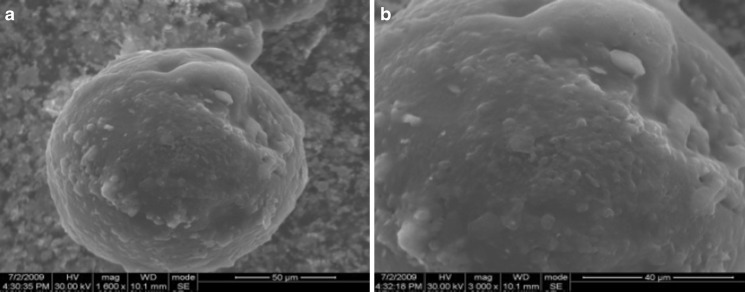
Using SEM, the surface properties of the microspheres were evaluated, and their spherical shape was confirmed. In Fig. 4a, the spherical shape of sample HG-2 is evident, while Fig. 4b shows the high porosity of the outside surface of the microspheres. Similar results were obtained for all the spherical samples. The results recorded of the morphological analyses suggest the potential use of the microspheres as drug delivery systems. The spherical shape indeed allows to eliminate the anisotropic swelling normally associated with others geometries, while the presence of micropores could facilitate the drug diffusion through the swollen polymeric network.
Fig. 4.
Scanning electron micrographs showing spherical shape a and porous surface b of HG-2. Similar results were recorded for all the samples
In our experiments, the diameter of the hydrogels was in the dimensional range 80–100 μm for HG-2, 90–120 μm for HG-1, and 110–130 μm for HG-3. The microsphere diameters were strictly dependent on the crosslinker amount in the polymeric networks; the values of mean diameter, in general, decrease as the crosslink density increases.
The swelling behavior of these hydrogels in the aqueous medium allows to investigate the applicability of the microspheres in controlled release field. The values of contained water percentages were determined in PBS solution (pH 7.0; 10−3 M) at 25 and 40°C, respectively. The data, reported in Table II, illustrate the water uptake at different temperatures, in grams per gram of dry copolymer, for each composition, and the ratio between the swelling at 25 and 40°C (Sr) was reported for all samples. Depending on the temperature of the surrounding medium, the hydrogel/solvent affinity can be modulated by the pendant hydrophobic groups in the polymeric chains. In particular, at 25°C, the interactions between the water molecules and the hydrophilic units of the macromolecular systems are predominant with a consequent enhancing of the hydrogel swelling. When the temperature increases to 40°C, a considerable lowering of the water content was observed, the hydrophobic interactions between hydrocarbon moieties on polymeric chains become predominant compared to the interaction with water molecules, and the diffusion of solvent outside the polymeric network occurs. The thermodynamics can explain this behavior with a balance between entropic effects ascribable to the dissolution process and to the ordered state of water molecules around the polymer. Enthalpic effects depend on the balance between intra- and intermolecular forces and on the solvation (hydrogen bonds and hydrophobic interactions) (27). The WR% data recorded at 25°C clearly showed that the polypeptide moieties, which represent the main hydrophilic portion of the macromolecular network, influence the hydrogel/solvent interactions more than the crosslinking degree of the matrices. As reported in Table II, HG-2 and HG-3 hydrogels, containing a greater amount of HGel-MA, exhibited the highest WR25 values. On the contrary, no remarkable differences were recorded for all polymers in the shrunken state (WR40) and the Sr values reflect the same trend of WR25 with the highest value for hydrogel HG-2 (Sr = 2.08).
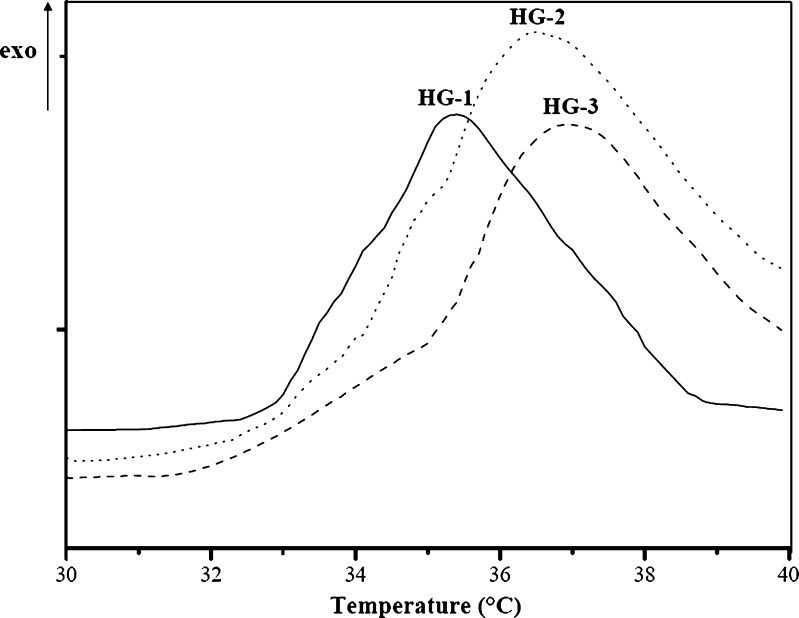
Finally, the transition temperature of the hydrogels was determined by performing thermal analyses from 25 to 55°C on the swollen hydrogels. As shown in Table II, all samples showed a higher LCST than the pure PNIPAAm hydrogel. This is due to the presence of hydrolyzed gelatin moieties which increase the hydrophilic content in the polymeric structure. Moreover, from HG-1 to HG-3, an increasing trend of the transition temperature was observed as a result of the enhancing HGel-MA content in the polymerization feed. The LCST values, indeed, were strictly dependent on functional monomer/crosslinker ratio in the polymerization feed. At temperatures below its LCST, the hydrophilic groups (amide groups) in the side chains of the PNIPAAm hydrogel bond the water molecules through hydrogen bonds. However, as the external temperature increases, the copolymer–water hydrogen bonds are broken. When the temperature raises the LCST, the water molecules, rigidly structured around the polymer chains, gain more freedom degrees and they diffuse in their bulk phase. As a result, hydrogen bonds between solvent molecules in the continuous phase are formed, while, inside the polymeric network, hydrophobic interactions among the isopropyl groups become dominant. Thus, a reduction of the HGel-MA amount in the polymeric structure results in a decrease of LCST values, while, as the hydrophilic moieties increased, the strength of hydrogen bonds between the water molecules and the hydrogels, and thus the transition temperature, enhanced from 35.4 to 36.9°C, close to the body temperature value (Fig. 5). Performing the calorimetric analyses on the samples containing the same amount of the hydrophilic crosslinker (HG-2 and HG-3), the LCST gap between the hydrogels was not found to be relevant.
Fig. 5.
DSC thermograms of the swollen stimuli-responsive hydrogels at a heating rate of 3°C (the temperatures at the maximum points of the exotherms were referred as volume phase transition temperature of the hydrogels)
In Vitro Release Studies
In literature, many papers report on the in vitro release profile analysis of therapeutics employing polymeric devices able to undergo volume phase transition in response to the variation of the environmental conditions (28–32). Thermo-sensitive hydrogels, acting as self-regulating systems, have attracted increasing attention in the drug delivery field because they can control the release of drug in response to change in the body temperature. In order to estimate the potential application of the hydrogels HG (1–3) as drug delivery devices, the microspheres were loaded with one of the most commonly used anti-inflammatory drug, diclofenac sodium salt. Diclofenac is a non-steroidal anti-inflammatory drug taken to reduce musculoskeletal complaints, especially arthritis, rheumatoid arthritis, poliomyelitis, dermatomyositis, osteoarthritis, spondylarthritis, ankylosing spondylitis, gout attacks, and pain management in cases of kidney stones and gallstones. An additional indication is the treatment of acute migraines. DC is commonly used to moderate post-operative or post-traumatic pain, particularly when inflammation is also present, and is effective against endometriosis. DC was loaded on the hydrogels by soaking procedure and the loading efficiency of all samples (LE %) was determined by HPLC analysis (Table II). DC was loaded on the microspheres with a LE (%) >98% for all samples in order to produce in each loaded matrix a DL% of approximately 10%. DC release experiments were carried out in PBS solution (pH 7.0, 10−3 M) at 25 and 40°C and the amount of released drug was expressed as percent of delivered drug (Mt) related to the effectively entrapped total mass (M0), as a function of time.
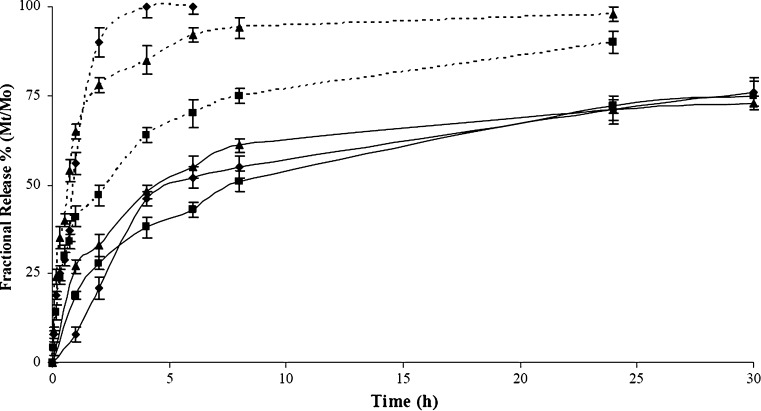
As reported in Fig. 6, a different release rate was recorded. In each experimental time and for all samples, indeed, the amount of drug molecules moving from the polymeric microspheres to the surrounding media was higher at 40 than 25°C, and a shape rise at the first 30 min was noted at 40°C, comparing to a slow increase at 25°C. This jump in the release profile was ascribed to rapid collapse of the hydrogel from swollen to collapsed state. The hydrogel HG-1, containing the lowest amount of both natural and synthetic crosslinkers, rapidly collapsed at 40°C to the shrunken state, completely releasing the entrapped DC after 3 h. Increasing the crosslinking degree of the network due to the reduced freedom degree of the polymeric chains, the Mt/M0 percent values ranged from 90 to 95% after 24 h, and after 3 h more than 60% of DC was delivered. If the temperature of the surrounding medium allows the swelling of the microspheres, the release profile of all the hydrogels showed a reduced burst effect, and after 30 h the amount of the drug in solution was about 75% for all the samples. Since the microspheres have a well-defined geometry and a narrow dimensional distribution, we determined the mechanism of drug release (Fickian or non-Fickian). In particular, the kinetics of DC release at 25°C under LCST value were analyzed by the semi-empirical Eq. (4) for Mt/M0 ≤ 0.6 (20)
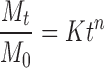 |
4 |
where Mt/M0 is the drug fraction released at time t, and K and n are a constant and the kinetic exponent of drug release, respectively. Although the use of this equation requires detailed statistical analysis, the calculated exponent, n, gives an indication of the release kinetics. If n = 0.43, the drug diffuses and releases out of the polymer matrix following a Fickian diffusion. For n > 0.43, anomalous or non-Fickian-type drug diffusion occurs. If n = 0.85, a completely non-Fickian or case II release kinetics is operative. The intermediary values ranging between 0.43 and 0.85 are attributed to anomalous-type diffusive transport. The least-squares estimations of the fractional release data along with the estimated correlation coefficient values, r, are presented in Table III. The empirical transport Eq. (4) represents an extension of the short time solutions for Fickian and non-Fickian diffusional release from a thin film. In theory, this equation should only be applicable to the first 60% of fractional release from thin slabs, for which the assumption of one-dimensional diffusion under perfect sink conditions is valid. In practice, however, the equation has been applied to systems of different geometries, to systems where one-dimensional diffusion cannot be assumed, and to systems where perfect sink boundary conditions are not maintained. In the experiments at 25°C, the exponent n was 0.38 and 0.41 for copolymers HG-1 and HG-2, respectively, indicating a predominantly diffusive mechanism in the release of the drug, while n value for HG-3 indicates an anomalous release profile. For these devices, at temperatures below the LCST, the diffusion plays a major role because it occurs through the available space between macromolecular chains, regarded as the “pore”.
Fig. 6.
Drug release expressed as percent of DDA delivered (M t) related to the effectively entrapped total dose (M 0), as a function of time for beads HG-1 (filled diamonds), HG-2 (filled squares), and HG-3 (filled triangles) at 25°C (solid lines) and 40°C (dashed lines) and at pH 7.0 (PBS solution 10−3 M)
Table III.
Release Kinetics Parameters of the Different Formulations by Fitting the Data with the Models Proposed by Peppas/Ritger and Peppas/Sahlin
| Sample | ||||||
|---|---|---|---|---|---|---|
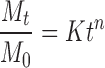 (4) (4) |
||||||
| K 103 (min−n) | n | r | ||||
| 25°C | 40°C | 25°C | 40°C | 25°C | 40°C | |
| HG-1 | 21.87 ± 4.72 | 3.36 ± 1.41 | 0.38 ± 0.08 | 0.66 ± 0.11 | 0.85 | 0.93 |
| HG-2 | 20.47 ± 1.36 | 6.69 ± 1.08 | 0.41 ± 0.02 | 0.41 ± 0.03 | 0.98 | 0.97 |
| HG-3 | 31.22 ± 2.59 | 5.99 ± 0.70 | 0.27 ± 0.03 | 0.58 ± 0.03 | 0.93 | 0.99 |
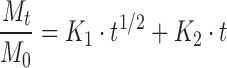 (5) (5) |
||||||
| K 1 103 (min−1/2) | K 2 103 (min−1) | R | ||||
| 25°C | 40°C | 25°C | 40°C | 25°C | 40°C | |
| HG-1 | 22.83 ± 2.99 | 2.88 ± 0.70 | −1.61 ± 0.65 | −1.52 ± 0.08 | 0.93 | 0.99 |
| HG-2 | 30.19 ± 0.83 | 5.70 ± 0.21 | −1.01 ± 0.18 | −0.11 ± 0.01 | 0.99 | 0.99 |
| HG-3 | 29.01 ± 0.88 | 3.18 ± 0.37 | −1.82 ± 0.19 | −1.22 ± 0.02 | 0.99 | 0.98 |
A more informative analysis can be obtained by fitting the data with the model proposed by Peppas and Sahlin (21). The equation for this model is:
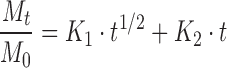 |
5 |
with Mt/M0 ≤ 0.95. In this equation, the first term is the Fickian contribution and the second term is the case II relaxational contribution. Table III reports K1 and K2 values according to Eq. (5). For all investigated samples, the term K1 is greater (the K1/K2 average value was 19.8) than the term K2, indicating that the predominant mechanism, at 25°C, for DC release is the Fickian diffusion through the swollen microspheres. Thus, the drug release was determined by two factors, the swelling rate of polymer and the diffusivity of the drug through the network. Because at the same temperature there are no marked differences of the diffusivity of drug in each polymer, the swelling rate of the polymer was the dominating factor. In swelling-controlled (and in general swellable) release systems, the dissolution medium surrounding the controlled release device may enter the polymer at a rate that controls the drug release, and under certain experimental conditions zero-order release can be achieved (20). The prevailing molecular mechanism is a coupling of diffusion and macromolecular relaxation as a result of which the drug diffuses outward with a kinetic behavior that is dependent on the relative ratio of diffusion and relaxation.
When the hydrogels are placed in an aqueous solution at 40°C, above their LCST, the release behaviors were complex and not only diffusional effects of drug through the polymer network has to be considered but also the squeezing effect of swelled polymer contributed to the apparent release rate. The n values of HG-1 and HG-3 samples (0.66 and 0.58, respectively) indicate a pronounced non-Fickian diffusion transport, while HG-2 (n = 0.41) seems to follow a Fickian trend of release profile. Fitting the experimental data using the Peppas–Sahlin equation, more information can be obtained; in particular, at 40°C and for HG-1 and HG-3 samples, the ratio between K1 and K2 values are considerably lower than that recorded at 25°C, as a result of the minor diffusional contribute to the release profile. On the contrary, the hydrogel HG-2 showed a K1/K2 confrontable to the fitting at 25°C as a consequence of the role of the diffusional component in the release mechanism at both these temperatures.
Pulsatile Drug Release Experiments
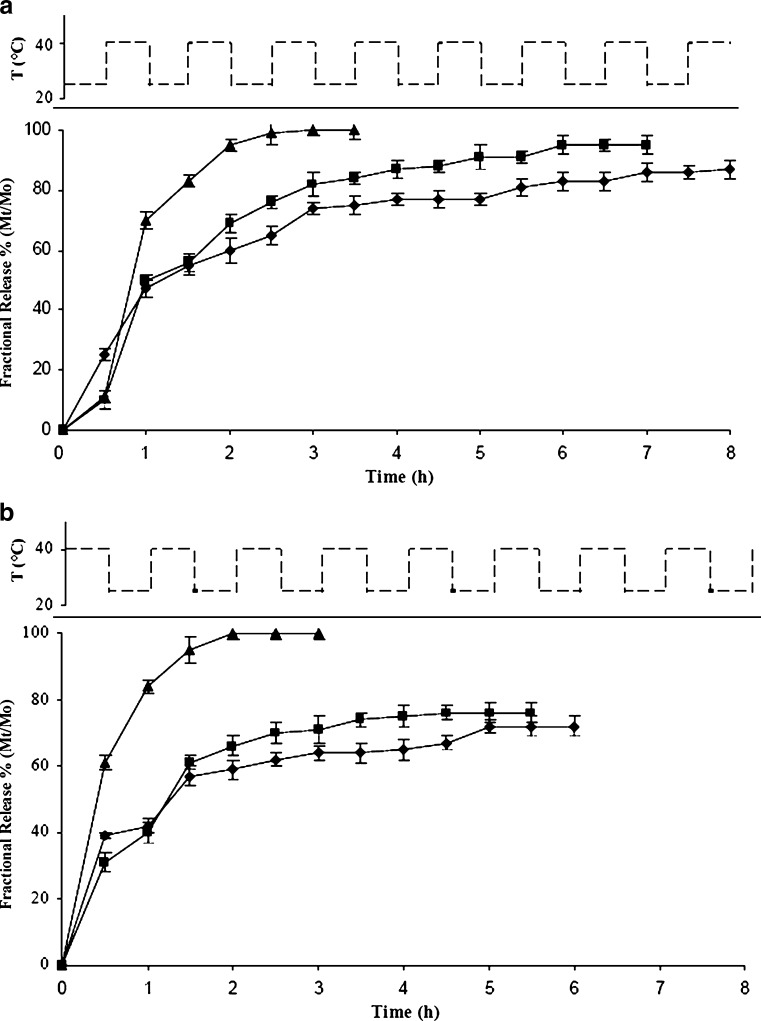
To extend the applications of the drug delivery devices in the areas of medicine, the rate of drug release is required to match the physiological requirements. The reversible on/off switching of DC release from the microspheres was demonstrated by repeated heating above and cooling below their LCST temperature in in vitro pulsatile release experiments. In these conditions, the release profiles during 5 h were found to have very good sustaining efficacy, and the effect of temperature cycling on drug release reflects a response rate to various environments. The experiments started placing the samples in a swelling (25°C) or in a collapsed state (40°C) and recording the amount of DC released, expressed as Mt/M0 percent, in the surrounding environment changing the temperature every 30 min. Figure 7a–b shows the DC release profile from HG hydrogels as a function of temperature cycling at fixed pH value (PBS solution 10−3 M, pH 7.0). Generally, for all samples, a pronounced burst effect was observed at 40°C with Mt/M0 percent values ranged from 39.0 to 61.0 after half an hour. This percentage decreases to values between 10.0 and 25.0 when the microspheres are placed to an initial temperature of 25°C. The results confirmed that, when the temperature reached the LCST, the strong hydrophobic interactions between the isopropyl groups of the macromolecular architecture produce the dispelling of a significant amount of aqueous drug solution from the collapsed hydrogels. Starting from the hydrogels in the collapsed state, a complete release of the drug took place in 3 h for HG-3, while the other samples released about 70% of DC after 6 h. On the contrary, placing the loaded hydrogel in a medium at temperatures below the LCST, the release profiles showed a slow release rate which produced Mt/M0 values in the range 75% and 85% for hydrogels HG-1 and HG-2, respectively, after 8 h, while HG-3 showed a complete release of DC after 3.5 h. These results clearly showed that the HG systems allow to obtain an effective modulation of the drug release rate.
Fig. 7.
Temperature-dependent stepwise DDA release profile expressed as percent of drug delivered (M t) related to the effectively entrapped total dose (M 0), as a function of time for beads HG-1 (filled diamonds), HG-2 (filled squares), and HG-3 (filled triangles) hydrogels first exposed to a temperature of 40°C a and 25°C b, with alternating temperature between 25 and 40°C at pH 7.0 (PBS solution 10−3 M)
CONCLUSIONS
In this work, methacrylated gelatin hydrolysates were employed as pro-hydrophilic macromer in the synthesis of thermo-responsive microspheres by free-radical suspension polymerization using NIPAAm and MBA as thermo-responsive functional monomer and crosslinking agent, respectively. Varying the amounts of the monomers in the feed composition, hydrogels with different hydrophobic/hydrophilic balance were synthesized. The spherical shape and the high degree of porosity of the swellable microspheres were confirmed by SEM analyses, while the negative thermo-responsive behavior by water uptake experiments at temperatures across the LCST was checked. Moreover, since the hydrolyzed gelatin moieties in the networks increase the strength of the interactions between the water molecules and polymers, a considerable enhancement of the transition temperatures, to values close to the body temperature, was recorded. The potential application of these materials as drug carriers was demonstrated by performing DC release experiments at temperatures above and below the LCST. Depending on the temperature of the surrounding environment, the release of the drug across the hydrogels takes place by rapid and reversible modification of volume hydrogels and by diffusion of the therapeutic through the polymeric network. In order to estimate the diffusional contribute on the drug delivery, semi-empirical equations were employed, showing the enhanced role of the diffusional component in the release mechanism principally in the swollen state, at a temperature below the LCST. Finally, the reversible on/off switching behavior of the microspheres was demonstrated by performing in vitro pulsatile release experiments in which the temperature of the surrounding medium cycles around the LCST value of the hydrogels.
Acknowledgments
This work was financially supported by MIUR (Programma di ricerca di rilevante interesse nazionale 2008) and University of Calabria funds.
References
- 1.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/S0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DG, Burdick JA, Langer R. Smart biomaterials. Science. 2004;305:1923–1924. doi: 10.1126/science.1099987. [DOI] [PubMed] [Google Scholar]
- 3.Lang Y, Jiang T, Li S, Zheng L. Study on physicochemical properties of thermosensitive hydrogels constructed using graft-copolymers of poly(N-isopropylacrylamide) and guar gum. J Appl Polym Sci. 2008;108:3473–3479. doi: 10.1002/app.27948. [DOI] [Google Scholar]
- 4.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 5.Bawa P, Pillay V, Choonara YE, Du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater. 2009 doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 6.De Las Heras Alarcón C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 7.Peppas NA, Leobandung W. Stimuli-sensitive hydrogels: ideal carriers for chronobiology and chronotherapy. J Biomater Sci Polym Ed. 2004;15:125–144. doi: 10.1163/156856204322793539. [DOI] [PubMed] [Google Scholar]
- 8.Iemma F, Spizzirri UG, Puoci F, Muzzalupo R, Trombino S, Cassano R, Leta S, Picci N. pH-Sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int J Pharm. 2006;312:151–157. doi: 10.1016/j.ijpharm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliver Rev. 2001;53:321–339. doi: 10.1016/S0169-409X(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrov I, Trzebicka B, Müller AHE, Dworak A, Tsvetanov CB. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Progr Polym Sci. 2007;32:1275–1343. doi: 10.1016/j.progpolymsci.2007.07.001. [DOI] [Google Scholar]
- 11.Bikram M, West JL. Thermo-responsive systems for controlled drug delivery. Exp Opin Drug Deliv. 2008;5:1077–1091. doi: 10.1517/17425247.5.10.1077. [DOI] [PubMed] [Google Scholar]
- 12.Iemma F, Spizzirri UG, Puoci F, Cirillo G, Curcio M, Parisi OI, Picci N. Synthesis and release profile analysis of thermo-sensitive albumin hydrogels. Colloid Polym Sci. 2009;287:779–787. doi: 10.1007/s00396-009-2027-y. [DOI] [Google Scholar]
- 13.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan C, Long Q, Yu D, Rao Y, Wu N, Li X. Swelling and drug releasing properties of poly(N-isopropylacrylamide) thermo-sensitive copolymer gels. Front Chem China. 2008;3:314–319. doi: 10.1007/s11458-008-0054-8. [DOI] [Google Scholar]
- 15.Chang Y, Xiao L, Tang Q. Preparation and characterization of a novel thermosensitive hydrogel based on chitosan and gelatin blends. J Appl Polym Sci. 2009;113:400–407. doi: 10.1002/app.29954. [DOI] [Google Scholar]
- 16.Joly-Duhamel C, Hellio D, Djabourov M. All gelatin networks: 1. Biodiversity and physical chemistry. Langmuir. 2002;18:7208–7217. doi: 10.1021/la020189n. [DOI] [Google Scholar]
- 17.Yang H, Kao WJ. Thermoresponsive gelatin/monomethoxy poly(ethylene glycol)-poly(D, L-lactide) hydrogels: formulation, characterization, and antibacterial drug delivery. Pharm Res. 2006;23:205–214. doi: 10.1007/s11095-005-8417-z. [DOI] [PubMed] [Google Scholar]
- 18.Chun S, Kim J. A novel hydrogel-dispersed composite membrane of poly(N-isopropylacrylamide) in a gelatin matrix and its thermally actuated permeation of 4-acetamidophen. J Control Release. 1996;38:39–47. doi: 10.1016/0168-3659(95)00097-6. [DOI] [Google Scholar]
- 19.Lee W, Lee S. Effect of gelatin on the drug release behaviors for the organic hybrid gels based on N-isopropylacrylamide and gelatine. J Mater Sci Mater Med. 2007;18:1089–1096. doi: 10.1007/s10856-007-0142-1. [DOI] [PubMed] [Google Scholar]
- 20.Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 21.Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm. 1989;57:169–172. doi: 10.1016/0378-5173(89)90306-2. [DOI] [Google Scholar]
- 22.Lin LH, Chen KM. Preparation and surface modification of gelatine derivative surfactants. Colloid Surf A. 2006;272:8–14. doi: 10.1016/j.colsurfa.2005.07.006. [DOI] [Google Scholar]
- 23.Iemma F, Spizzirri UG, Muzzalupo R, Puoci F, Trombino S, Picci N. Spherical hydrophilic microparticles by radical copolymerization of functionalized bovine serum albumin. Coll Polym Sci. 2004;283:250–256. doi: 10.1007/s00396-004-1071-x. [DOI] [Google Scholar]
- 24.Malliou ET, Markopoulou CK, Koundourellis JE. Simultaneous determination of clobutinol together with some anti-inflammatory drugs in urine by HPLC. J Liq Chromatogr Relat Technol. 2004;27:1565–1577. [Google Scholar]
- 25.Cirillo G, Iemma F, Puoci F, Parisi OI, Curcio M, Spizzirri UG, Picci N. Imprinted hydrophilic nanospheres as drug delivery system for 5-fluorouracil sustained release. J Drug Target. 2009;17:72–77. doi: 10.1080/10611860802455813. [DOI] [PubMed] [Google Scholar]
- 26.Kriwet B, Walter E, Kissel T. Synthesis of bioadhesive poly(acrylic acid) nano- and microparticles using an inverse emulsion polymerization method for the entrapment of hydrophilic drug candidates. J Control Release. 1998;56:149–158. doi: 10.1016/S0168-3659(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 27.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Zhao C, Zhuang X, He P, Xiao C, He C, Sun J, Chen X, Jing X. Synthesis of biodegradable thermo- and pH-responsive hydrogels for controlled drug release. Polymer. 2009;50:4308–4316. doi: 10.1016/j.polymer.2009.07.010. [DOI] [Google Scholar]
- 29.Spizzirri UG, Iemma F, Puoci F, Xue F, Gao W, Cirillo G, Curcio M, Parisi OI, Picci N. Synthesis of hydrophilic microspheres with LCST close to body temperature for controlled dual-sensitive drug release. Polym Adv Technol. 2010 [Google Scholar]
- 30.Mathews AS, Cho W-J, Kim I, Ha C-S. Thermally responsive poly[N-isopropylacrylamide-co-2-hydroxyethylacrylate] colloidal crystals included in β-cyclodextrin for controlled drug delivery. J Appl Polym Sci. 2009;113:1680–1689. doi: 10.1002/app.30164. [DOI] [Google Scholar]
- 31.Shi J, Liu L, Liu X, Sun X, Cao S. Inorganic–organic hybrid alginate beads with LCST near human body temperature for sustained dual-sensitive drug delivery. Polym Adv Technol. 2008;19:1467–1473. [Google Scholar]
- 32.Zhang X-Z, Chu C-C. Temperature-sensitive poly(N-isopropylacrylamide)/poly(ethylene glycol) diacrylate hydrogel microspheres: formulation and controlled drug release. Am J Drug Deliv. 2005;3:55–65. doi: 10.2165/00137696-200503010-00006. [DOI] [Google Scholar]