Abstract
The objectives of the present work were to prepare castor oil-based nano-sized emulsion containing cationic droplets stabilized by poloxamer–chitosan emulgator film and to assess the kinetic stability of the prepared cationic emulsion after subjecting it to thermal processing and freeze–thaw cycling. Presence of cryoprotectants (5%, w/w, sucrose +5%, w/w, sorbitol) improved the stability of emulsions to droplet aggregation during freeze–thaw cycling. After storing the emulsion at 4°C, 25°C, and 37°C over a period of up to 6 months, no significant change was noted in mean diameter of the dispersed oil droplets. However, the emulsion stored at the highest temperature did show a progressive decrease in the pH and zeta potential values, whereas the emulsion kept at the lowest temperatures did not. This indicates that at 37°C, free fatty acids were formed from the castor oil, and consequently, the liberated free fatty acids were responsible for the reduction in the emulsion pH and zeta potential values. Thus, the injectable castor oil-based nano-sized emulsion could be useful for incorporating various active pharmaceutical ingredients that are in size from small molecular drugs to large macromolecules such as oligonucleotides.
KEY WORDS: castor oil, chitosan, nano-sized emulsion, poloxamer, stability
INTRODUCTION
An oil-in-water (o/w) nano-sized emulsion is a heterogenous dispersion of two immiscible liquids. According to the second law of thermodynamics, the o/w nano-sized emulsion is subjected to various instability processes like aggregation, flocculation, coalescence, Ostwald ripening, and hence eventual phase separation (1,2). Unlike microemulsions which are thermodynamically stable and clear transparent liquid system, macro (coarse)- and nano-sized emulsions are meta-stable dispersions. However, the stability of the o/w macro (coarse)- and nano-sized emulsions can substantially be improved with the help of suitable emulsifiers or emulgators that are capable of forming a mono- or multilayer coating film around the dispersed oil droplets in order to reduce interfacial tension and to increase droplet–droplet repulsion (3). Depending on the concentrations of these three components (oil–emulgators–water) and the efficiency of the emulsification equipments used to reduce droplet size into a nano-sized and/or monodispersed size range, the o/w nano-sized emulsion with improved stability over the desired time span (in comparison to coarse emulsion) can be obtained. The advantages of the nano-sized emulsion system include: natural biodegradability, sub-micrometer droplet size range, sterilizability, substantial drug solubilization either at the innermost oil phase or at the o/w interface, minimized side effects, and improved bioavailability (4). Due to these advantages, the nano-sized emulsion is now recognized as a promising drug delivery vehicle or carrier for parenteral (5) and topical (ocular and percutaneous) (5,6) applications.
In general, the o/w type nano-sized emulsion (meant for parenteral and topical applications) consists of 0.5–20% of oil or oil mixture, 0.1–10% of an emulgator, 0.05–5% of a nonionic surfactant, and permitted amount of preservatives and osmotic agent (5). The remaining proportion comprises an aqueous component. To make anionic o/w nano-sized emulsion, phospholipid-like Lipoid E80 either alone or in combination with a water-soluble nonionic surfactant (poloxamer) and a stabilizer-like oleic acid/cholic acid/deoxycholic acid or its sodium salt was used as emulgator (3). In addition, a cationic o/w nano-sized emulsion was prepared and stabilized by forming a mixed emulgator film comprising phospholipids, poloxamer, and stearylamine/oleylamine at the o/w interface of the oil droplets (4,7). Antioxidants such as α-tocopherol was also included in most of the developed nano-sized emulsions in order to prevent the oxidative degradation of phospholipid or other emulsion components and thus the minimization of free fatty acid formation in phospholipid-based emulsions following autoclave sterilization and subsequent storage at stipulated conditions. However, even with the addition of antioxidants, the phospholipid-containing emulsion was prone to the liberation of free fatty acids (from phospholipid components), leading to the generation microclimate acidic pH in the vicinity of the oil phase, oil–water interface, and water phase of the emulsion (8,9). The microclimate pH thus generated might be harmful to the emulsion-incorporated drug molecules. Up to now, the adjustment of pH of the sterilized emulsion by adding mild alkali is the only viable option to prepare the emulsion with desired near neutral value (pH ≈7). If free fatty acid was released or mild alkali solution was added during the formulation of phospholipid-stabilized emulsion, then incorporation of peptide or protein into the emulsion might be questionable due to its possible structural degradation/modification. In this context, it becomes necessary to develop an emulsion without the addition of phospholipid emulgator but still shows the required kinetic stability for at least over months. Therefore, castor oil, poloxamer–chitosan, and water are being selected respectively as the model oil–emulgators–water components to prepare the phospholipidless nano-sized emulsion.
The objectives of the present work were to prepare castor oil-based nano-sized emulsion containing cationic droplets stabilized by poloxamer–chitosan emulgator film and to assess the kinetic stability of the prepared cationic emulsion after subjecting it to thermal processing and freeze–thaw cycling.
MATERIALS AND METHODS
Materials
Castor oil and α-tocopherol were purchased from Fluka (St. Louis, MO, USA). Chitosan (medium molecular weight; deacetylation, 81%; viscosity of 1 wt.% solution in 1 wt.% acetic acid, 286 cps; moisture, 4.6 wt.%; ash, 0.5 wt.%) was obtained from Aldrich Chemical Co. (St. Louis, MO, USA). Polyoxyethylene–poloxypropylene emulsifier, poloxamer 188 (Pluronic F68), was furnished by BASF (Parsippany, NJ, USA). All other chemicals were obtained commercially and used as received.
Methods
Preparation of Nano-sized Emulsion
The phospholipidless and castor oil-based nano-sized emulsion was prepared according to the method described elsewhere [6]. In brief, 5 ml of castor oil and 0.05 ml of α-tocopherol were taken in a beaker and heated up to 70°C. Two hundred fifty milligrams of chitosan was dissolved in 5 ml of 0.05 M acetic acid and heated up to 70°C. Five hundred milligrams of poloxamer 188 was dissolved in 50 ml double distilled water containing 1.125 g glycerin in a separate beaker and heated up to 70°C. Castor oil was added to chitosan solution and then poloxamer–glycerin solution was added and stirred well by means of a magnetic stirrer. The resulting mixture was further heated to a temperature of 85°C. At this temperature, the obtained crude emulsion was further mixed by a high shear mixer Polytron (Kinematica, Luzerne, Switzerland) for 5 min and rapidly cooled to below 20°C. After cooling, the emulsion was homogenized using a two-stage homogenizer valve assembly (Gaulin Homogenizer, APV Gaulin, Hilversum, the Netherlands) at 9000 £/in.2 for 5 min. The emulsion was then filtered through a TE membrane filter (Schuell and Schleicher, Dassel, Germany) with a pore size of 0.45 μm. The emulsion was packed under nitrogen atmosphere in siliconized glass bottles and then sterilized by steam autoclave at 121°C for 15 min. In total, nine replicate emulsion samples were prepared using the same ingredients with identical experimental conditions.
Zeta Potential, pH, and Viscosity Measurements
The zeta potential measurements were carried out using the Malvern Zetasizer 3000 (Malvern Instruments, Ltd., Malvern, UK). The samples were diluted in double distilled water and the measurements carried out in 10 mM NaCl solution. Each sample was analyzed twice, each analysis consisting of three replicates.
The pH was recorded at given time intervals using a pH meter under different storage temperatures (MP220 pH meter, Mettler Toledo, UK).
An Ubbelohde capillary viscometer (Schott, Hofheim, Germany) was used to measure the viscosity of the emulsion samples stored at three different storage temperatures over time.
Particle Size Analysis
Mean droplet diameter was determined utilizing an ALV Noninvasive Back Scattering High Performance Particle Sizer (ALV-NIBS HPPS; Langen, Germany) at 25°C and using water (refractive index, 1.332; viscosity, 0.894543) as the dilution medium. A laser beam at 632-nm wavelength was used. The sensitivity range was 0.5 nm–5 mm. Apart from the freshly prepared emulsion, the emulsions stored at 4 ± 2°C, 25 ± 2°C and 37 ± 2°C over up to 6 months were also followed up to notice the change in mean droplet diameters. Values reported were the mean droplet diameter of triplicate emulsion samples stored at three different storage temperatures.
Stability Assessment
The pH, zeta potential, and droplet mean diameter were monitored over up to 6 months’ time in the emulsions stored at 4 ± 2°C, 25 ± 2°C, and 37 ± 2°C. Three independent emulsion samples for each storage conditions (3 × 3 = 9 emulsions) were used. Possible phase separations were also assessed visually at given time intervals. All other visible changes were recorded.
Accelerated Stability Test
Since sterilization for 15 min is a drastic operation, it constitutes an accelerated stability test by itself. Creaming and phase separation were then assessed visually after sterilization. To evaluate the mechanical and physical resistance, the emulsion should be subjected to an accelerated mechanical stress. For this purpose, those emulsion bottles which were stored at the already stated temperature conditions for up to 6 months’ time were shaken at 100 strokes/minute by an oscillatory agitation motion over 24 h at 25°C. The droplet size was evaluated before and after this accelerated test.
Freeze–Thaw Cycling Stability
The freeze–thaw cycling stability of the emulsion in presence of cryoprotectants (5%, w/w, sucrose +5%, w/w, sorbitol) or in the absence of cryoprotectants was tested by transferring the emulsion sample (5 ml) into plastic test tubes and subsequently placing it in a −10°C freezer for 16 h and then thawing in a water bath at 30°C for 2 h. This freeze–thaw cycle was repeated five times, and its influence on emulsion property (particle mean diameter) was measured after each cycle.
Statistical Analysis
All the data were subjected to Student’s t test to find out the statistical difference. The alpha (α) value for statistical significance is 0.05 (i.e., p < 0.05).
RESULTS AND DISCUSSION
Excipient Selection
In the current investigation, a well-known combined emulsification and homogenization technique was used to prepare castor oil-based nano-sized emulsion. Without the addition of any phospholipid emulgator, the o/w nano-sized emulsion was stabilized with the poloxamer–chitosan emulgator film. In an arbitrary manner, the amount of castor oil, poloxamer, and chitosan was chosen and kept respectively at 5 ml, 500 mg, and 250 mg to prepare the nine replicate emulsion samples. In the medical field, most of the nano-sized emulsion was prepared utilizing the lower viscosity and digestible oils, derived from the family of triglycerides, like medium-chain triglycerides (MCT), soybean oil, sesame seed oil, cottonseed oil, and safflower oil. In our preliminary experiments, none of the aforementioned oils is able to produce a stable emulsion system when poloxamer–chitosan emulsifier combination is used. Previous literature references show that using corn oil as the oil phase and lecithin–chitosan as the emulsifier combination resulted in a stable emulsion system (10,11). In addition, it was shown previously in the phospholipid-stabilized emulsion that when using castor oil as a single oil phase, the final emulsion did not show stable behavior in the long-term stability (12). But it was our experience that in comparison to other oils, castor oil has higher solubilization capacity to dissolve peptidic molecule like cyclosporin A (9). Therefore, despite its higher viscosity, castor oil alone was used in that previous study to prepare the phospholipid-stabilized emulsion containing cyclosporin A. Among the different natural and synthetic antioxidant molecules that have been available (13), α-tocopherol was the widely used antioxidant to prepare phospholipid-stabilized nano-sized emulsion (6). The currently developed (phospholipidless) emulsion was prepared based on castor oil, stabilized with the poloxamer–chitosan emulgator film, and incorporated α-tocopherol and osmotic agent.
Particle Size and Emulsion Stabilization
Table I shows mean droplet diameter of the o/w nano-sized emulsion. The mean droplet diameter was in the range of 218–250 nm and presented a monodispersed droplet size distribution. As expected, the zeta potential of the developed emulsion was also in positive unit due to the presence of cationic polysaccharide (chitosan) at the mixed emulgator film. By comparing the positive zeta potential with mean particle diameter at nanoscale level, it could be deduced that chitosan molecules are localized at the oil–water interface and intercalated between the nonionic poloxamer molecules. Therefore, the combination of steric (by poloxamer) and electrostatic (by cationic chitosan) repulsions was responsible for the stabilization of dispersed castor oil droplets in the phospholipidless o/w nano-sized emulsion. In addition, the viscosity of the freshly prepared nano-sized emulsion formulation was measured and found to be 1.5 cps.
Table I.
Effect of Storage Temperature and Time on the Mean Droplet Diameter and Zeta Potential Values of Castor Oil-Based Nano-sized Emulsion
| Storage time (months) | Physicochemical properties | |||||
|---|---|---|---|---|---|---|
| Mean droplet diameter (nm) at different storage temperature (°C) | Zeta potential (mV) at different storage temperature (°C) | |||||
| 4 | 25 | 37 | 4 | 25 | 37 | |
| 0 | 243 (±45) | 243 (±48) | 243 (±38) | 41.4 (±1.24) | 40.5 (±1.80) | 47.3 (±1.91) |
| 1 | 250 (±56) | 238 (±50) | 240 (±35) | – | – | – |
| 3 | 235 (±45) | 230 (±46) | 228 (±46) | – | – | – |
| 6 | 221 (±52) | 221 (±40) | 218 (±50) | 37.8* (±0.89) | 37.2* (±2.35) | 30.6* (±1.37) |
Three independent emulsion samples for each storage temperatures (3 × 3 = 9 emulsions) were used
– Not determined
Figures in parentheses indicate standard deviation (n = 3)
*p < 0.05
Furthermore, the substantial stability shown by phospholipidless and castor oil-based nano-sized emulsion even after autoclave sterilization could reasonably be attributed to the following explanation. Castor oil is known to contain traces of free fatty acid (recinoleic acid), and even some amount of fatty acid is likely to be liberated after autoclave sterilization. After interaction with positively charged chitosan, the negatively charged fatty acids might acquire mild emulsification property (14). A similar effect of achievement of emulsion kinetic stability through the same poloxamer–chitosan emulgator film was reported (14,15). However, these authors used the oil mixture (castor oil and MCT) as an oil phase of the emulsion rather than single oil (castor oil) alone to prepare the o/w nano-sized emulsion. In this respect, our developed emulsion differs significantly from the report published previously by the other research groups.
Zeta Potential and Storage Conditions
The effect of storage temperature and time on the droplet mean diameter and zeta potential values of castor oil-based nano-sized emulsion was followed up to 6 months’ storage time at three different temperature conditions (Table I). It should be emphasized that in total, nine independent emulsion samples were used to perform the stability assessment. Up to 37°C, no significant changes were noted in mean droplet diameter. This indicates that over this 6-month period, castor oil-based nano-sized emulsion remained physically stable. However, depending on the storage temperatures, the zeta potential of the emulsion was found to decrease. Immediately after the preparation of fresh emulsion, the measured zeta potential was about + 41.4 mV (Table I). The observed high positive zeta potential value was sufficient enough to prevent droplet coalescence upon random collisions and even following autoclave sterilization at elevated temperature. Keeping the emulsion at 37°C for up to 6 months decreased the zeta potential to +30.6 ± 1.37 mV. The same emulsion stored at 25°C showed a lesser reduction in the zeta potential (+37.2 ± 2.35 mV). At 4°C, the zeta potential of the emulsion was still found to be +37.8 ± 0.89 mV. Statistical analysis of these data revealed significant differences (p < 0.05; Table I). From this result, it appears that the lowest temperature was more adequate for castor oil-based nano-sized emulsion storage in terms of the emulsified particle’s surface charge. It should be emphasized that an excessive shaking (100 strokes/minutes over 24 h) of the emulsion was performed following the completion of the storage conditions in order to verify the influence of the storage conditions and mechanical stress on the droplet diameter. No change in the mean droplet diameter was observed, indicating the lack of any influence before and after shaking of the emulsion.
Emulsion’s pH and Storage Conditions
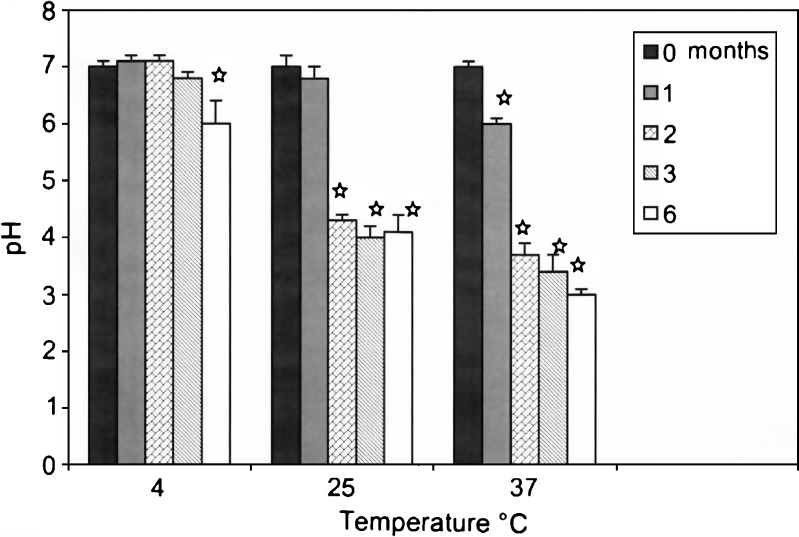
The change in pH of the castor oil-based nano-sized emulsion with time as a function of storage temperature is depicted in Fig. 1. The figure clearly shows the reduction of the emulsion’s pH at 25°C and 37°C. It should be noted that the initial pH of the emulsion was adjusted to 7.0 using a mild acid before subjecting the emulsion to sterilization. However, a progressive reduction in pH (1 to 4 units) of the emulsion was noticed when stored at 37°C. The higher is the storage temperature, the more is the liberation of free fatty acid from castor oil, resulting in the auto-alteration of the emulsion’s pH. At 4°C, it is interesting to see that the initial reduction of pH started only after 2 months, and even after its initiation, the progression in the reduction of pH was also not so rapid in the following months as compared to the higher storage temperatures. Thus, pH decreased only by one single unit.
Fig. 1.
Influence of temperature over 6 months storage on the pH of castor oil-based nano-sized emulsion ( p < 0.05)
p < 0.05)
The combined implications of decreasing zeta potential and pH within the studied storage conditions in the absence of significant droplet diameter increase could be explained on the basis of the mixed poloxamer–chitosan emulgator film and the observed high positive zeta potential value. Chitosan has a positive charge in acidic solutions due to the presence of protonated amino groups along its backbone that have pKa values between 6.3 and 7.0 (10,11). During emulsification, chitosan molecules are localized at the interface and intercalated between the nonionic surfactants. Hence, a mixed interfacial film comprising the poloxamer and chitosan molecules was formed at the o/w interface which resulted in an overall positive surface charge (zeta potential). The high positive zeta potential value observed even after the higher storage temperatures was sufficient enough to prevent droplet coalescence upon random collisions. Moreover, the combined steric (by poloxamer) and electrostatic (by cationic chitosan) repulsive forces were also operating onto the dispersed castor oil droplets to maintain the stability of the produced nano-sized particles. It should be added that the viscosity of the emulsion stored at three different temperatures did not show any significant change (data not shown). This result further substantiates the interfacial properties of the chitosan along with poloxamer. Consequently, no significant droplet diameter increase was noted in the emulsion.
Freeze–Thaw Cycling Stability and Cryoprotectants
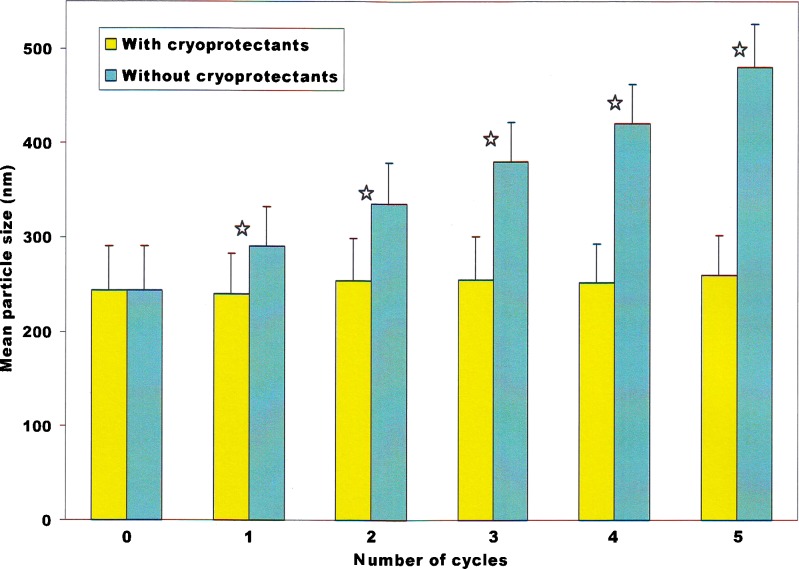
Preliminary experiments indicated that the emulsion was unstable for freeze–thaw cycling in the absence of cryoprotectants. Dependence of mean particle diameter of the castor oil-based nano-sized emulsion on the number of freeze–thaw cycles (−10°C for 22 h/30°C for 2 h) in the absence and presence of cryoprotectants is depicted in Fig. 2. In the absence of cryoprotectants, the mean particle size of the emulsion was found to increase progressively from 243 to 480 nm after five cycles. A number of physicochemical mechanisms may be responsible for the extensive droplet aggregation observed in the absence of cryoprotectants. First, when the emulsion was placed in the freezer, some of its water crystallized. This caused the dispersed oil droplets to come into closer proximity because the oil droplets were confined to the nonfrozen regions remaining in the aqueous phase (16). When there was no presence of sufficient free water to fully hydrate the oil droplet surfaces (17–19), the droplet–droplet interactions was forced closer together to effect coalescence to occur. Second, ice crystallization led to an increase in the ionic strength of the freeze-concentrated non-frozen aqueous phase surrounding the emulsion droplets (18). Third, it is possible that ice crystals formed during freezing may have penetrated into the oil droplets and disrupted their interfacial membranes. This allowed the oil droplets more prone to coalescence between them. Fourth, cooling may have caused some of the fat in the emulsion droplets to crystallize promoting partial coalescence due to penetration of a fat crystal from one droplet through the membrane of another droplet (20,21).
Fig. 2.
Dependence of mean particle diameter of the castor oil-based nano-sized emulsion on number of freeze–thaw cycles (−10°C for 22 h/30°C for 2 h) in the absence and presence of cryoprotectants ( p < 0.05)
p < 0.05)
In contrast, the presence of cryoprotectants improved the stability of emulsions to droplet aggregation during freeze–thaw cycling. For example, after one cycle, there was no significant change in the mean droplet diameter in the castor oil-based nano-sized emulsion containing 5% sucrose and 5% sorbitol (which remained the same around 240 nm), and even after five cycles, the mean droplet diameter increased to 260 nm only.
A number of mechanisms have been proposed to account for the ability of cryoprotectants to improve the stability of emulsions to aggregation during freeze–thaw cycling. First, cryoprotectants increased the osmolyte concentration in the aqueous phase, thereby reducing its crystallization temperature. Subsequently, the total amount of ice crystal formation was limited, and therefore, the volume of non-frozen aqueous phase available to the oil droplets was increased (18). Second, cryoprotectants formed hydrogen bonds with emulsifiers adsorbed to droplet surfaces, thereby reducing the tendency for interactions to occur between droplet surfaces when the free water content was reduced by ice crystallization (19).
CONCLUSIONS
The castor oil-based nano-sized emulsion was prepared following the well-known emulsification–homogenization technique. To eliminate the lecithin or phospholipid (anionic) components, the nonionic–cationic emulgator film was used to stabilize the o/w nano-sized emulsion. Regardless of the storage conditions, the poloxamer–chitosan emulgator film was able to stabilize the castor oil-based nano-sized emulsion in terms of mean droplet diameter. However, the emulsion’s zeta potential and pH were altered with storage at all temperatures. The addition of cryoprotectants stabilized the emulsion against droplet aggregation during freeze–thaw cycling. Further works are undergoing in our lab to investigate the stability of the castor oil-based emulsion following incorporation of various active pharmaceutical ingredients that range in size from small molecular drugs (acetazolamide, azithromycin, and celecoxib) to large macromolecules such as oligonucleotides.
References
- 1.Sarkar DK. Engineering of nanoemulsions for drug delivery. Curr Drug Deliv. 2005;2(4):1–14. doi: 10.2174/156720105774370267. [DOI] [PubMed] [Google Scholar]
- 2.Capek I. Degradation of kinetically-stable o/w emulsions. Adv Colloid Interface Sci. 2004;107:125–155. doi: 10.1016/S0001-8686(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 3.Grigoriev DO, Miller R. Mono- and multilayer covered drops as carriers. Curr Opin Colloid Interface Sci. 2009;14:48–59. doi: 10.1016/j.cocis.2008.03.003. [DOI] [Google Scholar]
- 4.Tamilvanan S. Formulation of multifunctional oil-in-water nanosized emulsions for active and passive targeting of drugs to otherwise inaccessible internal organs of the human body. Int J Pharm. 2009;381:62–76. doi: 10.1016/j.ijpharm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Tamilvanan S. Oil-in-water nanosized emulsions: medical applications. In: Gad SC, editor. Pharmaceutical manufacturing handbook, Chap. 7.4. New Jersey: Wiley; 2008. pp. 1329–1368. [Google Scholar]
- 6.Tamilvanan S, Benita S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm. 2004;58:357–368. doi: 10.1016/j.ejpb.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Korner D, Benita S, Albrecht G, Baszkin A. Surface properties of mixed phospholipid–stearylamine monolayers and their interaction with a nonionic surfactant (poloxamer) Colloids Surf. 1994;3:101–109. doi: 10.1016/0927-7765(93)01111-4. [DOI] [Google Scholar]
- 8.Rabinovich-Guilatt L, Couvreur P, Lambert G, Goldstein D, Benita S, Dubernet G. Extensive surface studies help to analyse zeta potential data: the case of cationic emulsions. Chem Phys Lipids. 2004;131:1–13. doi: 10.1016/j.chemphyslip.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Tamilvanan S, Khoury K, Gilhar D, Benita S. Ocular delivery of cyclosporin A. I. Design and characterization of cyclosporin A-loaded positively-charged submicron emulsion. STP Pharma Sci. 2001;11:421–426. [Google Scholar]
- 10.Ogawa S, Decker EA, McClements DJ. Influence of environmental conditions on the stability of oil in water emulsions containing droplets stabilized by lecithin–chitosan membranes. J Agric Food Chem. 2003;51(18):5522–5527. doi: 10.1021/jf026103d. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Decker EA, McClements DJ. Production and characterization of o/w emulsions containing cationic droplets stabilized by lecithin–chitosan membranes. J Agric Food Chem. 2003;51(9):2806–2812. doi: 10.1021/jf020590f. [DOI] [PubMed] [Google Scholar]
- 12.Jumaa M. Ph.D thesis, Christian Albrecht University for Kiel, Kiel, Germany; 1999.
- 13.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jumaa M, Müller BW. Physicochemical properties of chitosan–lipid emulsions and their stability during the autoclaving process. Int J Pharm. 1999;183:175–184. doi: 10.1016/S0378-5173(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 15.Calvo P, Remuñá-López C, Vila-Jato JL, Alonso MJ. Development of positively charged colloidal drug carriers: chitosan-coated polyester nanocapsules and submicro-emulsions. Coll Polym Sci. 1997;275:46–53. doi: 10.1007/s003960050050. [DOI] [Google Scholar]
- 16.Saito H, Kawagishi A, Tanaka M, Tanimoto T, Okada S, Komatsu H, et al. Coalescence of lipid emulsions in floating and freeze-thawing processes: examination of the coalescence transition state theory. J Colloid Interface Sci. 1999;219:129–134. doi: 10.1006/jcis.1999.6452. [DOI] [PubMed] [Google Scholar]
- 17.Ausborn M, Schreier H, Brezesinski G, Fabian H, Meyer HW, Nuhn P. The protective effect of free and membrane bound cryoprotectants during freezing and freeze-drying of liposomes. J Control Release. 1994;30:105–116. doi: 10.1016/0168-3659(94)90257-7. [DOI] [Google Scholar]
- 18.Komatsu H, Okada S, Handa T. Suppressive effects of salts on droplet coalescence in a commercially available fat emulsion during freezing for storage. J Pharm Sci. 1997;86:497–502. doi: 10.1021/js960166r. [DOI] [PubMed] [Google Scholar]
- 19.Strauss G, Hauser H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc Natl Acad Sci USA. 1986;83:2422–2426. doi: 10.1073/pnas.83.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanapalli SA, Palanuwech J, Coupland JN. Stability of emulsions to dispersed phase crystallization: effect of oil type, dispersed phase volume fraction, and cooling rate. Colloid Surf A. 2002;204:227–237. doi: 10.1016/S0927-7757(01)01135-9. [DOI] [Google Scholar]
- 21.Harada T, Yokomizo K. Demulsification of oil-in-water emulsion under freezing conditions: effect of crystal structure modifier. J Am Oil Chem Soc. 2000;77:859–863. doi: 10.1007/s11746-000-0137-y. [DOI] [Google Scholar]