Abstract
In the present study attempt was made for preparation of isotretinoin-hydroxypropyl β cyclodextrin (HP-β-CD) inclusion complex and encapsulate this complex in elastic liposomes to study the effect of dual carrier approach on skin targeting of isotretinoin. The isotretinoin HP-β-CD complex was prepared by freeze-drying method and characterized by IR spectroscopy. The drug and drug-CD complex loaded elastic liposomal formulation were prepared and characterized in vitro, ex-vivo and in vivo for shape, size, entrapment efficiency, no. of vesicles per cubic mm, in vitro skin permeation and deposition study, photodegradation and skin toxicity assay. The transdermal flux for different vesicular formulations was observed between 10.5 ± 0.5 to 13.9 ± 1.6 μg/cm2/h. This is about 15-21 folds higher than that obtained from drug solution (0.7 ± 0.1 μg/cm2/h) and 4-5 folds higher than obtained with drug-CD complex solution (2.7 ± 0.1 μg/cm2/h). The amount of drug deposit was found to increase significantly (p < 0.05) by cyclodextrin complexation (30.1 ± 0.1 μg). The encapsulation of this complex in elastic liposomal formulation further increases its skin deposition (262.2 ± 21 μg). The results of skin irritation study using Draize test also showed the significant reduction in skin irritation potential of isotretinoin elastic liposomal formulation in comparison to free drug. The results of the present study demonstrated that isotretinoin elastic liposomal formulation possesses great potential for skin targeting, prolonging drug release, reduction of photodegradation, reducing skin irritation and improving topical delivery of isotretinoin.
Key words: cyclodextrin vesicles dual approach, skin irritation; elastic liposomes; isotretinoin; skin targeting
INTRODUCTION
Isotretinoin is recommended for topical treatment of different dermatological disorders such as acne vulgaris, icthyosis, and psoriasis (1). This drug is also under therapeutic investigation for various types of cancer (2). Its anti-acne and chemopreventative effects require chronic oral administration for 15-20 and 5-8 weeks, respectively. Unfortunately, oral use of isotretinoin is unacceptable due to the severe side effects such as hypertriglyceridemia, dry skin, ocular side effects, hypercalcemia, and mood disorders (3). Isotretinoin is a highly lipophilic drug and has poor oral bioavailability (25%) (4). Due to associated systemic side effects with oral administration, topical administration of isotretinoin is preferred (4–6). Presently available marketed topical formulations for isotretinoin are cream and ointment.
Topical application of isotretinoin is limited by several drawbacks, such as skin irritation, very low water solubility, difficulty to incorporate in topical base, and photodegradation. Photodegradation render the drug ineffective and also causes allergy due to degradation products (7,8). In order to overcome the limitations of present topical therapy, carrier approach such as liposomes (9), niosomes (10) and solid lipid nanoparticles (11) for effective delivery of isotretinoin have been reported. It has been found that, topically applied liposomes increase the residence time of isotretinoin in stratum corneum and reduces its systemic absorption (9). Although, many authors have proposed the use of tretinoin liposomal formulations, but no work has been carried out on tretinoin elastic liposomal formulations. Elastic liposomes seem to be a very promising vehicle in dermal and transdermal drug delivery (12–15). Elastic liposomes are reported to have five- to tenfold higher skin permeation and deposition in comparison to conventional liposomal formulation. In the present study, an attempt was made to prepare isotretinoin elastic liposomal formulation with the aim of developing new topical formulation for delivery of isotretinoin.
Cyclodextrin complexation of drug is known to increase the solubility, stability, sustaining the release and minimize the photodegradation of complexed drug (16). Inclusion of isotretinoin in cyclodextrin could possibly reduce the problem of poor aqueous solubility, skin irritation, poor incorporation in vehicle, and photodegradation problems associated with currently used topical formulation. In the present study, attempt was also made for preparation of isotretinoin-hydroxypropyl β cyclodextrin (HP-β-CD) inclusion complex and furthers its encapsulation in elastic liposomal formulation and studying its skin targeting potential. Recently, encapsulation of drug in the form of cyclodextrin-drug complex in vesicular formulation has been investigated as new strategy for merging the relative advantages of the two types of carrier into a single system (17).
In particular, the focus of investigation was to see that combination of vesicular approach with cyclodextrin complexation would help in increasing the solubility, skin permeation, deposition, and reducing the photodegradation of isotretinoin.
MATERIALS AND METHODS
Materials
Isotretinoin and soya phosphatidylcholine were received as gift sample from Indchemie Health Specialties Pvt. Ltd., Daman, India and Lifecare Innovation Ltd., Gurgaon, India, respectively. Sephadex G-50, 6-carboxyfluorescein, HP β-Cyclodextrin were purchased from sigma Chemicals, St Louis, MO, USA. Span 60, span 80, and cholesterol were purchased from Hi Media Ltd., Mumbai, India. Ethanol, acetonitrile, acetic acid, hematoxylin and eosin were procured from E. Merck, Mumbai, India. All reagents used in this study were of analytical grade. All experiments were performed using amber color glassware.
Complexation of Cyclodextrin with Isotretinoin
The solid inclusion complex of isotretinoin was prepared by freeze-drying method as reported by Yap et al. (18). Briefly, 10 mg/ml suspension of isotretinoin in 0.30 M of HP-β-CD solution in phosphate buffer solution (pH = 8.1) was prepared. The suspension was shaken at 400 rpm on a horizontal rotary shaker (Remi instruments, Mumbai, India) at room temperature (25°C) for 2 days and then filtered to remove the excess drug. The resulting solution in glass vials was frozen at −80°C and then subjected to lyophilization in a freeze-dryer (ModulyoD, NewYork, USA) for 24 h to obtain a powder. Physical mixture of isotretinoin/HP-β-CD was prepared by mixing together both compounds to obtain a ratio of 1:1. The inclusion complex and physical mixture together with pure isotretinoin and HP-β-CD were then subjected to physiochemical analysis.
Fourier Transform Infrared Spectroscopy
Physical mixture of cyclodextrin and drug, cyclodextrin, drug, and cyclodextrin-drug complex was subjected to infrared spectroscopy (IR) analysis (Perkin Elmer, Spectrum RX-I, Waltham, MA, USA). The samples were grounded and mixed thoroughly with potassium bromide (2:98 w/w). The disks obtained were used to generate the IR spectra from 4,000 to 400 cm−1.
Preparation of Elastic Liposomal Formulations
The elastic liposomes were prepared by conventional rotary evaporation sonication method as described by Cevc et al. (19). Different batches of elastic liposomes were prepared using surfactant, phospholipids, and different concentrations of drug or drug-cyclodextrin complex. The composition of elastic liposomal formulations is summarized in Table I. The accurately weighed amount of drug, phospholipid, and surfactant were taken in a clean, dry, amber-colored round-bottom flask and this lipid mixture was dissolved in small quantity of chloroform. The organic solvent was removed by rotary evaporator (rotary evaporator, Perfit, India) under reduced pressure at 40 ± 1°C. Final traces of solvent were removed under vacuum overnight (Perfit, Ambala, India). The deposited lipid film was hydrated using 7% ethanol by rotation at 60 rev/min for 1 h. The resulting vesicles were swollen for 2 h at room temperature to get large multilamellar vesicles (LMLVs). To prepare smaller vesicles, LMLVs were sonicated for 12 min at 40% frequency. The same method was used for preparation of niosomal formulation that was used for comparison purpose.
Table I.
Composition and Characterizations of Elastic Liposomal Formulations
| S.No. | Formulation code | PCa | Span 80 (mg) | Span 60 (mg) | Cholesterol (mg) | Drug (mg) | Vesicle shape | Entrapment efficiency (%) | No. of vesicles per cubic mm | Particleb size (μm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ELP10 | 85 | 15 | - | - | 10 | Spherical vesicular | 81.2 ± 7.3 | 47 ± 5.0 | 5.2 ± 0.3 |
| 2 | ELP15 | 85 | 15 | - | - | 15 | Spherical vesicular | 75.6 ± 6.4 | 50 ± 6.0 | 5.3 ± 0.4 |
| 3 | ELP20 | 85 | 15 | - | - | 20 | Spherical vesicular | 62.1 ± 4.3 | 52 ± 5.0 | 5.2 ± 0.2 |
| 4 | ELP-COMPb | 85 | 15 | - | - | 10 | Spherical vesicular | 90.1 ± 8.3 | 49 ± 4.0 | 6.2 ± 0.5 |
| 5 | NIO | 10 | - | 60 | 40 | 10 | Spherical vesicular | 70.5 ± 6.9 | 50 ± 4.0 | 5.2 ± 0.3 |
Values represented as mean ± SD (n = 3)
ELP elastic liposomal formulation, ELP-COMP elastic liposome formulation with isotretinoin–cyclodextrin complex, NIO niosomal formulation
a PC phosphatidylcholine
bParticle-size measurement before sonication
Optimization of Drug Loading
To determine the maximum amount of drug that can be loaded in vesicles, elastic liposomal formulations were prepared with increasing amounts of isotretinoin (10, 15, and 20 mg). Drug-loaded formulations were examined over a period of 14 days using optical microscope for the appearance of drug crystals, entrapment efficiency, and in vitro skin permeation study.
Solubility Studies
For quantitative solubility study, a defined quantity (10 mg/ml) of drug and drug-cyclodextrin complex was taken in thoroughly cleaned amber-colored glass vials. Different investigated solvents were added and glass vials were shaken for 24 h on horizontal rotary shaker. The mixture obtained was filtered on a 0.45 μm membrane filter and drug content in filtrate was determined spectrophotometrically.
Characterizations of Elastic Liposomal Formulation
The vesicle size and distribution were determined by dynamic light scattering method (Mastersizer 2000, Malvern, Worcestershire, UK). Measurements were carried out at an angle of 90° at 25°C. Dispersions were diluted with double-distilled water to ensure that the light scattering intensity was within the instrument’s sensitivity range. Vesicle shape (without sonication) was determined by visualization of vesicular dispersion under optical microscope at 400× (Olympus, DX31, Japan). The entrapment efficiency was determined after separating unentrapped drug using Sephadex G-50 column. The eluted vesicles were lysed using Triton-× 100 (0.1% v/v) and subsequently analyzed for drug content spectrophotometrically. Elastic liposomal formulation (without sonication) was diluted five times with 0.9% NaCl solution and number of vesicles per cubic millimeter was counted by optical microscopy using hemocytometer (Marienfeld, Germany).
Skin Permeation Study
The in vitro skin permeation of isotretinoin from elastic liposomal formulations, niosomes, and drug solution was studied using Franz glass diffusion cell maintained at 37 ± 1°C under non-occlusive conditions. The effective permeation area of the diffusion cell was 2.303 cm2. The receptor compartment contained 22.5 ml of 0.5% v/v Tween 80 in phosphate buffer saline (PBS 6.8) and was constantly stirred at 100 rpm. Excised albino abdomen rat skin was mounted between the donor and the receptor compartment. Elastic liposomal formulation (2.0 ml) was applied to the epidermal surface of skin. The samples (0.5 ml) were withdrawn through the sampling port of the diffusion cell at 1, 3, 6, 10, 12, 16, 20, and 24 h time intervals and analyzed by high performance liquid chromatography (HPLC) assay. An equal volume of fresh phosphate buffer maintained at 37 ± 1°C was replaced into the receptor compartment after each sampling. Diffusion cells were covered with aluminum foil to prevent light exposure. All the experiments were performed in triplicate.
Skin Deposition Study
Skin deposition study was carried out using same protocol as discussed above for skin permeation study. At the end of the permeation experiments (24 h), the surface of the skin was washed five times with 50% ethanol to remove excess drug from the surface. The washing protocol was verified and found to remove >95% of the applied dose at zero time. The skin was then cut into small pieces. The tissue was further homogenized with 50% ethanol (10 ml) and left for 24 h at room temperature. After shaking for 5 min and centrifugation for 5 min at 3,000 rpm, the isotretinoin content in the upper phase was determined by HPLC assay (20). The method was previously validated by our group for estimation of amount of drug deposited in skin for sumatriptan, colchicine, and found to have the efficiency of 90-92% (14,15).
Photo Degradation Studies
The photodegradation of isotretinoin was studied using a ultraviolet (UV) lamp set at 366 nm. The isotretinoin methanolic solution (1.0 mg/ml), isotretinoin-cyclodextrin solution (1.0 mg/ml) and isotretinoin and isotretinoin-cyclodextrin-loaded elastic liposomal formulations in a 10-ml glass vials were maintained at room temperature and exposed to UV irradiation from a 30 W lamp (366 nm, Perfit, Ambala, India) for 1 h at a fixed distance of 10 cm. At regular time intervals (0.25, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 h) samples were first stirred and then 0.5 ml aliquots of the dispersions were removed and quantify for isotretinoin content. All experiments were carried out at 25 ± 1°C. The results were expressed as percentages of the remaining isotretinoin. Each test was carried out in triplicate.
Skin Irritation Study Using Draize Test
The irritancy of different formulations was determined in male albino rabbits (1.9-2 kg) based on the method described by Draize et al. (21). Rabbit was used as test animal as per requirements of US Food and Drug Administration (FDA). The animals were housed in an air-conditioned room (20 ± 2.0°C) and hair of the back was trimmed short, 24 h before the beginning of the assay. The animals were divided into six groups each consisting of three animals. The first group (control) received topical application of normal saline solution. Second and third group received topical application of isotretinoin solution in propylene glycol: ethanol (75:25 v/v) and isotretinoin-cyclodextrin complex solution, respectively. Fourth, fifth, and sixth group received topical application of elastic liposomes without drug, isotretinoin elastic liposomes and isotretinoin-cyclodextrin-loaded elastic liposomes formulation, respectively. Three squares were drawn on each side of the back of each rabbit, and 0.5 ml formulation was applied on each square. The treated animal was protected by using nylon mesh, supported by the plastic squares having small pores, kept above the treated area. At different time intervals 1, 24, 48, and 72 h after application, exposed area was scored for the erythema and edema on grade of 0-4 as described by US FDA guidelines. All investigations were performed after approval of the Institutional Animal Ethics Committee of the Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala and in accordance with the disciplinary principles and guidelines of the Committee for the Purpose of Control and Supervision on Experiments on Animals.
Skin Toxicity Studies
Sprague Dawley rats weighing 100-150 g were divided in five groups each consisting of three animals. The first group (control) received topical application of normal saline solution. Second, third, fourth, and fifth group received topical application of isotretinoin solution in propylene glycol: ethanol (75:25 v/v), isotretinoin-cyclodextrin solution, isotretinoin elastic liposomes and isotretinoin-cyclodextrin-loaded elastic liposomal formulation, respectively. After 24 h of application the rats were sacrificed. The skin was removed, cut into small pieces, fixed, microtomed (5 μm thickness), and sections were fixed on glass slide. These sections were stained with eosin-hematoxylin and observed under optical microscope (DX 31, Olympus, Tokyo, Japan) for integrity and structural changes of epidermis (22).
HPLC Assay
Isotretinoin was estimated in skin permeation and deposition study by the HPLC method as reported by Tashtoush et al. (20). Trifluoroacetic acid (0.01%) and acetonitrile (15: 85 v/v%) was used as mobile phase and delivered at 1.0 mL min–1. The injected fluid (20 μL) was eluted in C 18 column at room temperature and isotretinoin was monitored at 349 nm using a UV detector (Waters, MA, USA). The calibration curve with in a concentration range from 0.5 to 10.0 μg/mL was used to measure the isotretinoin concentration. The relative standard deviation around the calibration line ranged from 1.0% to 4.5% and the squared correlation coefficient was 0.9972.
Statistical Analysis
Data are expressed as the mean ± SD of obtained results. Data were statistically analyzed by one-way ANOVA for more than two groups and Student’s t test was applied for comparison to two groups. The data were analyzed using software GraphPad Prism (version 2.0, San Diego, CA, USA) and results were considered statistically significant at 95% confidence (p < 0.05).
RESULTS AND DISCUSSION
Preparation of Elastic Liposomal Formulations
The compositions of elastic liposomal formulations are summarized in Table I. Different batches of elastic liposomes were prepared with Span 80 and phosphatidylcholine (PC) using conventional rotary evaporation sonication method (19). Span 80 was selected as edge activator surfactant because it is biocompatible and pharmaceutically acceptable (23). Phosphatidylcholine is used as bilayer forming agent. Niosomes was used as control and prepared by optimized composition as reported by our group (22). Niosomes consist of non-ionic surfactant as bilayer forming agent along with cholesterol and PC as stabilizing agent. In comparison, elastic liposomes consists of PC as bilayer forming agent and surfactant used at sublytic concentration as edge activator for providing the flexibility to vesicle membrane (23,24). These elastic liposomal formulations were colloidal dispersions having average diameter in range of 4-5 µm (without sonication) and 100-200 nm after sonication (Table I).
Attempt was also made to prepare the cyclodextrin-isotretinoin inclusion complex and study its effect on skin permeation and deposition with or without encapsulating in elastic liposomes. HP-β-CD was selected as complexing agent due to its high aqueous solubility, inclusion capacity and skin penetrability (25). HP-β-CD has been subjected to extensive toxicological studies and is considered safe, with no carcinogenic effects (26). HP-β-CD is the most widely reported CD for topical/transdermal delivery of drug (16).
Cyclodextrin-isotretinoin complex was prepared using lyophilization method as reported by Yap et al. (18). Optimized ratio of HP-β-CD and isotretinoin (0.3: 1.0 M) was selected for formation of inclusion complex as reported by Yap et al. (18) and Caddeo et al. (27). Formation of complex was confirmed by carrying out the IR analysis of physical mixture of cyclodextrin and drug, cyclodextrin, drug, and cyclodextrin-drug complex. Isotretinoin is highly lipophilic drug and its water solubility is very low (1.1 ± 0.2 μg/ml) that contribute to its poor bioavailability and poor incorporation in topical vehicle. Solubility of isotretinoin by cyclodextrin complexation was found to increase nine folds (9.2 ± 1.1 μg/ml) and this shall be helpful for its inclusion in topical vehicle. Similar increase in isotretinoin solubility by formation of cyclodextrin inclusion complex was reported in literature by many authors (18,27,28).
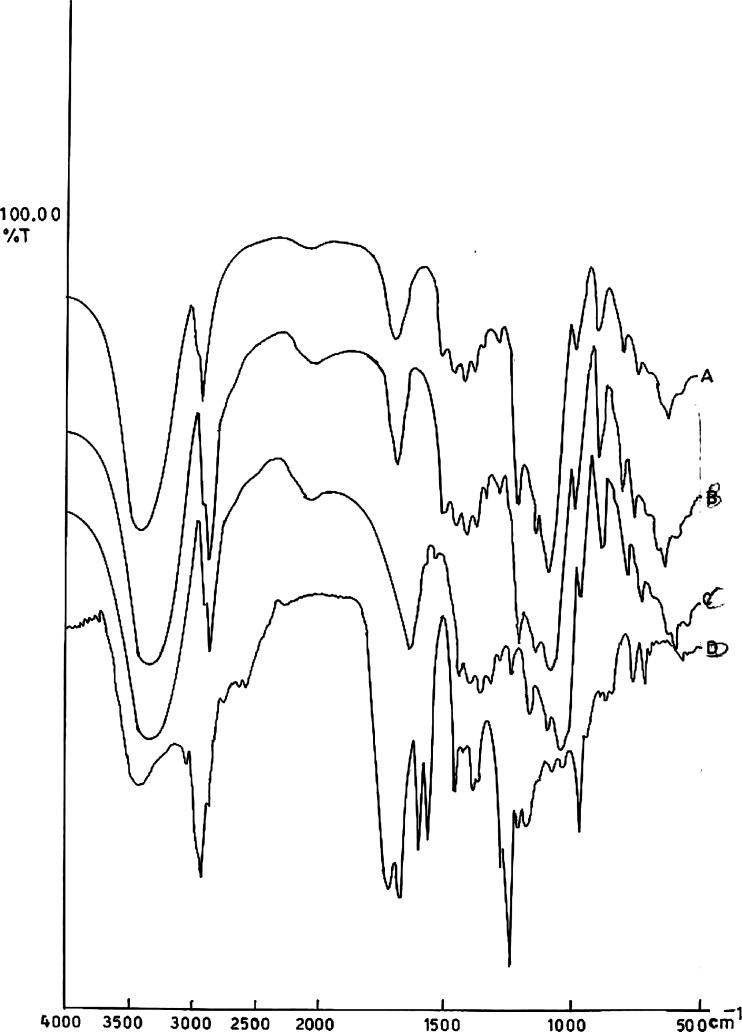
Figure 1a-d shows the IR spectra of drug (a), physical mixture of cyclodextrin and drug (b), cyclodextrin (c), and cyclodextrin-drug complex (d), respectively. Isotretinoin is characterized by peak appearing between 1,700 and 1,500 cm−1 that is corresponding to C = O stretching vibration, whereas the spectra of cyclodextrin is characterized by band at 3,600-3,000 cm−1 due to O-H stretching vibration (18). In the physical mixture, the spectra are the superposition of those of pure products with attenuation of the isotretinoin peak. For the complex, the isotretinoin peak mostly disappears and the excess of free cyclodextrin is still visible (Fig. 1d). These results well-correlated with previous reports (18,28).
Fig. 1.
IR spectra of a Isotretinoin b Physical mixture of isotretinoin and HP-β-CD c HP-β-CD and d isotretinoin-HP-β-CD complex
In Vitro Characterizations
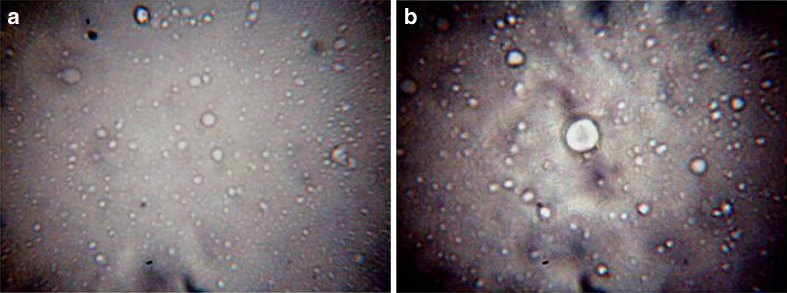
Figure 2a, b shows the optical microscopy photomicrographs of isotretinoin and isotretinoin-cyclodextrin complex-loaded elastic liposomal formulation. Elastic liposomes appeared as spherical shape vesicular structure and isotretinoin-cyclodextrin-loaded elastic liposomes also showed the similar structure. This indicates that isotretinoin-cyclodextrin complex successfully encapsulated in elastic liposomes and did not affect the normal morphology of vesicles. Vesicle size of elastic liposomes formulation before sonication was found between 5.2 ± 0.3 and 6.2 ± 0.5 μm and after sonication it was between 100 and 200 nm. No significant difference (p < 0.05) was found in no. of vesicles per cubic millimeter of isotretinoin elastic liposomes and isotretinoin-cyclodextrin elastic liposomes formulations (Table I).
Fig. 2.
Optical microscopy photomicrograph of isotretinoin elastic liposomes (a ×450) and isotretinoin-cyclodextrin elastic liposomes formulation (b ×450)
To optimize the maximum amount of drug that can be entrapped in elastic liposomal formulation, increasing amount of isotretinoin ranging from 10 to 20 mg was added in the vesicular formulations. The optical microscopy observation showed that no drug crystals were observed in 10 mg formulation and increasing amount of drug (15-20 mg) lead to precipitation. Maximum amount of drug that could be incorporated in formulations was found to be 10 mg (Table II). The entrapment efficiency data shows that elastic liposomal formulation prepared with 10 mg drug have maximum entrapment efficiency (81.2 ± 7.3%). The entrapment efficiency in case of inclusion complex of cyclodextrin with isotretinoin was found 90.1 ± 8.3%. The reason behind the increase in entrapment efficiency with cyclodextrin complexation is due to better retention of drug-cyclodextrin complex in vesicle bilayers.
Table II.
Optimization of Isotretinoin Loading In Elastic Liposomal Formulation
| Formulation Code | Amount of drug (mg) | Appearance of drug crystal | Entrapment efficiency |
|---|---|---|---|
| ELP10 | 10 | Not appeared | 81.2 ± 7.3 |
| ELP15 | 15 | Appeared | 75.6 ± 6.4 |
| ELP20 | 20 | Appeared | 62.1 ± 4.3 |
Values represented as mean ± SD (n = 3)
Skin Permeation and Deposition Studies
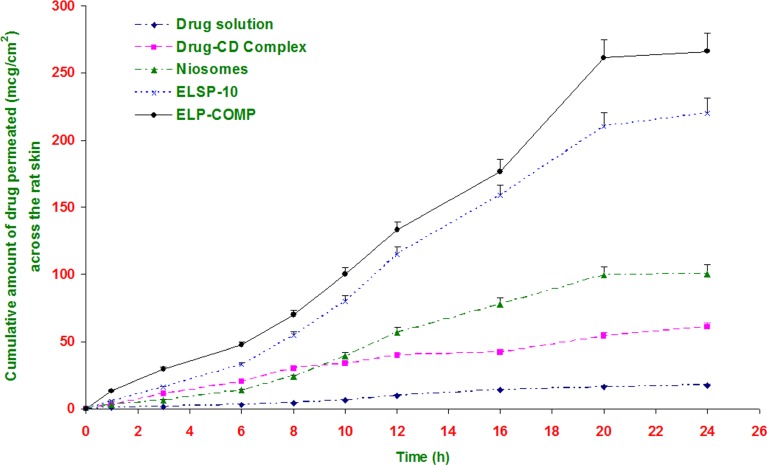
Figure 3 shows the cumulative amount of isotretinoin permeated across the rat skin as a function of time from different formulations. The in vitro skin permeation of drug is mainly assessed by its flux, Jss (µg/cm2/h). This was calculated from the slope of the linear steady portion of cumulative amount release vs. time (h) plot. The value of transdermal flux for different formulations is reported in Table III. The isotretinoin solution in propylene glycol/ethanol (75:25 v/v) mixture was used as a control to evaluate the skin-targeting ability of the elastic liposomal formulations.
Fig. 3.
Cumulative amount of isotretinoin permeated across rat epidermal sheet from different formulations. All values shown as mean ± SD (n = 3)
Table III.
Transdermal Permeation Parameters of Different Isotretinoin Formulations Across the Rat Skin
| Formulation code | Jss (µg/cm2/h) | De (µg) | % Drug deposition | ER | ER |
|---|---|---|---|---|---|
| ELSP10 | 10.5 ± 0.9 | 160.6 ± 9.0 | 16.1 | 12.9 | 15.9 |
| ELSP15 | 10.7 ± 1.2 | 166.2 ± 10 | 8.3 | 6.7 | 16.2 |
| ELSP20 | 10.9 ± 1.2 | 172.1 ± 14 | 8.6 | 6.9 | 16.5 |
| ELP-COMP | 13.9 ± 1.6 | 262.2 ± 21 | 26.2 | 21.1 | 21.0 |
| NIO | 4.9 ± 0.5 | 68.7 ± 8.0 | 6.9 | 5.5 | 7.5 |
| CYD-COMP Solution | 2.7 ± 0.3 | 30.1 ± 40 | 3.0 | 2.4 | 4.1 |
| Drug Solution | 0.7 ± 0.1 | 12.4 ± 2.0 | 1.2 | - | - |
Values represented as mean ± SD (n = 3)
Jss transdermal flux, calculated from the slop of Cartesian plot of cumulative amount of drug present in receptor compartment vs. time; De amount of drug deposited in skin; ER enhancement ratio of drug deposition in skin; ER enhancement ratio of drug permeation in skin
Phosphate buffer saline of pH 6.8 or 7.4 are generally used as the receptor phase in skin permeation studies (12). Isotretinoin solubility in PBS is very low (1.2 ± 0.2 μg/ml) and could not provide the perfect sink condition. In such a condition, an aqueous phase containing co-solvents or solubilizers becomes necessary. To find a suitable receptor phase, preliminary permeation study with isotretinoin using three receptor phases Tween 20 PBS 6.8 solutions (0.5 and 1.0%, w/v) and PBS 6.8 alone. Results showed that both Tween 20 solutions increase the solubility of isotretinoin and provide a perfect sink condition (Table IV). To minimize the possible interactions of this nonionic surfactant with skin, the lower concentration (0.5% solution) was chosen as the receptor phase. Solubility of isotretinoin in this receptor phase was found 47.1 ± 3.8 μg/ml.
Table IV.
Quantitative Solubility Data of Isotretinoin
| Solvent | Solubility (μg/ml) |
|---|---|
| Distilled water | 1.1 ± 0.2 |
| Phosphate buffer saline (pH 6.8) | 1.2 ± 0.2 |
| Propylene glycol: ethanol (75/25) | 76.2 ± 2.4 |
| Drug-cyclodextrin complex in distilled water | 9.2 ± 1.1 |
| Tween 20: PBS (0.5% w/v) | 47.1 ± 3.8 |
| Tween 20: PBS (1.0% w/v) | 59.7 ± 4.2 |
Values represented as mean ± SD (n = 3)
The value of transdermal flux for different vesicular formulations was observed between 10.5 ± 0.5 and 13.9 ± 1.6 µg/cm2/h. This is about 15 to 21-folds higher than that obtained from drug solution (0.7 ± 0.1 µg/cm2/h) and threefolds higher than obtained with niosomal formulation. Elastic liposomal formulation containing cyclodextrin-drug complex showed significant increase (p < 0.05) in transdermal flux to 13.9 ± 1.6 µg/cm2/h indicating synergistic effect of combination of vesicular approach with cyclodextrin (Table III).
Skin retention study was carried out with the objective to determine the depot effect of elastic liposomes in the deeper layer of skin. For isotretinoin to be an effective anti-acne agent, it must deliver the drug to deeper layers of skin. Table III also compare the percent skin deposition of isotretinoin after the application with elastic liposomes, niosomes, and elastic liposomal formulation loaded with isotretinoin-cyclodextrin complex and drug solution with or without cyclodextrin. The amount of drug deposited was 21-folds higher in case of elastic liposomes of isotretinoin-cyclodextrin complex than drug solution. This showed better deposition and skin-targeting effect of elastic liposomal formulation. Higher skin deposition of elastic liposomes could be attributed to the difference in the mechanism of drug transport across the skin from vesicles and drug solution. In contrast to isotretinoin molecule, elastic liposome is too large to enter into the cutaneous blood circulation directly; locally, they bypass the cutaneous capillary bed and subcutaneous tissue and here they act as depot and sustain the drug release. The intradermal capillary plexus, allowing more of drug to reach the deep subcutaneous tissue, consequently clears the drug from the elastic liposomes less efficiently. In the case of drug solution, free drug movement occurs, allowing drug absorption by intradermal capillary plexus once it reaches into the dermis region of the skin and then into the systemic circulation (12,13,19). The higher drug deposition in case of elastic liposomes may be due to creation of reservoir of drug in deeper layer of skin. There are some similar results indicating that elastic liposomes can increase the uptake of drug in skin (14). In our previous work, the skin-targeting effect was proved by fluorescence microscopy (12) and confocal laser scanning microscopy study (15).
The clinical efficacy of a topically applied drug depends not only on pharmacological properties but also on the availability of drug at target site. Commercial available topical formulations of isotretinoin have limited skin permeation due to its highly hydrophobic nature, difficult to mix with vehicle, and degradation on application. Cyclodextrin is mainly used as permeation enhancer in pharmaceutical preparations. Result of skin permeation and deposition study showed that complexation of isotretinoin with cyclodextrin increases its skin deposition significantly (p < 0.05). Further, encapsulation of this complex in elastic liposomal formulation increases the skin deposition 21-folds (Table III). The observed significant (p < 0.05) higher skin deposition of HP-β-CD-loaded formulation was due to increase in solubility and partitioning, higher entrapment efficiency and sustain release of drug. In case of drug-cyclodextrin complex, size is again significantly higher. Molecular weight of isotretinoin is 300 and of cyclodextrin is 1,135 that may retard permeation and increase depot forming property of inclusion complex (29).
Photodegradation Study
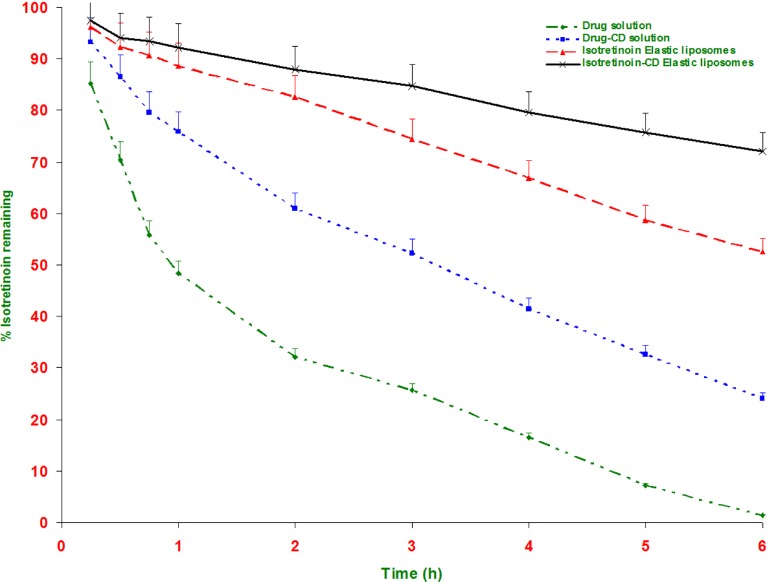
It is known that a large amount of isotretinoin, locally applied on skin surface, degrades when exposed to sunlight for 1-2 h (8). This will leads to loss of potency during storage or application (7), resulting in skin irritation and allergy (7,30). Vesicular formulations are known to provide the protection for photolabile drug. Figure 4 shows the result of photostability measurement of isotretinoin elastic liposomal formulation in comparison to plain isotretinoin solution. The isotretinoin decomposition was very fast in solution form and after 1 h of UV light exposure solution form showed 52.2% decomposition. In comparison, isotretinoin elastic liposomal formulation showed 11.4% and isotretinoin-cyclodextrin complex-loaded formulation showed only 7.8% degradation. After 6 h isotretinoin-elastic liposomes and isotretinoin-cyclodextrin elastic liposomal formulation showed 47.5% and 27.9% degradation; and in comparison, drug solution showed 98.5% degradation of isotretinoin. These results demonstrated the protective effect of elastic liposomal formulation due to encapsulation of drug. Further dual carrier approach provides significantly (p < 0.05) higher protection from photodegradation and only 27.9% degradation was observed. Cyclodextrin is well-reported for protection from photodegradation of isotretinoin due to formation of inclusion complex (27). Yap et al. (18) reported the molecular modelling of the isotretinoin-cyclodextrin complex and showed that the side chain of isotretinoin was included into the oligosaccharide ring of cyclodextrin, thus minimizing the photo degradation due to the stearic hindrance imposed by the CD cavity. Another reason for better photostability of isotretinoin-cyclodextrin complex loaded elastic liposomal formulation is significantly higher entrapment efficiency (90.1 ± 8.3%) in comparison to isotretinoin-elastic liposomal formulation (81.2 ± 7.3%). The relationship between the photodegradation rate versus the drug concentration has been well-reported (27,31).
Fig. 4.
Photodegradation curves of isotretinoin in different formulations as a function of time. All values shown as mean ± SD (n = 3)
Skin Irritation Study
Isotretinoin is known for causing skin irritation after topical application and this is the major disadvantage associated with topical isotretinoin therapy. Ideally, delivery vehicle should be able to minimize the skin irritation potential of isotretinoin. Presently, most of the commercially available topical dosage forms of isotretinoin such as cream, lotion, gels, and ointment are not able to reduce the skin irritation potential of isotretinoin (7). The skin irritation scores as defined by the code of federal regulation (32) following application of different formulation at 72 h are shown in Table V. The edema was almost absent in all the cases. The mild to well-defined erythema was however observed in case of plain drug, showing mean score of 2.3 after 24 h and 2.7 after 48 h of administration. The isotretinoin elastic liposomal formulation caused comparatively less irritation showing mean scores of 0.3 in comparison drug solution shows mean score of 1.3 after 72 h of exposure. The skin irritation study indicated that isotretinoin elastic liposomal formulation has less skin irritation in comparison to free drug. The decrease in the drug irritation is may be due to the encapsulation of isotretinoin in the lipid bilayer of elastic liposomes that reduces the contact of free drug to stratum corneum. It was reported that contact of acidic functional group (-COOH) of isotretinoin and stratum corneum are responsible for skin irritation of isotretinoin (33). Elastic liposomal formulation mainly consists of phospholipids that are known to improve the safety of their co-applied agent and likely to minimize the danger of skin irritation that may be induced by topical isotretinoin (7). National institute for occupational safety and health (34) interprets the score of 0-0.9 as non-irritant and safe for intact human skin contact, while scores of 1-1.9 is interpreted as mild irritant and requiring protective measure. Thus, the isotretinoin elastic liposomal formulation can be considered to be less irritating than free drug, although this conclusion should be confirmed by a larger sample size.
Table V.
Skin Irritation Score as Per Draize Method After Application of Different Isotretinoin Formulations
| Formulation | Erythema | Odema | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 48 h | 72 h | 1 h | 24 h | 48 h | 72 h | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drug solution | 2.3 | 2.7 | 2 | 1.3 | 0 | 1 | 0.7 | 0 |
| Drug-HP-β-CD solution | 0 | 1.33 | 1 | 0 | 0 | 0 | 0 | 0 |
| Elastic liposomes without drug | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Isotretinoin elastic liposomes | 0 | 0.7 | 0.5 | 0.3 | 0 | 0 | 0 | 0 |
| Isotretinoin-HP-β-CD elastic liposomes | 0 | 0.3 | 0.3 | 0 | 0 | 0 | 0 | 0 |
Values represented as mean (n = 3)
Score are define as 0 = no erythema, 1 = very slight erythema (light pink), 2 = well-defined erythema (dark pink), 3 = moderate to severe erythema (light red), 4 = severe erythema (extreme redness) similarly defined for edema
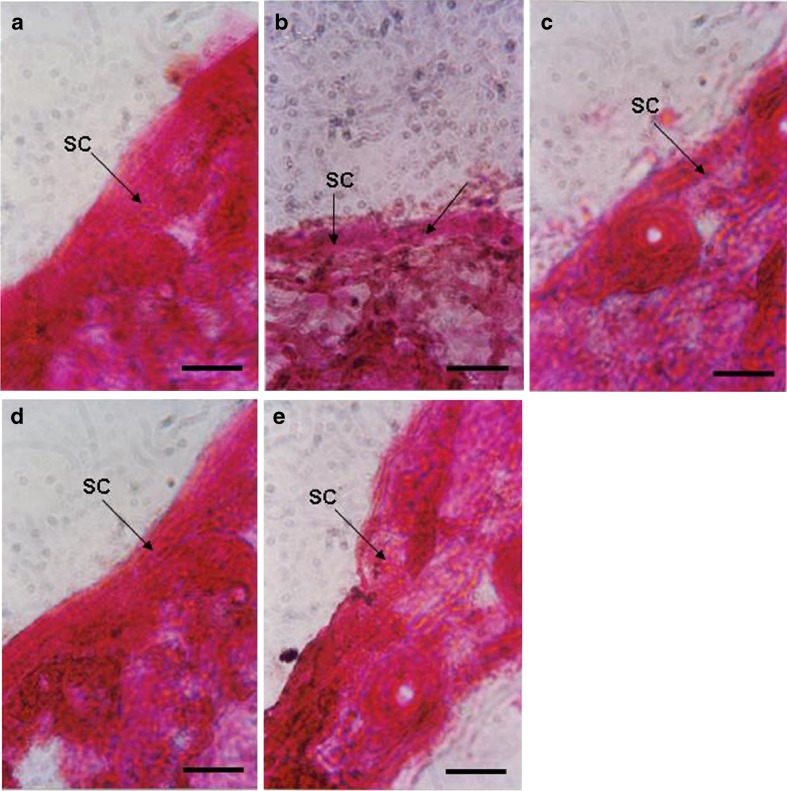
Figure 5a–e shows that eosin-hematoxylin stained photomicrograph of rat skin treated with solvent control, drug solution, drug-HP-β-CD complex solution, and isotretinoin and isotretinoin-HP-β-CD-loaded elastic liposomal formulation, respectively. Rat skin treated with isotretinoin solution showed partial damage of SC (indicated by arrow) and separation of lower layer due to skin irritation potential of isotretinoin. Some inflammatory cells were also observed. In comparison, skin treated with elastic liposomal formulations did not show any significant damage and photomicrograph was similar to the control. This is perhaps due to encapsulation of isotretinoin in elastic liposomal formulation that avoids the direct contact of drug with skin surface and release drug gradually at target site. These results are well in agreement with those reported by Shah et al. (11) that solid lipid nanoparticle-based formulation of tretinoin has less toxicity to skin as compared to free drug.
Fig. 5.
Eosin-hematoxylin stained photomicrograph of rat skin treated with a PBS (pH 6.5), ×450; b drug solution (×450); c drug-cyclodextrin complex solution (×450), d isotretinoin elastic liposomes, ×450; and e isotretinoin cyclodextrin elastic liposomes for 24 h. Scale bar 500 μM, SC stratum corneum
CONCLUSION
The results of the present study demonstrated that elastic liposomal formulation of isotretinoin could be easily made for its topical delivery due to its skin targeting potential and easy preparation method. Dual carrier approach isotretinoin in CD in elastic liposomes was found to increase the skin deposition 26-folds in comparison to drug solution. The encapsulation of isotretinoin-CD complex in elastic liposomes also found to dramatically increase its photostability. Only 27.9% degradation was observed after 6 h of exposure in case of elastic liposomal formulation in comparison 98.5% degradation was observed with drug solution. Skin histopathology study also showed the significant reduction in skin toxicity of isotretinoin after encapsulation in elastic liposomes. The encapsulation of isotretinoin in elastic liposomes was found to dramatically increase its photostability, skin targeting, and decrease its skin irritation. These are the major drawbacks associated with currently used commercial formulations. This formulation seems to represent an attractive strategy for topical delivery of isotretinoin.
ACKNOWLEDGMENT
The authors are grateful to the Director, Electron Microscopy Section, AIIMS, New Delhi, India for providing the facilities for transmission electron microscopy study.
REFERENCES
- 1.Ward A, Brogden RN, Heel RC, Speight TM, Avery GS. Isotretinoin. A review of its pharmacological properties and therapeutic efficacy in acne and other skin disorders. Drugs. 1984;28:6–37. doi: 10.2165/00003495-198428010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Aass N, De Mulder PH, Mickisch GH, Mulders P, van Oosterom AT, van Poppel H, Fossa SD, de Prijck L, Sylvester RJ. Randomized phase II/III trial of interferon Alfa-2a with and without 13-cis-retinoic acid in patients with progressive metastatic renal cell carcinoma: the European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group (EORTC 30951) J Clin Oncol. 2005;23:4172–4178. doi: 10.1200/JCO.2005.07.114. [DOI] [PubMed] [Google Scholar]
- 3.McLane J. Analysis of common side effects of isotretinoin. J Am Acad Dermatol. 2001;45:S187–194. doi: 10.1067/mjd.2001.113719. [DOI] [PubMed] [Google Scholar]
- 4.Gollnick H, Ehlert R, Rinck G, Orfanos CE. Retinoids: an overview of pharmacokinetics and therapeutic value. Methods Enzymol. 1990;190:291–304. doi: 10.1016/0076-6879(90)90034-X. [DOI] [PubMed] [Google Scholar]
- 5.Peinni C, Vigolti M. Drug and cosmetics in relation to the topical treatment of acne: data from a nationwide enquiry. Cosmet Dermatol. 1991;2:17–26. [Google Scholar]
- 6.Layton AM, Cunlife WJ. Guidelines for optimal use of isotretinoin in acne. J Am Acad Dermatol. 1992;27:S2–7. doi: 10.1016/S0190-9622(08)80252-6. [DOI] [PubMed] [Google Scholar]
- 7.Elbaum DJ. Comparison of the stability of topical isotretinoin and topical tretinoin and their efficacy in acne. J Am Acad Dermatol. 1988;19:486–491. doi: 10.1016/S0190-9622(88)70202-9. [DOI] [PubMed] [Google Scholar]
- 8.Lehman PA, John JT, Franz TJ. Percutaneous absorption of retinoids: influence of vehicle, light exposure and dose. J Invest Dermatol. 1990;91:56–61. doi: 10.1111/1523-1747.ep12463289. [DOI] [PubMed] [Google Scholar]
- 9.Masini V, Bonte F, Meybeck A, Wepierre J. Cutaneous bioavailability in hairless rats of tretinoin in liposomes or gel. J Pharm Sci. 1990;82:17–21. doi: 10.1002/jps.2600820104. [DOI] [PubMed] [Google Scholar]
- 10.Manconi M, Sinico C, Valenti D, Loy G, Fadda AM. Niosomes as carrier for tretinoin I. Preparation and properties. Int J Pharm. 2002;234:237–248. doi: 10.1016/S0378-5173(01)00971-1. [DOI] [PubMed] [Google Scholar]
- 11.Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticle (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007;345:163–171. doi: 10.1016/j.ijpharm.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29:1013–1026. doi: 10.1081/DDC-120025458. [DOI] [PubMed] [Google Scholar]
- 13.Jain S, Jain N, Bhadra D, Tiwary AK, Jain NK. Transdermal delivery of an analgesic agent using elastic liposomes: preparation, characterization and performance evaluation. Curr Drug Deliv. 2005;2:223–233. doi: 10.2174/1567201054368020. [DOI] [PubMed] [Google Scholar]
- 14.Garg T, Jain S, Singh HP, Sharma S, Tiwary AK. Elastic liposomal formulation for sustained delivery of anti-migraine drug: In vitro characterization and biological evaluation. Drug Dev Ind Pharm. 2008;34(10):1100–1110. doi: 10.1080/03639040801965079. [DOI] [PubMed] [Google Scholar]
- 15.Singh HP, Utreja P, Tiwary AK, Jain S. Elastic liposomal formulation for sustained delivery of colchicine: in vitro characterization and in vivo evaluation of anti-gout activity. AAPS J. 2009;11(1):54–64. doi: 10.1208/s12248-008-9078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cal K, Centkowska K. Use of cyclodextrins in topical formulations: practical aspects. Eur J Pharm Biopharm. 2008;68:467–478. doi: 10.1016/j.ejpb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Maestrelli F, González-Rodríguez ML, Rabasco AM, Mura P. Effect of preparation technique on the properties of liposomes encapsulating ketoprofen–cyclodextrin complexes aimed for transdermal delivery. Int J Pharm. 2006;312:53–60. doi: 10.1016/j.ijpharm.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Yap KL, Liu X, Thenmozhiyal JC, Ho PC. Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexs by phase solubility, photostability, physicochemical and computational analysis. Eur J Pharm Sci. 2005;25:49–56. doi: 10.1016/j.ejps.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Cevc G, Blume G, Schatzlein A. Transfersomes mediated transepidermal delivery improves the regiospecificity and biological activity of corticosteroids in vivo. J Control Release. 1997;45:211–226. doi: 10.1016/S0168-3659(96)01566-0. [DOI] [Google Scholar]
- 20.Tashtoush BM, Jacobson EL, Jacobson MK. A rapid HPLC method for simultaneous determination of tretinoin and isotretinoin in dermatological formulations. J Pharm Biomed Anal. 2007;43:859–864. doi: 10.1016/j.jpba.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Draize JH, Woodward G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;8:377–390. [Google Scholar]
- 22.Kaur K, Jain S, Sapra B, Tiwary AK. Niosomal gel for site specific sustained delivery of anti-arthritic drug: in vitro in vivo evaluation. Curr Drug Deliv. 2007;4(4):276–282. doi: 10.2174/156720107782151250. [DOI] [PubMed] [Google Scholar]
- 23.El Maghraby GMM, Williams AC, Barry BW. Skin delivery from ultradeformable liposomes: refinement of surfactant concentration. J Pharm Pharmacol. 1999;51:1123–1134. doi: 10.1211/0022357991776813. [DOI] [PubMed] [Google Scholar]
- 24.Jain S, Tiwary AK, Jain NK. Sustained and targeted delivery of an anti-HIV agent using elastic liposomal formulation: mechanism of action. Curr Drug Deliv. 2006;3(2):157–166. doi: 10.2174/156720106776359221. [DOI] [PubMed] [Google Scholar]
- 25.Williams AC, Shatri SR, Barry BW. Transdermal permeation modulation by cyclodextrins: a mechanistic study. Pharm Dev Technol. 1998;3:283–296. doi: 10.3109/10837459809009856. [DOI] [PubMed] [Google Scholar]
- 26.Rowe RC, Sheskey PJ, Owen SC (2006) Handbook of pharmaceutical excipients, 5th ed., American Pharmaceutical Association. 217–21.
- 27.Caddeo C, Manconi M, Valenti D, Pini E, Sinico C. Photostability and solubility improvement of β-cyclodextrin-included tretinoin. J Incl Phenom Macrocycl Chem. 2007;59:293–300. doi: 10.1007/s10847-007-9326-z. [DOI] [Google Scholar]
- 28.Montassier P, Duchene D, Poieman MC. Inclusion complex of tretinoin with cyclodextrins. Int J Pharm. 1997;153:199–209. doi: 10.1016/S0378-5173(97)00104-X. [DOI] [Google Scholar]
- 29.Dalmora ME, Dalmora SL, Oliveira AG. Inclusion complex of piroxicam with beta-cyclodextrin and incorporation in cationic microemulsion. In vitro drug release and in vivo topical anti-inflammatory effect. Int J Pharm. 2001;222:45–55. doi: 10.1016/S0378-5173(01)00692-5. [DOI] [PubMed] [Google Scholar]
- 30.Dromgoole SH, Maibach HI. Sunscreening agent intolerance: contact and photocontact sensitization and contact urticaria. J Am Acad Dermatol. 1990;22:1068–1078. doi: 10.1016/0190-9622(90)70154-A. [DOI] [PubMed] [Google Scholar]
- 31.Tan X, Meltzer N, Lindenbaum S. Determination of the kinetics of degradation of 13-cis retinoic acid and all-trans retinoic acid in solution. J Pharm Biomed Anal. 1993;11:817–822. doi: 10.1016/0731-7085(93)80074-B. [DOI] [PubMed] [Google Scholar]
- 32.Code of Federal Regulation (1976) Title 16, Part 173.210.
- 33.Yamaguchi Y, Nagasawa T, Nakamura N, Takenaga M, Mizoguchi M, Kawai S, Mizushima Y, Igarashi R. Successful treatment of photo-damaged skin of nano-scale at RA particles using a novel transdermal delivery. J Control Release. 2005;104:29–40. doi: 10.1016/j.jconrel.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Campbell KI, George EL, Hall LL, Stara JF. Dermal irritancy of metal compounds. Studies with palladium, platinum, lead, and manganese compounds. Arch Enviorn Health. 1975;30:168–170. doi: 10.1080/00039896.1975.10666669. [DOI] [PubMed] [Google Scholar]