Abstract
Δ9-Tetrahydrocannabinol hemisuccinate (THC-HS), an ester prodrug of Δ9-tetrahydrocannabinol (THC) has been investigated for its potential to form inclusion complexes with modified synthetic beta-cyclodextrins (CDs). Phase solubility studies were performed to determine the stoichiometric ratio of complexation of THC-HS with random methylated beta-cyclodextrin (RAMEB) and 2-hydroxypropyl beta-cyclodextrin (HPBCD). THC-HS/RAMEB and THC-HS/HPBCD solid systems were prepared by lyophilization and the lyophilized complexes were characterized by Fourier transform infrared (FT-IR) spectroscopy, proton nuclear magnetic spectroscopy, and molecular modeling techniques. The formation of inclusion complexes of THC-HS/RAMEB and THC-HS/HPBCD was demonstrated by an AL type curve with the slopes less than unity by the phase solubility method. The association constants for THC-HS/RAMEB and THC-HS/HPBCD were found to be 562.48 and 238.83 M−1, respectively. The stoichiometry of both of the complexes was found to be 1:1 as determined from the Job's plot. This was confirmed by 1H NMR and FT-IR techniques. The results obtained from the molecular modeling studies were in accordance with the data obtained from nuclear magnetic resonance and FT-IR. The docking studies revealed the most probable mode of binding of THC-HS with RAMEB in which the alkyl chain was submerged in the hydrophobic pocket of the CD molecule and hydrogen bonding interactions were observed between the hemisuccinate ester side chain of THC-HS and the rim hydroxy groups of RAMEB. The solubility of THC-HS was significantly higher in RAMEB compared to HPBCD. Solid dispersions of THC-HS with CDs will be further utilized to develop oral formulations of THC-HS with enhanced bioavailability.
Key words: Δ9-tetrahydrocannabinol hemisuccinate, job's plot, lyophilization, molecular modeling, random methylated beta-cyclodextrin
INTRODUCTION
Δ9-Tetrahydrocannabinol (THC) is the primary active ingredient of the plant Cannabis sativa (marijuana) and is responsible for the majority of the pharmacological effects (1). While THC in marijuana is mainly known for its abuse potential, it also exhibits therapeutic effects in the treatment of nausea and vomiting during cancer chemotherapy, in appetite stimulation, cachexia associated with AIDS, glaucoma, analgesia, and other indications (2–6). To date, the most promising clinical applications approved by the Food and Drug Administration (FDA) are for the control of nausea and vomiting associated with chemotherapy and for appetite stimulation of AIDS patients suffering from anorexia and wasting syndrome. It is significant to note that the only dosage form currently approved by FDA is an oral, soft gelatin capsule (e.g., Marinol®). In addition, orally administered THC has shown slow and variable absorption due to low oral solubility/permeability and first-pass metabolism (6). The alternative routes of administration such as inhalation/smoking and parenteral delivery of THC have demonstrated more consistent and higher plasma drug levels; however, these methods are not desirable as they bear the potential of drug abuse. These limitations of the alternative routes of administration underline the need to explore various drug delivery strategies for oral administration of THC. The recent advances in drug delivery technologies make oral administration an attractive approach to redevelop THC formulations without its earlier limitations. A variety of formulation approaches under the consortium of supersaturating delivery systems for intestinal absorption are being applied to solubilize and maintain the supersaturation of water-insoluble drugs for a time period sufficient for absorption (7). They include solubilized formulations such as cosolvent systems, lipid-based formulations as well as high-energy solids like amorphous, crystalline salt forms, cocrystals, and prodrugs.
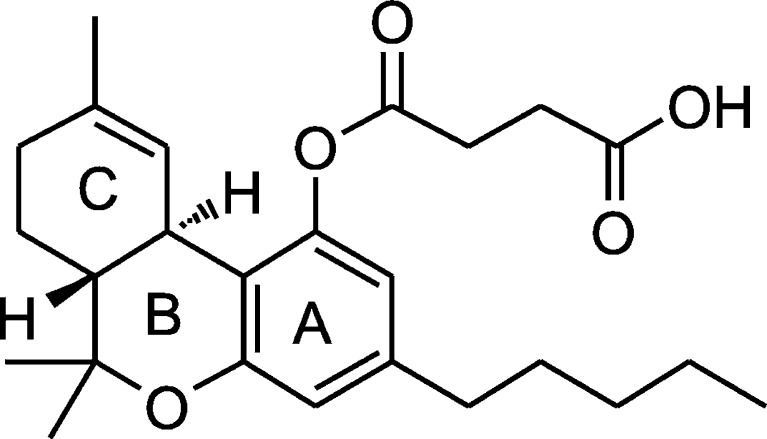
Our strategy towards developing THC oral formulations uses a two-pronged approach: (a) development of a new generation of hydrophilic prodrugs of THC with our collaborators at ElSohly Laboratories Inc. (use of high-energy solids) and (b) solubility enhancement and stabilization of prodrugs in oral formulations. The approach of synthesizing more hydrophilic prodrugs of the molecule has been successfully used in modifying the physicochemical properties of the parent drug without changing the core structure of the compound (8,9). Thus, a first-generation prodrug of THC, the hemisuccinate ester of THC (THC-HS; Fig. 1) was synthesized at ElSohly Laboratories Inc. It is a light-yellow, viscous liquid that is sticky at room temperature and hardens upon refrigeration, suggesting that the glass transition temperature is below 25°C, thereby necessitating its storage under freezing conditions for stability purposes.
Fig. 1.
Structure of THC-HS
THC-HS has a molecular weight of 414.53 and logP of 3.33 (Moriguchi's method), is sparingly soluble in water, and mostly acidic with a pKa of 4.25 (ACD/Labs, Toronto, Ontario, Canada) (10,11). Indeed, the prodrug itself is not without problems; one of the main issues being its high hydrolytic potential.
The other problems associated with transmucosal oral delivery of THC-HS are: (a) high instability to heat and hydrolysis and prone to oxidation, (b) the poor solubility of the prodrug (better than the parent drug) making its absorption and dissolution rate limited, and (c) since the prodrug is sticky and resinous in nature, handling issues are a major concern in the formulation of the drug. Hence, suitable methods need to be employed to make it free-flowing. At this stage, a solubilization approach was adopted to enhance the solubility of THC-HS and maintain a higher concentration in the gastrointestinal tract (GIT) in order to enhance absorption. Earlier, attempts have been made by Repka et al. to enhance the solubility and stability of THC-HS using different polymers, solubilizers, antioxidants, and pH modifiers (10–13). These solubilization methods were only moderately successful in increasing the solubility of THC-HS, warranting a better solubilization strategy. One of the most commonly explored technologies to enhance the solubility and, in turn, the absorption of water-insoluble drug molecules from the GIT is the use of cyclodextrin complexation of drugs.
Cyclodextrins (CDs) are cyclic oligosaccharides composed of six to eight dextrose units (alpha-, beta-, and gamma-CDs, respectively) joined through one to four bonds. Because the interior of these molecules is relatively hydrophobic and the exterior relatively hydrophilic, they tend to form inclusion complexes of the type shown in Fig. 2. The synthetically modified CDs such as random methylated beta-cyclodextrin (RAMEB), 2-hydroxypropyl beta-cyclodextrin (HPBCD), sulfobutyl ether beta-cyclodextrin (SBECD) have better solubility and excellent safety profiles compared to the parent CDs. They have been used in parenteral, oral, transdermal, and ocular formulations (14–19) and approved by regulatory agencies in the USA and Europe (20).
Fig. 2.
Equilibrium binding of a drug with a CD to form a 1:1 inclusion complex
By the virtue of being able to form inclusion complexes with the drugs, CDs are known to enhance the solubility and protect the ester linkage of the compounds from hydrolysis (21). A vast amount of literature is published supporting the enhancement of bioavailability for oral formulations (22–27) due to the inclusion complex formation with CDs. However, in very few cases, the absorption was hindered due to CD binding to the free drug and thus reducing its free concentration (28). The drugs can also be lyophilized with the modified CDs to yield free-flowing powders. As a result, it was determined worthwhile to investigate these advantages offered by CDs to develop an oral formulation of THC-HS. The range of effects produced by CD complexation on the absorption of water-insoluble molecules is variable due to the chemical nature of the drugs, dose, and drug/CD ratio. Hence, it is difficult to predict a priori or create a generalized model of efficacy of CD with every drug molecule. Yet, it is important to understand the physical and chemical interactions of CDs with the individual drug molecule to predict the efficacy of the complexation process on that particular drug. A number of methods have been reported in the literature to determine the nature of complexation between CD and the drug. Some of these methods include phase solubility studies, nuclear magnetic resonance (NMR), ultraviolet (UV) and Fourier transform infrared (FT-IR) spectroscopy, molecular modeling, and thermal techniques such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (29–31). As a first step towards the development of oral controlled formulations of THC-HS, we are reporting the preformulation studies of CD inclusion complexes with THC-HS. The objective of this work is to determine the feasibility of inclusion complex formation of THC-HS with two beta-CD derivatives to enhance the solubility of THC-HS. For this purpose, solid dispersions of THC-HS–beta-CD derivatives were prepared by different techniques (coevaporation, adsorption, and lyophilization) and characterized by proton NMR, FT-IR spectroscopy, Job's plot, and molecular modeling.
MATERIALS
The following chemicals were used as received: RAMEB and HPBCD were purchased from Sigma-Aldrich with a degree of substitution of 1.7 and 0.6, respectively. High-performance liquid chromatography (HPLC)-grade water was freshly prepared in the laboratory (by Nanopure Systems, Barnstead, Dubuque, IA). HPLC-grade acetonitrile and methanol were obtained from Fisher Scientific, Fair Lawn, NJ; THC-HS (in hexane) and THC (in absolute ethanol) were provided by ElSohly Laboratories Inc., Oxford, MS.
METHODS
For the preparation of the complexes, aqueous solutions of RAMEB/HPBCD and THC-HS were equilibrated for 24 h at 25°C (±2°C) under constant shaking at 100 rpm. The samples containing THC-HS and RAMEB/HPBCD were lyophilized at −50°C in a lyophilizer (Labconco Freeze Dry Systems/Freezone 2.5). The freeze-dried powders of the THC-HS–RAMEB/HPBCD complex were used for NMR and FT-IR studies.
Phase Solubility Studies
The phase solubility study was performed by employing a previously reported procedure by Higuchi (32). Briefly, THC-HS–RAMEB/THC-HS–HPBCD complexes in aqueous solutions were prepared by adding an excess amount of THC-HS to RAMEB/HPBCD solutions of different concentrations (10–200 mM). The suspensions were equilibrated for 24 h at 25°C (±2°C) at 100 rpm under constant shaking. After equilibration, undissolved THC-HS was removed from the suspensions by centrifugation. Intrinsic solubility (S0) of THC-HS in pure water was determined by following the same protocol, but without the addition of CDs. All of the samples were prepared in triplicate. The concentration of THC-HS in the inclusion complexes was determined by HPLC assay. Stability constants for the formation of inclusion complexes between THC-HS and RAMEB were determined from the phase solubility data using Eq. 1:
 |
1 |
NMR Spectroscopy
The 1H NMR spectra were recorded at 25°C (±2°C) on a Bruker 400 MHz spectrometer using a 5-mm NMR probe. Since the aqueous solubility of THC-HS is extremely low, the spectra had to be recorded in deuterated water/methanol (1:1). For proton NMR, 128 scans were recorded with the following parameters: 32 K data points, pulse width of 4.0 µs, and relaxation delay of 1 s. Digital zero filling to 64 K and a 0.5-Hz exponential were applied before Fourier transformation. 1H NMR chemical shifts variations (∆δ) were calculated according to the Eq. 2 (33,34):
 |
2 |
Job's Plot
The Job's plot of THC-HS was determined from 1H NMR and UV data, according to the continuous variation method (35). The NMR experiment was carried out with solutions of THC-HS and RAMEB/HPBCD in deuterated water/methanol (1:1). The total molar concentrations of THC-HS and RAMEB/HPBCD were kept constant at 20 mM, but their mole fractions were varied. The chemical shift in the proton signals due to the complex formation was plotted against the mole fraction of the two components. Solutions of the same composition, but in unbuffered water only, were used for UV spectrophotometric determination of the stoichiometry. In this case, the shift of λmax of the UV spectrum of THC-HS was plotted against the mole fraction of the two components. Spectra were obtained with a Shimadzu UV–Vis spectrophotometer.
Fourier Transform Infrared Spectroscopy
The FT-IR spectra were acquired for the lyophilized powders of THC-HS–RAMEB/HPBCD complexes (1:1). FT-IR spectra were recorded using a universal attenuated total reflection sampling accessory with a zinc selenide crystal on a Perkin-Elmer Spectrum 100 FT-IR spectrometer. The results were the means of three determinations. The physical mixtures of drug alone and RAMEB/HPBCD were used as blanks.
Molecular Modeling
Molecular modeling studies were carried out using the Maestro molecular modeling suite (Schrödinger Inc.). The three-dimensional structure of beta-CD was obtained from the protein complex of alpha-amylase (pdb id:1jl8.pdb) available from the protein data bank. The CD was then extracted and dimethylated using the Maestro molecular modeling suite (Schrödinger, LLC, New York, NY, 2005). Modified CD aka dimethyl beta-cyclodextrin (DIMEB) was then energy minimized to remove the steric hindrance and clashes that can appear in the building process due to addition of two methyl groups. DIMEB was adopted as a substitute for RAMEB to facilitate modeling studies (36). A similar protocol was used to generate the three-dimensional structure of HPBCD. The structure of THC and THC-HS were sketched and energy minimized using Maestro. Molecular docking experiments were carried out using the Glide (Glide, version 4.0, Schrödinger, LLC, New York, NY, 2005) module of the Schrödinger molecular modeling suite. Glide searches for favorable interactions between small molecules (ligand, THC-HS in this case) and a receptor molecule (DIMEB/HPBCD in this case). The flexible docking procedure using Glide automatically generates conformations for each input ligand. Each conformer generated is then positioned into the cavity of DIMEB/HPBCD to find the preferred binding geometries. The most likely conformation of the complex was the one with the lowest energy. The parent THC structure was also used in docking studies for comparison with THC-HS. The docked conformation of the THC and THC-HS were merged into the DIMEB and/or HPBCD. The resultant complexes were then minimized with the help of OPLS2002 force field using the Macromodel module of the Maestro molecular modeling suite. The minimized energy of the complex represents E(complex). The binding energy (∆E) upon complexation between THC-HS and DIMEB/HPBCD calculated for the minimum energy structure is defined in Eq. 3 (37):
 |
3 |
where E(complex) represents the minimized energy of the complex. ETHC-HS and ECD represent the energies of the guest (THC-HS) and host (CD), respectively, for the configuration extracted from the optimized complex geometry. The magnitude of the energy change would be a sign of the driving force towards complexation. The more negative the binding energy is, the more thermodynamically favorable is the inclusion complex.
RESULTS AND DISCUSSION
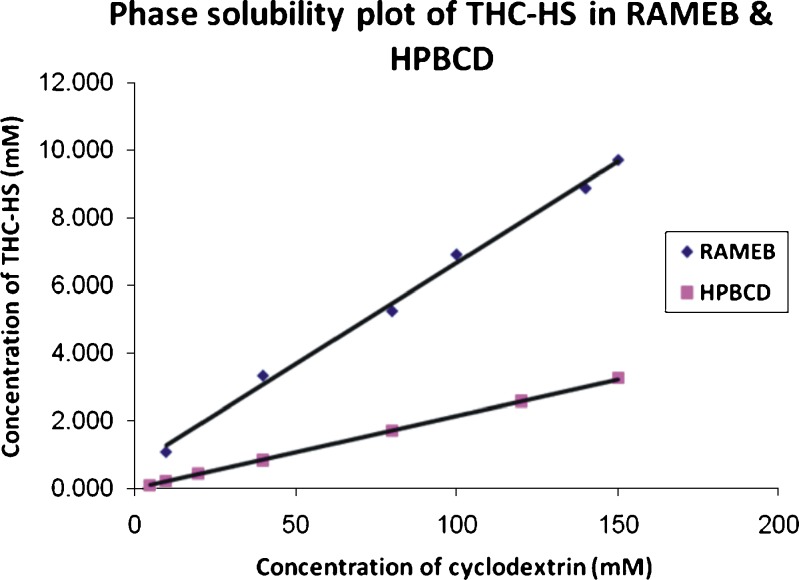
The apparent solubility of THC-HS increases linearly with increasing concentrations of RAMEB as well as HPBCD. The aqueous solubility of THC-HS was increased 100-fold from an intrinsic aqueous solubility of 0.092 to 9 mM, in the presence of 0.18 mM RAMEB. The phase solubility plot of THC-HS with RAMEB and HPBCD is shown in Fig. 3. The slope of the phase solubility diagram is smaller than unity over the entire concentration range studied, indicating an AL type diagram as seen in Fig. 3 with the formation of a complex with 1:1 stoichiometry. The calculated association constant (KS) from Table I for the THC-HS–RAMEB complex is 562.48 M−1, indicating that THC-HS–RAMEB complexes (1:1 molar ratio) are moderately stable. Actually, lower values of KS indicate a weak interaction between drug and CD, while higher values mean an incomplete drug release from the inclusion complex. The association constant with HPBCD (238.83 M−1) is lower than that obtained with RAMEB. The higher solubilization in the case of RAMEB is attributed to the presence of hydrophobic methyl groups which extend the hydrophobic cavity of the CD molecule. The values of the association constants of THC-HS indicate a moderately strong complex with RAMEB and a weak complex with HPBCD. The association constant for the parent THC molecule with RAMEB and HPBCD were determined to be 21,137.44 and 15,589.88 M−1, respectively. These values were in agreement with those reported by Manilla et al. (38). The higher association constants of CDs with THC are indicative of a tighter binding with the CDs and thus lower free drug concentration.
Fig. 3.
Phase solubility plot of THC-HS (RAMEB and HPBCD)
Table I.
Solubility and Association Constants of THC-HS and THC (in Millimolars) in RAMEB and HPBCD
| Compound | Solubility (mM) | K s 1:1 (M−1) | ||
|---|---|---|---|---|
| RAMEB (150 mM) | HPBCD (150 mM) | RAMEB | HPBCD | |
| THC-HS | 8.088 ± 0.65 | 3.24 ± 0.89 | 562.48 ± 5.2 | 238.83 ± 4.3 |
| THC | 5.63 ± 0.9 | 4.49 ± 1.2 | 21,137.44 ± 15.6 | 15,589.88 ± 23.0 |
NMR Spectroscopy
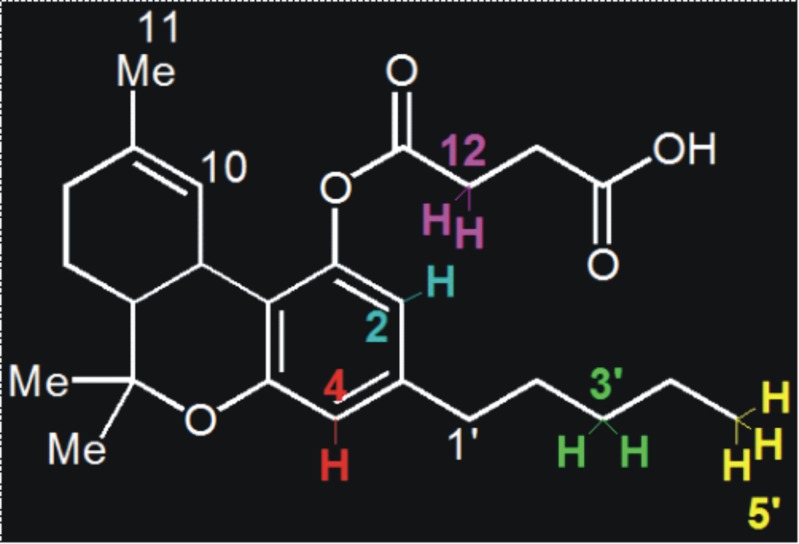
NMR studies with RAMEB are not without difficulty because any collection of modified beta-CD molecules is a highly complex mixture of different isomeric forms of variously substituted beta-CD derivatives. Hence, only several of the NMR signals for THC-HS could be unambiguously identified as shown in Fig. 4. The signals from RAMEB were inconclusive and could, therefore, not be interpreted with confidence. Therefore, the prediction of the orientation of THC-HS in the complex with RAMEB/HPBCD is a fair approximation using an array of different techniques to make definite conclusions. In 1H NMR, significant changes in chemical shifts of protons of the free acid group of the hemisuccinate side chain of THC-HS as well as the protons of the phytyl side chain and the aromatic ring of THC-HS were observed (the shifts in the carboxylic acid protons of the hemisuccinate ester side chain of THC-HS were observed when the NMR was determined in an aprotic solvent such as dimethyl sulfoxide). Especially the protons of the side chain were found to shift to the maximum extent followed by the carboxylic acid proton, indicating that the hydrogen bonding and van der Waals interactions are involved in the inclusion complex formation (39,40). It was predicted that the molecule could be entering the CD cavity with its aliphatic side chain inserted into the cavity followed by the aromatic ring. The largest chemical shift in signals of H2, H4, H3′, H5′, and H12 for THC-HS was observed, while that of H7, H8, and H11 remained unaffected (Fig. 4). These data indicate an inclusion of ring A and the alkyl side chain of THC-HS into the RAMEB cavity. This may be explained in that the alkyl side chain completely enters the CD cavity and protrudes from the opposite end, exposing H5′ to the solvent. Protons in ring C were mostly unaffected, indicating that the C ring might not be included in the hydrophobic cavity of RAMEB. This is also in good agreement with the observations of Hazekamp et al. (33) who reported non-inclusion of ring C of THC in RAMEB. Aromatic protons at H2 and H4 undergo an expected upfield shift upon inclusion in the complex. The hemisuccinate H12 proton exhibited a downfield shift possibly due to its interaction with the solvent. The relatively small ∆δ values observed for all signals indicate a relatively weak association.
Fig. 4.
Structure of THC-HS (protons showing significant changes in chemical shifts shown in different colors)
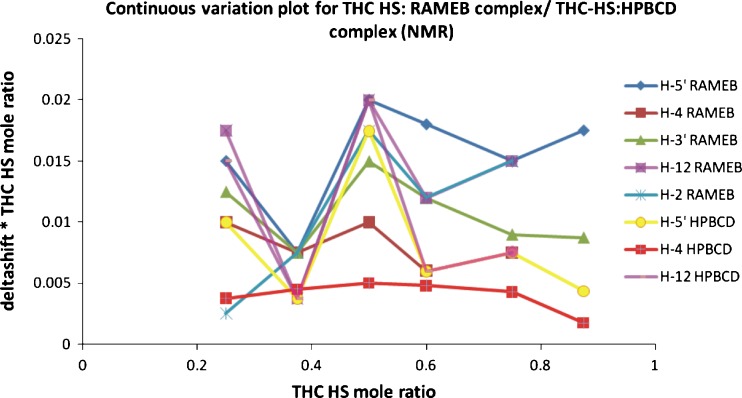
Job's Plot
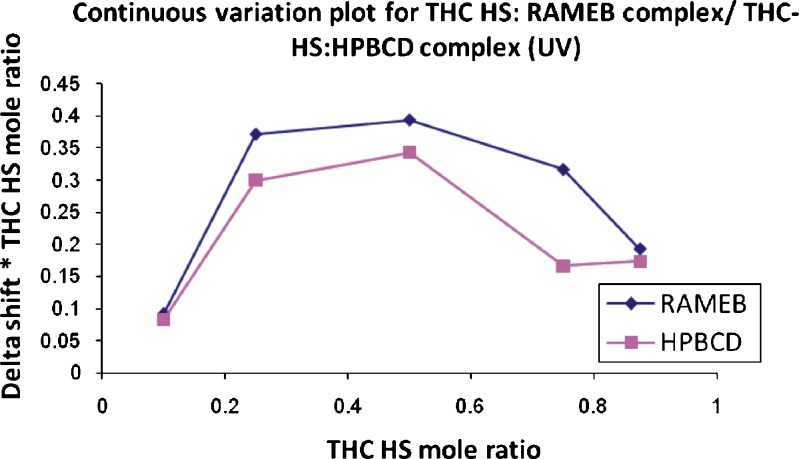
Several techniques such as DSC, IR, and UV–Vis spectroscopy can establish whether guest molecules form an inclusion complex with CDs; however, they cannot provide information about the structural configuration of the complex (41,42). In contrast, NMR is a technique that provides the most useful evidence for the inclusion of a guest into the hydrophobic CD cavity in solution. Two different techniques were used for the preparation of a Job's plot in order to determine the stoichiometry of the inclusion complex. Thus, the ratio of RAMEB/HPBCD and THC-HS was varied, while the sum of their concentrations was kept constant and a continuous variation plot was prepared. The chemical shift in the proton signals in NMR/shift of λmax due to the complex formation in UV spectroscopy was plotted against the mole fraction of the two components (Figs. 5 and 6). Using this method, the value for ∆δ reaches a maximum at the stoichiometric point. The results from UV as well as NMR spectroscopy yielded a 1:1 stoichiometry of THC-HS to RAMEB/HPBCD. In a linear 1:1 stable complex, the plot usually has a triangular form with a maximum, while the formation of weak complexes results in curved plots, indicating that the complex formed between THC-HS and RAMEB/HPBCD is not a strong one. The values obtained of association constants also suggest the same.
Fig. 5.
Job's plot (NMR spectroscopy)
Fig. 6.
Job's plot (UV spectroscopy)
Infrared Spectroscopy
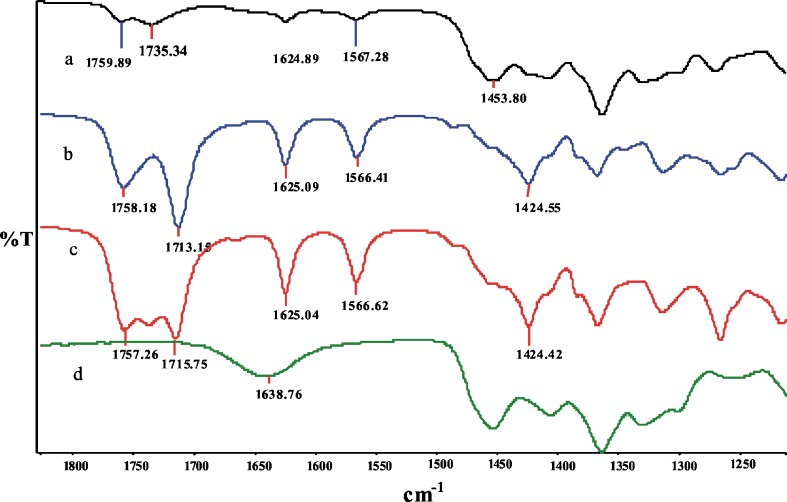
THC-HS was characterized by bands around 1,700–1,500 and 1,300–1,100 cm−1 (Fig. 7). The FT-IR spectra of CDs showed intense bands at 3,600–3,200 cm−1, corresponding to absorption by hydroxy groups. The bands that appeared at 3,000–2,800 cm−1 were assigned to stretching vibrations of the bonds in –CH and –CH2 groups. In THC-HS spectra, C=O stretching vibrations (1,758 and 1,713 cm−1) and –CH2 bending vibration (1,424 cm−1) were used to assess the interaction between RAMEB and the guest molecule (THC-HS) in the solid state. The spectra of the physical mixture as well as the lyophilized mixture did not show any new peaks, indicating that no new chemical bonds were created in the formed complex. In the physical mixtures, the spectra were the exact superposition of those of the pure compounds, except that the intensities of the peaks had reduced to some extent (Fig. 7). However, in the spectra of inclusion complexes, especially with RAMEB (Fig. 7), the bands arising from all of the major interactions like C=O stretching vibrations (1,758 and 1,713 cm−1) and –CH2 bending vibration (1,424 cm−1) have decreased in intensity and moved toward the right. This is likely due to the restrictions in the vibrations from hydrogen bonding during CD complexation. The bands located at 1,758 and 1,713 cm−1 are correlated to the stretching vibrations of the carbonyl group of the ester and the free carboxylic acid, respectively. In the lyophilized complex of THC-HS and RAMEB, the band from the free carboxylic acid at 1,713 cm−1 (shifted to 1,735 cm−1 in the lyophilized complex) has broadened and decreased in intensity due to the potential hydrogen bonding. The band from the ester carbonyl group did not show any shift in the wavelength upon complexation but was visible at a very low intensity, indicating that the hydrogen bonding interactions with RAMEB are primarily occurring at the free carboxylic acid end of the guest molecule (THC-HS). Another important interaction observed at 1,424 cm−1 is –CH2 bending vibrations. This band is also minimized in intensity as it relates to the hydrocarbon chain of THC-HS. Compared to RAMEB, the HPBCD inclusion complex of THC-HS did not show any significant interaction and the differences in the IR spectrum of the physical mixture and lyophilized complexes were too subtle to be identified. It is likely that the molecule enters the CD molecule with its hydrocarbon tail inserted into its hydrophobic cavity first with the hemisuccinate ester side chain in close proximity to the rim hydroxy groups of RAMEB. These results support the assumption that the inclusion complex was formed when the lyophilization was used in contrast to the physical mixture of THC-HS and RAMEB/HPBCD (Fig. 7). The FT-IR spectrum for the THC-HS–HPBCD complex could not be clearly deciphered due to the low binding affinity of THC-HS for HPBCD (data not shown).
Fig. 7.
FT-IR spectra of a lyophilized complex of THC-HS and RAMEB, b THC-HS, c physical mixture of THC-HS and RAMEB, and d RAMEB
Molecular Modeling
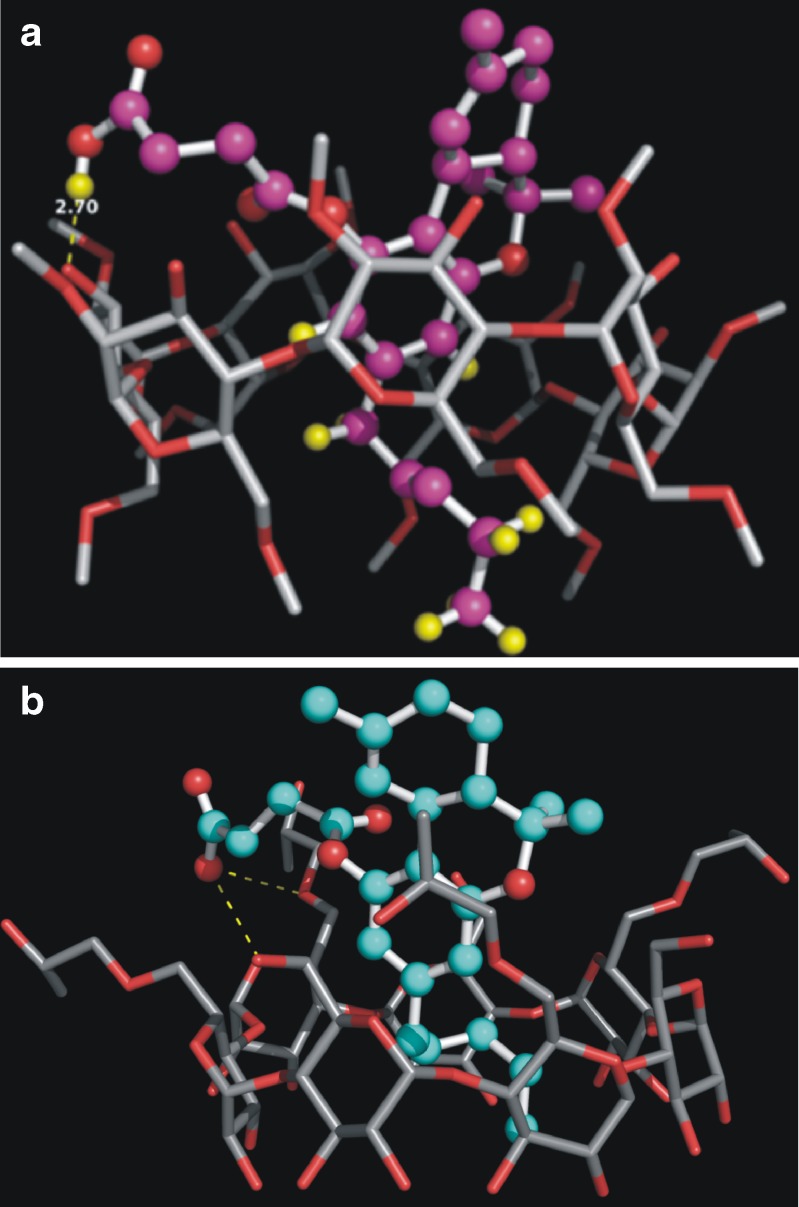
The docking studies indicated that the aromatic ring and the appended hydrophobic phytyl chain of the THC-HS molecule are oriented inside the cavity of DIMEB (Fig. 8a). From this figure, the tricyclic ring of THC-HS shows van der Waals interactions with the hydrophobic cavity of DIMEB. It is quite significant to note that no fixed constraints were imposed during the docking calculations and the docking results corroborated with the data obtained from NMR and FT-IR spectroscopy. The terminal carboxylic acid functional group of the hemisuccinate ester side chain is involved in hydrogen bonding interactions with one of the hydroxy groups on the surface of DIMEB. The NMR results demonstrated changes in the chemical shift values of a few protons, including the aromatic and the phytyl chain protons. As can be further seen from Fig. 8a, the protons shown in yellow are submerged inside the DIMEB cavity. Similarly, IR studies showed shifting of a carboxylic acid stretch due to hydrogen bonding interactions with one of the hydroxy groups of CD. The docking studies revealed that the carboxyl group of the hemisuccinate side chain lies within hydrogen bonding distance from the hydroxy of DIMEB (2.7 Å). The docking studies of THC-HS with HPBCD demonstrated a lower binding affinity of the ligand for HPBCD (Fig. 8b). In Table II, the binding energies, along with the van der Waals energies and the docking scores of the various inclusion complexes, of THC-HS and THC with DIMEB and HPBCD are provided. THC and THC-HS showed similar docking scores (−5.42 and −5.67, respectively). Hence, the binding energy calculations were performed to understand the stability of the two complexes. It is evident that the THC-HS showed better binding and thus complexation (−182.9 kJ/mol) with DIMEB compared to the parent molecule THC (−137.73 kJ/mol).
Fig. 8.
a Binding pose of THC-HS within the DIMEB cavity during the formation of the THC-HS–RAMEB complex, as predicted by the Glide docking program. b Binding pose of THC-HS within the HPBCD cavity during the formation of the THC-HS–HPBCD complex, as predicted by the Glide docking program (The protons shown in yellow show significant changes in proton NMR as well as docking studies)
Table II.
Binding Energies and Docking Scores of the Inclusion Complexes of THC-HS and THC
| Complex ID | Binding energy | van der Waal energy | Docking score |
|---|---|---|---|
| THC-HS–DIMEB | −182.9 | −66.69 | −5.42 |
| THC-HS–HPBCD | −125.23 | 40.43 | −2.96 |
| THC–DIMEB | −137.74 | −42.47 | −5.65 |
| THC–HPBCD | −125.07 | 40.43 | −3.33 |
The THC-HS–DIMEB complex shows lower binding energy and docking score compared to the THC-HS–HPBCD complex, suggesting that the latter is energetically less favored. This data is also supported by strong van der Waals interactions reflecting the snug fit of THC-HS obtained while complexation with DIMEB (−66.69 kJ/mol) compared to HPBCD (40.43 kJ/mol) (Table II) (43). When compared with THC, the binding energy of the THC–DIMEB complex (∆E = −137.74 kJ/mol) is significantly higher than that of the THC-HS–DIMEB complex (∆E = −182.9 kJ/mol). The molecular modeling results correlated very well with the phase solubility and NMR spectroscopic data in that RAMEB was able to better solubilize THC-HS compared to HPBCD. The best possible mode of binding of THC-HS with DIMEB is shown in Fig. 9. From this figure, it is very clear that the alkyl chain is embedded in the hydrophobic cavity of DIMEB, whereas the hemisuccinate ester side chain forms hydrogen bonding interactions with one of the surface hydroxyl groups of the DIMEB molecule.
Fig. 9.
Orientation of THC-HS molecule in the DIMEB cavity, the polar portion of THC-HS oriented outside the cavity, as predicted by the Glide docking program
CONCLUSION
Inclusion complexes of THC-HS with various modified CDs were prepared by lyophilization and characterized using several techniques such as phase solubility, NMR, FT-IR spectroscopy, and molecular modeling. The aqueous solubility of THC-HS was increased by almost 100-fold due to the inclusion complex formation with RAMEB. HPBCD was unable to form a stable complex with THC-HS and subsequently enhance its solubility in aqueous medium. The results obtained from phase solubility and Job's plot suggest the formation of a 1:1 inclusion complex of THC-HS and RAMEB. NMR and FT-IR spectroscopy were useful in the characterization of the structure of the inclusion complex formed. Higher association constants were obtained and, in turn, better solubilization of THC-HS was achieved by RAMEB compared to HPBCD. Our experimental data was well supported by molecular modeling studies suggesting energetically favorable inclusion complex formation of the THC-HS–RAMEB complex compared to the THC-HS–HPBCD complex. The complex formation of THC-HS with RAMEB and HPBCD predicted by molecular docking studies corroborated well with the results of NMR and FT-IR spectroscopy. CDs have aided in the solubilization of THC-HS in vitro and the solid dispersions of THC-HS and RAMEB will be further incorporated into solid dosage forms for the development of stable oral controlled-release THC formulations.
Acknowledgements
The work was supported by grant numbers P20RR021929 (NCRR/NIH) and 2R42GM067304-02 (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors also acknowledge the technical expertise provided by Mr. Pankaj Daga from the Department of Medicinal Chemistry for the molecular modeling studies.
References
- 1.Archer RA. The cannabinoids: therapeutic potentials. Annu Rep Med Chem. 1974;9:253–9. doi: 10.1016/S0065-7743(08)61448-7. [DOI] [PubMed] [Google Scholar]
- 2.Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003;139(4):258–66. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- 3.Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007;82(5):572–8. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- 4.Adam J, Cowley PM, Kiyoi T, Morrison AJ, Mort CJ. Recent progress in cannabinoid research. Prog Med Chem. 2006;44:207–329. doi: 10.1016/S0079-6468(05)44406-9. [DOI] [PubMed] [Google Scholar]
- 5.Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91(11):1585–614. doi: 10.1111/j.1360-0443.1996.tb02264.x. [DOI] [PubMed] [Google Scholar]
- 6.Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38(1):21–43. [PubMed] [Google Scholar]
- 7.Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72. doi: 10.1002/jps.21650. [DOI] [PubMed] [Google Scholar]
- 8.Anderson BD, Conradi RA, Knuth KE. Strategies in the design of solution-stable, water-soluble prodrugs I: a physical-organic approach to pro-moiety selection for 21-esters of corticosteroids. J Pharm Sci. 1985;74(4):365–74. doi: 10.1002/jps.2600740402. [DOI] [PubMed] [Google Scholar]
- 9.Aungst BJ, Blake JA, Rogers NJ, Saitoh H, Hussain MA, Ensinger CL, et al. Prodrugs to improve the oral bioavailability of a diacidic nonpeptide angiotensin II antagonist. Pharm Res. 1995;12(5):763–7. doi: 10.1023/A:1016228129729. [DOI] [PubMed] [Google Scholar]
- 10.Munjal M, Elsohly MA, Repka MA. Polymeric systems for amorphous delta9-tetrahydrocannabinol produced by a hot-melt method. Part II: effect of oxidation mechanisms and chemical interactions on stability. J Pharm Sci. 2006;95(11):2473–85. doi: 10.1002/jps.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munjal M, ElSohly MA, Repka MA. Chemical stabilization of a delta9-tetrahydrocannabinol prodrug in polymeric matrix systems produced by a hot-melt method: role of microenvironment pH. AAPS PharmSciTech. 2006;7(3):71. doi: 10.1208/pt070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munjal M, Stodghill SP, Elsohly MA, Repka MA. Polymeric systems for amorphous delta 9-tetrahydrocannabinol produced by a hot-melt method. Part I: chemical and thermal stability during processing. J Pharm Sci. 2006;95(8):1841–53. doi: 10.1002/jps.20667. [DOI] [PubMed] [Google Scholar]
- 13.Repka MA, ElSohly MA, Munjal M, Ross SA. Temperature stability and bioadhesive properties of delta9-tetrahydrocannabinol incorporated hydroxypropylcellulose polymer matrix systems. Drug Dev Ind Pharm. 2006;32(1):21–32. doi: 10.1080/03639040500387914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammar HO, Salama HA, El-Nahhas SA, Elmotasem H. Design and evaluation of chitosan films for transdermal delivery of glimepiride. Curr Drug Deliv. 2008;5(4):290–8. doi: 10.2174/156720108785915005. [DOI] [PubMed] [Google Scholar]
- 15.Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Implication of inclusion complexation of glimepiride in cyclodextrin-polymer systems on its dissolution, stability and therapeutic efficacy. Int J Pharm. 2006;320(1–2):53–7. doi: 10.1016/j.ijpharm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Angelova A, Ringard-Lefebvre C, Baszkin A. Drug-cyclodextrin association constants determined by surface tension and surface pressure measurements. J Colloid Interface Sci. 1999;212(2):275–9. doi: 10.1006/jcis.1999.6088. [DOI] [PubMed] [Google Scholar]
- 17.Arima H, Hagiwara Y, Hirayama F, Uekama K. Enhancement of antitumor effect of doxorubicin by its complexation with gamma-cyclodextrin in pegylated liposomes. J Drug Target. 2006;14(4):225–32. doi: 10.1080/10611860600711136. [DOI] [PubMed] [Google Scholar]
- 18.Arima H, Kondo T, Irie T, Uekama K. Enhanced rectal absorption and reduced local irritation of the anti-inflammatory drug ethyl 4-biphenylylacetate in rats by complexation with water-soluble beta-cyclodextrin derivatives and formulation as oleaginous suppository. J Pharm Sci. 1992;81(11):1119–25. doi: 10.1002/jps.2600811116. [DOI] [PubMed] [Google Scholar]
- 19.Bilensoy E, Rouf MA, Vural I, Sen M, Hincal AA. Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole:beta-cyclodextrin complex. AAPS PharmSciTech. 2006;7(2):E38. doi: 10.1208/pt070238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36(1):30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro JB, Chiaradia LD, Brandao TA, Magro JD, Yunes RA. Enzymatic hydrolysis of diloxanide furoate in the presence of beta-cyclodextrin and its methylated derivatives. Int J Pharm. 2003;267(1–2):93–100. doi: 10.1016/j.ijpharm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6(2):E329–57. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uekama K, Fujinaga T, Hirayama F, Otagiri M, Yamasaki M, Seo H, et al. Improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation. J Pharm Sci. 1983;72(11):1338–41. doi: 10.1002/jps.2600721125. [DOI] [PubMed] [Google Scholar]
- 24.Uekama K, Hirayama F, Yamada Y, Inaba K, Ikeda K. Improvements of dissolution characteristics and chemical stability of 16,16-dimethyl-trans-delta 2-prostaglandin E1 methyl ester by cyclodextrin complexation. J Pharm Sci. 1979;68(8):1059–60. doi: 10.1002/jps.2600680838. [DOI] [PubMed] [Google Scholar]
- 25.Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123(2):78–99. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Hirayama F, Minami K, Uekama K. In-vitro evaluation of biphenylyl acetic acid-beta-cyclodextrin conjugates as colon-targeting prodrugs: drug release behaviour in rat biological media. J Pharm Pharmacol. 1996;48(1):27–31. doi: 10.1111/j.2042-7158.1996.tb05871.x. [DOI] [PubMed] [Google Scholar]
- 27.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–25. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 28.Savolainen J, Jarvinen K, Taipale H, Jarho P, Loftsson T, Jarvinen T. Co-administration of a water-soluble polymer increases the usefulness of cyclodextrins in solid oral dosage forms. Pharm Res. 1998;15(11):1696–701. doi: 10.1023/A:1011900527021. [DOI] [PubMed] [Google Scholar]
- 29.Al-Marzouqi AH, Elwy HM, Shehadi I, Adem A. Physicochemical properties of antifungal drug–cyclodextrin complexes prepared by supercritical carbon dioxide and by conventional techniques. J Pharm Biomed Anal. 2009;49(2):227–33. doi: 10.1016/j.jpba.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Gillet A, Grammenos A, Compere P, Evrard B, Piel G. Development of a new topical system: drug-in-cyclodextrin-in-deformable liposome. Int J Pharm. 2009;380(1–2):174–80. doi: 10.1016/j.ijpharm.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Phillip Lee YH, Sathigari S, Jean Lin YJ, Ravis WR, Chadha G, Parsons DL, et al. Gefitinib–cyclodextrin inclusion complexes: physico-chemical characterization and dissolution studies. Drug Dev Ind Pharm. 2009;35(9):1113–20. doi: 10.1080/03639040902783074. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 33.Hazekamp A, Verpoorte R. Structure elucidation of the tetrahydrocannabinol complex with randomly methylated beta-cyclodextrin. Eur J Pharm Sci. 2006;29(5):340–7. doi: 10.1016/j.ejps.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Figueiras A, Sarraguca JM, Carvalho RA, Pais AA, Veiga FJ. Interaction of omeprazole with a methylated derivative of beta-cyclodextrin: phase solubility, NMR spectroscopy and molecular simulation. Pharm Res. 2007;24(2):377–89. doi: 10.1007/s11095-006-9161-8. [DOI] [PubMed] [Google Scholar]
- 35.Job P. Formation and stability of inorganic complexes in solution. Ann Chim. 1928;9:113–203. [Google Scholar]
- 36.Liu X, Lin HS, Thenmozhiyal JC, Chan SY, Ho PC. Inclusion of acitretin into cyclodextrins: phase solubility, photostability, and physicochemical characterization. J Pharm Sci. 2003;92(12):2449–57. doi: 10.1002/jps.10495. [DOI] [PubMed] [Google Scholar]
- 37.Barbiric DJ, Castro EA, De Rossi RH. A molecular mechanics study of 1:1 complexes between azobenzene derivatives and beta-cyclodextrin. J Mol Struct Theochem. 2000;532:171–81. doi: 10.1016/S0166-1280(00)00516-9. [DOI] [Google Scholar]
- 38.Mannila J, Jarvinen T, Jarvinen K, Tarvainen M, Jarho P. Effects of RM-beta-CD on sublingual bioavailability of delta9-tetrahydrocannabinol in rabbits. Eur J Pharm Sci. 2005;26(1):71–7. doi: 10.1016/j.ejps.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Bradshaw JS, Yi G, Pyo D, Black DR, Zimmerman SS, et al. Self-inclusion complexes derived from cyclodextrins: synthesis and characterization of 6A, 6B-Bis-O-[p-(allyloxy)phenyl]-substituted beta-cyclodextrins. J Org Chem. 1996;61(25):8949–55. doi: 10.1021/jo960679i. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann J, Kleinpeter E. 1H NMR spectroscopy as a probe of intermolecular interactions in β-cyclodextrin inclusion compounds. J Incl Phenom Macrocycl Chem. 1991;10(2):233. doi: 10.1007/BF01066207. [DOI] [Google Scholar]
- 41.Ali SM, Upadhyay SK. Complexation study of midazolam hydrochloride with beta-cyclodextrin: NMR spectroscopic study in solution. Magn Reson Chem. 2008;46(7):676–9. doi: 10.1002/mrc.2231. [DOI] [PubMed] [Google Scholar]
- 42.Cruz JR, Becker BA, Morris KF, Larive CK. NMR characterization of the host–guest inclusion complex between beta-cyclodextrin and doxepin. Magn Reson Chem. 2008;46(9):838–45. doi: 10.1002/mrc.2267. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Jeong K, Park H, Jung S. Preference prediction for the stable inclusion complexes between cyclodextrins and monocyclic insoluble chemicals based on Monte Carlo docking simulations. J Incl Phenom Macrocycl Chem. 2006;54(3–4):165–70. doi: 10.1007/s10847-005-6288-x. [DOI] [Google Scholar]