Abstract
Brucea javanica oil-loaded liposomes (BJOL) were prepared through thin film hydration method and characterized by transmission electron microscope, dynamic light scattering, and differential scanning calorimetry. Acute toxicity of B. javanica oil (BJO) in liposomes was assessed by determining the number of deaths of Kunming mice over intravenous treatment for 2 weeks. The pharmacokinetic behavior of the main active component (oleic acid) was studied in SD rats. The pharmacodynamics of BJOL was investigated using MMC-7721 cell lines and mice with Lewis lung cancer. The commercial emulsion of BJO (BJOE) was used as a reference. The data showed that BJOL had an average diameter of 108.2 nm with a zeta potential of −57.0 mV, drug loading of 3.60%, and entrapment efficiency of 92.40%. The area under curve of BJO in liposomes and emulsions were 2.31 and 1.15 mg min/ml, respectively. Compared with BJOE, mean residence time and elimination half-time (t1/2) increased 2.8- and 4.0-fold, respectively, and the clearance (CL) decreased 0.5-fold. In the acute toxicity test, the median lethal dose (LD50) of BJOE was 7.35 g/kg. In contrast, all mice treated with liposomes survived even at the highest dosage (12.70 g/kg). The IC50 value of BJOL group was one third of that of BJOE group (p < 0.01), and a less weight loss was observed in the BJOL-treated animals (p < 0.05). In conclusion, the present study suggests that BJOL significantly decreased toxicity of BJO and enhance the antitumor activity. Therefore, liposomes may be a potential effective delivery vehicle for this lipophilic antitumor drug.
KEY WORDS: antitumor, Brucea javanica oil (BJO), liposomes, pharmacodynamics, pharmacokinetics
INTRODUCTION
Brucea javanica oil (BJO), extracted from the nucleoli of B. javanica which widely distributes from Southeast Asia to northern Australia, has been used in treatment of various ailments including cancer, amoebic dysentery, and malaria. BJO is a complex mixture of fatty acids and fatty acid derivatives, and its main activity components are oleic acid and linoleic acid with content of 63.3% and 21.2%, respectively (1). The mechanisms of antitumor activity of BJO include inhibiting DNA polymerase activity (2), overcoming tumor multidrug resistance (3), and destructing cancer cell membrane system (4). It has been used clinically to treat hepatic, esophageal, rectal, pulmonic, renal, and prostatic carcinomas (5,6), commonly administered as BJO emulsion (BJOE) alone or in combination with chemotherapy. However, the long-term use of BJOE could induce phlebitis, sclerosis of blood vessels, hemangioma (7), anaphylactic response (8), and some irritation on muscles and blood vessels, which possibly resulted from the abundant surfactant required to stabilize the BJOE in the formulation and the administration of a comparatively high dose. Moreover, due to the thermodynamic instability of emulsions, BJOE easily delaminates, flocculates, and rancidifies during the storage.
Liposomes have been developed as effective drug carriers, particularly in anticancer therapy, due to their ability to deliver encapsulated drugs to specific target sites, provide sustained drug release (9,10), and protect encapsulated therapeutic agents. Compared to emulsions, less surfactants are used in the liposomes mainly consisting of phospholipids and cholesterol, which are biocompatible and biodegradable. Therefore, liposomes were chosen as a potential delivery system of BJO in antitumor therapy though intravenous administration.
The aim of the current study was to develop liposome formulation (B. javanica oil-loaded liposomes (BJOL)) by thin film hydration method with the objectives to optimize the pharmacokinetic profiles of BJO, enhance antitumor activity, and reduce toxicity of BJO associated with the emulsion formulation. Commercial product, BJOE was used as a control.
MATERIALS AND METHODS
Materials
BJO (63.3% oleic acid) and bruceolic oil emulsions were obtained from Mingxing Pharmaceutical Co. Ltd. (Guangzhou, China). Soybean phospholipid was purchased from Degussa Co. Ltd. (Germany). Cholesterol was supplied by Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Oleinic acid (9-octadecenoic acid) and daturic acid standards were purchased from Sigma–Aldrich Chemical Co. Ltd. (St. Louis, MO, USA). Cyclophosphamide (CTX) was obtained from Hengrui Pharmaceutical Co. Ltd. (Jiangsu, China). All reagents used were of analytical grade except methanol and acetonitrile of chromatographic grade.
Human hepatoma cell line SMMC-7721 and mice Lewis lung cancer cells were offered by Pharmacology Laboratories of China Pharmaceutical University (Nanjing, China). Fetal bovine serum was from Gibco (Grand Island, NY, USA).
Kunming mice (weight, 18–22 g; half male and half female) and male SD rats (200 ± 20 g) were purchased from Experimental Animal Center of China Pharmaceutical University. The animal studies were in the compliance of the university conduct and adhered to the principles of Institutional Animal Care and Use Committee Guidebook (http://en.wikipedia.org).
Preparation of BJO Liposomes
BJOL were prepared by thin film hydration method (11). Briefly, the lipid phase, a mixture of 8% (w/w) BJO, 80% (w/w) phospholipid, and 12% (w/w) cholesteryl, was dissolved in sufficient dehydrated alcohol by sonication (DL-720, Shanghai, China), then transferred to a round bottom flask and submitted to a rotary evaporator (RE501, Nanjing, China) at 50°C to remove the dehydrated alcohol. The film obtained from the flask wall was then hydrated by adding to phosphate buffer saline with a pH of 7.4 under vigorous mechanical shaking with a vortex mixer. After being hydrated thoroughly followed by being sonicated for 45 min, BJOL were obtained.
Quantification of BJO
Since oleic acid (9-octadecenoic acid) has been reported to be the main active component in BJO (12), it was taken as the essential component for determination of BJO. The compound has only ultraviolet (UV) end absorption; pre-column derivation was employed in combination with reversed-phase high-performance liquid chromatography (HPLC) (13–15).
Before analysis, Na2SO4 was added to liposome suspensions, and then heated for 30 min in an 80°C water-bath in order to demulsify the liposome suspensions. Then, 9-octadecenoic acid was extracted by adding a mixture of dimethyl carbinol-skellysolve C-glacial acetic acid (40:10:1, v/v/v) to the above-mentioned suspensions followed by 50 µl internal standard solution (1 mg/ml). Fifty-microliter ω-phenacyl bromide (20 mg/ml) and 50-µl triethanolamine (TEA, 25 mg/ml) were employed to connect a UV-absorption group to the molecular structure at 100°C. The resultant was dried by N2 and redissolved in 500-µl methyl alcohol. The supernate fluid was obtained and determined by reversed-phase HPLC (Shimadzu LC-10A, Kyoto, Japan) equipped with a UV detector set at 242 nm and a Shim-pack VP-ODS column (150 mm × 4.6 mm) maintained at 28°C. The injection volume was 20 µl. The mobile phase was a mixture of methanol–methyl cyanide–water (65:27:8, v/v/v) with a flow rate of 1 ml/min. Drug and excipients were validated to have no interference with each other.
Quantification of 9-octadecenoic acid was done by using a calibration curve. The straight-line equation was
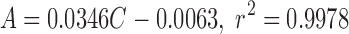 |
1 |
where C is drug concentration (micrograms per milliliter), and A is the peak area ratio (sample/internal standard). The linearity of the method was studied in the range of 9.83–196.50 µg/ml.
Characterization of BJO Liposomes
Visualization by Transmission Electron Microscopy (TEM)
The ultra-structure of BJOL was examined by transmission electron microscopy (TEM; H-7000, Hitachi, Japan) with negative stain method (16). A drop of each sample was applied to a copper grid coated with carbon film and air-dried; 2% (w/v) phosphotungstic acid solution was then added onto the grids. After being negative stained and air-dried at room temperature, the samples were accomplished for the TEM investigation.
Measurement of Size and Zeta Potential
Size and zeta potential of liposomes were measured by dynamic light scattering with a Zetasizer 3000 HAS (Malvern, UK). Samples were diluted appropriately with double distilled water for the measurements.
Determination of Entrapment Efficiency (EE)
BJOL suspensions (0.5 ml) were eluted by phosphate buffer (pH 7.4) in a Sephadex-G50 column (1.5 × 20 cm), and then the opalescence part of the eluate was collected (17). 9-octadecenoic acid in the eluate and in the suspensions was determined by HPLC. Entrapment efficiency (EE) was calculated with the following formula:
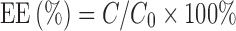 |
2 |
where C is the amount of drug in eluate, and C0 is the total amount of drug in liposome suspensions.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) thermograms were obtained using a differential scanning calorimeter DSC 204 (NETZSCH, Germany). Samples were weighted individually into a standard aluminum sample pan and heated from 20°C to 250°C at a rate of 10°C/min under a constant nitrogen stream (30 ml/min).
Acute Toxicity in Mice
To determine the acute toxicity of BJOL, the values of LD50 of both BJOL and BJOE were calculated using the Bliss method (18).
Kunming mice (half male and half female, 18–22 g) were housed under normal condition with free access to food and water. Mice were randomly divided into two treatment groups, BJOL and BJOE. Each group was further divided into six subgroups according to the dose (n = 10 each subgroup). The BJOL and BJOE were injected via tail vein with various doses of 3.01, 4.02, 5.36, 7.14, 9.53, and 12.70 g/kg (BJO). Mice were observed for 2 weeks in all groups, and the number of mice surviving was recorded. The LD50 of BJOL and BJOE were calculated using LD50CALC Software Version 2.0 (JSS Software Studio, China).
Pharmacokinetic Studies in Rats
Pharmacokinetics of BJO liposomes was investigated in rats following intravenous administration at a dose of 200 mg/kg (BJO), while BJOE were chosen as a reference formulation.
Animal Experiments
Twelve male SD rats (200 ± 20 g) were fasted with water allowed for 12 h prior to initiation of the study and randomly divided into two groups. On the first day of the experiment, blank blood samples (0.50 ml) were obtained by retro-orbital puncture at 5, 15, 30, and 45 min and 1, 1.5, 2, 3, 4, and 6 h from both groups. On the seventh day, rats were anesthetized by light ether and intravenously administered BJOL or BJOE at the same dose of 200 mg/kg by femoral vein injection. Time taken for administration was 30 s. Blood samples (0.5 ml) were obtained with the above-mentioned method after intravenous injection. Plasma was separated by centrifugation at 3,000 rpm for 15 min and stored at −20°C until analysis.
Extraction of 9-Octadecenoic Acid
Prior to extraction of 9-octadecenoic acid, frozen plasma samples were thawed at ambient temperature. One hundred microliters of serum was pipetted into a 10-ml glass centrifuge tube containing 50 µl internal standard solution (1 mg/ml). Then, the 9-octadecenoic acid was extracted by adding a mixture of dimethyl carbinol-skellysolve C-glacial acetic acid (40:10:1, v/v/v).
Determination of 9-Octadecenoic Acid in Plasma
The derivatization and determination of 9-octadecenoic acid in plasma were conducted with the method as described above. Blank rat plasma was extracted and analyzed for the assessment of the level of endogenous oleic acid. At each time point, the oleic acid concentration measured was corrected by the oleic acid concentration in blank plasma.
Quantitative analysis of 9-octadecenoic acid was performed using the internal standard method. The standard curves ranging from 2.0 to 0.10 × 103 μg/ml were linear (r2 > 0.99). Each solution was injected three times. The linear regression equation is C = 135.78A − 24.381 (n = 3, r = 0.9982), where A is the peak area ratio (sample/internal standard), and C is the concentration of 9-octadecenoic acid. No significant difference was observed in the slopes of standard curves (analysis of variance (ANOVA), p > 0.05). Intra- and inter-day variabilities were less than 10%. All of the absolute recoveries were above 80%, with all coefficients of variation less than 15%.
Pharmacokinetic Analysis
Standard compartmental pharmacokinetic parameters (±SD) were calculated using the pharmacokinetic programs 3p97, edited by the Committee of the Mathematic Pharmacology, the Chinese Society of Pharmacology. Statistical comparisons between groups were performed by an overall ANOVA. Values were reported as mean ± SD, and the data were considered as statistically significant as p < 0.05 or p < 0.01.
Pharmacodynamic Investigation
Inhibition Effects on Human Hepatocellular Carcinoma SMMC-7721 Cell Lines
Human hepatocellular carcinoma SMMC-7721 cells were cultured in RPMI-1640 containing 10% fetal bovine serum at 37°C in an incubator containing 0.5% CO2 (19). When the cultured cells went into logarithmic growth phase, the cells were detached by trypsinization, centrifuged at 1,000 rpm/min for 5 min, and re-suspended in fresh complete medium at a density of 2 × 105 cells/ml. Then, 100 µl of cells suspension per well was distributed in a 96-well plate and incubated in 5% CO2–air mixture at 37°C. After 12 h, 100 µl of BJOL or BJOE diluted in RPMI-1640 (at concentrations of 0.18, 0.35, 0.70, 1.40, and 2.80 mg/ml, respectively) per well was added to plates, respectively, whereas only 100 µl of RPMI-1640 was added in the control cells. After incubation of 48 h, 20 µl MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 5 mg/ml) was added, and then cultivated for 4 h before 100 µl DMSO was added to dissolve crystal and oscillated for 5 min. Absorbance readings were performed at 570 nm that serves as a measure of cell viability (20,21). The percentage of tumor cell inhibition was calculated according to the following formula:
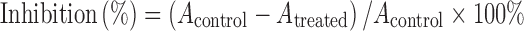 |
3 |
where Acontrol is the absorbance at 570 nm of the control group, and Atreated is that of treated cells.
Inhibition Effects on Mice Lewis Lung Cancer
Sixty Kunming mice were inoculated with transplantable Lewis lung cancer cells. Twenty-four hours later, the mice were randomly divided into six groups (n = 10, half male and half female). Cyclophosphamide (CTX) was used as positive control. As shown in Table I, drugs were administrated once daily for 10 days via tail vein. In the 11th day, tumor-bearing mice were weighed, sacrificed, and tumors were excised for being weighed immediately. The tumor inhibition ratio was calculated according to the following formula:
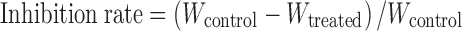 |
4 |
where Wcontrol is average tumor weight of control group, and Wtreated is average tumor weight of treated group.
Table I.
The Dosage Regimen of BJOL and BJOE on Mice Transplanted Lewis Lung Cancer by Intravenous Administration (n = 10)
| Groups | Number | Dose (mg/kg) |
|---|---|---|
| Control (blank) | 10 | RPMI-1640 (100 μl) |
| CTX | 10 | 25 |
| Emulsion | 10 | 180 |
| 10 | 60 | |
| Liposomes | 10 | 120 |
| 10 | 180 |
RESULTS AND DISCUSSION
Characterization of BJO Liposomes
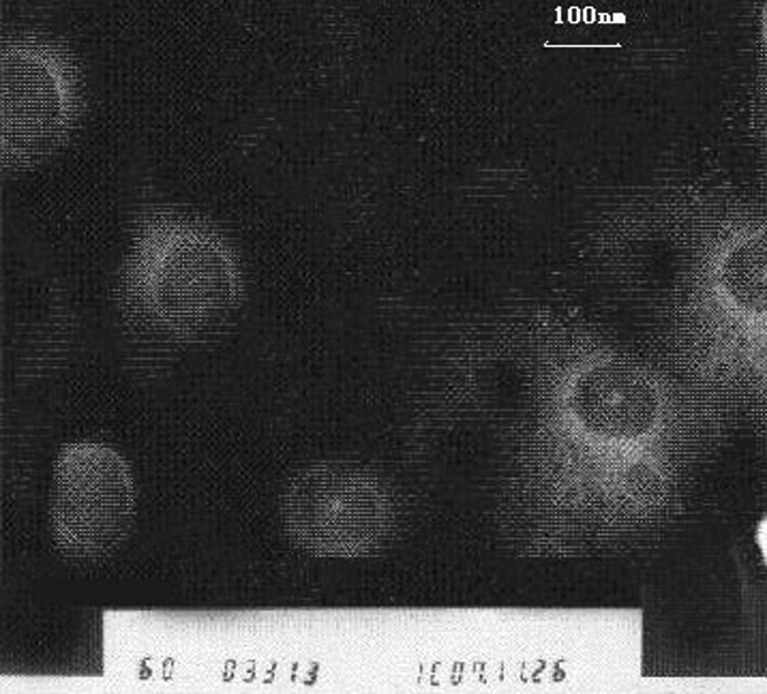
Preliminary experiments were performed to determine the optimal component for the liposomes. The ratio of drug:cholesterol:lipid of 2:3:20 was selected for the final BJOL formulation. As shown in Fig. 1, the BJOL was found to be regular spherical shapes under a TEM, the size was uniform, and the bilayer membrane can be clearly seen. BJOL had an average diameter of 108.2 nm with a zeta potential of −57.0 mV, drug loading of 3.60% with a high drug EE of 92.40%.
Fig. 1.
Microphotograph of BJOL by transmission electron microscope (original magnification ×60,000). Under electron microscope, BJOL showed a global, regular contour with homogeneous size and distribution
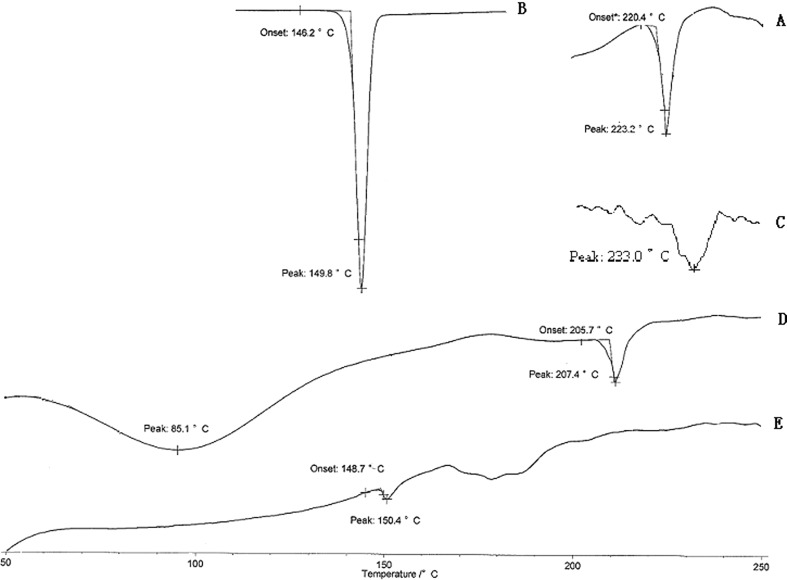
DSC (Fig. 2) of cholesterol and soybean phospholipid showed a main transition peak (Tm) at 149.8°C and 223.2°C, respectively, corresponding to their gel-to-liquid crystalline transition temperature. While with unloaded liposomes, a single main Tm was observed at 207.4°C, signifying that the phospholipid and cholesterol interacted with each other to a great extent when forming the lipid bilayer. In comparison, the drug-loaded liposomes (BJOL) produced substantial change in the DSC thermogram with no peak of BJO (233.0°C) observed. The enthalpy decreased because of the interaction between the hydrocarbon chains of the phospholipid and the drug. These results suggest that the lipophilic drug interacted strongly with the lipid bilayer of liposomes and may be potentially released slowly in vivo.
Fig. 2.
Overlaid DSC thermograms of soybean phospholipid A, cholesterol B, BJO C, unloaded liposomes D, and BJO liposomes E
Acute Toxicity in Mice
The LD50 was used to determine the acute toxicity as described in methods.
The toxic response such as severe prostration, respiratory distress, and catatonia were observed. After 7 days of dosing, the survivors were gradually recovered. The mice mortality and LD50 of BJOE were shown in Table II. The LD50 of BJOE and its 95% confidence limits were 7.35 and 6.22–8.84 g/kg, respectively. In contrast, the LD50 of BJOL was not detected at the investigated dose range, as no animal death was observed in any subgroups following the treatment with the liposomes. Maximal tolerance limit of BJOL will be determined in further study. These parameters indicated that the BJOL were less toxic than BJOE.
Table II.
LD50 for BJOE After Intravenous Administration to Mice and Its Confidence Interval (n = 10)
| Dose (mg/kg) | Log dose (x) | Death number | Mortality (%) | Regression probit (Y) | LD50 and 95% CI (g/kg) |
|---|---|---|---|---|---|
| 12,700 | 4.1038 | 10 | 100 | 6.4094 | |
| 9,525 | 3.9789 | 6 | 60 | 5.6679 | |
| 7,144 | 3.8539 | 5 | 50 | 4.9265 | 7.3510 |
| 5,358 | 3.7290 | 2 | 20 | 4.1851 | 6.2170∼8.8420 |
| 4,018 | 3.6040 | 1 | 10 | 3.4433 | |
| 3,014 | 3.4791 | 0 | 0 | 2.7022 |
Pharmacokinetics in Rats
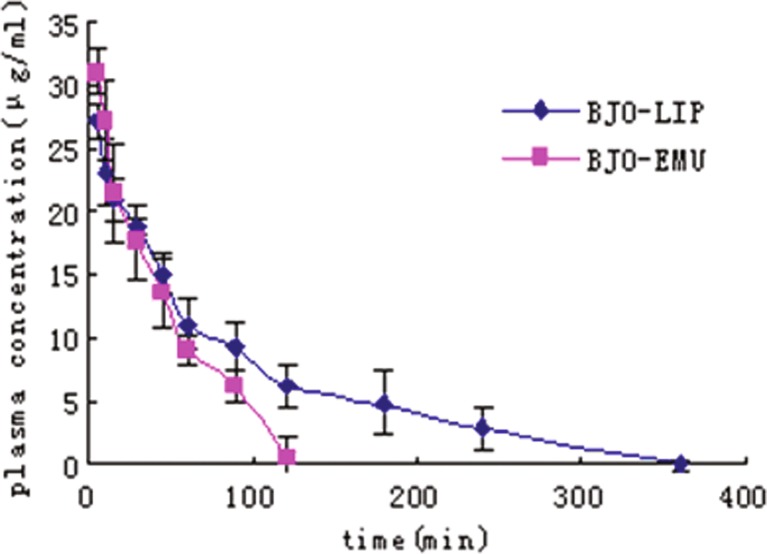
The plasma drug concentration curves versus time after intravenous administration were shown in Fig. 3. All pharmacokinetic parameters evaluated were reported in Tables III and IV.
Fig. 3.
Mean plasma concentration–time curves of BJO after intravenous administration in rats. Diamonds, BJOL. Squares, BJOE. Each point represents the mean ± SD (n = 5)
Table III.
Pharmacokinetic Parameters of BJO Liposomes Following Intravenous Administration in Rats (n = 6)
| Parameter | Unit | Value (mean ± standard error) |
|---|---|---|
| A | μg/ml | 16.58 ± 0.331 |
| α | 1/min | 0.054 ± 0.0224 |
| B | 16.79 ± 0.241 | |
| β | 1/min | 0.0084 ± 0.00811 |
| V(d) | L/kg | 6.00 |
| T 1/2α | min | 12.9 |
| T 1/2β | min | 82.8 |
| K 21 | 1/min | 0.031 |
| K 10 | 1/min | 0.018 |
| K 12 | 1/min | 0.017 |
| AUC | mg min/ml | 2.31 |
| CL(s) | L/(kg min) | 0.086 |
| AUMC | mg min2/ml | 150.62 |
| MRT | min | 102.5 |
Table IV.
Pharmacokinetic Parameters of BJO Emulsion Following Intravenous Administration in rats (n = 6)
| Parameter | Unit | Value (mean ± standard error) |
|---|---|---|
| C 0 | μg/ml | 35.71 ± 0.632 |
| Ke | 1/min | 0.031 ± 0.00285 |
| V(d) | L/kg | 5.60 |
| T 1/2(Ke) | min | 22.3 |
| AUC | mg min/ml | 1.15 |
| CL(s) | L/(kg min) | 0.17 |
| AUMC | mg min2/ml | 47.26 |
| MRT | min | 36.3 |
Encapsulation of BJO in the liposomes produced a significant change in drug pharmacokinetic parameters compared to the emulsion formulation. After bolus intravenous administration, the pharmacokinetic data of BJOE can be best described using one-compartment open model. In comparison, the data of BJO liposomes were processed following a two-compartment open model with a distribution phase (t1/2α = 12.9 min). The area under curve (AUC)0-t value of the BJOL (2.31 mg min/ml) was approximately twofold higher than that of BJOE. Compared with BJOE, higher mean residence time (MRT; 2.8-fold), t1/2 (4.0-fold), and lower CL (∼50%) were obtained after the administration of BJOL. These results indicated that the encapsulation of BJO in the liposomes could prolong the drug circulation in the blood and tissues for a longer period of time. Vd of BJOL was slightly larger than that of BJOE, although not statistically significant (p > 0.05).
The obvious increase in AUC0-t and the prolonged circulating time of BJOL may be attributed to delayed elimination of BJO when incorporated into liposomes and the reduction in uptake by the RES in the liver and spleen due to the small size, as reported by other researchers (22,23). In addition, the significant increase in elimination half life and the obvious decrease in CL could be ascribed to the protection of the lipid bilayer membranes and the slow release of BJO from liposomes. The slight increase of Vd indicated that more BJO could distribute to peripheral tissue or organ in liposomes than in emulsions.
Pharmacodynamic Investigation
Inhibition Effects on Human Hepatoma Cell Line SMMC-7721
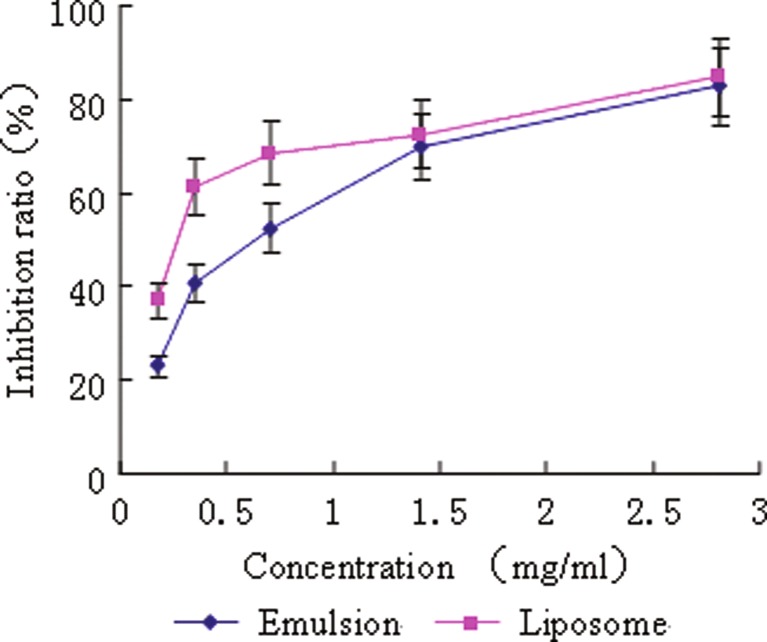
In vitro, both formulations showed a dose-dependent cytotoxicity effect against human hepatoma cell line SMMC-7721 in the MTT assay (Fig. 4). However, the inhibition ratios of liposomes were higher than that of emulsions except for that at the highest dose, at which both formulations showed the maximum cytotoxicity. The IC50 values obtained in liposomes group and emulsions group were 0.20 ± 0.02 and 0.59 ± 0.12 mg/ml, respectively (n = 3). The IC50 value of liposomes group was one third of that of emulsions group with statistical significance (p < 0.01). The higher inhibitory effect of the liposomes to the cells than emulsions might be resulted from their enhancement of intracellular delivery of BJO by fusing with the cell membrane (24). Furthermore, the tiny size of liposomes could increase their contact areas with tumor cell significantly, which might generate an apparent improvement in tumor inhibition effect.
Fig. 4.
Inhibition effects of BJOL and BJOE on 7721 human liver tumor cell. Diamonds, BJOE. Squares, BJOL. Each point represents the mean ± SD (n = 3)
Inhibition Effects on Mice Transplantable Lewis Lung Cancer
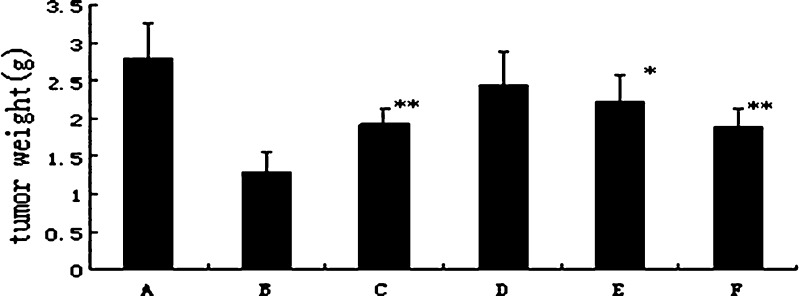
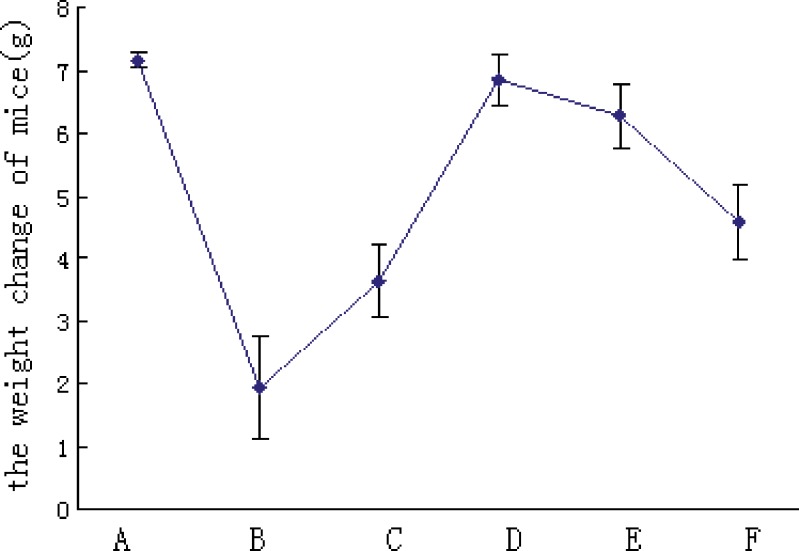
Figure 5 shows the inhibition effects of BJOL and BJOE on mice transplantable Lewis lung cancer. Compared with blank control group, significant inhibition effects on Lewis lung cancer was observed in liposomes groups with middle dose (p < 0.05) and high dose (p < 0.01), whereas in emulsions groups, significant effect was only observed with high dose (p < 0.05). Moreover, the liposomes groups had less weight loss than emulsions groups (p < 0.05, Fig. 6). These results indicated that BJOL had stronger antitumor activity with a lower toxicity than BJOE. Moreover, given the increase of Vd coupled with the enhanced antitumor activity compared to BJOE, it may conclude the targeting effect of BJOL to tumor tissue in some extent (25).
Fig. 5.
The weight of transplantable tumor on mice after treatment with BJOL and BJOE at different doses of BJO. Each point represents the mean ± SD (n = 10). A Control. B Cyclophosphamide (CTX). C Emulsions 180. D Liposomes 60. E Liposomes 120. F Liposomes 180. Results were tested for significance using t test. Significance levels: single asterisk, p < 0.05; double asterisk, p < 0.01
Fig. 6.
The weight change of mice (grams) after treatment of BJOL and BJOE. Each point represents the mean ± SD (n = 10). A Control. B Cyclophosphamide (CTX). C Emulsions 180. D Liposomes 60. E Liposomes 120. F Liposomes 180
The likely explanations for the improved therapeutic activity and decreased toxicity of BJOL were as follows. Generally, tumor tissues proliferate rapidly with abundant vasculogenesis, which may strongly increase the uptake of BJOL to the tumor. It has been reported that the cytocidal effect of anticancer drug depends on its concentration and duration of exposure to tumor tissues (26). Accumulation of biodegradable liposomes containing BJO in tumor tissues created not only a high concentration in tumor tissues but also a greater gradient of drug concentration for a massive and prolonged diffusion of the free drug towards neoplastic tissues, which remarkably enhance the inhibition of proliferation of tumor tissues. In addition to the targeting ability of liposomes, the decreased toxicity might also be largely ascribed to the slow release of BJO from liposomes (9,27). In rats, the BJOL produced a more favorable pharmacokinetic profile compared to the emulsion. The comparative pharmacokinetic data showed that BJO distributed to the body following the two-compartment model from the liposomal system compared to the one-compartment model in the emulsion. This suggests that distribution of the liposome-associated lipophilic drug to the interstitial fluids was restricted due to the limited tissue accessibility of the particular carriers before the drug was released (9). As a result, BJOL gave a larger AUC and longer half life, which could have increased the drug contact time with the tumors and consequently enhanced the antitumor activity. Therefore, liposomes may provide highly desirable characteristics as delivery vehicles of lipophilic antitumor drugs like BJO.
CONCLUSIONS
In conclusion, BJOL were successfully prepared for intravenous administration and investigated in vitro and in vivo. Compared to the emulsion formulation, the liposomal BJO showed more efficacious antitumor activity, more favorable pharmacokinetic parameters, and a decreased toxicity to animals. Data obtained from human subjects will be required to support the conclusion of this study in clinical settings. Particle aggregation or hydrolysis of liposomal preparations could occur during long-term storage due to the existence of BJOL in the aqueous medium; further study will be carried out to investigate the stability of BJO liposomes, and technology using freeze drying may be used to enhance the shelf life of BJOL.
Acknowledgments
This study is financially supported by the major project of National Science and Technology of China for new drugs development (No. 2009ZX09310-004) and “The Six Major Talent” peak project in Jiangsu Province, China (No. 07-C-013).
References
- 1.Bi YL. Quality analysis of Brucea javanica oil. J Zhengzhou Inst Technol. 2001;22(4):72–4. [Google Scholar]
- 2.Wang F, Cao Y, Liu HY, Fu ZD, Han R. Experimental studies on the apoptosis of HL-60 cells induced by Brucea javanica oil emulsion. Zhongguo Zhong Yao Za Zhi. 2003;28(8):759–61. [PubMed] [Google Scholar]
- 3.Tang T, Meng LH, Chen LJ, Ding J. Reversal of multidrug resistance and inhibition of DNA topoisomerase by emulsion of seed oil of Brucea. Chin Pharmacol Bull. 2001;17(5):534–9. [Google Scholar]
- 4.Tan HY, Li Y, Zhu SJ, Wan DG, Cui HJ, Jia LQ, et al. The treatment of 25 cases of elderly patients with advanced non-small cell lung cancer using gemcitabine combined with Brucea joint oil emulsion treatment. China tumor. 2007;16(6):474–5. [Google Scholar]
- 5.Li XG, Nan XY, Dang JG, Li X, Yang SY. An experimental study of effect of Brucea javanica oil emulsion on human renal carcinoma. J Clin Urol. 1998;13(2):82–4. [Google Scholar]
- 6.Nan XY, He DL, Dang JG, Zhang Y, Wang MZ, Yang ZS. Treatment of prostatic carcinoma (Stage C to D) with fructus bruceae emulsion. J Clin Urol. 1998;13(12):531–3. [Google Scholar]
- 7.Xiang Q, Zhou LL, Zhang H, Zhang HB, Yao CS, Hang YD. The pharmacokinetic studies of Brucea javanica oil microemulsions in rats. J Chin Med Mater. 2007;30(9):1113–5. [Google Scholar]
- 8.Ji XY. One cases of anaphylactic response induced by Brucea javanica oil emulsion injection. J Mod Oncol. 2009;17(8):1537. [Google Scholar]
- 9.Allen TM, Hansen CB, Meneze DE. Pharmacokinetics of long-circulating liposomes. Adv Drug Deliv Rev. 1995;16:267–84. doi: 10.1016/0169-409X(95)00029-7. [DOI] [Google Scholar]
- 10.Chiu GN, Bally MB, Mayer LD. Targeting of antibody conjugated, phosphatidylserine-containing liposomes to vascular cell adhesion molecule 1 for controlled thrombogenesis. Biochim Biophys Acta. 2003;1613:115–21. doi: 10.1016/S0005-2736(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 11.Vemuri S, Rhodes CT. Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharmaceutics Acta Helvetiae. 1995;70:95–111. doi: 10.1016/0031-6865(95)00010-7. [DOI] [PubMed] [Google Scholar]
- 12.Yu YL, Lu Y, Tang X, Cui FD. Formulation, preparation and evaluation of an intravenous emulsion containing Brucea javanica oil and Coix seed oil for anti-tumor application. Biol Pharm Bull. 2008;31(4):673–80. doi: 10.1248/bpb.31.673. [DOI] [PubMed] [Google Scholar]
- 13.Mehta A, Oeser AM, Carlson MG. Rapid quantitation of free fatty acids in human plasma by high-performance liquid chromatography. J Chromatogr B. 1998;719:9–23. doi: 10.1016/S0378-4347(98)00403-4. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Yue CL, Xiang Q, Yao CS, Zhou LL. Determination of fatty acids in Brucea jananica oil injection by high-performance liquid chromatography. Chin J Mod Appl Pharm. 2006;23(4):329–31. [Google Scholar]
- 15.Weinkove C, Reed P, Abushufa R. Fatty acids in erythocytes measured by isocratic HPLC. Clin Chem. 1994;40(9):1707–12. [PubMed] [Google Scholar]
- 16.Dayan N, Touitou E. Carriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. liposomes. Biomaterials. 2000;21:1879–85. doi: 10.1016/S0142-9612(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 17.Gao XL, Ji XM. Determining the trap efficiency of liposome using sephadex column chromatography. China Pharm J. 2003;38(7):515–7. [Google Scholar]
- 18.Li QX, Wang H, Xiao QQ, Kong R. The evaluation and calculation of median lethal dose (LD50) using Bliss method. J Math Med. 1995;4:318–20. [Google Scholar]
- 19.Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8(3):483–7. doi: 10.3748/wjg.v8.i3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sargent JM, Taylor CG. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Brit J Cancer. 1989;60(2):206–10. doi: 10.1038/bjc.1989.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barba I, Cabanas ME, Arus C. The relationship between nuclear magnetic resonance-visible lipids, lipid droplets, and cell proliferation in cultured C6 cells. Cancer Res. 1999;59(8):1861–8. [PubMed] [Google Scholar]
- 22.Takeuchi H, Kojima H, Toyoda T, Yamamoto H, Hino T, Kawashima Y. Prolonged circulation time of doxorubicin-loaded liposomes coated with a modified polyvinyl alcohol after intravenous injection in rats. Eur J Pharm Biopharm. 1999;48:123–9. doi: 10.1016/S0939-6411(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 23.Nagayas A, Uchiyama K, Kiwada H. The size of liposomes: a factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv Drug Deliv Rev. 1999;40:75–87. doi: 10.1016/S0169-409X(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 24.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–75. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 25.Zhu JB, Wen N, Xiong F, Xiong F. Stability and pharmacokinetic studies of O-palmitoyl amylopectin anchored dipyridamole liposomes. Int J Pharm. 2006;313:136–43. doi: 10.1016/j.ijpharm.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Nam NH, Kim Y, You YJ, Hong DH, Kim HM, Ahn BZ. Combretoxazolones: synthesis, cytotoxicity and antitumor activity. Bioorg Med Chem Lett. 2001;11(23):3073–6. doi: 10.1016/S0960-894X(01)00622-9. [DOI] [PubMed] [Google Scholar]
- 27.Chang CC, Liu DZ, Lin SY, Liang HJ, Hou WC, Huang WJ, et al. Liposome encapsulation reduces cantharidin toxicity. Food Chem Toxicol. 2008;46:3116–21. doi: 10.1016/j.fct.2008.06.084. [DOI] [PubMed] [Google Scholar]