INTRODUCTION
Selective inhibition of the cyclooxygenase-1 (COX-1) isoform has been shown to reduce inflammation and tumorigenesis and lack the gastrointestinal toxicity of traditional nonsteroidal anti-inflammatory drugs. The COX-1-selective inhibitor 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole (SC-560), shown in Fig. 1, was identified as one of several structurally related compounds with varying selectivity for the COX isoforms during the development of the COX-2-selective inhibitor celecoxib (1). From this work, SC-560 was found to be a highly selective and potent inhibitor of COX-1, with in vitro studies showing an IC50 of 0.007 μM for COX-1 and 75 μM for COX-2. In animal studies, SC-560 has been shown to be effective in treating neuroinflammation when administered by injection (2). However, the poor bioavailability that results following oral administration of SC-560 may limit its effectives in treating diseases where COX-1 inhibition would provide efficacy. When administered orally to rats as a suspension in 1% methylcellulose, the mean bioavailability is only 5% of the dose (3). Altering the oral dosage formulation by dissolving in polyethylene glycol 600 improves the bioavailability to only 15% (3). Therefore, additional studies are needed to identify dosage formulations which may improve oral bioavailability. To develop dosage forms and products of a potential drug compound, solid-state properties of the chemical need to be thoroughly examined. One of the basic attributes is the crystal structure of the compound, which determines important physical and chemical properties of the substance, including solubility and dissolution rate. We have solved the crystal structure for SC-560 and reported it elsewhere (4). Herein, we describe additional solid-state properties, including its solubility in water.
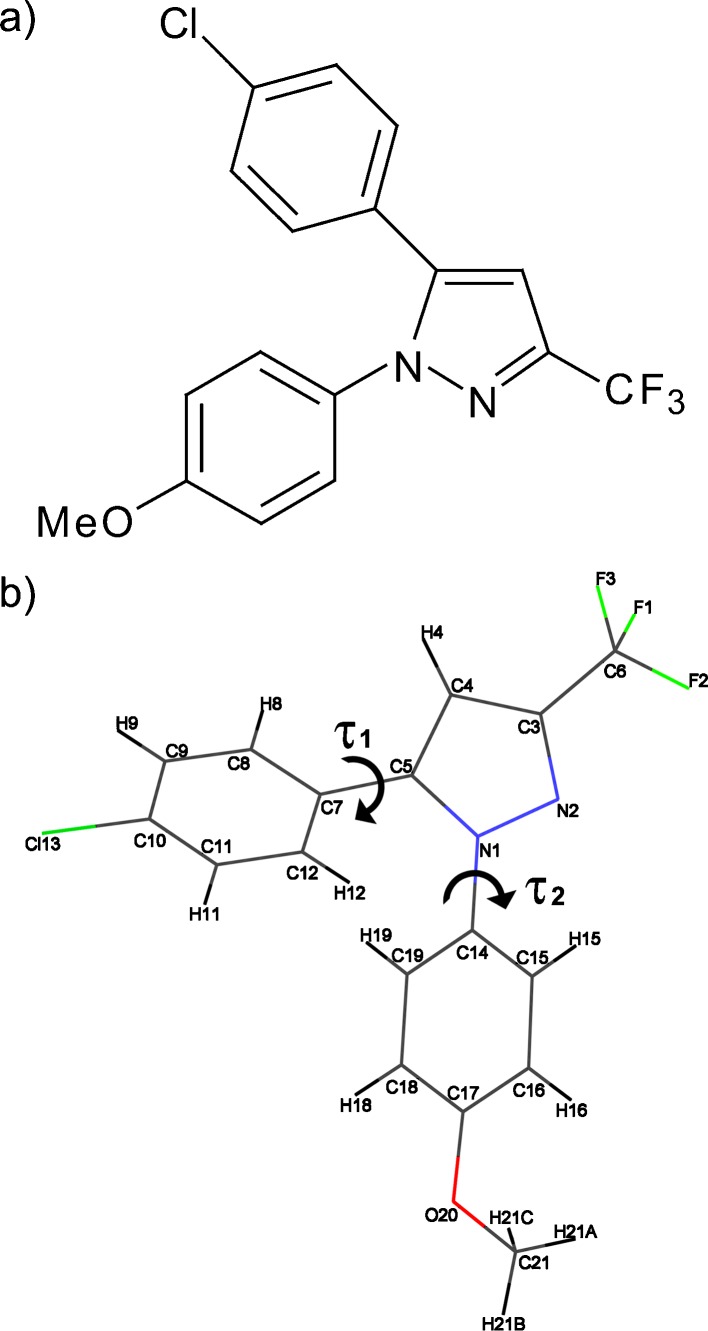
Fig. 1.
Molecular structure of SC-560 and its labeling in the crystal. The two torsion angles are also marked
MATERIALS AND METHODS
Materials
SC-560 was from Cayman Chemical Company (Ann Arbor, MI, USA). Organic solvents (high-performance liquid chromatography grade) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) without further purification.
Crystal Growth
Crystallization was carried out in several selected organic solvents including methanol, ethanol, ethyl acetate, hexane, and acetone. A typical crystallization experiment involved dissolving 31 mg of SC-560 in solvent in a glass vial at room temperature. The vial was sealed with Parafilm® and punctured with numerous pin-size holes to allow for evaporation of the solvent. Colorless block crystals were obtained following approximately 1 week of slow evaporation. Although similar procedures were repeated, utilizing the selected organic solvents described above, only methanol produced sufficient high-quality crystals of SC-560 for single crystal X-ray diffraction measurements.
Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) was conducted for the harvested crystals. Thermal analyses were performed on TA Instruments 2920 MDSC. The sample (3.68 mg of SC-560) was placed in a hermetically sealed aluminum DSC pan. A heating rate of 5°C/min was employed for a temperature scan of 40–75°C after initial confirmations of the sole melting point. N2 was used to purge the sample at 50 ml/min.
Conformational Search
The energy of the single molecule of SC-560 in different conformations in gas phase was evaluated as a function of either of the two torsion angles, τ1 and τ2 (Fig. 1b), with Gaussian 03 (Gaussian, Inc., Wallingford, CT, USA). The molecule was optimized from various initial structures in order to identify the most stable conformation, which was then used for scanning each torsion angle with the bond lengths, bond angles, and other torsion angles all being fixed. The method used for the structural optimization and conformational search was B3LYP/6-31++G(d, p).
Solubility Measurements
The solubility of SC-560 was measured gravimetrically in deionized water at 25°C. An aqueous solution with an excess, known amount of drug was equilibrated in a temperature-controlled shaker at 100 rpm for 24 h, filtered with 0.2 μm membrane, and left to dry for several hours. The solid obtained was weighed using a microbalance. The value represents the average of three samples.
RESULTS AND DISCUSSION
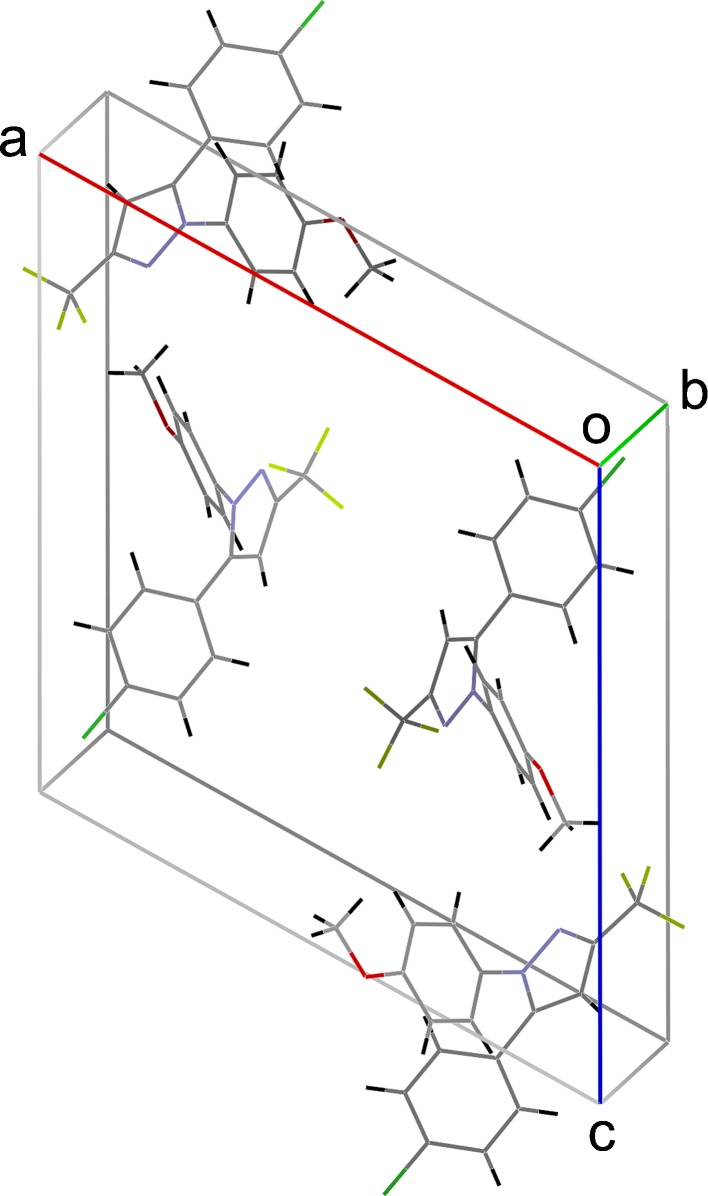
Crystals of SC-560 grown from methanol solution are shown in Fig. 2. The majority of the crystals was smaller than 0.2 mm, typically formed prisms, and grew as aggregates in the growth medium. Single crystal X-ray diffraction experiments were used to solve the structure of this material, which belongs to the monoclinic space group with four molecules in a unit cell (4). Table I lists its crystallographic data. As shown in Fig. 3, the molecule lacks the moieties necessary to form intermolecular hydrogen bonding. Thus, the lattice energy mainly consists of dispersion energies, which typically result in a low melting point because of the weak intermolecular interactions. Despite the entire chemical structure being fused together by three aromatic rings, a large conjugate system between the rings is not seen due to steric repulsion between the two phenyl rings. Each of the aryl rings, however, maintains its planer conformation. The relative orientation or the twisting between a phenyl and the pyrazolyl ring is indicated by either τ1 or τ2, respectively (Fig. 1b). In the crystal, these two angles are 42.87° (τ1) and 45.44° (τ2). Due to the glide plane, one of four symmetry operators of the crystal system, there exists another mirroring conformation, which gives τ1 and τ2 as −42.87° and −45.44°, respectively. The two conformations are believed to be isoenergetic and can be imagined as mirror images against the plane of the pyrazolyl ring (Table I).
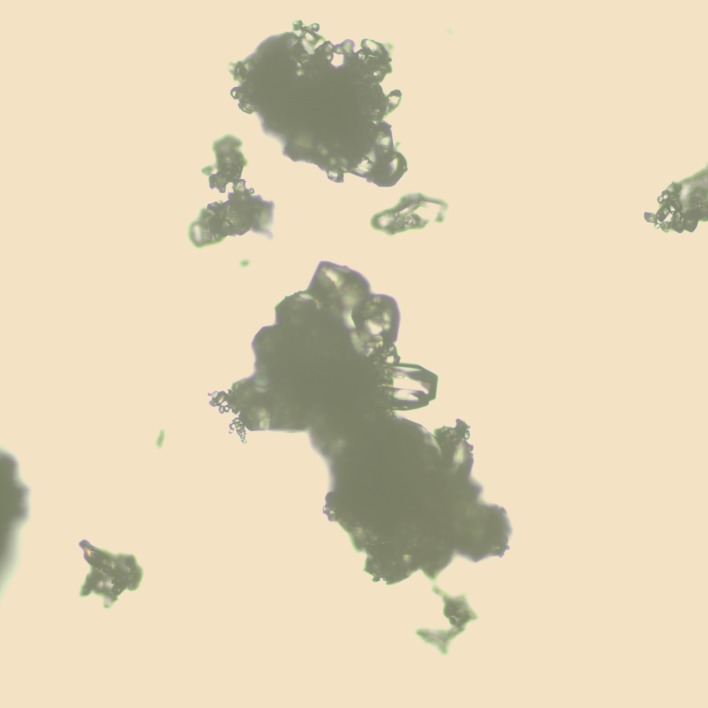
Fig. 2.
Photomicrograph of SC-560 crystals grown from methanol. Scale bar: 0.2 mm
Table I.
Crystallographic Data of SC-560
| Morphology | Colorless block |
| Space group | P21/n |
| a/Å | 15.585 (3) |
| b/Å | 7.1671 (14) |
| c/Å | 15.789 (3) |
| α/° | 90.00 |
| β/° | 116.81 (3) |
| γ/° | 90.00 |
| Z, Z′ | 4, 1 |
| V/Å3 | 1,574.1 (5) |
| D cal/g cm−3 | 1.488 |
| R 1 | 0.0352 |
| T/K | 90 (2) |
Fig. 3.
Crystal packing of SC-560
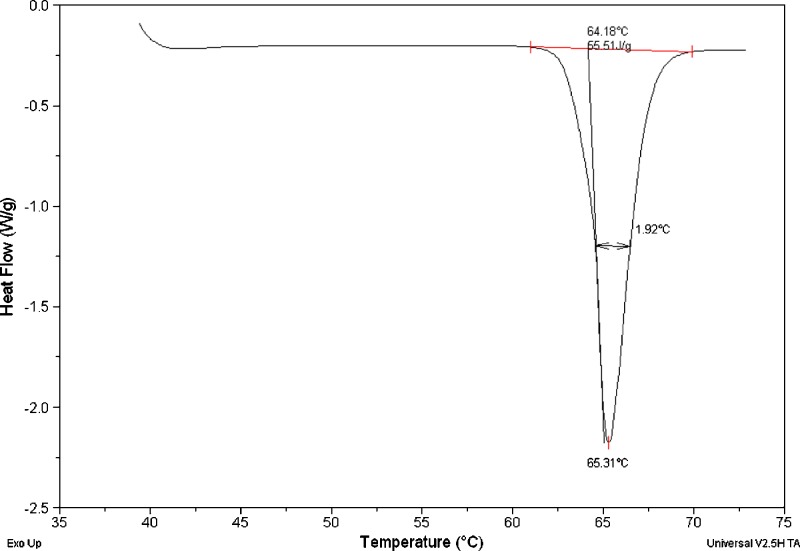
The melting point of SC-560 was measured by DSC and the thermograph is shown in Fig. 4. Only a single melting peak was observed and its onset temperature was 64.18°C with the heat of fusion determined as 55.51 J/g. Such a low melting point echoes the earlier analysis of crystal structure in which only weak dispersion energies form between molecules.
Fig. 4.
Differential scanning calorimetry thermogram of SC-560. The negativity of heat flow indicates endotherm
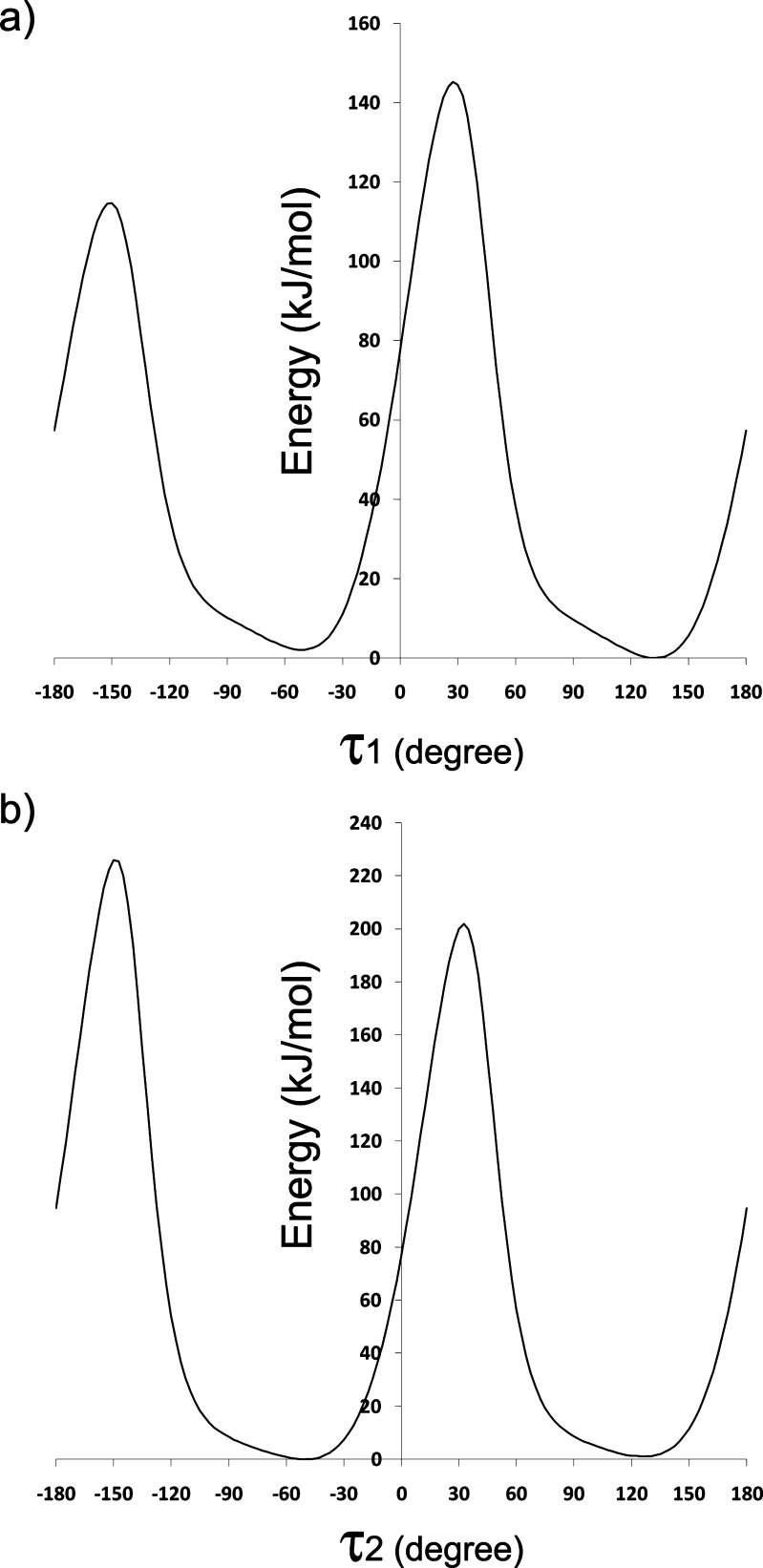
To further understand the structure-packing relationship of the crystal system, the conformational flexibility of the SC-560 molecule was analyzed by scanning the potential energy space of either of the two torsion angles, τ1 and τ2, which define the overall variation of the molecule’s conformation. The results are shown in Fig. 5. Because of the C2 symmetry of each phenyl ring about the axis linking the ring with the pyrazolyl group, any data point on the two curves in Fig. 5 should repeat itself when the ring or the torsion angle rotates ±180°. The less symmetric feature of each curve, especially the considerable difference between two maxima, is merely an artifact caused by the calculation where the torsion angle was allowed to change while other angles and bond distances were kept constant. For the τ1 curve, the two isoenergetic minima were found at −52.5° and 132.5° with their difference not being 180° due to the calculation approach. For τ2, the two minima were −50.0° and 125.0°. As such, the conformation in the crystal with τ1 and τ2 of −42.87° and −45.44°, respectively, approximates the global minimum of the potential energy of the single molecule. Moreover, different scans of τ1 and τ2 reveal two potential energy curves that closely resemble mirror images of Fig. 5 against the y-axis (results not shown), indicating the isoenergetic symmetry of the molecule through the reflection on the pyrazolyl plane. This may explain the existence of the mirror conformation in the crystal structure with τ1 and τ2 of 42.87° and 45.44°, respectively. It is reasonable to expect, therefore, that the molecular structure in the crystal resides in the only energy minimum defined by τ1 and τ2. With the lack of hydrogen bonding between molecules, the crystal packing is mainly regulated by the molecular conformation itself, thereby making it unlikely that there exist other polymorphic structures.
Fig. 5.
Conformational energy scans by B3LYP/6-311++G(d, p)//B3LYP/6-311G(d, p) of the torsion angles τ1 a and τ2 b with the lowest energy of each scan shifted to zero
Solubility of SC-560 in water was estimated to be 0.3 ± 0.1 μg/ml. The large standard deviation was due to the gravimetric method used for determining the solubility. The low aqueous solubility is expected from the aromaticity of the compound and lack of polar functional groups. It also explains the poor bioavailability of oral dosage forms.
CONCLUSION
There exists no hydrogen bonding in the SC-560 crystal structure and, consequently, the major contribution to the crystal stability stems from weak dispersion energies leading to its low melting point at 62.5°C. This relatively low melting temperature may complicate the development of solid dosage forms of SC-560. Electronic energy calculations further indicate that the molecular structure in the crystal is at the only energy minimum in the molecules’ conformational space. In addition, the solubility of SC-560 in water was extremely low which accounts for the poor bioavailability.
Acknowledgment
We are thankful for the financial support by NSF (DMR-0449633).
References
- 1.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1, 5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1 h-pyrazol-1-yl]benzenesulfonamide (sc-58635, celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 2.Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teng XW, Abu-Mellal AKM, Davies NM. Formulation dependent pharmacokinetics, bioavailability and renal toxicity of a selective cyclooxygenase-1 inhibitor SC-560 in the rat. J Pharm Pharm Sci. 2003;6:205–210. [PubMed] [Google Scholar]
- 4.Long S, Theiss KL, Li T, Loftin CD. Cyclooxygenase-1-selective inhibitor sc-560. Acta Cryst. 2009;E65:o360. doi: 10.1107/S1600536809001779. [DOI] [PMC free article] [PubMed] [Google Scholar]