Abstract
An epinephrine (E) tablet is under development for sublingual (SL) administration for the first-aid treatment of anaphylaxis; however, the inherent bitterness of E may hinder acceptability by patients, especially children. To assess the degree of E bitterness and to predict the masking effects of sweetening and/or flavoring non-medicinal ingredients (NMIs), the potential usefulness of an electronic tongue (e-Tongue) was evaluated. The e-Tongue sensors were conditioned, calibrated, and tested for taste discrimination. Six standard active pharmaceutical ingredients were used to build and validate a bitterness model which was then used to assess E bitartrate (EB) solutions from 0.3–9 mM. Taste-masking efficiency of aspartame (ASP), acesulfame potassium (ASK), and citric acid (CA) each at 0.5 mM was evaluated. Using EB 9 mM, the bitterness score was 20 on a scale of 20 (unacceptable) down to 1 (not detected). When NMIs 0.5 mM were added, neither ASK (17.2, unacceptable) nor was ASP (14.0, limit acceptable) effective in masking the bitter taste. When the combination of ASK and ASP was used, the bitterness score was reduced to 9.2 (acceptable). However, the addition of CA alone resulted in the best reduction of the bitterness score to 3.3 (not detected). Using the e-Tongue, the incorporation of a variety of sweetening and/or flavoring NMIs into a SL tablet of E could be shown to mask its bitter taste by up to 80%. These results should be confirmed by in vivo studies.
KEY WORDS: anaphylaxis, artificial sweeteners, electronic tongue, epinephrine, sublingual tablet
INTRODUCTION
Medications that enter the oral cavity, whether orally administered, sublingually administered, or inhaled, should have an acceptable taste. One of the major barriers that prevent patients from following a prescribed medication regimen has been identified as the unpleasant taste of active pharmaceutical ingredients (APIs) in these dosage forms (1).
For a prescription sublingual (SL) tablet, the recommended residence time in the mouth is 2 min or until dissolved (2). Taste may affect the length of time a patient holds a tablet within the SL cavity which in turn may affect compliance. In order to achieve optimal compliance, the taste of a SL tablet should be assessed and improved if necessary to ensure that it is palatable, especially for children (3). Taste assessment is usually performed in the early stages of drug development of a new chemical entity (NCE). The taste of the NCE or API may require the addition of sweetening and/or flavoring non-medicinal ingredients (NMIs) to the final formulation.
Epinephrine (E), a potent vasoconstrictor and bronchodilator with a narrow therapeutic index, is the drug of choice for the treatment of anaphylaxis (4,5). For the first-aid, pre-hospital treatment of anaphylaxis, E is available in auto-injectors including EpiPen®, EpiPen Jr® (Dey LP, Nappa, CA, USA), Twinject 0.3 mg®, Twinject 0.15 mg® (Sciele Pharma, Inc., a Shionogi Company, Atlanta, GA, USA), Anapen 0.15 mg ®, Anapen 0.3 mg®, and Anapen 0.5 mg®(Lincoln Medical, Salisbury, UK). A fast disintegrating SL tablet formulation of E has been successfully formulated in our laboratory (6). The bioavailability profile of this E formulation is similar to that of an IM injection of E (7,8). The SL tablet formulation has not been approved for administration to humans. Accordingly, at this early stage of development, assessment of its taste by using human sensory analysis panels (SAPs) is not an option.
Taste assessment using a multichannel taste sensor, an instrument commonly named the electronic tongue (e-Tongue), is becoming established as a novel alternative to human SAPs. A number of pharmaceutical laboratories around the world are using this instrument to assess the bitterness of NCEs/APIs and the masking efficiency of NMIs. In addition, it is used in placebo development, in taste matching of formulations, and in unknown-to-reference comparisons (9–15). The e-Tongue consists of an array of liquid electrochemical sensors coated with an organic membrane that governs the sensitivity and selectivity of each individual sensor. The αAstree e-Tongue (Alpha M.O.S., France) is a fully automated taste analyzer equipped with a seven-sensor probe assembly that is based on the chemical modified field-effect transistor (ChemFET) technology for liquid sample analysis (16,17).
The degree of bitter taste of the E SL tablet has not yet been evaluated. The purpose of this study was to assess the potential of the e-Tongue to determine the degree of E bitterness and to evaluate the taste-masking effect of sweetening and/or flavoring NMIs.
METHODS
Materials
Epinephrine bitartrate (EB) and acesulfame potassium were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Aspartame and citric acid anhydrous were purchased from Fisher Scientific (Nepean, ON, Canada). Acetaminophen, caffeine monohydrate, quinine hydrochloride (HCl), loperamide HCl, and famotidine were purchased from MP Biomedicals (Solon, OH, USA). Prednisolone metasulfobenzoate sodium was purchased from Science Lab (Houston, TX, USA). Hydrochloric acid (HCl 0.1 and 1 M), sodium chloride (NaCl 0.1 and 1 M), and monosodium glutamate (MSG 0.1 M) solutions were provided by Alpha M.O.S. All chemicals were of analytical grade and used without further purification.
Equipment
The αAstree e-Tongue (Alpha M.O.S., France) used in all experiments is equipped with a 48-position auto-sampler, a bitterness prediction module (BPM) software, and a seven-sensor probe assembly (reference number 803-0070: sensors BD, EB, JA, JG, KA, OA, and OB), specifically developed to detect and predict bitter taste, with the Ag/AgCl reference electrode from Metrohm AG.
Selection of an Appropriate Concentration Unit Based on the Molecular Assumption
Since potentiometric differences created by the sensors and the Ag/AgCl reference electrode are based on molecular interactions between the molecules in solution and the molecules of the sensor membrane material; concentrations were presented as millimole per liter (mM). One mole of any substance contains the Avogadro’s number of atoms or molecules. By calculating the quantities of samples based on molar concentrations, precise molecular ratios can be calculated giving accurate estimates of quantities of flavors and/or sweeteners needed for masking effects.
Sample Preparation and e-Tongue Operational Conditions
Each series of experiments consisted of three main procedures: e-Tongue preparation and training, sample preparation and analysis, and data processing and statistical analysis (Table I). All samples were weighed using an analytical balance (±0.5 mg precision) and completely dissolved in appropriate volumes of non-deionized distilled water at 25°C to obtain the desired concentrations and taste attributes (Table II). Each of the e-Tongue testing beakers was loaded with 25 mL of the appropriate, particle-free solution. The reference electrode and the seven-sensor assembly were immersed into each testing beaker for an acquisition time of 120 s. This was followed by sequential immersion into two rinsing beakers containing fresh non-deionized distilled water for 10 s each to prevent any cross-contamination or carry-over residues from previous samples. This series of tests was repeated six times in rotation. The first two replicate measurements of the test solution were for sensor training purposes and the readings from the last four replicates were used for data analysis. The potentiometric difference created between each individual sensor and the reference electrode was measured and recorded by the e-Tongue BPM software. All samples were analyzed at room temperature.
Table I.
Summary of the Procedure Followed for Each Series of Experiments
| Major steps | Sub-steps |
|---|---|
| I. e-Tongue preparation and training | A. Sensors conditioning and calibration. |
| B. Sensors taste discrimination ability. | |
| C. Building and validating the bitterness standard model. | |
| II. Sample preparation and analysis | D. Preparation of EB (0, 0.3, 3, 9 mM) solutions. |
| E. Predicting EB bitter taste. | |
| F. Preparation of EB 9 mM + NMIs 0.5 mM (ASP, ASK and CA) solutions. | |
| G. Assessment of NMIs masking effect on EB. | |
| III. Data processing and statistical analysis | H. Building data libraries. |
| I. Data analysis using multivariate algorithms: | |
| 1. Principle component analysis (PCA). | |
| 2. Partial least-squares (PLS). |
EB epinephrine bitartrate, ASP aspartame, ASK acesulfame potassium, CA citric acid, NMIs non-medicinal ingredients
Table II.
Formulations Prepared for Taste Analysis by the e-Tongue
| Samples | Contents (concentration in mM) | Taste attribute(s) in order |
|---|---|---|
| API | EB (0.3, 3, or 9) | Bitter |
| Formulation 1 | EB (9), ASK (0.5) | Bitter, sweet |
| Formulation 2 | EB (9), ASP (0.5) | Bitter, sweet |
| Formulation 3 | EB (9), ASK (0.5), ASP (0.5) | Bitter, sweet, sweet |
| Formulation 4 | EB (9), ASK (0.5), ASP (0.5), CA (0.5) | Bitter, sweet, sweet, sour |
| Formulation 5 | EB (9), CA (0.5) | Bitter, sour |
API active pharmaceutical ingredient, EB epinephrine bitartrate, ASK acesulfame potassium, ASP aspartame, CA citric acid
Sensor Array Conditioning and Calibration
The best long-term storage environment for the sensitive e-Tongue sensors is in the dry state so they must be conditioned and hydrated before each use. Sensor conditioning is needed to check the signal stability of each individual sensor. Following a procedure prescribed by Alpha M.O.S., three beakers each containing 25 mL of 10−2 M HCl reference solution were used to condition the sensors and the reference electrode for 300 s in each immersion. The pass criterion was to achieve stable signals for all seven sensors with minimal or no noise or drift. This was a prerequisite prior to the calibration procedure. Due to the chemical nature of the samples and the sensitivity of the sensor array used in this study, the conditioning step was repeated 12 times at the beginning of every working week following ≥2 days of sensor storage in the dry state.
To ensure consistency and reproducibility of data produced from the e-Tongue, each individual sensor was calibrated to a known numerical value before use. Each sensor required its own target value and a previously defined error limit. The calibration step ensured that the output response of each sensor did not exceed the maximum error allowed. According to the calibration procedure prescribed by Alpha M.O.S., one beaker containing 25 mL of 10−2 M HCl reference solution was used to calibrate the sensors for 120 s for each immersion. The calibration step was performed after every successful conditioning step. The pass criterion for the calibration step was to have all sensors adjusted to their target values within the specified error limit.
Taste Discrimination Ability of the Sensor Array
The e-Tongue must be trained to identify distinctive tastes to ensure it is working optimally. A diagnostic procedure using HCl, NaCl and MSG each at a concentration of 10−1 M representing sourness, saltness, and umami tastes, respectively, was performed. The pass criterion required a discrimination index of at least 0.94 with compound clusters being visibly separated from each other on a principal component analysis (PCA) map.
Building and Validating a Bitterness Standard Model
A 1 to 20 range was used to associate the bitterness intensity of different APIs with scores (Table III). The specific type of sensors used in this study was designed to detect the bitter taste of APIs and correlate their measurements with the bitterness intensities of these standardized APIs. For this purpose, several APIs as references have been tasted in vivo at several concentrations by human SAPs and the bitterness scores were provided by Alpha M.O.S. (Table IV). To examine the correlation between in vivo measurements and the e-Tongue measurements, the same APIs were analyzed by the e-Tongue in the current experiments. The correlation of both data measurements was achieved using an inverse standard model based on partial least-squares (PLS) analysis. This bitterness standard model should have a correlation coefficient (r2) of 0.8 or more (16). As shown in Table IV, caffeine, paracetamol, prednisolone, and quinine each at two different concentrations were used to build the bitterness standard model. This model was validated using loperamide and famotidine, each at two different concentrations (Table IV).
Table III.
Bitterness Intensity Levels with Corresponding Scores Used in Building the Bitterness Standard Model
| Bitterness intensity level | Corresponding score | |
|---|---|---|
| From | To | |
| Taste not detected | 1 | 4.5 |
| Slight taste | 4.5 | 8.5 |
| Acceptable | 8.5 | 12.5 |
| Limit acceptable | 12.5 | 16.5 |
| Not acceptable | 16.5 | 20 |
Table IV.
The in vivo Sensory Analysis Panel (SAP) Scores Obtained for Reference Active Pharmaceutical Ingredients (APIs) at Each Concentration Used Either to Build or Validate the Bitterness Standard Model as Provided by Alpha M.O.S.
| Reference APIs | Used to builda | Used to validatea | Concentration (mM) | In vivo score |
|---|---|---|---|---|
| Caffeine | √ | 0.24 | 2.5 | |
| 2.36 | 8.5 | |||
| Paracetamol | √ | 3.31 | 4 | |
| 19.85 | 11 | |||
| Quinine | √ | 0.03 | 9 | |
| 0.12 | 15.5 | |||
| Prednisolone | √ | 0.44 | 13.5 | |
| 0.88 | 17 | |||
| Loperamide | √ | 0.002 | 7.5 | |
| 0.01 | 14 | |||
| Famotidine | √ | 0.06 | 4.2 | |
| 0.15 | 9 |
aThe reference active pharmaceutical ingredients (APIs) that were used either to build or to validate the bitterness standard model
Predicting the Bitter Taste of Epinephrine
The E base was only slightly soluble in water (18) therefore E water-soluble salts (hydrochloride and bitartrate) were used instead for medical applications (19). The bitartrate salt of E (EB) was used in our continuing studies because it is readily obtainable as the pure L-isomer, the pharmacologically active form used in the E SL tablet formulations. Numerous studies of various concentrations of EB were carried out to determine the e-Tongue threshold of EB. Ultimately in order to assess the degree of E bitterness, three solutions with increasing concentrations of EB (0.3, 3, and 9 mM) were analyzed by the e-Tongue and compared to a negative control of water containing no EB. Analysis of each solution was repeated at least three times.
Bitterness Masking of Epinephrine
To mask the bitter taste of EB, different NMIs were added to EB solutions. Based on critical and extensive review of the available NMIs used for taste-masking/improvement, aspartame (ASP) and acesulfame potassium (ASK) were selected as artificial sweeteners and citric acid (CA) as a flavor. Numerous studies of EB 9 mM plus various concentrations of NMIs were carried out to select the optimal ratio of these agents. In the definitive studies, all NMIs, each alone or in combination, were used at a concentration of 0.5 mM and added to EB 9 mM using the same sample analysis procedure described in the e-Tongue operational conditions. Analysis of each solution was repeated at least three times.
Data Processing and Statistical Analysis
Due to the complexities of analyzing the output data from several sensors for more than two samples, all data were processed and analyzed using the αAstree software provided by Alpha M.O.S. except for some primary data interpretation that was done using Microsoft Excell software following Alpha M.O.S. recommendations. The αAstree software reduces the number of variables created by the sensors when analyzing a given sample. Data reduction allows responses of the seven sensors to be processed and displayed in two- or three-dimensional maps. These tools are known as multivariate statistic algorithms to determine which of the differences between samples are important to identify unknown samples, to predict sensory intensities, or to quantify substance concentration of unknown samples. The principal component analysis (PCA) and the partial least squares (PLS) multivariate statistic techniques were used in this study. The PCA technique was used to assess discrimination performances of the sensors when examining their taste discrimination abilities. The PCA summarizes the information contained in the database into individual principle components (PCs) which are linear combinations of the original variables. For every sample analysis, the two PCs with most informative results are used to create the PCA map. The efficiency of the PCA map of a given group of samples is measured with the discrimination index. The closer the index to 100%, the more efficient the PCA map is. The PLS technique was used to quantify the intensity of the bitter taste of the samples assessed including the references and the samples. The PLS map is considered valid if the correlation coefficient is greater than 0.8 (16). This PLS map is then used to predict the bitterness intensities of unknowns. To obtain reproducible data, the relative standard deviations of each individual sensor type and in every analysis and experiment were confirmed to be below 3%.
RESULTS AND DISCUSSION
Sensor Array Conditioning and Calibration
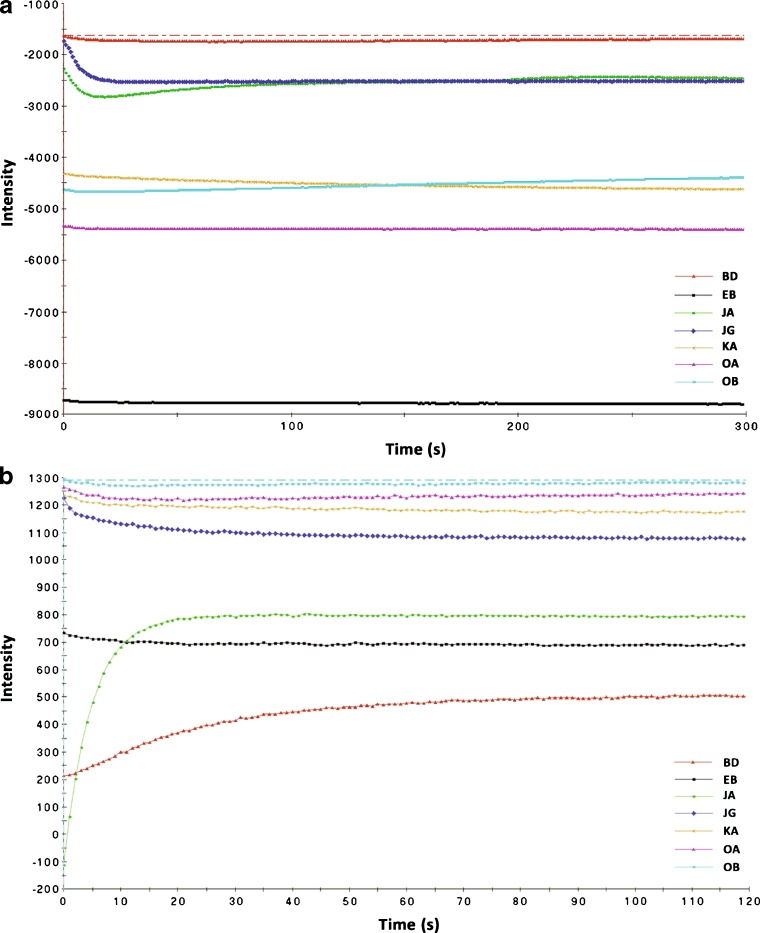
The organic coating membrane of the sensors must be completely hydrated in order to allow possible interactions between the sample molecules dissolved in liquid and the sensitized molecules of the coating membrane covalently bound to the solid electrochemical sensor. All sensors showed stable signals (Fig. 1a) in the sensor array conditioning step and among the experiments with minimal noise and drift. The output from the seven sensors was successfully adjusted to the default target intensity value (Fig. 1b). These are predetermined values that were set by default for every individual sensor (Table V).
Fig. 1.
Sensor array conditioning and calibration. a Example of a stable signal for the sensor array used in this study. b Example of a successful calibration (hydration) step in which the numerical values of all sensors were adjusted to their target values. BD, EB, JA, JG, KA, OA, and OB are sensor types (Letter designations are Alpha M.O.S. identification codes)
Table V.
The Target and Actual Achieved Values for Each Individual Sensor Used in this Study
| Sensor typea | Target value (mV) | Achieved value (mV) | Difference (mV) | Error (%) | Pass/fail |
|---|---|---|---|---|---|
| BD | 500 | 504.00 | −4.0 | 0.80 | Pass |
| EB | 700 | 690.30 | 9.7 | 1.39 | Pass |
| JA | 800 | 794.68 | 5.32 | 0.67 | Pass |
| JG | 1,080 | 1,079.05 | 0.95 | 0.09 | Pass |
| KA | 1,200 | 1,174.52 | 25.48 | 2.12 | Pass |
| OA | 1,250 | 1,239.74 | 10.26 | 0.82 | Pass |
| OB | 1,300 | 1,281.54 | 18.46 | 1.42 | Pass |
aLetter designations are company identification codes
Taste Discrimination Ability of the Sensor Array
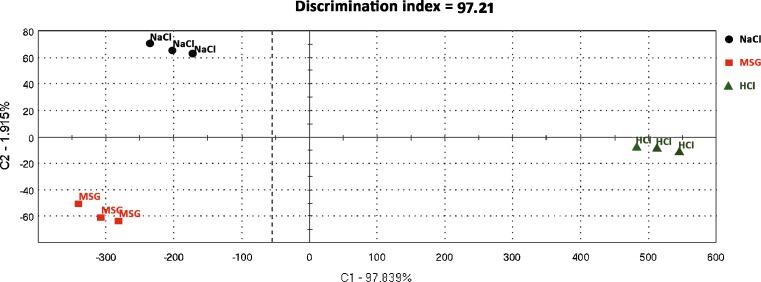
The human tongue can recognize five basic tastes: salt, sour, sweet, bitter, and umami. The umami taste is commonly referred to the taste of MSG first described by Kikunae Ikeda (20) and widely used as a flavor enhancer. These taste attributes were tested using the e-Tongue in order to determine its ability to differentiate between tastes: salt, sour, and umami. A mean discrimination index of 97.8% was obtained from 11 repetitions throughout all experiments performed to achieve the objectives of this study using the e-Tongue (Fig. 2).
Fig. 2.
Example of a successful taste discrimination test having a discrimination index of 97.2%. The three different taste compounds (NaCl, HCl, and MSG) were discriminated from each other into separate space locations in a two-dimensional principal component analysis (PCA) map
Building and Validating a Bitterness Standard Model
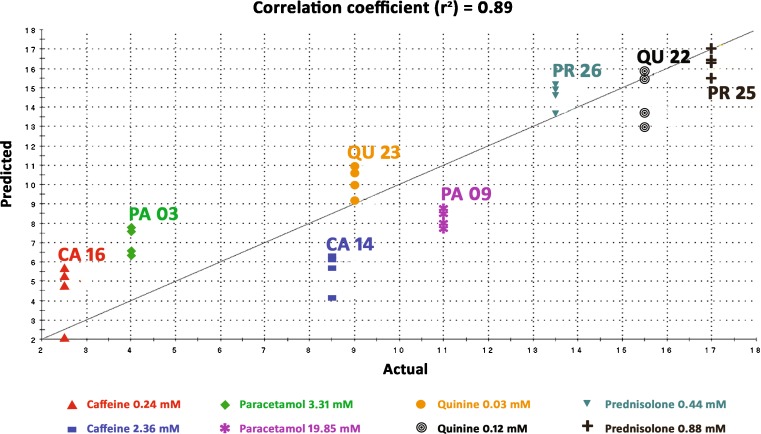
Actual bitterness scores (Table IV) from in vivo studies (Alpha M.O.S.) were compared to predicted bitterness scores from the e-Tongue (Fig. 3). In all standard models built throughout the experiments, the correlation coefficient (r2) obtained was always above 80% which is the acceptable criterion for a successful model (16). Absolute differences (∆) between actual and predicted scores were calculated and were always within the limits specified by Alpha M.O.S. For the four standard drugs used to build the bitterness standard model, ∆ was always <2.5; and for those two standard drugs used to validate the model, ∆ was always <5.
Fig. 3.
Example of a successful bitterness standard model (r 2 = 89%). The straight line shown represents an ideal 100% correlation and the colored points are the predicted e-Tongue measurements in comparison with the actual in vivo sensory analysis panel (SAP) measurements for four standards (caffeine, paracetamol, quinine, prednisolone). The other two standards (loperamide, famotidine) were used to validate the bitterness standard model (validation results are not shown)
e-Tongue Threshold and Concentration Determination of EB
A number of studies were performed to determine the threshold of the e-Tongue sensors to an appropriate range of EB concentrations that could be evaluated using this instrument. Based on the results from studies of other bitter APIs, an initial concentration of EB 60 mM was tested using the e-Tongue which resulted in consistent bitterness scores of >20, above the maximum level, indicating that EB has an intensely bitter taste. The concentration was reduced systematically until within-range bitterness scores of ≤20 were obtained for EB. These relatively low concentrations (0.3, 3, 9 mM) were selected for evaluation. The EB 9 mM concentration was selected as the maximum strength to evaluate a series of bitterness-masking NMIs.
Reproducibility and Method Modifications
Lack of reproducibility of results obtained from the e-Tongue was observed after several taste-masking studies of EB 9 mM with all NMIs. In order to obtain reproducible results from the e-Tongue, major adjustments were made to several procedural steps. The one time hydration or conditioning of e-Tongue sensors was increased to 12 times. Instead of immersion in one rinsing beaker of water between active samples for evaluation, the sensors were sequentially immersed in two beakers of water for 10 s in each, following each sample analysis. The intense bitterness of EB almost overwhelmed the sensors and the offset sensor values had to be readjusted using strict, default, and large calibration levels. These levels were evaluated for the parameters that best reproduced the results from the e-Tongue. The parameters define the maximum allowed dispersion or drift of within and across each sensor’s responses.
Predicting the Bitter Taste of EB
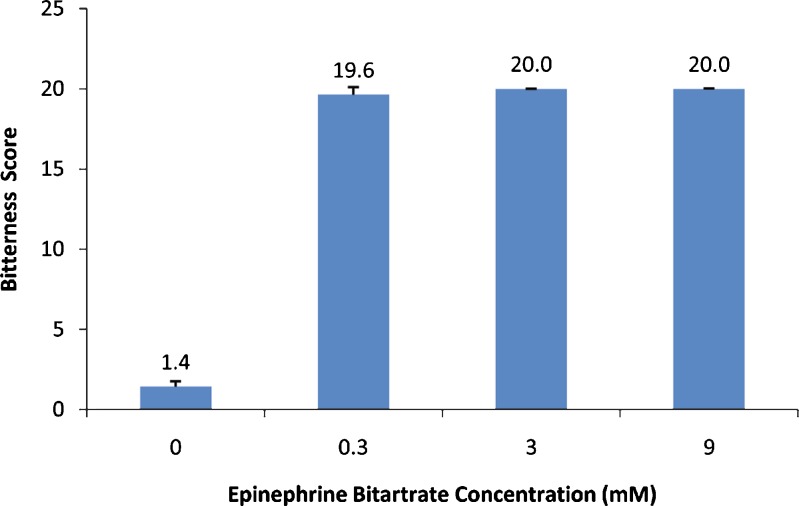
As expected, EB solutions resulted in high scores of bitterness (Fig. 4). Even the lowest, EB 0.3 mM, resulted in a 19.6 ± 0.5 bitterness score which indicated an unacceptable bitter taste. The intensely bitter taste of EB required an efficient taste-masking approach to enable the formulation of palatable SL tablets. This approach should lack any heating or moistening process that may affect the chemical stability of the heat and moisture-labile EB. The addition of intense sweeteners and/or flavors to EB was identified as the most suitable approach to mask EB bitterness and was assessed in this study.
Fig. 4.
Bitterness scores for epinephrine bitartrate (EB) at three different concentrations. A blank of water (containing no EB) was used as a negative control
Bitterness Masking of Epinephrine
Two artificial sweeteners (ASP and ASK) were selected to mask the unacceptable highly bitter taste of EB. ASP and ASK have an approximate sweetening power of 180–200 times that of sucrose (21). ASP, a first-generation artificial sweetener, enhances flavor systems and can be used to mask some unpleasant taste characteristics. It is widely used in medications including Feldene Melt (piroxicam), Maxalt-MLT (rizatriptan), Pepcid RPD (famotidine), Zyprexa Zydis (olanzapine), Zofran ODT (ondansetron), and Nulev (hyoscyamine) (22). ASK, a second- or new-generation artificial sweetener, is widely used as a sugar substitute in compounded formulations and as a toothpaste sweetener (21). Although artificial sweeteners have been reported to show toxic, mutagenic, or carcinogenic effects, results are inconsistent due to poor study design (23–25). Toxicities related to ASP or ASK were observed at doses many fold greater than those proposed here (26–28). Minute quantities of these sweeteners could be safely incorporated into a SL tablet formulation of EB developed in our laboratory with minimal effect on the in vitro characteristics of these tablets.
CA was selected as a flavoring agent to be added to EB due to its wide use, safety, and acceptance by children who prefer the sour “lemon” taste over the sweet (29). CA is widely used in a number of FDA approved products available in the market like Remeron Soltab (mirtazepine) and Zoming ZMT (zolmitriptan) (22).
The ratio of EB to these NMIs must be appropriate for use in formulation of E SL tablets. Accordingly, ASP was first used for taste-masking studies at 0.1, 0.5, and 5 mM reducing the bitterness score of EB 9 mM to 14.4, 14.0, and 13.9, respectively. From these results, it seemed that ASP 0.1 mM partially masked the bitterness score of EB 9 mM but there was no apparent increased effect upon increasing the concentration of ASP. Similar concentration-independent masking trends were observed with either ASK or CA alone when added to EB 9 mM. The NMI 0.5 mM concentration was selected for further studies because when tested against EB 9 mM, a ratio of around 1:30 (NMI:API) was achieved for either CA:EB or ASK:EB and of around 1:20 for ASP:EB, (based on a milligram scale) which was feasible to achieve in the tablet formulation.
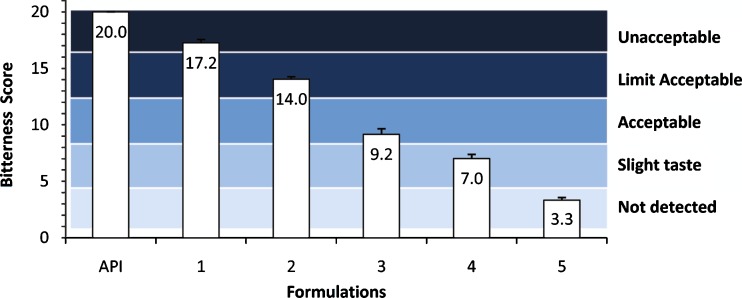
ASP and ASK, alone or in combination, at a concentration of 0.5 mM were added to a EB 9 mM solution resulting in a maximum bitterness-masking effect of more than 54% when both sweeteners (Formulation 3) were used (Fig. 5). Neither of the sweeteners alone at the concentration 0.5 mM (Formulations 1 and 2) improved the bitterness intensity from “unacceptable” to “acceptable” but did in combination (Formulation 3) suggesting a synergistic effect. This combination of ASP and ASK was much more effective in reducing the bitterness score of EB 9 mM than increasing the concentration of either of them when used alone.
Fig. 5.
Bitterness scores and intensity levels of the formulations in comparison with epinephrine bitartrate (EB) 9 mM as a positive control. Formulations contained EB alone or in combination with acesulfame potassium (ASK), aspartame (ASP), and/or citric acid (CA) as following: API (EB 9 mM), 1 (EB 9 mM, ASK 0.5 mM), 2 (EB 9 mM, ASP 0.5 mM), 3 (EB 9 mM, ASK 0.5 mM, ASP 0.5 mM), 4 (EB 9 mM, ASK 0.5 mM, ASP 0.5 mM, CA 0.5 mM), 5 (EB 9 mM, CA 0.5 mM)
When CA 0.5 mM was added to the combination of ASP and ASK (Formulation 4) the masking effect increased to almost 65% reducing the bitter taste of EB 9 mM from “unacceptable” to “slight taste”. CA alone (Formulation 5) was able to inhibit the intense bitter taste of EB 9 mM by more than 80% from “unacceptable” to “not detected” (Fig. 5). The CA results were consistent with previous reports in that bitter taste-masking effects of acidic substances are pH- rather than concentration-dependent. It was also found that acidic substances have an inhibitory effect on one of the human bitter taste receptors found in the tongue (30).
Based on the assumption that interactions occur among molecules in solution and with molecules of the sensor coatings, every molecule of EB could interact with one molecule of the masking agent for complete masking efficacy, e.g., EB 9 mM would require NMI 9 mM for 100% masking effect. However, this assumption alone cannot explain the results reported above, so other mechanisms of masking effect are likely involved. In addition to the molecular interaction assumption, the masking effects could be explained by the different affinities EB and the NMIs might have toward each other and toward the sensor coatings.
From our results, it can be seen that neither ASK (Formulation 1) nor ASP (Formulation 2) alone at 0.5 mM was effective in masking the bitter taste of EB 9 mM. Even the combination of ASK and ASP (Formulation 3) did not reduce the bitterness intensity level of EB 9 mM to “not detected”. These results could be explained by the slightly bitter aftertaste these sweeteners have (21) which appeared to be masked by the addition of CA 0.5 mM (Formulation 4). However, CA 0.5 mM alone (Formulation 5) resulted in the best masking of the bitterness of EB 9 mM of >80% from “unacceptable” to “not detected”.
CONCLUSION
We have demonstrated that the e-Tongue is a useful tool in taste assessment, enhancement, and masking studies for an intensely bitter substance such as EB. The e-Tongue has the potential to screen different NMIs to determine the agent that best masks the unpleasant taste of the API, especially in the early stage of drug and formulation development. To our knowledge, this is the first study showing the degree of EB bitterness and the taste-masking effect of sweetening and/or flavoring NMIs using the e-Tongue.
ACKNOWLEDGMENT
The e-Tongue was provided by the Richardson Centre for Functional Foods and Nutraceuticals (RCFFN), University of Manitoba, Canada.
Ousama Rachid would like to acknowledge the financial support received from the Manitoba Health Research Council/Manitoba Institute of Child Health (MHRC/MICH) Studentship Award, the University of Manitoba Graduate Fellowship (UMGF), and the Pfizer Canada Inc. Centennial Pharmacy Research Award.
REFERENCES
- 1.Ayenew Z, Puri V, Kumar L, Bansal AK. Trends in pharmaceutical taste masking technologies: a patent review. Recent Pat Drug Deliv Formul. 2009;3(1):26–39. doi: 10.2174/187221109787158364. [DOI] [PubMed] [Google Scholar]
- 2.Repchinsky C. Compendium of pharmaceuticals and specialties: the Canadian drug reference for health professionals. 44. Toronto: Canadian Pharmacists Association; 2009. [Google Scholar]
- 3.Mennella JA, Beauchamp GK. Optimizing oral medications for children. Clin Ther. 2008;30(11):2120–2132. doi: 10.1016/j.clinthera.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberman DB, Teach SJ. Management of anaphylaxis in children. Pediatr Emerg Care. 2008;24(12):861–866. doi: 10.1097/PEC.0b013e31818ea116. [DOI] [PubMed] [Google Scholar]
- 5.Simons FER. Anaphylaxis: recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124(4):625–636. doi: 10.1016/j.jaci.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Rawas-Qalaji MM, Simons FER, Simons KJ. Fast-Disintegrating Sublingual Tablets: Effect of Epinephrine Load on Tablet characteristics. AAPS PharmSciTech. 2006;7(2): Article 41. [DOI] [PMC free article] [PubMed]
- 7.Rawas-Qalaji MM, Simons FER, Simons KJ. Sublingual epinephrine tablets versus intramuscular injection of epinephrine: dose equivalence for potential treatment of anaphylaxis. J Allergy Clin Immunol. 2006;117(2):398–403. doi: 10.1016/j.jaci.2005.12.1310. [DOI] [PubMed] [Google Scholar]
- 8.Rawas-Qalaji MM, Simons FER, Simons KJ. Epinephrine for the treatment of anaphylaxis: do all 40 mg sublingual epinephrine tablet formulations with similar in vitro characteristics have the same bioavailability? Biopharm Drug Dispos. 2006;27(9):427–435. doi: 10.1002/bdd.519. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz JK, Reo JP, Hendl O, Worthington JH, Petrossian VD. Evaluation of a taste sensor instrument (electronic tongue) for use in formulation development. Int J Pharm. 2009;367(1–2):65–72. doi: 10.1016/j.ijpharm.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Zheng JY, Keeney MP. Taste masking analysis in pharmaceutical formulation development using an electronic tongue. Int J Pharm. 2006;310(1–2):118–124. doi: 10.1016/j.ijpharm.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 11.Takagi S, Toko K, Wada K, Ohki T. Quantification of suppression of bitterness using an electronic tongue. J Pharm Sci. 2001;90(12):2042–2048. doi: 10.1002/jps.1155. [DOI] [PubMed] [Google Scholar]
- 12.Sadrieh N, Brower J, Yu L, Doub W, Straughn A, Machado S, Pelsor F, Martin ES, Moore T, Reepmeyer J, Toler D, Nguyenpho A, Roberts R, Schuirmann DJ, Nasr M, Buhse L. Stability, dose uniformity, and palatability of three counterterrorism drugs-human subject and electronic tongue studies. Pharm Res. 2005;22(10):1747–1756. doi: 10.1007/s11095-005-6387-x. [DOI] [PubMed] [Google Scholar]
- 13.Kayumba PC, Huyghebaert N, Cordella C, Ntawukuliryayo JD, Vervaet C, Remon JP. Quinine sulphate pellets for flexible pediatric drug dosing: formulation development and evaluation of taste-masking efficiency using the electronic tongue. Eur J Pharm Biopharm. 2007;66(3):460–465. doi: 10.1016/j.ejpb.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Naini V, Ahmed SU. Utilization of a modified special-cubic design and an electronic tongue for bitterness masking formulation optimization. J Pharm Sci. 2007;96(10):2723–2734. doi: 10.1002/jps.20900. [DOI] [PubMed] [Google Scholar]
- 15.Tokuyama E, Matsunaga C, Yoshida K, Mifsud JC, Irie T, Yoshida M, Uchida T. Famotidine orally disintegrating tablets: bitterness comparison of original and generic products. Chem Pharm Bull (Tokyo) 2009;57(4):382–387. doi: 10.1248/cpb.57.382. [DOI] [PubMed] [Google Scholar]
- 16.Alpha MOS. Astree electrochemical sensor technology—Technical note: T-SAS-04. 2004.
- 17.Mifsud J-C, Lucas Q. Alpha M.O.S. Apparatus and method for characterizing liquids. US Patent. 2003;6,290,838. Application No. 380663. Filed November 22, 1999.
- 18.Keef KA. Sympathomimetic drugs. In: Hendrickson R, chairman of the editorial board. Remington: The science and practice of pharmacy. 21st ed. Baltimore, Md: Lippincott Williams & Wilkins; 2005. p. 1386.
- 19.Sciarra JJ, Patel JM, Kapoor AL. Synthesis and formulation of several epinephrine salts as an aerosol dosage form. J Pharm Sci. 1972;61(2):219–223. doi: 10.1002/jps.2600610216. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K. New seasonings. Chem Senses. 2002;27(9):847–849. doi: 10.1093/chemse/27.9.847. [DOI] [PubMed] [Google Scholar]
- 21.Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. 4. London: The Pharmaceutical Press; 2003. [Google Scholar]
- 22.Goel H, Rai P, Rana V, Tiwary AK. Orally disintegrating systems: innovations in formulation and technology. Recent Pat Drug Deliv Formul. 2008;2(3):258–274. doi: 10.2174/187221108786241660. [DOI] [PubMed] [Google Scholar]
- 23.Hagiwara A, Fukushima S, Kitaori M, Shibata M, Ito N. Effects of three sweeteners on rat urinary bladder carcinogenesis initiated by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Gann. 1984;75:763–768. [PubMed] [Google Scholar]
- 24.Ishii H. Incidence of brain tumors in rats fed aspartame. Toxicol Lett. 1981;7:433–437. doi: 10.1016/0378-4274(81)90089-8. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson B, Williams GM. Carcinogenicity of aspartame in rats not proven. Environ Health Perspect. 2008;116(6):A239–240. doi: 10.1289/ehp.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehouse CR, Boullata J, McCauley LA. The potential toxicity of artificial sweeteners. AAOHN J. 2008;56(6):251–259. doi: 10.3928/08910162-20080601-02. [DOI] [PubMed] [Google Scholar]
- 27.Bandyopadhyay A, Ghoshal S, Mukherjee A. Genotoxicity testing of low-calorie sweeteners: aspartame, acesulfame-K, and saccharin. Drug Chem Toxicol. 2008;31(4):447–457. doi: 10.1080/01480540802390270. [DOI] [PubMed] [Google Scholar]
- 28.Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, Pariza MW, Spencer PS, Waddell WJ, Walker R, Williams GM. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol. 2007;37(8):629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- 29.Liem DG, Westerbeek A, Wolterink S, Kok FJ, de Graaf C. Sour taste preferences of children relate to preference for novel and intense stimuli. Chem Senses. 2004;29:713–720. doi: 10.1093/chemse/bjh077. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai T, Misaka T, Nagai T, Ishimaru Y, Matsuo S, Asakura T, Abe K. pH-Dependent inhibition of the human bitter taste receptor hTAS2R16 by a variety of acidic substances. J Agric Food Chem. 2009;57(6):2508–2514. doi: 10.1021/jf8040148. [DOI] [PubMed] [Google Scholar]