Abstract
This study aimed at preparation of a sustained-release steroidal treatment for chronic inflammatory conditions, such as rheumatoid arthritis. To achieve such a goal, biodegradable poly-lactide-co-glycolide prednisolone-loaded microspheres were prepared using o/w emulsion solvent evaporation method. Formulation parameters were adjusted so as to optimize the microsphere characteristics. The prepared microspheres exhibited smooth and intact surfaces, with average size range not exceeding 65 µm. The encapsulation efficiency percent of most microsphere formulations fell within the range of 25–68%. Drug release from these microspheres took place over 4 weeks, with near-to-zero-order patterns. Two successful formulations were chosen for the treatment of unilateral arthritis, induced in mice using Freund's complete adjuvant (FCA). Inflammatory signs of adjuvant arthritis included severe swelling of the FCA-injected limbs, in addition to many histopathological lesions. These included inflammatory cell infiltration, synovial hyperplasia, cartilage, and bone erosion. Parenteral administration of the selected formulae dramatically reduced the swelling of the FCA-injected limbs. In addition, histological examination revealed that the microsphere treatment protocol efficiently protected cartilages and bones of mice, injected with FCA initial and booster doses, from erosion. These results could not be achieved by a single prednisolone dose of 5 mg/kg.
KEY WORDS: adjuvant arthritis, histological investigation, microspheres, PLGA, prednisolone
INTRODUCTION
Arthritis is a wide-range term that describes various conditions. Rheumatoid arthritis (RA), a common autoimmune disease, is characterized by chronic inflammation of synovial joints and progressive destruction of articular tissue (1). Cartilage destruction appears as narrowing of the joint space and is followed by destruction of the underlying bone (2,3). The disease is thought to affect approximately 1% of the population worldwide (2). The most commonly prescribed medication for rheumatoid arthritis is steroidal, nonsteroidal anti-inflammatory, disease-modifying anti-rheumatic, and immunosuppressant drugs (4).
Experimentally, arthritis could be induced in different animal models by the administration of various agents, namely Freund's complete adjuvant (FCA), type II collagen, and streptococcal wall (5). Adjuvant arthritis (AA) is one of the most widely used models to study the pathogenesis of RA and for screening the new drugs for treatment of rheumatoid diseases, as it shares some features with human RA (6). Therefore, it is an ideal model to study anti-inflammatory effects (7).
Adjuvant-induced arthritis model was established for mice (2,8,9). Briefly, the animals are injected intradermally with FCA, which is composed of heat-killed Mycobacterium tuberculosis, suspended in paraffin oil, either in the tail base (10–12) or in the hind paw [plantar (13) or subplantar regions(14), tibiotarsal joint (9), or stifle joint (2)]. Early manifestations that follow injection of FCA in the foot pads appear as swelling and edema, followed by inflammatory cell infiltration. Hyperplasia of synovial lining cells and proliferation of granulation tissue take place later. This may be followed by damage of bone and cartilage, accompanied by rapid new bone formation in the adjacent periosteum (2,8,15).
In 1950, the Noble Prize in Medicine and Physiology was awarded to Kendall, Reichstein, who independently isolated and synthesized cortisol, and to Hench, who first applied it and described its dramatic efficacy on patients with developed RA in 1949 (16–18). Since then, and despite their long-term unfavorable side effects, corticosteroids are still used extensively and successfully in treatment of many inflammatory conditions, including RA, either alone or co-administered with other drugs (17–19).
Recent reports highlighted the effectiveness of corticosteroids in reducing inflammation and slowing joint damage, especially when administered at an early stage of disease propagation (20). In addition, it was also reported that prednisolone given for an extended period is capable of ameliorating joint damage in both collagen- and antigen-induced murine arthritis (2). So, a sustained-release biocompatible and biodegradable drug delivery system, containing a steroid, such as prednisolone, is believed to be beneficial in management of RA, especially at its early stages.
In this paper, prednisolone is encapsulated in poly-lactide-co-glycolide (PLGA) microspheres to provide a sustained delivery of the drug, thus, reducing the frequency of administration and consequently, enhancing patient compliance. It is well-known that poor compliance is one of the reasons for treatment failure in RA (21).
Microspheres loaded with prednisolone or prednisolone derivatives have been manufactured and described previously in the literature. Polymers such as PLA (22,23), PGA (24), hyaluronan (25), gelatin (26), and chitosan (27–29) have been commonly used for encapsulation of prednisolone.
The use of PLGA as a biodegradable polymer in microsphere production is common in the literature due to its attractive properties, including the availability of various co-polymer compositions and molecular weights, which makes the manufacture of microspheres with tailored characteristics accessible (30). Formulation parameters of the produced microspheres, such as particle size, encapsulation efficiency percent (EE%), and release characteristics, were studied, in order to optimize the microsphere characteristics. These microspheres were applied in an adjuvant-induced arthritis model in mice (9). This model involved the induction of localized mono-arthritis, enabling the study of the arthritic lesion without the complicating factors of poor animal mobility, altered weight gain, and systemic disease associated with poly-arthritis (9). Different methods were used to evaluate the induced adjuvant arthritis along with the effect of the microspheres, including morphological examination, diameter measurement of the affected joint, and histopathological evaluation.
The aim of this study can be summarized as preparation of prednisolone-loaded PLGA microspheres with optimized characteristics and in vivo evaluation of this extended-release steroid delivery system in AA in mice.
EXPERIMENTAL
Materials
Poly dl-lactide-co-glycolide, PLGA (Resomer® RG503H, intrinsic viscosity of 0.32 to 0.44 dL/g, molecular weight of 34 kd) was purchased from Boehringer Ingelheim, GmBH, Germany). Prednisolone (PD) was kindly granted from Al-Kahira Pharmaceutical Company (Cairo, Egypt). Polyvinyl alcohol (PVA; partially hydrolyzed, degree of hydrolysis 88%) was obtained from Fluka (Fluka Chemie, GmBH, Germany). Tween 80, gelatin, and methyl cellulose (MC) were purchased from ADWIC (El-Nasr Pharmaceutical Co., Egypt). Sodium dodecyl (lauryl) sulfate (SDS) was obtained from Winlab (Winlab, Market Harborough, Leicestershire, UK). FCA (cell suspension of heat-killed M. tuberculosis in sterile paraffin oil) was purchased from Sigma (Sigma-Aldrich Inc., St. Louis, Missouri, USA). All other reagents and solvents were of analytical grade and used as received.
Methodology
Preparation of the Microspheres
Microspheres were prepared using oil-in-water (o/w) emulsion–solvent evaporation technique. Different weights of PLGA were dissolved in 1.5 ml of dichloromethane (DCM) in a screw-capped test tube to make solutions of 7.5–12.5% w/v. Weighed amounts of PD, to make drug/PLGA ratio as 1:4, were then dispersed in the organic phase using a sonicated water bath (BranSonic 220, Zurich, Switzerland) for 10 min. The organic phase was then added drop by drop using a Pasteur pipette to 50 mL of an aqueous solution of the emulsifying agent in a beaker stirred at 2,000 rpm by an over-head stirrer (Janke and Kunkel KG, Germany) for 10 min. The formed emulsion was then stirred under a slower speed (500 rpm) using a magnetic-type stirrer (Heidolph MR 3001, Heidolph, Germany) for 3 h to evaporate the organic solvent. Microspheres were then harvested by centrifugation (Fischer Centrific® Centrifuge, USA) at 8,000 rpm, washed three times with distilled water, and freeze-dried overnight (FreeZone 180, Labconco Corporation, Kansas City, Missouri, USA). Dried microspheres were stored at −20°C pending investigation.
Characterization of the Microspheres
Morphology
The morphology of the microsphere surfaces was investigated using scanning electron microscopy (SEM). Microspheres were spread on a carbon double-adhesive layer on a metal holder and gold-coated using an ion-sputtering device (Jeol Fine-Coat JFC 1100E, Jeol LTD, Tokyo, Japan). The microspheres were scanned by SEM (Jeol JSM-5400 LV, Jeol LTD, Tokyo, Japan).
Particle Size Analysis
The size distribution of the PLGA microspheres was investigated using laser light diffraction (Cilas particle size analyzer, model 1064 liquid, France). For a typical experiment, about 30 mg of microspheres were suspended in 100 ml water, sonicated for 30 s, and analyzed. The sizes below which 10% (D10), 50% (D50), and 90% (D90) of the microspheres fell were used to characterize the microspheres size distribution. The mean diameter was taken as the average of D10, D50, and D90 values.
Span value was used to represent size uniformity and dispersity of the microspheres; it was calculated from the following formula (31):
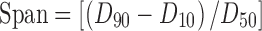 |
1 |
Encapsulation Efficiency
Weighed amounts of the microspheres were dissolved in 25 ml of DCM by sonication (for 15 min); the drug was analyzed in DCM using UV/Vis spectrophotometer (Spectronic Genesys 2PC, Milton Roy Co., USA) at 241 nm provided with Winspec software. Drug-free microspheres were prepared and subjected to the same procedure, and the solution obtained from which was used as a blank. PLGA did not show any significant UV absorbance in the selected wavelength range. All experiments were carried out in triplicate.
Drug EE% was calculated using the following formula:
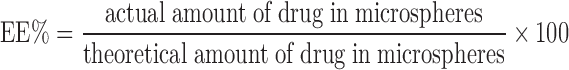 |
2 |
Release Study
Approximately 10 mg of PD-loaded microspheres were suspended in 6 ml phosphate--buffered saline (PBS, pH 7.4) in capped test tubes. The tubes were kept under constant shaking (60 rpm) in a shaking water bath (PolyScience, model 20 L-M, Niles, Illinois, USA) at 37°C. The release experiments were carried out under sink condition, where the drug concentration in the release medium was not exceeding 10% of the saturation concentration (∼0.4 mg/ml). At time intervals, the tubes were centrifuged, and 5 ml was withdrawn from each tube and replaced with 5 ml of fresh buffer (kept at the same temperature). The drug concentration was determined spectrophotometrically in the withdrawn samples at 247 nm. Blank microspheres were subjected to the same procedure, and the supernatant obtained was used as a blank at the same time intervals. All release experiments were carried out in triplicate.
PLGA Degradation Study
pH Measurements
A weighed amount of the drug-free PLGA microspheres (50 mg) was immersed in 20 ml PBS (pH 7.4) in a 100-ml beaker. The beaker was shaken at 37°C at 60 rpm. pH values were measured at predetermined time intervals.
Scanning Electron Microscopy
A weighed amount of the drug-free PLGA microspheres (10 mg) were placed in a screw-capped test tube containing 6 ml PBS 7.4. The test tubes were put in a shaking water bath at 37°C at 60 rpm. At predetermined time intervals, a tube was randomly selected and centrifuged at 4,000 rpm. The supernatant was removed, and the residue was dried by lyophilization. These samples were visualized using SEM as previously described.
Glass Transition Temperature Measurements
Glass transition temperatures (Tg) of the lyophilized microspheres, obtained from the previous experiment were measured using a differential scanning calorimeter (DSC, Perkin Elmer, 2-C, New York, USA) instrument with a thermal analysis data station system. Procedures included heating ∼5 mg samples from 30–300°C at a scanning rate of 5°C/min in sealed aluminum pans under a stream of nitrogen gas at flow rate of 40 ml/min. The instrument was calibrated using indium standard.
Animal Study
Adjuvant Arthritis Induction
Female Swiss Albino mice weighing 20–25 g (8 weeks old) were obtained from the university animal house. They were acclimatized for 1 week. Food and water were supplied ad libitum. All animal experiments carried out in this study were in accordance with guidelines of the Institutional Animal Ethics Committee.
Animal were allocated in five groups, ten animals each. Group I was left without neither arthritis induction nor drug treatment (negative control). Meanwhile, AA was induced in all other mice by injecting them with 0.1 ml FCA (1 gm/ml) subdermally around the tibiotarsal joint of the left hind limb on day 0. One week later, the FCA-injected mice were also injected with a booster dose of FCA of 0.05 ml subcutaneously in the subplantar side of the left hind paw. The other leg of the animal was used as control. Group II was used as positive control, in which no drug treatment was given. Instead, they received a volume of the vehicle (sterile isotonic saline solution containing 0.5% w/v Tween 80 and Na carboxymethyl cellulose), equal to that used for microspheres administration. One week after the booster dose, group III was injected subcutaneously with a sterile solution of prednisolone in the vehicle at a dose of 5 mg/kg in the backs of the animals (single dose-treated). On the other hand, mice from group IV were injected subcutaneously with a suspension of the microspheres from F#3 in the vehicle (suspended at the time of injection), so as to provide a daily dose of 5 mg/kg of prednisolone, in the same region. Group V mice were similarly injected with a suspension of F#6 microspheres in the vehicle to provide the same dose of prednisolone, i.e., 5 mg/kg/day.
Assessment of the Induced Arthritis
Morphological Examination
Photographs of both arthritic and contralateral un-immunized hind paws of mice from different groups were taken using a digital camera (Nikon D80, Nikon, Japan) on the 15th day of starting drug treatment.
Measurement of Ankle Joint Diameter
Diameters of both left (ipsilateral) and right (contralateral) ankle joints of animals from different groups were measured at anterior–posterior position using a digital micrometer (Mitutuyu, Tokyo, Japan, accuracy 0.01 mm) at days 0, 2, 4, 9, 14, and 24.
Histopathological Examination
After 24 days of drug treatment, animals were sacrificed by cervical dislocation. Their hind limbs were removed and fixed in phosphate-buffered saline solution containing 10% formaldehyde at 4°C for 7 days. Then, they were moved to 10% ethylenediaminetetraacetic acid solution for decalcification for another 7 days. Finally, the specimens were washed thoroughly in distilled water, cut longitudinally, and then embedded in paraffin blocks. Sections of 10-µm thickness were mounted on glass slides and stained with hematoxylin–eosin for histopathological examination using light microscopy with the aid of a digital camera (Olympus, Japan) connected to a computer.
Statistical Analysis
Results were expressed as mean ± SD. Student's t test was used to make comparisons between the groups. P values <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Microsphere Size and Morphology
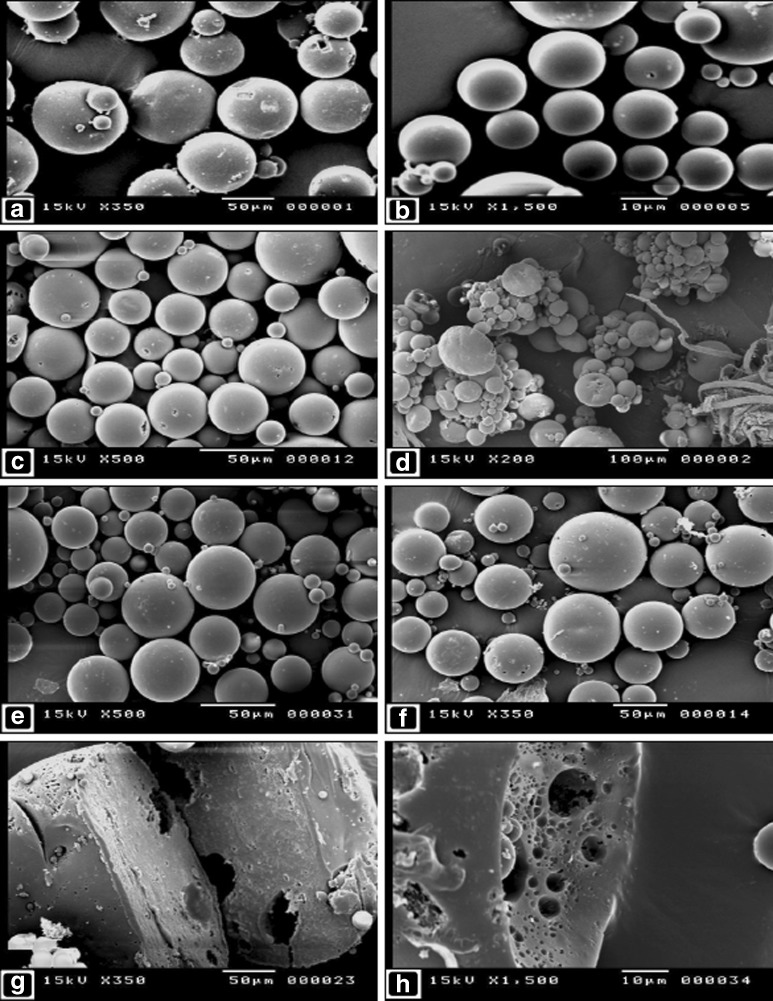
Table I shows different formulation parameters and characteristics, including particle size results and span values of the prepared microspheres formulae. The mean particle size of the microspheres ranged from ∼18 to 65 µm, according to the type and concentration of the emulsifier used. The SEM images showed that the microspheres are spherical in shape, with smooth and intact surfaces (Fig. 1a–c, f). The effect of PVA concentration on the size of the microspheres can be seen by comparing between the images of F#3 and 5 (Fig. 1a, b, respectively). It is obvious that increasing PVA concentration markedly lowered the mean particle size of the produced microspheres. When Tween 80 was used as an emulsifier (F#7), massive aggregations of small microspheres, in addition to large and irregular particles, could be seen (Fig. 1d). This indicates the inability of Tween 80, under such conditions, to maintain the stability of the emulsion during emulsification (32). On the other hand, upon using SDS as an emulsifier (F#8 and 10), the produced microspheres were spherical and intact, with no evidence of microspheres aggregation (Fig. 1e). Porous internal structures of the microspheres (F#11 and 12) can be seen in Fig. 1g, h, respectively.
Table I.
Formulation Parameters and Properties of Different Formulae of Prednisolone-Loaded PLGA Microspheres
| F# | Polymer conc. (% w/v) | Emulsifier type | Emulsifier conc. (% w/v) | Yield (% w/w) | D10 (µm) | D50 (µm) | D90 (µm) | Average diameter (µm) | Span value | Drug content % (w/w ±SD)a | EE % (w/w ±SD)a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.5 | PVA | 1 | 54.1 | 17.18 | 48.33 | 76.1 | 47.11 | 1.22 | 5.27 ± 0.12 | 26.3 ± 0.59 |
| 2 | 10 | PVA | 1 | 48.5 | 18.87 | 51.23 | 74.84 | 49.2 | 1.09 | 7.43 ± 0.31 | 37.17 ± 1.56 |
| 3 | 12.5 | PVA | 1 | 50.4 | 20.3 | 52.01 | 86.15 | 53.42 | 1.27 | 13.6 ± 0.27 | 68.0 ± 1.35 |
| 4 | 12.5 | PVA | 3 | 58.97 | 15.38 | 37.85 | 65.72 | 39.37 | 1.33 | 6.88 ± 0.37 | 34.42 ± 1.85 |
| 5 | 12.5 | PVA | 5 | 54.8 | 1.92 | 17.34 | 28.95 | 17.04 | 1.56 | 5.145 ± 0.29 | 25.73 ± 1.42 |
| 6 | 12.5 | Gelatin | 1 | 64.53 | 18.91 | 38.79 | 59.60 | 39.1 | 1.05 | 10.15 ± 0.48 | 50.75 ± 2.37 |
| 7 | 12.5 | Tween 80 | 1 | 14.96 | ND | ND | ND | ND | ND | ND | ND |
| 8 | 12.5 | SDS | 1 | 66.24 | 10.53 | 32.05 | 47.44 | 30.35 | 1.15 | Nil | Nil |
| 9 | 12.5 | MC | 1 | 70.09 | 31.97 | 65.17 | 96.70 | 64.84 | 0.99 | 8.82 ± 0.33 | 44.1 ± 1.64 |
| 10 | 12.5 | SDS | 0.5 | 64.96 | 9.41 | 31.6 | 50.70 | 30.56 | 1.31 | 0.9 ± 0.22 | 4.48 ± 1.09 |
| 11 | 12.5 | Gelatin | 0.5 | 59.83 | 19.78 | 55.85 | 90.38 | 55.89 | 1.26 | 8.16 ± 0.14 | 40.78 ± 0.67 |
| 12 | 12.5 | MC | 0.5 | 75.2 | 30.87 | 65.14 | 95.36 | 64.55 | 0.99 | 10.99 ± 0.38 | 54.94 ± 1.88 |
aMean ± standard deviation (SD; n = 3)
PVA indicates polyvinyl alcohol, ND not determined, SDS sodium dodecyl sulfate, and MC methyl cellulose
Fig. 1.
Scanning electron micrographs of microspheres from formulae # 3, 5, 6, 7, 8, 9, 11, and 12, as represented in images a, b, c, d, e, f, g, and h, respectively. Cut sections of microspheres from F#11 and 12 are represented
Encapsulation Efficiency
When PLGA concentration was increased from 7.5% to 12.5% w/v, EE% was increased likewise from 26.3 to 68.0, respectively (Table I). This marked improvement in EE% can be due to the increase in the amount of the added drug, to keep drug/polymer ratio as constant. In addition, the denser microspheres matrix formed upon using higher polymer concentrations (33) may hinder the drug diffusion out of the microspheres during solvent evaporation and washing steps.
Table I shows that, as PVA concentration decreased from 5%, to 3%, and 1%, the EE% was markedly increased from 25.73%, to 34.42%, and 68.0%, respectively. It is obvious that this enhancement of EE% can be correlated with the increase in size of the microspheres upon using lower concentration of PVA. As the average size of the microspheres increases, the path required for the drug to escape to the external phase is also increased. This results in delaying the drug escaping process. In addition, drug crystals, which could still be found in crystalline state in the microspheres, varied in size from submicron ranges to about 8–10 µm (unpublished data). Knowing that drug crystals were dispersed as a suspension in the oil phase, it can be suggested that as the average size of the microspheres becomes smaller, the size range of drug crystals suitable to be encapsulated becomes narrower, leading to low EE%.
When SDS was used as an emulsifier, although the morphology, size, and yield of the microspheres were promising, almost no drug was encapsulated (Table I). It is believed that SDS enhanced the aqueous solubility of the drug, making it unfavorable for drug crystals to stay in the organic water-immiscible internal phase. On the other hand, upon using MC and gelatin to prepare the microspheres, EE% values ranged from ∼41% to 55% w/w, as shown in Table I.
In vitro Drug Release Profiles
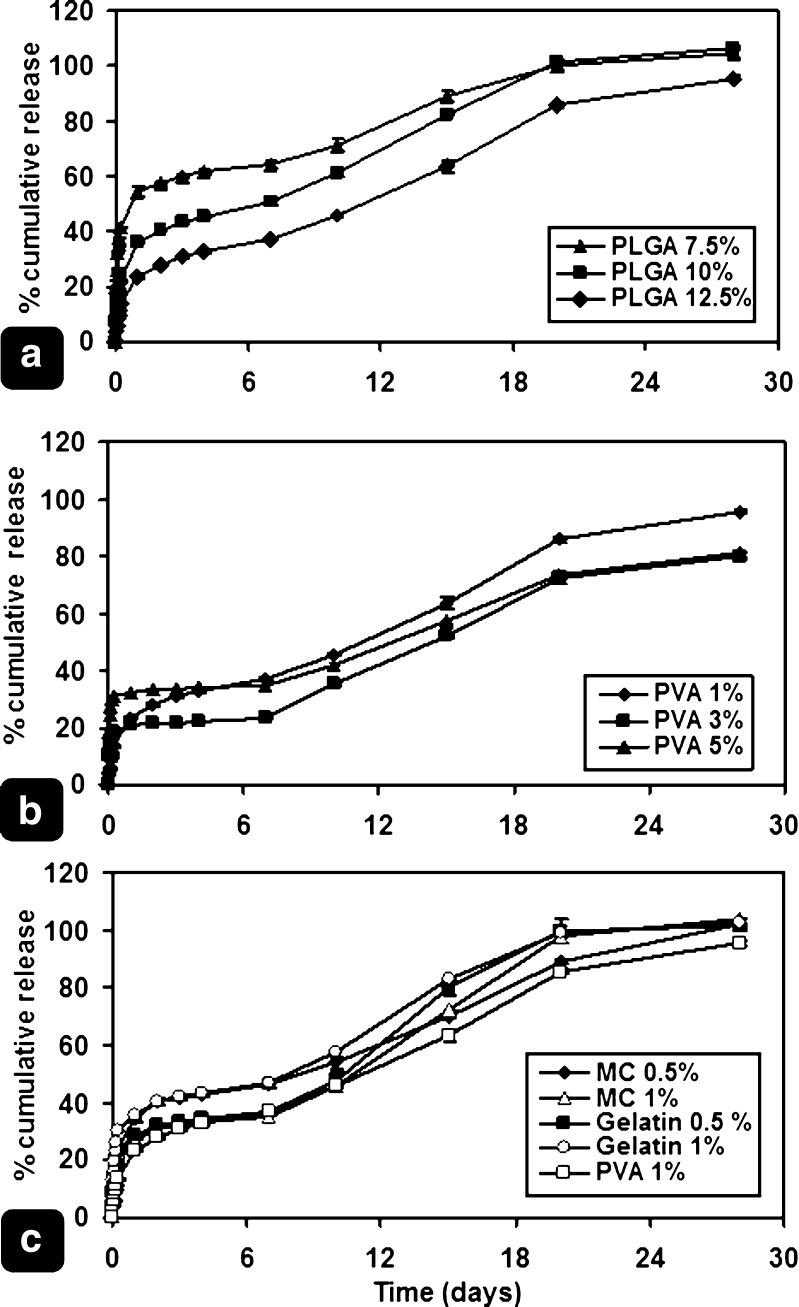
Drug release from the prepared microspheres followed the triphasic pattern previously described by other authors (34), resulting in a near-to-zero-order release pattern. Optimization of the release properties of the prepared microspheres took place through the variation of formulation parameters, such as PLGA concentration, in addition to emulsifier type and concentration.
PLGA Concentration
PLGA concentration in the organic phase was expected to alter release pattern as it affects density of PLGA matrix and the presence of drug onto or near the surface of the microspheres. Increasing initial polymer concentration in the organic phase was assumed to increase the polymeric matrix density, leading to slowing down drug diffusion rate (33,35). In addition, the drug that escaped to the external aqueous phase during solvent evaporation, or migrated/deposited to/onto the surface, may be affected by changing polymer concentration and matrix density. Accordingly, drug deposition onto or near the surface is expected to be greater in low-PLGA concentration formulae than that in those with higher concentrations. For these reasons, the decrease in both drug release rates and the burst amounts, due to increasing PLGA concentration, can be justified (Fig. 2a).
Fig. 2.
Effect of: a PLGA concentration, b PVA concentration, and c emulsifier type, on prednisolone release from different formulae. Bar represents average ±SD (n = 3)
PVA Concentration
Variation of PVA concentration was expected to change release patterns, as it affects both particle size and EE%. When the PVA concentration was lowered from 5%, to 3%, and 1% (% w/v), the average particle size increased from 17.04, to 39.37, and 53.42 µm, respectively. As a result, the specific surface area decreased resulting in slower drug release rates during the first 6 h. On the other hand, decreasing PVA concentration enhanced EE% (Table I). Consequently, the percentage drug content was markedly increased. It is believed that, in case of high-percentage drug content, the release of drug from the matrix results in the formation of small pores and holes that were previously occupied by the drug. This leads to increasing the porosity of the matrix, and, hence, introducing the release medium into the deeper regions (35). The release rates were expected to increase after the burst period. Therefore, increased percentage cumulative drug released at the end of the experiment was expected, too. These effects outweigh the slower release rates that resulted from decreasing microsphere surface areas. Figure 2b shows drug release patterns from F#3, 4, and 5. Formula #5 exhibited the fastest drug release rates during the first 6 h, due to its smaller average particle size. Meanwhile, F#3 showed accelerating release rates, with minimal lag period between days 2 and 7.
Effect of Emulsifier Type
With the application of different emulsifiers other than PVA, namely, gelatin and MC (0.5 and 1% w/v of each), the release patterns exhibited the same triphasic fashion. Starting with initial burst, followed by slow release period, then another near-zero-order burst period, representing polymer erosion phase. However, the primary release stage appears slightly higher than those obtained with the corresponding concentrations of PVA (Fig. 2c). Similar results were reported in the literature during the preparation of acyclovir–PLGA microspheres, where the addition of gelatin to the external aqueous phase gave faster drug release from the first day of release (36).
Microsphere Degradation
Blank PLGA microspheres were subjected to conditions similar/close to release conditions and characterized by SEM, pH measurement, and Tg detection using DSC.
According to many authors, PLGA is a polymer characterized by bulk erosion (37). This is due to the presence of less reactive functional groups on the surface (38); as a result, the polymer chains inside the bulk of the matrix start to be cleaved by hydrolysis, producing acidic shorter-chain moieties, which start a series of autocatalytic hydrolysis reactions. These random hydrolytic cleavage reactions result in a decrease of the average molecular weight of the polymer (39). Polymer weight loss is not expected in this early stage because the acidic degradation products are still entrapped within the bulk of the polymer; this is emphasized by absence of any decrease in pH of the degradation medium (40). Chain cleavage continues, along with increasing porosity, until molecular weights of these shorter-chain products reach critical values that allow for their dissolution in the aqueous medium. Diffusion starts within the whole bulk, until the trapped, more-soluble, acidic moieties are released to the surrounding aqueous medium (40–42). At this point, the medium pH starts to drop.
Poly-lactide-co-glycolide degradation was investigated using SEM as shown in Fig. 3. This figure shows PLGA microspheres after incubation in PBS 7.4 at various periods at 37°C. The first morphological change was represented by the increased porosity and roughness of the surface, though the microspheres kept their spherical shape (Fig. 3b). Microsphere morphology became significantly different after 15 days in PBS as the surfaces show marked erosion and pitting (Fig. 3c). Even so, some of the microspheres, though still spheroid, became degraded to a large extent (marked mass loss), with their internal structures revealed. After 28 days, microspheres lost their spherical shapes. Their surfaces became largely crumpled, wrinkled, and collapsed (severe mass loss), leaving only eroded polymeric structures (Fig. 3d).
Fig. 3.
Scanning electron micrographs of blank PLGA microspheres after incubation in PBS 7.4 at 37°C for a 0, b 5, c 15, and d 28 days
When the pH of the medium (PBS 7.4) was plotted against time, the pH started to drop slightly after 10 days. However, the pH started to drop dramatically after 20 days until it reached about 4.5 by the 28th day. It is believed that the medium pH did not start to drop until a reasonable amount of the acidic shorter-chain moieties was released. Also, glass transition temperature (Tg) of the polymeric microspheres was plotted against time of degradation. It was found that Tg started to decrease markedly after 20 days of experiment, to reach a value of 42.9°C by the 35th day, compared with 53.8°C before the experiment. This lowering in Tg values can be attributed to the plasticizing effect of water on PLGA (40,41,43) that caused a physical-state transition of the PLGA matrix into an elastic solid mass (43). This facilitated further drop in the average molecular weight, along with a consequent increase in the macromolecular mobility (44).
Animal Experiment
Upon studying PLGA drug-loaded microspheres, three formulae showed promising results, from the perspective of production yield, EE%, and release patterns. These formulae were F#3, F#6, and F#12. Drug release studies showed that F#3 and F#12 exhibited closely related release profiles, where F#6 showed higher drug release rate. Accordingly, F#3 and F#6 were selected for the in vivo study.
Morphological Changes
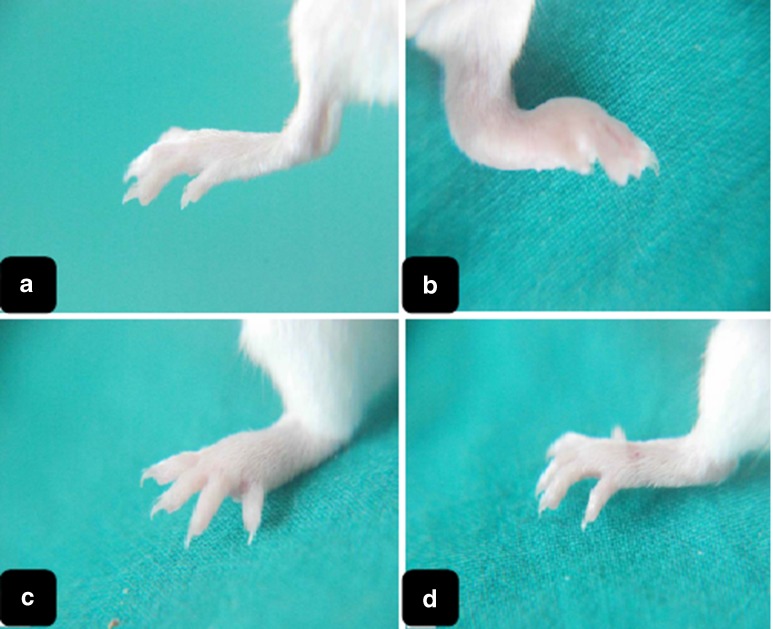
Inflammation signs started to appear on the ankle joint of the left hind paw of mice from all groups 5 days after the first FCA injection. These signs were aggravated after 5 days from the second FCA injection (booster dose), with plantar side of the paw involved. The signs included redness (slight to severe) of the paw and severe swelling around the left ankle joint and the plantar region of the paw. The toes were swollen and parted in some animals. In group III, which received a single prednisolone dose, paw redness almost disappeared. The ankle joint and the paw plantar side, although partially ameliorated, showed evident swelling. These inflammatory signs disappeared either completely or almost-completely in microsphere-treated animals (groups IV and V). Figure 4 shows photographs of hind paws of the animals from different groups.
Fig. 4.
Digital photographs representing the left (ipsilateral) hind limbs of mice after 22 days of injection with FCA booster dose from a group I, b group II, c group III, and d group IV
Diameter of the Arthritic Ankle Joints
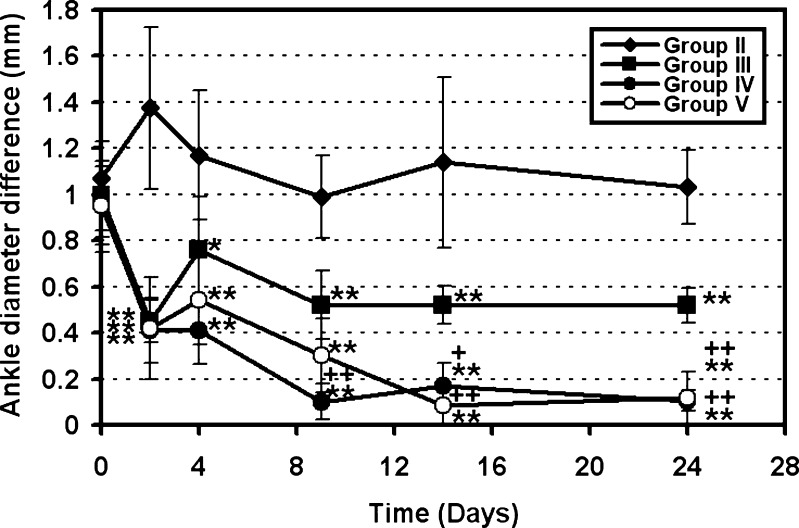
Drug treatment was started after 2 weeks of the first FCA injection and 1 week from the booster FCA dose. The day of treatment was assigned as “day 0”. The diameter of the contralateral joint did not show any significant difference (P > 0.05) between day 0 and day 24 of the experiment. Hence, it was used as an internal control (2,14). The diameter difference between the ankle joints of two hind paws (left and right) was measured at predetermined time intervals and plotted against time (Fig. 5). It is worth mentioning that diameters of right and left ankles were similar in all animals prior to the experiment. The inflammation was restricted to the FCA-injected limb, with the contralateral limbs unaffected, as previously reported (9).
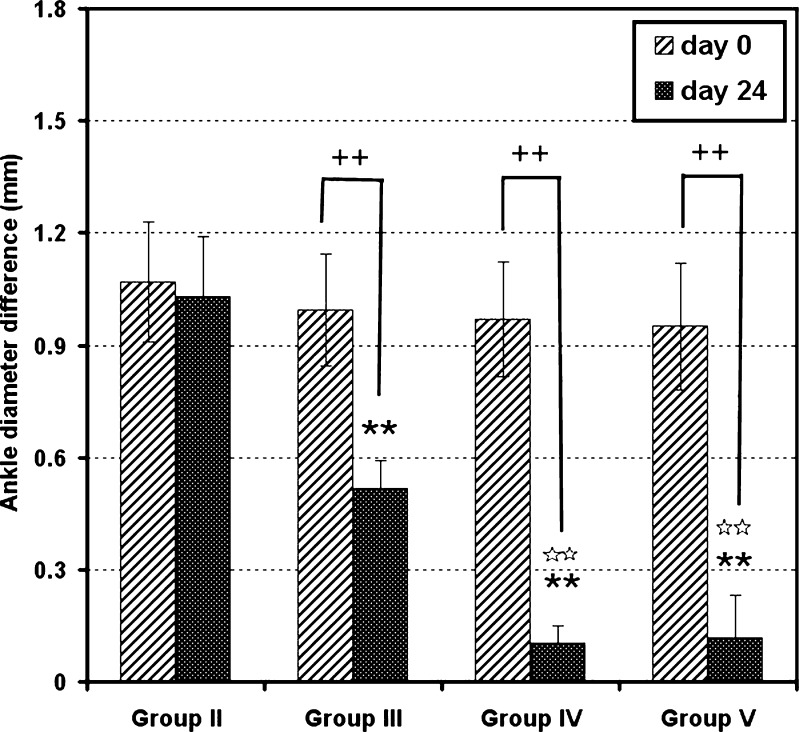
Fig. 5.
Ankle diameter differences between left (ipsilateral) and right (contralateral) hind limbs of FCA-injected mice as a function of time. Data represent the mean ± SD (n = 10). Statistically significant differences were denoted as follows: **P < 0.01 compared with control, ++P < 0.01 compared with single-dose prednisolone (5 mg/kg), *P < 0.05 compared with control, and +P < 0.05 compared with single-dose prednisolone (5 mg/kg)
At day 2 of treatment, group II (positive control) showed a marked increase in joint diameter difference, as shown in Fig. 5, from ∼1.1 to 1.37 mm. On the contrary, both the single dose-treated (group III) and the microsphere-treated (group IV and V) mice exhibited significant reduction in average joint diameter difference from 0.95 to ∼0.42 mm (P < 0.01). This marked reduction of the joint swelling from the first day of prednisolone treatment was also previously reported in rats (45) and mice (2).
According to Gauldie et al. (2), the FCA-induced inflammation of the stifle joint of mice was markedly reduced after 7 days of daily 1 mg/kg prednisolone injection. However, inflammation returned after stopping of the treatment in the form of an increase in the knee diameter. In our study, ankle swelling returned in day 4 in group III, which received a single 5 mg/kg prednisolone dose. The ankle diameter difference increased significantly (P < 0.05) from 0.42 ± 0.09 mm (day 2) to 0.76 ± 0.23 mm (day 4). However, diameter difference decreased again on day 9 to reach 0.52 ± 0.15 mm and did not show any significant change from the day 9 to day 24 (P > 0.05).
Joint diameter differences in microsphere-injected groups (IV and V) were reduced significantly (P < 0.01) from 0.97 ± 0.21 to 0.45 ± 0.14 mm with F#3 and from 0.95 ± 0.21 to 0.42 ± 0.22 mm with F#6 after 2 days. The reduction in diameter difference continued until it reached values of 0.105 ± 0.04 mm and 0.116 ± 0.115 mm for F#3 and F#6, respectively, at the end of the experiment (day 24). The continued reduction of paw swelling with microspheres treatment, compared with single-dose treatment, can be attributed to the prolonged drug release from the microspheres.
Changes in joint diameter difference between day 0 and day 24 were compared for all groups (Fig. 6). It was found that the mean ankle diameter difference of the positive control group (group II) did not show any significant change (P > 0.05). Meanwhile, group III mice showed a significant decrease (P < 0.01) in diameter difference from 0.95 ± 0.21 at day 0 to 0.52 ± 0.07 mm at the end of the experiment. Regarding groups IV and V animals, diameters difference decreased from 0.97 ± 0.15 and 0.95 ± 0.17 mm to 0.105 ± 0.044 and 0.116 ± 0.115 mm, respectively. Therefore, it is clear that, although the diameter difference in group III was significantly reduced in day 24 (P < 0.01) when compared with that at day 0, the reduction did not exceed 45%, compared with 89% and 88% reduction with groups IV and V, respectively. This finding indicates that the use of PD-loaded microspheres treatment is much better than the use of the free drug.
Fig. 6.
A plot comparing between ankle diameter differences on day 0 and day 24 in groups II, III, IV, and V. Data represent mean ± SD (n = 10). Statistically significant differences were denoted as follows: **P < 0.01 against control, ☆☆P < 0.01 against single-dose prednisolone (5 mg/kg), and ++P < 0.01 against ankle diameter difference of the same limb at day 0
Histopathological Examination
Tissue samples were taken as mentioned previously and investigated for histopathological findings. The following is a brief description of histopathological findings of each group.
-
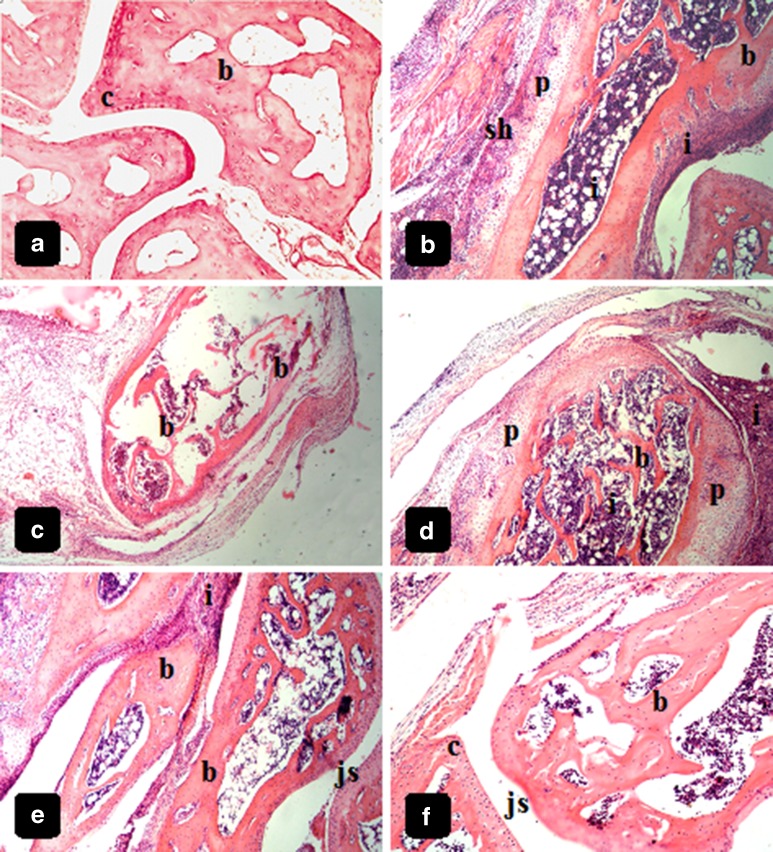
Group I (negative control)
Light microscopy photographs of intact ankle joints of normal animals show intact bone and articular cartilages (Fig. 7a), with no sign of inflammation or erosion.
-
Group II (positive control)
All mice selected from group II showed inflammatory lesions, with high degrees of severity. Many inflammatory findings can be seen, including severe and dense inflammatory infiltration around bone and in bone marrow, along with pannus invasion, in addition to synovial hyperplasia (Fig. 7b, c) and narrowing of joint space (not shown). Bone and cartilage erosion are markedly seen in different bone tissues of the ankle joints of all animals investigated (Fig. 7b, c).
-
Group III (single-dose prednisolone-treated)
Different inflammatory findings found in group II mice were also seen in group III mice. Inflammatory infiltration is dense and severe, and joint spaces are narrowed. In addition, tissue samples exhibited bone and cartilage erosion (Fig. 7d).
-
Group IV and V (microsphere-treated)
Some inflammatory cell infiltration can still be visible in photographs of microsphere-treated joints from groups IV and V (Fig. 7e, f). However, joint spaces appear normal in most ankle joints, and no pannus invasion can be seen. In addition, bones and cartilages are almost intact and preserved. This is a very important finding that indicates the success of treatment.
Fig. 7.
Histological photography examination of ankle joints of mice from different groups showing: a uninjected ankle joints of mice (group I) with intact bone (b) and articular cartilages (c), and normal joint space (js), H&E ×100. b, c FCA-injected ankle joints of the mice (group II) with heavy Inflammatory cell infiltration (i) of the bone and bone marrow, pannus invasion (p), synovial hyperplasia (sh), and severe cartilage and bone erosion (b), H&E ×40. d FCA-injected single dose-treated ankle joints of the mice (group III) with dense inflammatory infiltration around the bone and into the bone marrow (i), bone erosion (b), and pannus invasion of the bone matrix (p), H&E ×40. e, f arthritic, microspheres-treated mice with nearly normal histological features with almost intact bone (b) and cartilage (c), in addition to normal joint space (js). Cellular infiltration is still evident but markedly decreased (i), H&E, ×100 for e and ×200 for f
CONCLUSION
An o/w emulsion solvent evaporation technique was used successfully to prepare prednisolone-loaded PLGA microspheres. Formulation parameters could be optimized so as to obtain microspheres with an average size of ∼18 up to 65 µm and EE% values up to 68%. The prepared formulations gave almost-steady drug release during the release experiment, which would be of great value for in vivo application in treatment of arthritis.
Unilateral arthritis was induced successfully in the hind limbs of mice after two successive doses of FCA, separated by 1 week. Prednisolone-loaded PLGA microsphere treatment protocol not only significantly suppressed the swollen FCA-injected joints, but also protected bone and cartilage from erosion, owing to its sustained drug delivery. This system can comprise an important step in the management of RA in its early stages and can be a successful alternate to steroid multiple dosage regimens.
ACKNOWLEDGEMENTS
The authors would like to thank Al-Kahira Pharmaceutical Company for supplying prednisolone. This research was not financially supported by any institution or any funding agency.
REFERENCES
- 1.Bao L, Zhu Y, ElHassan AM, Wu Q, Xiao B, Zhu J, Lindgren U. Adjuvant-induced arthritis: IL-1â, IL-6 and TNF-α are up-regulated in the spinal cord. Neuroimmunology (Neuroreport) 2001;12(18):3905–3908. doi: 10.1097/00001756-200112210-00010. [DOI] [PubMed] [Google Scholar]
- 2.Gauldie SD, McQueen DS, Clarke CJ, Chessell IP. A robust model of adjuvant-induced chronic unilateral arthritis in two mouse strains. J Neurosci Methods. 2004;139:281–291. doi: 10.1016/j.jneumeth.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Rannou F, Francois M, Corvol MT, Berenbaum F. Cartilage breakdown in rheumatoid arthritis. Joint Bone Spine. 2006;73:29–36. doi: 10.1016/j.jbspin.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Mythilypriya R, Shanthi P, Sachdanandam P. Salubrious effect of kalpaamruthaa, a modified indigenous preparation in adjuvant induced arthritis in rats—a biochemical approach. Chem Biol Interact. 2008;173(2):148–158. doi: 10.1016/j.cbi.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Kumar VL, Roy S, Schgal R, Padhy BM. A comparative study on the efficacy of rofecoxib in monoarticular arthritis induced by latex of Calotropis procera and Freund's complete adjuvant. Inflammopharmacol. 2006;14:17–21. doi: 10.1007/s10787-006-1512-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Li J, Yu S-C, Jin Y, Lv X-W, Zou Y-H, Li Y. Therapeutic effects and mechanisms of total flavonoids of Turpinia arguta seen on adjuvant arthritis in rats. J Ethnopharmacol. 2008;116:167–172. doi: 10.1016/j.jep.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Ding CH, Li Q, Xiang ZY, Zhou AW, Jones G, Xu SY. Oral administration of type II collagen suppresses pro-inflammatory mediator production by synoviocytes in rats with adjuvant arthritis. Clin Exp Immunol. 2003;132:416–423. doi: 10.1046/j.1365-2249.2003.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight B, Katz DR, Isenberg DA, Ibrahim MA, LePage S, Huchings P, Schwartz P, Cooke A. Induction of adjuvant arthritis in mice. Clin Exp Immunol. 1992;90:459–465. doi: 10.1111/j.1365-2249.1992.tb05868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chillingworth NL, Donaldson NF. Characterisation of a Freund's complete adjuvant-induced model of chronic arthritis in mice. J Neurosci Methods. 2003;128:45–52. doi: 10.1016/S0165-0270(03)00147-X. [DOI] [PubMed] [Google Scholar]
- 10.Bulej P, Kuchar M, Panajotova V, Jegorov A. Pharmacological profile of 4-(2', 4'-difluorobiphenyl-4-yl)- 2-methylbutyric acid (deoxoflobufen) Arzneimittelforschung. 2005;55(8):466–472. doi: 10.1055/s-0031-1296890. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt G, Jahns J, Hindemith M, Spranger S, Sack U, Kinne RW, Madaj-Sterba P, Wolf U, Kamprad F. Effects of low dose radiation therapy on adjuvant induced arthritis in rats. Int J Radiat Biol. 2000;76(8):1143–1153. doi: 10.1080/09553000050111613. [DOI] [PubMed] [Google Scholar]
- 12.Hambleton P, McMahon S. Drug actions on delayed-type hypersensitivity in rats with developing and established adjuvant arthritis. Agents Actions. 1990;29(3/4):328–332. doi: 10.1007/BF01966465. [DOI] [PubMed] [Google Scholar]
- 13.Tachibana M, Inoue N, Yoshida E, Matsui M, Ukai Y, Yanu J. Anti-inflammatory effect and low ulcerogenic activity of etodolac, a cyclooxygenase-2 selective non-steroidal anti-inflammatory drug, on adjuvant-induced arthritis in rats. Pharmacology. 2003;68:96–104. doi: 10.1159/000069536. [DOI] [PubMed] [Google Scholar]
- 14.Palakurthi S, Vyas SP, Diwan PV. Biodisposition of PEG-coated lipid microspheres of indomethacin in arthritic rats. Int J Pharm. 2005;290:55–62. doi: 10.1016/j.ijpharm.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Yordanov M, Deleva A, Ivanovska N. Host resistance against Candida albicans infection in mice with adjuvant induced arthritis. Mycopathologia. 2001;153:77–82. doi: 10.1023/A:1014463122641. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg IE, Grundtman C, Larsson E, Klareskog L. Corticosteroids—from an idea to clinical use. Best Pract Res Clin Rheumatol. 2004;18(1):7–19. doi: 10.1016/j.berh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Boers M. Glucocorticoids in rheumatoid arthritis: a senescent research agenda on the brink of rejuvenation? Best Pract Res Clin Rheumatol. 2004;18(1):21–29. doi: 10.1016/j.berh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ. Corticosteroids: the drugs to beat. Eur J Pharmacol. 2006;533:2–14. doi: 10.1016/j.ejphar.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 19.Adcock IM. Corticosteroids: limitations and future prospects for treatment of severe inflammatory disease. Drug Discov Today Ther Strat. 2004;1(3):321–328. doi: 10.1016/j.ddstr.2004.11.015. [DOI] [Google Scholar]
- 20.Vanniasinghe AS, Bender V, Manolios N. The potential of liposomal drug delivery for the treatment of inflammatory arthritis. Semin Arthritis Rheum. 2009;39(3):182–196. doi: 10.1016/j.semarthrit.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F. The epidemiology of drug treatment failure in rheumatoid arthritis. Baillières Clin Rheumatol. 1995;9(4):619–632. doi: 10.1016/S0950-3579(05)80305-X. [DOI] [PubMed] [Google Scholar]
- 22.Smith A, Hunneyball I. Evaluation of poly(lactic acid) as a biodegradable drug delivery system for parenteral administration. Int J Pharm. 1986;30:215–220. doi: 10.1016/0378-5173(86)90081-5. [DOI] [Google Scholar]
- 23.Khaled AK, Sarhan HA, Ibrahim MA, Naguib YW. Controlled-release prednisolone poly (dl-lactide) microspheres: impact of formulation parameters, characterization and release mechanism. Bull Pharm Sci, Assiut Univ. 2008;31(1):49–67. [Google Scholar]
- 24.Redmon MP, Hickey AJ, DeLuca PP. Prednisolone-21-acetate (poly glycolic) acid microspheres: influence of matrix characteristics on release. J Control Release. 1989;9:99–109. doi: 10.1016/0168-3659(89)90001-1. [DOI] [Google Scholar]
- 25.Esposito E, Meregatti E, Cortesi R. Hyaluronan-based microspheres for drug delivery: a comparative study. Int J Pharm. 2005;288:35–49. doi: 10.1016/j.ijpharm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Burgess DJ, Davis SS, Tomlinson E. Potential use of albumin microspheres as a drug delivery system. 1. Preparation and in vitro release of steroids. Int J Pharm. 1987;39:129–136. doi: 10.1016/0378-5173(87)90207-9. [DOI] [Google Scholar]
- 27.Onishi H, Oosegi T, Machida Y, Ku S, McGinity JW. Preparation and in vitro evaluation of chitosan microspheres containing prednisolone: comparison of simple and conjugate microspheres. Drug Dev Ind Pharm. 2005;31:597–605. doi: 10.1080/03639040500216063. [DOI] [PubMed] [Google Scholar]
- 28.Oosegi T, Onishi H, Machida Y. Gastrointestinal distribution and absorption behavior of Eudragit-coated chitosan–prednisolone conjugate microspheres in rats with TNBS-induced colitis. Int J Pharm. 2008;348:80–88. doi: 10.1016/j.ijpharm.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Oosegi T, Onishi H, Machida Y. Novel preparation of enteric-coated chitosan-prednisolone conjugate microspheres and in vitro evaluation of their potential as a colonic delivery system. Eur J Pharm Biopharm. 2008;68:260–266. doi: 10.1016/j.ejpb.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Brannon-Peppas L, Vert M. Polylactic and polyglycolic acids as drug delivery carriers. In: Wise D, editor. Handbook of pharmaceutical controlled release technology. New York: Marcel Dekker Inc.; 2000. pp. 99–130. [Google Scholar]
- 31.Park S-B, Jeon Y-J, Haam S, Park H-Y, Kim Y-S. Preparation of chitosan microspheres using membrane emulsification and its size modeling. J Microencapsul. 2004;21(5):539–552. doi: 10.1080/02652040410001729304. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery H, Davis SS, O'Hagan DT. The preparation and characterization of poly(lactide-co-glycolide) microparticles. I: Oil-in-water emulsion solvent evaporation. Int J Pharm. 1991;77:169–175. doi: 10.1016/0378-5173(91)90314-E. [DOI] [PubMed] [Google Scholar]
- 33.Chen PC, Park YJ, Chang LC, Kohane DS, Bartlett RR, Langer R, Yang VC. Injectable microparticle–gel system for prolonged and localized lidocaine release. I. In vitro characterization. J Biomed Mater Res A. 2004;70:412–419. doi: 10.1002/jbm.a.30086. [DOI] [PubMed] [Google Scholar]
- 34.Luan X, Bodmeier R. Modification of the tri-phasic drug release pattern of leuprolide acetate-loaded poly(lactide-co-glycolide) microparticles. Eur J Pharm Biopharm. 2006;63:205–214. doi: 10.1016/j.ejpb.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Bodmeier R, McGinity JW. The preparation and evaluation of drug-containing poly (dl-lactide) microspheres formed by the solvent evaporation method. Pharm Res. 1987;4(6):465–471. doi: 10.1023/A:1016419303727. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Sancho C, Herrero-Vanrell R, Negro S. Poly (d,l-lactide-co-glycolide) microspheres for long-term intravitreal delivery of aciclovir: influence of fatty and non-fatty additives. J Microencapsul. 2003;20(6):799–810. doi: 10.1080/02652040310001600532. [DOI] [PubMed] [Google Scholar]
- 37.Siepmann J, Goepferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev. 2001;48:229–247. doi: 10.1016/S0169-409X(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 38.Arifin DY, Lee LY, Wang C-H. Mathematical modeling and simulation of drug release from microspheres: implications to drug delivery systems. Adv Drug Deliv Rev. 2006;58:1274–1325. doi: 10.1016/j.addr.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Lewis DH. Controlled release of bioactive agents from lactide/glycolide polymers. In: Chasin M, Langer R, editors. Biodegradable polymers as drug delivery systems. New York, USA: Marcel Dekker; 1990. pp. 1–40. [Google Scholar]
- 40.Chen X, Ooi CP. Hydrolytic degradation and drug release properties of ganciclovir-loaded biodegradable microspheres. Acta Biomater. 2008;4(4):1046–1056. doi: 10.1016/j.actbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Blanco MD, Sastre RL, Teijon C, Olmo R, Teijon JM. Degradation behaviour of microspheres prepared by spray-drying poly(d, l-lactide) and poly(d, l-lactide-co-glycolide) polymers. Int J Pharm. 2006;326:139–147. doi: 10.1016/j.ijpharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1125–1130. doi: 10.1016/0142-9612(95)93575-X. [DOI] [PubMed] [Google Scholar]
- 43.Blasi P, D'Souza SS, Selmin F, DeLuca PP. Plasticizing effect of water on poly(lactide-co-glycolide) J Control Release. 2005;108:1–9. doi: 10.1016/j.jconrel.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Klose D, Siepmann F, Elkharraz K, Siepmann J. PLGA-based drug delivery systems: importance of the type of drug and device geometry. Int J Pharm. 2008;354:95–103. doi: 10.1016/j.ijpharm.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Blackham A, Burns JW, Farmer JB, Radziwonik H, Westwick J. An X-ray analysis of adjuvant arthritis in the rat. The effect of prednisolone and indomethacin. Agents Actions. 1977;71:145–151. doi: 10.1007/BF01964912. [DOI] [PubMed] [Google Scholar]