Abstract
In this study, solid lipid nanoparticles (SLNs) were successfully prepared by an ultrasonic and high-pressure homogenization method to improve the oral bioavailability of the poorly water-soluble drug cryptotanshinone (CTS). The particle size and distribution, drug loading capacity, drug entrapment efficiency, zeta potential, and long-term physical stability of the SLNs were characterized in detail. A pharmacokinetic study was conducted in rats after oral administration of CTS in different SLNs, and it was found that the relative bioavailability of CTS in the SLNs was significantly increased compared with that of a CTS-suspension. The incorporation of CTS in SLNs also markedly changes the metabolism behavior of CTS to tanshinone IIA. These results indicate that CTS absorption is enhanced significantly by employing SLN formulations, and SLNs represent a powerful approach for improving the oral absorption of poorly soluble drugs.
Key words: absorption, bioavailability, cryptotanshinone, high-pressure homogenization, solid lipid nanoparticles
INTRODUCTION
It has been reported that many newly discovered drug candidates are poorly water-soluble. A lot of failures in new drug development have been attributed to poor biopharmaceutical properties, including water insolubility. Poor water solubility leads to ineffective absorption in the site of administration, which has been designated as one of the important factors attributed to the high clinical failure. Attempts to solve the solubility problem and improve oral absorption have been investigated in many recent studies (1–6). One of the promising approaches for enhancing the oral bioavailability of poorly soluble drugs is the incorporation of these drugs in solid lipid nanoparticles (SLNs; 7–9).
SLNs offer an attractive means of drug delivery, particularly for poorly water-soluble drugs. SLNs are in the submicron size range, and they are composed of physiologically tolerated lipid components, which at room temperature remains in the solid state (10). SLNs combine the advantages of polymeric nanoparticles, fat emulsions, and liposomes (11). Our previous research showed that SLNs can enhance the absorption and bioavailability of all-trans-retinoic acid (12). When all-trans-retinoic acid was loaded into SLNs, the oral bioavailability in rats was increased four- to fivefold compared with that of suspension. The mechanisms of enhanced oral absorption in ATRA-SLNs are mainly attributed in reduction in the particles size and the lipid protection of the drug from chemicals as well as enzymatic degradation.
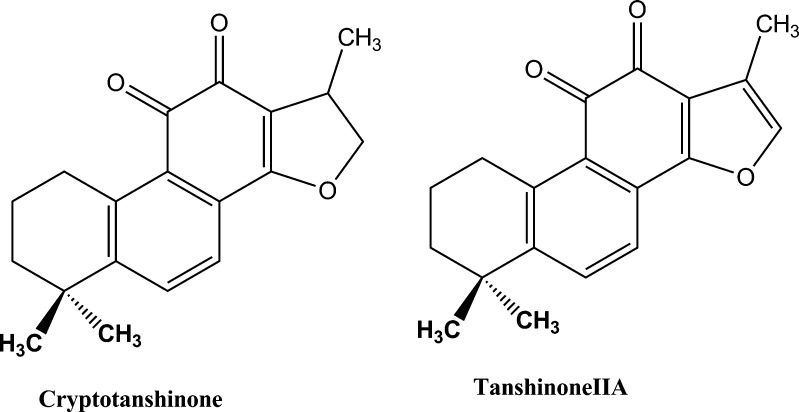
Danshen, a traditional Chinese medicine, derived from the dried roots of Salvia miltiorrhiza Bunge, have been extensively used for the treatment of many kinds of disease, such as coronary heart disease and hyperlipidemia, stroke, hepatitis, and chronic renal failure (13). Cryptotanshinone (CTS; Fig. 1) is the major active ingredient of danshen. Preclinical studies have shown that CTS possesses multiple pharmacological activities including anti-inflammation, cytotoxic, anti-bacterial, anti-parasite, anti-angiogenic, and anti-oxidative effects (14–18). It exhibits inhibitory effects on chemotactic migration in macrophages, tumor necrosis factor-induced matrix metalloproteinase-9 production, interleukin-12 and interferon-production, and mast cell degranulation (14).
Fig. 1.
Chemical structures of cryptotanshinone and tanshinone IIA
In spite of the attractive biological activities, CTS presents a poor oral bioavailability due to its extremely low water solubility. The absolute oral bioavailability of CTS is only about 2% in rats (19). The low oral bioavailability decreases its clinical effect. To improve the oral bioavailability of CTS, CTS-loaded solid lipid nanoparticles (CTS-SLNs) were successfully developed, and the physicochemical characteristics were investigated. The oral bioavailability of CTS-SLNs was compared with that in a suspension to assess the feasibility of SLN to enhance the oral bioavailability of CTS. CTS was rapidly metabolized to its active metabolite tanshinone IIA (20,21). The effect of SLNs on the metabolism change of CTS was also studied. The absorption mechanism of the SLNs was discussed.
MATERIALS AND METHODS
Materials
Soy lecithin was provided by TaiWei Pharmaceutical Co. (Shanghai, China). Tween 80 was bought from Beijing Yili Fine Chemicals Co. Ltd. (Beijing, China). Sodium dehydrocholate (SD) was obtained from Sigma Co. Ltd. (Shanghai, China). We extracted CTS ourselves based on the method reported (22), with a purity higher than 94.6% by high performance liquid chromatography (HPLC). Glyceryl monostearate was purchased from Tianjin kemio Chemical Reagent Exploitation Center (Tianjin, China). Compritol 888 ATO was bought from Gattefosse Co. (Shanghai, China). All other chemicals were of analytical grade. Tanshinone IIA, CTS, and diazepam standard (internal standard) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Preparation of CTS-SLNs
CTS-SLNs were prepared with an ultrasonic and high-pressure homogenization method. The optimal formulation containing 0.25 g of CTS, 3.0 g soy lecithin, and 3.0 g lipid matrix were dissolved completely in absolute alcohol in a water bath at 80°C to obtain a clear melting organic phase. One gram Tween 80 and desired amounts of SD were dissolved in 50 ml water and then heated to 80°C which was then used as a water phase. The water phase was added dropwise to the organic phase with magnetic stirring for 5 min, and then coarse premix was subjected to ultrasonic treatment for 10 min using a high-intensity probe ultrasonicator at 80°C. After the organic solvent had completely evaporated, the coarse emulsion was passed through a high-pressure homogenizer at 800 bar for three homogenization cycles. The dispersions were immediately filtered through a 0.45-μm membrane, and the final volume was adjusted to 100 ml with distilled water, stored at 4 ± 2°C. CTS-SLNs obtained using glyceryl monostearate and Compritol 888 ATO as lipid matrix were abbreviated as GMS-SLNs and CP-SLNs, respectively.
Particle Size and Zeta Potential
The morphology of CTS-SLNs was examined using a transmission electron microscopy (TEM, JEM-1200EX, and JEOL, Tokyo, Japan). After dilution with doubly distilled water, the samples were negatively stained with 2% (w/v) osmium tetroxide for observation.
The particle size of CTS-SLNs was measured by photon correlation spectroscopy using a NICOMP particle sizing system (CW380, Santa Barbara, CA, USA) at a fixed angle of 90° and at a temperature of 25°C. The particle size analysis data were evaluated using the volume distribution. Zeta potential measurements were operated using the same instrument at electrical field strength of 10 v/cm and at the same temperature. Prior to measurement, SLN dispersions were diluted 20-fold and 50-fold with the original dispersion preparation medium for size determination and zeta potential measurements, respectively. All the measurements were performed in triplicate.
Drug Encapsulation Efficiency and Drug Loading
The SLNs were dissolved in methanol to preferentially precipitate the lipid. After centrifugation (4,000 rpm for 15 min), the drug content in the supernatant was measured by HPLC analysis using a HPLC pump (L-7100, Hitachi) and a UV-Vis detector (L-7420, Hitachi, Japan) with a C18 reverse phase column (Diamonsil TM 5 μ C18, 200 × 4.6 mm, Beijing, China). The mobile phase composed of acetonitrile and water (80:20, v/v) at a flow rate of 1 mL/min, and the effluent was monitored at 270 nm.
The SLNs were subjected to ultracentrifugation (Hitachi CS120GXL Micro Ultracentrifuge, Japan) at 60,000 rpm for 4 h at 4°C in vacuum. The supernatant containing the free drug was withdrawn for HPLC analysis as described above. The precipitate in the ultracentrifuge tube was desiccated to give an exact weight.
The equations for the drug content and entrapment efficiency are as follows:
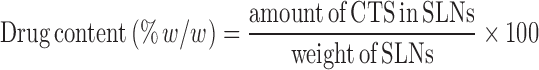 |
1 |
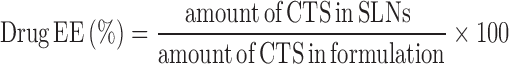 |
2 |
In Vitro Release Studies
In vitro release studies were performed by using the dialysis bag method. Distilled water was used as dissolution medium. The dialysis bag (molecular weight cutoff 10,000–12,000 Da) could retain nanoparticles and allow the diffusion of free drug into dissolution media. The bags were soaked in ionized water for 12 h before use. A 1-ml SLN dispersion was poured into the bag with the two ends fixed by clamps. The bags were placed in a conical flask and 10 ml dissolution media was added. The conical flasks were placed into a thermostatic shaker at 37°C and 50 strokes per minute. At 1, 2, 4, 6, 8, 12, 24, 48, 72 h after test, the medium in the conical flask was completely removed for analysis and fresh dialysis medium was then added to maintain sink conditions. The drug contents in samples were analyzed by the HPLC method mentioned above. All the operations were carried out in triplicate.
Storage Stability
The CTS-SLNs were studied for stability at 4 ± 2°C. These formulations were determined at regular time intervals for any change in particle size, zeta potential, and drug content.
Pharmacokinetic Studies in Rats
Healthy male Sprague-Dawley rats (250 ± 20 g) were supplied from the Laboratory Animal Center of Hebei University. Prior to use, the rats were kept in a temperature and humidity controlled animal observation room (25°C, 55–60% air humidity). All animal experiments complied with the requirements of the National Act of the People's Republic of China on the use of experimental animals. All rats were kept for overnight fasting but allowed free access to water. The rats were orally administrated at a drug single dose of 16 mg/kg of GMS-SLNs, CP-SLNs, and CTS-suspension, respectively. About 0.3 ml of blood samples via the jugular vein were collected into 1.0-ml heparinized tubes immediately at 0, 0.25, 0.5, 1, 2, 4, 6, 8, and 12 h after administration. Samples were centrifuged immediately to separate plasma 0.1 ml, which were stored at −20°C until analysis.
The plasma concentrations of CTS and tanshinone IIA were simultaneously determined by liquid chromatography/tandem mass spectrometry (LC–MS/MS) previously reported (23). Briefly, an aliquot of 0.1 ml plasma was mixed with 10 μl diazepam methanol solution (internal standard) and 0.4 ml ethyl acetate. The samples were vortex-mixed for 3 min and centrifuged at 3,000×g for 10 min. The organic portion was separated and evaporated to dryness under a gentle stream of nitrogen at 40°C. The residues were then reconstituted in 100 μl acetonitrile followed by centrifugation at 3,000×g for 10 min before LC–MS/MS analysis. An aliquot (10 μl) was injected for analysis.
The area under the concentration–time curve from time zero to time t (AUC0−t) was calculated using the trapezoidal method. Peak concentration (Cmax) and time of peak concentration were obtained directly from the individual plasma concentration–time profiles. The area from time zero to infinity was calculated by: AUC0−∞ = AUC0−t + Ct/Ke, where Ct is the CTS concentration observed at last time, and Ke is the apparent elimination rate constant obtained from the terminal slope of the individual plasma concentration–time curves after logarithmic transformation of the plasma concentration values and application of linear regression. The relative bioavailability Fr at infinity at the same dose was calculated as: Fr = AUCSLN, 0−∞/AUCsus, 0−∞.
Statistical Analysis
The data obtained from the release rate and pharmacokinetic parameters were analyzed statistically by ANOVA; a value of P < 0.05 was used to determine significance.
Results
Formulation Optimism
The specific characteristics of the lipid matrix chosen for SLNs can also have an important effect on the stability, and this should be taken into consideration during formulation screening and development. To investigate the effect of the degree of lipid material on drug absorption, three different lipids, stearic acid, glyceryl monostearate, and Compritol 888 ATO, were employed as a solid lipid matrix to investigate their influence on the drug stability and particle size of SLNs (Table I). The results indicated that stable formulations were observed with GMS-SLNs and CP-SLNs, respectively. SLNs prepared using stearic acid as a lipid matrix were unstable with flocculation and drug precipitation observed after 5 days of storage. The results indicate that lipid excipients are responsible for the stability of SLNs. In our study, Tween 80, soy lecithin, as well as SD were chosen as emulsifiers to stabilize SLNs. It was found that soy lecithin was an important factor under our operation conditions and without it the drug readily separated out from drug-loaded SLNs irrespective of the lipid used. Using soy lecithin alone can produce SLNs with a size above 400 nm. The addition of Tween 80 can effectively decrease the size. With 1% (w/v) amount of Tween 80, the size was about 150 nm. So the emulsifiers were fixed as soy lecithin/Tween 80 (3:1) and a lipid matrix amount 3%. The drug concentration in the SLN was 2.5 mg/ml.
Table I.
Composition of the SLN Formulations
| Component | Formulation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | |
| CTS (g) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Compritol 888 ATO (g) | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | |||||||
| glyceryl monostearate (g) | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | |||||||
| stearic acid (g) | 3.0 | 3.0 | ||||||||||
| Tween 80 (g) | 1.0 | 1.0 | 1.0 | 0.75 | 0.5 | 1.0 | 1.0 | 1.0 | 0.75 | 0.5 | 1.0 | 1.0 |
| Soy lecithin (g) | 3.0 | 4.0 | 2.0 | 3.0 | 3.0 | 3.0 | 4.0 | 2.0 | 3.0 | 3.0 | 3.0 | 3.0 |
SLNs solid lipid nanoparticles, CTS cryptotanshinone
Characterization of SLNs
TEM shows that the particles had round and uniform shapes. The mean diameters of GMS-SLNs and CP-SLNs were 121.4 ± 6.3 nm, 137.5 ± 7.1 nm, respectively. The zeta potential of GMS-SLNs and CP-SLNs were −25.2 ± 1.3 mV, −27.6 ± 1.2 mV, respectively. SLNs were found to be more stable after 6 months of storage at 4 ± 2°C, no dramatic increase in the size of GMS-SLNs and CP-SLNs occurred (Table II).
Table II.
Stability Studies of SLNs at 4 ± 2°C (n = 3)
| Formulation | Size (nm) | Zeta potential (mV) | Entrapment efficiency (%) |
|---|---|---|---|
| GMS-SLNs | |||
| 0 day | 121.4 ± 6.3 | −25.2 ± 1.3 | 94.2 ± 0.48 |
| 3 months | 123.5 ± 7.5 | −24.7 ± 1.2 | 93.6 ± 0.59 |
| 6 months | 127.3 ± 8.1 | −24.1 ± 0.9 | 93.7 ± 0.47 |
| CP-SLNs | |||
| 0 day | 137.5 ± 7.1 | −27.6 ± 1.2 | 96.3 ± 0.55 |
| 3 months | 136.3 ± 8.4 | −27.3 ± 1.1 | 95.9 ± 0.46 |
| 6 months | 138.5 ± 10.1 | −27.9 ± 1.4 | 95.5 ± 0.51 |
SLNs solid lipid nanoparticles, GMS-SLNs glyceryl monostearate-solid lipid nanoparticles, CP-SLNs Compritol 888 ATO-solid lipid nanoparticles
In Vitro Drug Release
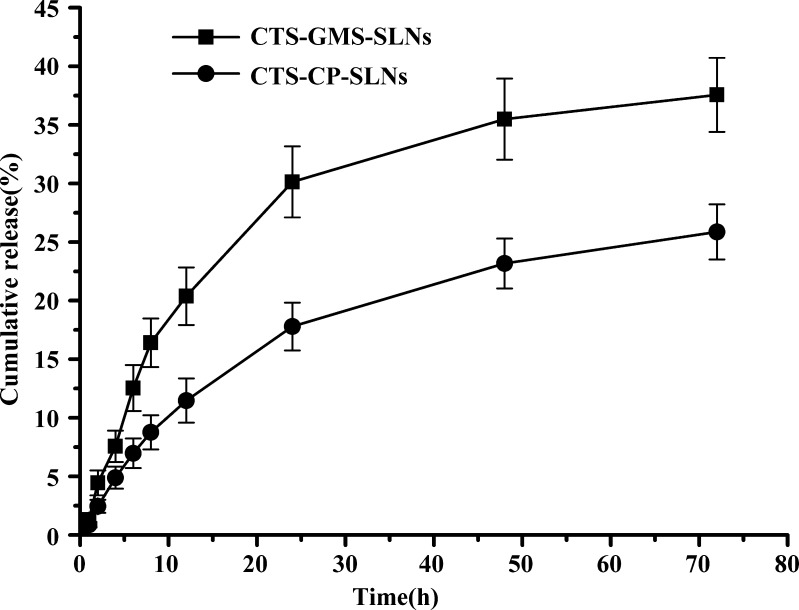
Figure 2 shows the cumulative percent release of CTS from CP-SLNs and GMS-SLNs. Both formulations exhibited a controlled release with <40% drug released up to 72 h. The percentage of CTS released from SLN formulations up to 72 h was 25.87% with CP-SLNs and 37.56 % with GMS-SLNs. The drug release was affected by nature of the lipid matrix. It was clear that CP had more sustained release than the GMS. From the release profiles, it was found that these SLN resembled the drug-enriched core model. In such a model, the drug-enriched core is surrounded by a practically drug-free lipid shell (24). Due to the increased diffusional distance and hindering effects by the surrounding solid lipid shell, the drug has a sustained release profile.
Fig. 2.
In vitro drug release of CTS-GMS-SLNs and CTS-CP-SLNs (n = 3)
Pharmacokinetics Studies
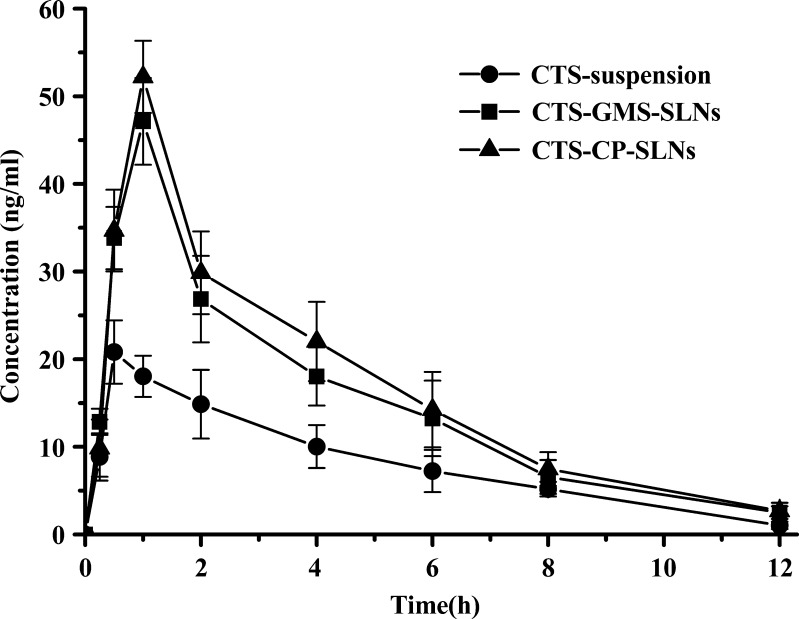
In this study, good linearity was obtained from 0.2 to 100 ng/ml for CTS and from 0.5 to 50 ng/ml for tanshinone IIA. The lower limit of quantifications for CTS and tanshinone IIA were 0.2 and 0.5 ng/ml, respectively. The recovery of CTS from plasma at the three concentrations (1.0, 10.0, and 100 ng/ml) ranged from 81.2% to 93.8%. The recovery of tanshinone IIA from plasma at the three concentrations (1.0, 10.0, and 50 ng/ml) ranged from 83.4% to 91.7%. The pharmacokinetic parameters could be described using non-compartmental model (Table II), and the pharmacokinetic profiles are shown in Fig. 3. In this study, the GMS-SLNs and CP-SLNs resulted in higher Cmax (49.82 and 53.68 μg/ml, respectively.) of CTS as compared with the CTS-suspension that showed a lower Cmax (20.89 μg/ml). The AUC0−∞ of CTS after oral administration of GMS-SLNs (187.9 ± 13.7 ng·h·mL-1) and CP-SLNs (209.5 ± 17.2 ng·h·mL-1) were 1.86 and 2.05 times higher than those obtained with the CTS-suspension (101.1 ± 12.1 ng·h·mL-1). These results showed CTS absorption was enhanced significantly by employing the SLN formulations.
Fig. 3.
Mean plasma concentration–time curves after oral administration of CTS at a dose of 16 mg/kg to rats GMS-SLNs (without SD), CP-SLNs (without SD), and CTS-suspension (n = 6)
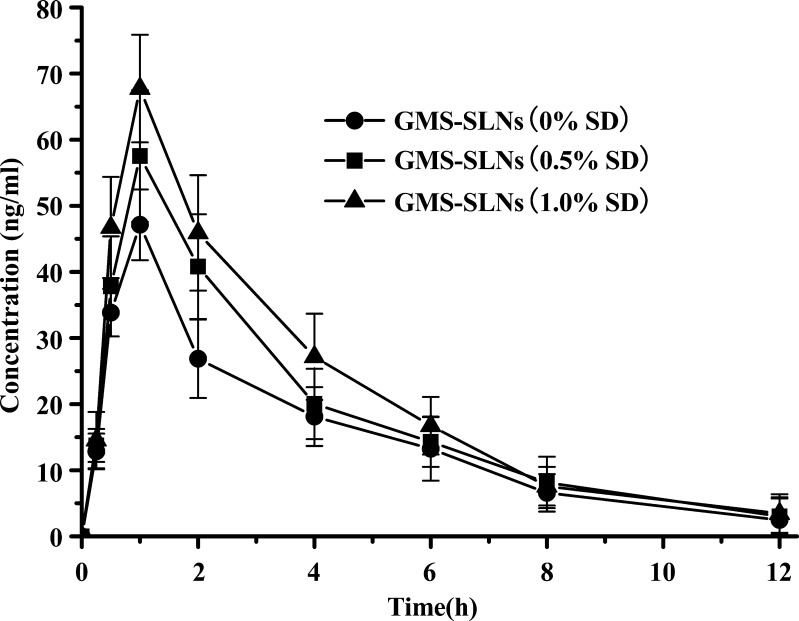
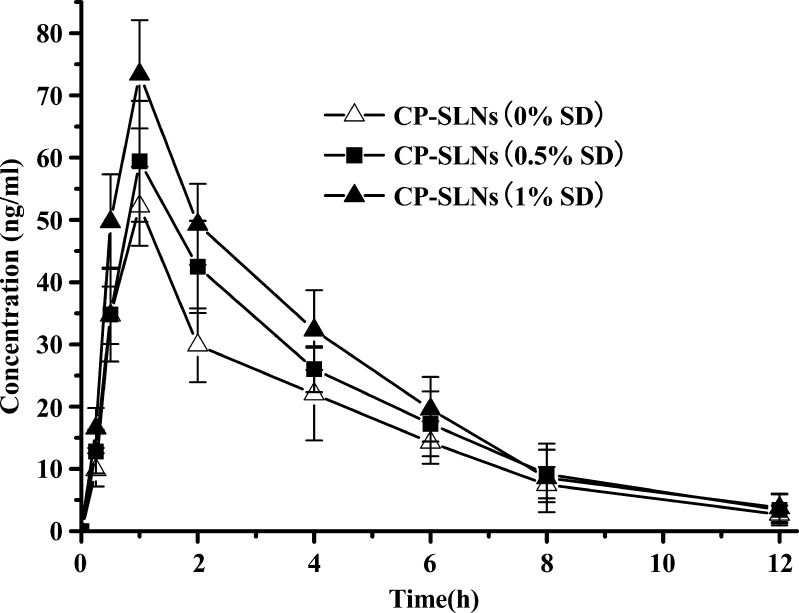
In this study, an interesting result is that SLN formulations containing SD improve the absorption when compared with SLNs without SD. The differences in the pharmacokinetic profiles exhibited in Figs. 4 and 5 are impressive because these SLNs differ only in the content of SD (0% vs. 0.5%, 1.0%), respectively. The SLNs (with SD) resulted in a relatively very high Cmax and oral bioavailability (Table III). This result suggests that incorporation of SD in the SLNs can result in enhanced bioavailability.
Fig. 4.
Mean plasma concentration–time curves after oral administration of CTS-GMS-SLNs containing different amount of SD at a CTS dose of 16 mg/kg (n = 6)
Fig. 5.
Mean plasma concentration–time curves after oral administration of CTS-CP-SLNs containing different amount of SD at a CTS dose of 16 mg/kg (n = 6)
Table III.
Pharmacokinetic Parameters of CTS after Oral Administration to Rats GMS-SLNs, CP-SLNs, and CTS-suspension (n = 6)
| Parameter | GMS-SLNs | CP-SLNs | CTS-suspension | ||||
|---|---|---|---|---|---|---|---|
| 0% SD | 0.5% SD | 1.0% SD | 0% SD | 0.5% SD | 1.0% SD | ||
| Ke (h-1) | 0.273 ± 0.06 | 0.258 ± 0.07 | 0.249 ± 0.07 | 0.275 ± 0.05 | 0.263 ± 0.06 | 0.261 ± 0.04 | 0.241 ± 0.04 |
| T1/2 (h) | 2.54 ± 0.37 | 2.72 ± 0.61 | 2.79 ± 0.34 | 2.53 ± 0.48 | 2.65 ± 0.34 | 2.72 ± 0.49 | 2.88 ± 0.21 |
| Tmax(h) | 0.75 ± 0.27 | 1.08 ± 0.40 | 1.25 ± 0.61 | 0.83 ± 0.25 | 1.16 ± 0.41 | 1.33 ± 0.52 | 0.58 ± 0.20 |
| Cmax (ng/ml) | 49.82 ± 3.71 | 57.13 ± 4.74 | 67.32 ± 4.62 | 53.68 ± 6.14 | 59.45 ± 4.31 | 73.45 ± 6.78 | 20.89 ± 1.96 |
| MRT (h) | 4.19 ± 0.58 | 4.12 ± 0.40 | 4.01 ± 0.38 | 4.22 ± 0.59 | 4.48 ± 0.39 | 3.97 ± 0.42 | 4.20 ± 0.36 |
| AUC0–∞(ng·h· mL-1) | 187.9 ± 13.7 | 232.5 ± 17.4 | 270.3 ± 19.2 | 209.5 ± 17.2 | 258.17 ± 16.5 | 302.3 ± 19.2 | 101.1 ± 12.1 |
| Fr (%) | 186.0 ± 11.8 | 230.2 ± 15.3 | 267 ± 20.3 | 205 ± 23.7 | 256 ± 22.7 | 299 ± 24.8 | |
CTS cryptotanshinone, GMS-SLNs glyceryl monostearate-solid lipid nanoparticles, CP-SLNs Compritol 888 ATO-solid lipid nanoparticles, Cmax peak concentration, Tmax time of peak concentration, MRT mean residence time, Ke apparent elimination rate constant, Fr relative bioavailability
DISCUSSION
SLNs may improve the oral bioavailability of poorly soluble drugs by a number of possible mechanisms. SLNs can increase the solubilization capacity of drug in the GI tract and blood; the apparent drug concentration in the SLNs was far higher than the aqueous solubility of CTS. So, in SLNs drug existed in a supersaturatable state, which is thermodynamically stable and no drug crystallized out of the dispersion during storage. The increased CTS concentration increases drug absorption from the GI tract. While with CTS in suspension, we predicted that the drug might precipitate at the gut wall after administration and thus result in a reduced oral absorption.
The surfactants used in SLNs, such as Tween 80 and SD, also have contributed to both stability and improved bioavailability to SLNs as permeability enhancers. They could increase the permeability of the intestinal membrane, reduce the activity of intestinal efflux transporters, and the P glycoprotein efflux pump. Intestinal drug efflux by P glycoprotein and other transporters is widely recognized as a major determinant for low or variable oral absorption and bioavailability (25).
The decreased first-pass metabolism of CTS is considered another factor for its low oral bioavailability. In this study, an interesting result is that SLNs change the metabolism behavior of CTS. It was proved that CTS could be metabolized to tanshinone IIA in vivo (20). In this study, when CTS was orally administrated to rats, tanshinone IIA was also found in the plasma. The tanshinone IIA pharmacokinetics found in CTS-SLNs and CTS-suspension was significantly different. The Cmax of tanshinone IIA in suspension was about 2.5 times higher than those found in GMS-SLNs and CP-SLNs, and the AUC was about two times higher (Table IV). So, we could conclude that the low oral bioavailability of CTS is due in part to a high first-pass metabolism that happens in the liver and intestine. The decreased metabolism of CTS to tanshinone IIA may be attributed to the protection of the drug from metabolism by embedding the drug into a solid lipid matrix or the interference with intestinal efflux thereby delaying the in vivo metabolism and improving oral bioavailability.
Table IV.
Pharmacokinetic Parameters of Tanshinone IIA after Oral Administration to Rats GMS-SLNs, CP-SLNs, and CTS-suspension (n = 6)
| Parameter | GMS-SLNs | CP-SLNs | CTS-suspension | ||||
|---|---|---|---|---|---|---|---|
| 0% SD | 0.5% SD | 1.0% SD | 0% SD | 0.5% SD | 1.0% SD | ||
| Ke (h-1) | 0.221 ± 0.06 | 0.215 ± 0.06 | 0.209 ± 0.07 | 0.216 ± 0.04 | 0.204 ± 0.05 | 0.198 ± 0.04 | 0.251 ± 0.07 |
| T1/2 (h) | 3.12 ± 0.35 | 3.24 ± 0.41 | 3.28 ± 0.35 | 3.23 ± 0.43 | 3.42 ± 0.46 | 3.55 ± 0.41 | 2.87 ± 0.66 |
| Tmax(h) | 0.46 ± 0.10 | 0.46 ± 0.10 | 0.67 ± 0.26 | 0.58 ± 0.20 | 0.54 ± 0.25 | 0.67 ± 0.26 | 0.42 ± 0.13 |
| Cmax (ng/ml) | 7.17 ± 1.52 | 7.78 ± 1.57 | 7.39 ± 1.31 | 6.86 ± 1.82 | 7.35 ± 1.28 | 7.94 ± 1.52 | 18.43 ± 1.36 |
| AUC0–∞(ng·h· mL-1) | 9.39 ± 1.38 | 10.76 ± 1.51 | 11.43 ± 1.65 | 10.71 ± 1.43 | 10.86 ± 1.25 | 11.93 ± 1.54 | 23.9 ± 3.52 |
CTS cryptotanshinone, GMS-SLNs glyceryl monostearate-solid lipid nanoparticles, CP-SLNs Compritol 888 ATO-solid lipid nanoparticles, Cmax peak concentration, Tmax time of peak concentration, MRT mean residence time, Ke apparent elimination rate constant, Fr relative bioavailability
CONCLUSIONS
In the present study, CTS-SLNs were successfully prepared by an ultrasonic and high-pressure homogenization method using different lipids. The CTS-SLNs were spherically shaped when observed under TEM. The mean diameters and zeta potential of GMS-SLNs and CP-SLNs were 121.4 ± 6.3 nm, 137.5 ± 7.1 nm, and −25.2 ± 1.3 mV, −27.6 ± 1.2 mV, respectively. The in vivo study indicated that SLNs change the metabolism behavior of CTS to tanshinone IIA and the bioavailability of CTS was significantly enhanced by incorporation into SLNs as compared with that in suspension. SLNs are useful in enhancing the oral bioavailability of poorly soluble drugs.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- 1.Yang S, Gursoy RN, Lambert G, Benita S. Enhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors. Pharm Res. 2004;21:261–270. doi: 10.1023/B:PHAM.0000016238.44452.f1. [DOI] [PubMed] [Google Scholar]
- 2.Itoh K, Matsui S, Tozuka Y, Oguchi T, Yamamoto K. Improvement of physicochemical properties of N-4472. Part II: characterization of N-4472 microemulsion and the enhanced oral absorption. Int J Pharm. 2002;246:75–83. doi: 10.1016/S0378-5173(02)00346-0. [DOI] [PubMed] [Google Scholar]
- 3.Brocks DR, Betageri GV. Enhanced oral absorption of halofantrine enantiomers after encapsulation in a proliposomal formulation. J Pharm Pharmacol. 2002;54:1049–1053. doi: 10.1211/002235702320266190. [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan P, Lee BJ, Oh DH, Kim JO, Lee YI, Kim DD, Lee JJP, YB WJS, Yong CS, Choi HG. Enhanced oral bioavailability of coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374:66–72. doi: 10.1016/j.ijpharm.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy M, Hu J, Gao P, Li L, Ali-Reynolds A, Chal B, Gupta V, Ma C, Mahajan N, Akrami A, Surapaneni S. Enhanced bioavailability of a poorly soluble VR1 antagonist using an amorphous solid dispersion approach: a case study. Mol Pharm. 2008;5:981–993. doi: 10.1021/mp800061r. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Lu Y, Chen J, Lai J, Sun J, Hu F, Wu W. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int J Pharm. 2009;376:53–60. doi: 10.1016/j.ijpharm.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Geng Y, Li H, Zhang Y, You J, Chang Y. Enhancement the oral bioavailability of praziquantel by incorporation into solid lipid nanoparticles. Pharmazie. 2009;64:86–89. [PubMed] [Google Scholar]
- 8.Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133:238–244. doi: 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Ugazio E, Cavalli R. Gasco M.R. Incorporation of cyclosporin A in solid lipid nanoparticles (SLN) Int J Pharm. 2002;241:341–344. doi: 10.1016/S0378-5173(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery. II. Drug incorporation and physicochemical characterization. J Microencapsul. 1999;16:205–213. doi: 10.1080/026520499289185. [DOI] [PubMed] [Google Scholar]
- 11.Manjunath K, Reddy JS, Venkateswarlu V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol. 2005;27(2):127–144. doi: 10.1358/mf.2005.27.2.876286. [DOI] [PubMed] [Google Scholar]
- 12.Hu L, Tang X, Cui F. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J Pharm Pharmacol. 2004;56:1527–1535. doi: 10.1211/0022357044959. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Feng X, Kestell P, Paxton JW, Baguley BC, Chan E. Transport of the investigational anti-cancer drug 5, 6-dimethylxanthenone-4-acetic acid and its acyl glucuronide by human intestinal Caco-2 cells. Eur J Pharm Sci. 2005;24:513–524. doi: 10.1016/j.ejps.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Kang BY, Chung SW, Kim SH, Ryu SY, Kim TS. Inhibition of interleukin-12 and interferon-γ production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacol. 2000;49:355–361. doi: 10.1016/S0162-3109(00)00256-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang AM, Sha SH, Lesniak W, Schacht J. Tanshinone (Salviae miltiorrhizae extract) preparations attenuate aminoglycoside-induced free radical formation in vitro and ototoxicity in vivo. Antimicrob Agents Chemother. 2003;47:1836–1841. doi: 10.1128/AAC.47.6.1836-1841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hur JM, Shim JS, Jung HJ, Kwon HJ. Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in vitro. Exp Mol Med. 2005;37:133–137. doi: 10.1038/emm.2005.18. [DOI] [PubMed] [Google Scholar]
- 17.Jin DZ, Yin LL, Ji XQ, Zhu XZ, Zhou S, Feng X, Kestell P, Paxton JW, Baguley BC, Chan E. Cryptotanshinone inhibits cyclooxygenase-2 enzyme activity but not its expression. Eur J Pharmacol. 2006;549:166–172. doi: 10.1016/j.ejphar.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 18.Don MJ, Shen CC, Syu WJ, Ding YH, Sun CM. Cytotoxic and aromatic constituents from Salvia miltiorrhiza. Phytochem. 2006;67:497–503. doi: 10.1016/j.phytochem.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Huang M, Guan S, Bi HC, Pan Y, Duan W, Chan SY, Chen X, Hong YH, Bian JS, Yang HY, Zhou S. A mechanistic study of the intestinal absorption of cryptotanshinone, the major active constituent of Salvia miltiorrhiza. J Pharmacol Exp Ther. 2006;317:1285–1294. doi: 10.1124/jpet.105.100701. [DOI] [PubMed] [Google Scholar]
- 20.Xue M, Cui Y, Wang HQ. Pharmacokinetics of cryptotanshinone and its metabolite in pigs. Acta Pharm Sin. 1999;34:81–84. [Google Scholar]
- 21.Song M, Hang TJ, Zhang ZX, Du R, Chen J. Determination of cryptotanshinone and its metabolite in rat plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:205–209. doi: 10.1016/j.jchromb.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Xue F, Jia XN, Zhang YY, Wang JX. The research of extraction parameters of cryptotanshinone from Salvia miltiorrhiza Bunge (danshen) Northwest Pharm J. 2008;23:145–147. [Google Scholar]
- 23.Hao H, Wang G, Li P, Li J, Ding Z. Simultaneous quantification of cryptotanshinone and its active metabolite tanshinone IIA in plasma by liquid chromatography/tandem mass spectrometry (LC–MS/MS) J Pharm and Biomedical Anal. 2006;40:382–388. doi: 10.1016/j.jpba.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Wacher VJ, Salphati L, Benet LZ. Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv Drug Deliv Rev. 2001;46:89–102. doi: 10.1016/S0169-409X(00)00126-5. [DOI] [PubMed] [Google Scholar]