Abstract
The purpose of this study was to compare relative precision of two different abbreviated impactor measurement (AIM) systems and a traditional multi-stage cascade impactor (CI). The experimental design was chosen to provide separate estimates of variability for each impactor type. Full-resolution CIs are useful for characterizing the aerosol aerodynamic particle size distribution of orally inhaled products during development but are too cumbersome, time-consuming, and resource-intensive for other applications, such as routine quality control (QC). This article presents a proof-of-concept experiment, where two AIM systems configured to provide metrics pertinent to QC (QC-system) and human respiratory tract (HRT-system) were evaluated using a hydrofluoroalkane-albuterol pressurized metered dose inhaler. The Andersen eight-stage CI (ACI) served as the benchmark apparatus. The statistical design allowed estimation of precision with each CI configuration. Apart from one source of systematic error affecting extra-fine particle fraction from the HRT-system, no other bias was detected with either abbreviated system. The observed bias was shown to be caused by particle bounce following the displacement of surfactant by the shear force of the airflow diverging above the collection plate of the second impaction stage. A procedure was subsequently developed that eliminated this source of error, as described in the second article of this series (submitted to AAPS PharmSciTech). Measurements obtained with both abbreviated impactors were very similar in precision to the ACI for all measures of in vitro performance evaluated. Such abbreviated impactors can therefore be substituted for the ACI in certain situations, such as inhaler QC or add-on device testing.
KEY WORDS: AIM, APSD, impactor, inhaler, simplified
INTRODUCTION
Aerosol aerodynamic particle size distributions (APSDs) of orally inhaled products have traditionally been assessed by multi-stage cascade impactors (CIs) or liquid impingers (1). However, such methods are time-consuming, labor-intensive, and require exceptional skill to obtain consistent results, with many opportunities for measurement failure, including causes arising from the operator, the analytical method, the apparatus itself, and the product being evaluated (2). For many applications, including product quality control (QC), it can be argued that an abbreviated impactor comprising as few as two stages is as effective as a multi-stage system for the determination of metrics that are critical quality attributes of inhaler performance (3).
A paper published in 2003 aimed to provide users with a systematic guide towards good CI measurement practices (4). Since the publication of that paper, a clear objective to develop a simpler and more rapid method that would allow the appropriate assessment of critical changes in APSD with equal or improved accuracy and precision has been articulated in the scientific community. In 2008, the abbreviated impactor measurement (AIM) concept was set forth as a means to achieve such simplification for the purpose of testing inhalers in a quality-by-design environment (5,6). At the same time, the Cascade Impaction Working Group of the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS) analyzed an extensive database of retrospective CI data and developed a theoretical basis for improved decision making in product QC based on measures of so-called large and small particle mass, where the boundary size between these fractions is selected close to the mass median aerodynamic diameter (MMAD) of the sampled aerosol (7).
Previous experimental work on the AIM concept (8) focused on the evaluation of two abbreviated systems based on the Andersen eight-stage non-viable impactor (ACI) and intended to determine possible metrics for an AIM system relevant for likely particle deposition in the human respiratory tract (HRT). Such a configuration might be especially suited for the evaluation of add-on devices (e.g., spacers and valved holding chambers) that are widely used with pressurized metered dose inhalers (pMDIs). That study established that AIM measurements can provide comparable precision to that of the full resolution CI and also demonstrated the need for mitigating dry particle bounce. A further investigation (9) showed that it is also important to match the dead volume before the first impaction stage of the abbreviated impactor to that of the same section of the full resolution CI for the most accurate measurements when sampling aerosols containing a volatile excipient.
The objective of the present experiment was to assess whether two similar but not identical abbreviated ACI configurations have comparable precision to the full-resolution CI, used as defined in the pharmaceutical compendia (10,11). The first system was intended for use in inhaler development and QC and is designated as a “QC” impactor. The second system was developed to provide metrics more useful in terms of predicting likely deposition in the HRT, hence termed the “HRT” impactor. This study was conducted to assess the precision of two abbreviated CIs relative to the precision of the full-resolution CI and to obtain preliminary evidence to support the proposal that under specific circumstances that use of an abbreviated CI could be substituted for the full-resolution CI. In this context, the AIM approach offers distinct advantages because of the capability for a more rapid measurement. For example, the potential exists to increase the number of inhalers tested within a given time period, which may be beneficial both for a more thorough characterization of the product and method in development, and for more accurate and confident decision making in QC (6).
MATERIALS AND METHODS
The experiment was undertaken as a 6-day intensive study at one location (Trudell Medical International, London, Ontario, Canada) in early August 2009, in which three operators each performed the same specific tasks contributing to a given complete measurement sequence in order to minimize the risk of introducing operator-linked bias (4). A US commercial pMDI hydrofluoroalkane-albuterol (salbutamol) was used as test product. The following three impactor configurations were investigated:
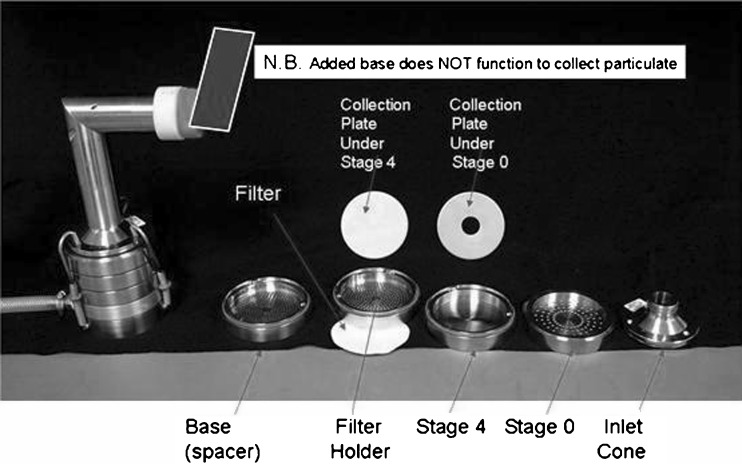
A full resolution ACI as benchmark system (Fig. 1),
A QC metric abbreviated system (Fig. 2),
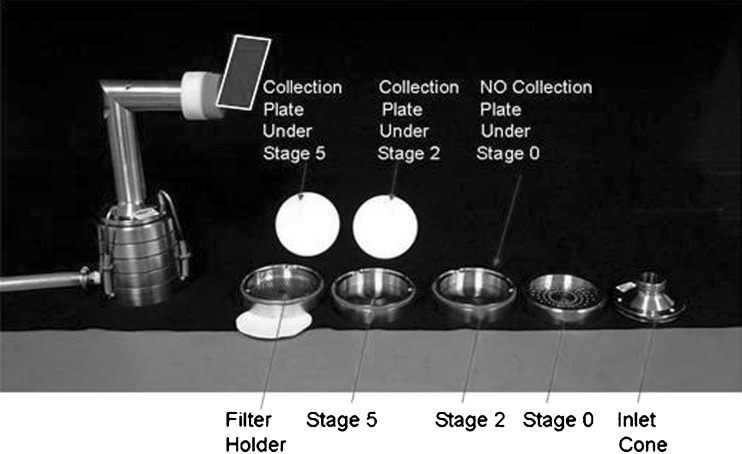
An HRT metric abbreviated system (Fig. 3).
Fig. 1.
Configuration of the full resolution Andersen 8-stage cascade impactor (ACI) (benchmark system)
Fig. 2.
Configuration of quality control metric (QC) abbreviated system. N.B. Nota Bene, or “note well”
Fig. 3.
Configuration of human respiratory tract metric (HRT) abbreviated system
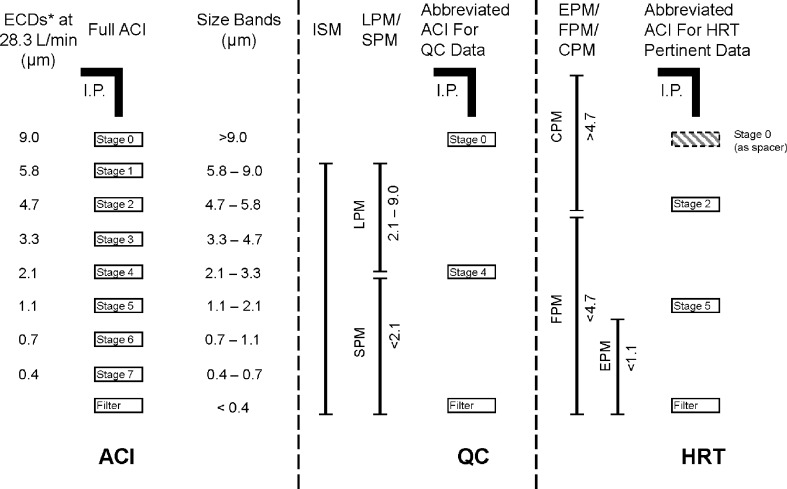
Each configuration was equipped with the standard US Pharmacopeia/European Pharmacopeia induction port (11,12), and the same type of glass microfiber back-up filter (product 934-AH, Whatman Inc., Florham Park, New Jersey, USA) was used in each system. These configurations are depicted schematically in Fig. 4.
Fig. 4.
Impactor configurations used in the designed experiment. ACI Andersen 8-stage cascade impactor, CPM Coarse particle mass, ECD Effective Cut-Off Diameter, EPM Extra-fine particle mass, FPM Fine particle mass, HRT Human respiratory tract, abbreviated impactor to provide information relating to mass fractions inhalable into the human respiratory tract, I.P. Induction Port, ISM Impactor sized mass, i.e., mass entering the impactor excluding non-sizing portions, LPM Large particle mass, QC Quality control, abbreviated impactor for orally inhaled drug product quality control, SPM Small particle mass
The boundary size for the QC impactor was chosen to be 2.1 µm aerodynamic diameter, set at the stage cut-off diameter closest to the MMAD of the formulation used in the investigation (7). This single-stage configuration enabled both small (SPM) and large (LPM) particle mass to be determined in a single process. A non-functioning stage (i.e., without a collection plate) was inserted as a spacer below the filter stage to permit this system to be used with the same short-length spring retainer set as was employed with the two-stage HRT impactor (Fig. 4). The HRT-system was configured to evaluate simultaneously values of coarse (CPM), fine (FPM), and extra-fine (EPM) particle mass (12). The boundary size for the first stage defining FPM was fixed at 4.7 µm aerodynamic diameter, matching the cut-point size of stage 2 for the full resolution ACI at the test flow rate. The second boundary demarcating EPM was chosen to be 1.1 µm aerodynamic diameter, equivalent to stage 5 of the full ACI configuration. A non-functioning stage (stage “0” without its collection plate) was inserted between the inlet cone and first impaction stage (Fig. 4) in order to maintain a comparable internal volume to that of the ACI before size-separation takes place (8).
Following inspection for scratches and flatness, the stainless steel collection plate located beneath each impaction stage of each configuration was thoroughly coated with a surfactant (Brij-35, polyoxyethylene 23 lauryl ether, Fisher Canada, Nepean, Ontario, Canada) before each use in order to mitigate possible bias from particle bounce and re-entrainment (1). Five actuations from the pre-primed inhaler were delivered at 30-s intervals per measurement with each impactor configuration. Afterwards, the mass of albuterol recovered from each component was assayed by a validated high-performance liquid chromatography-ultraviolet absorbance spectrophotometric method (13).
Since the objective of the experiment was to compare the relative precision of the three measurement methods, the sample collection process was designed to minimize the effect of product variation; the operation of the measurement equipment was conducted to minimize the effect of environmental measurement variation, and the analytical testing scheme (i.e., experimental study) was designed to enable estimation of the intrinsic variability (precision) of each impactor system in isolation from other potentially confounding effects by controlling for inhaler-to-inhaler variation, through-life trends of pMDIs, inter-operator variation, and inter-impactor system variation. However, variability in valve/valve delivery and actuator orifice was not specifically addressed in this study.
The samples were specifically selected from a population of sequential canisters, with a theoretical dose of 90 µg/actuation albuterol base equivalent ex-inhaler mouthpiece spanning approximately 1 min of production from a single manufacturing run so as to obtain the most uniform samples possible. The measurement equipment was operated under tightly controlled ambient conditions, with temperature and relative humidity confined to the ranges 21.0 ± 1.0°C and 45 ± 5% RH, respectively, while each impactor was operated at a constant flow rate of 28.3 L/min ± 5%. The experimental design as described in Table I consisted of generating a total of 54 sets of CI measurements across the three impactor configurations and six inhalers. The analytical testing scheme allowed for three repeat measurements from each of the six inhalers.
Table I.
Experimental Design with Three Cascade Impactor Configurations, Six Inhalers, Three Dosing Sets by Inhalers, and Three Replicates of the Same Inhalers
| Inhaler | Replicate | Cascade impactor configuration [dosing set] | ||
|---|---|---|---|---|
| 1 | 1 | ACI [1] | QC [2] | HRT [3] |
| 2 | HRT [5] | ACI [6] | QC [4] | |
| 3 | QC [9] | HRT [7] | ACI [8] | |
| 2 | 1 | QC [2] | ACI [1] | HRT [3] |
| 2 | ACI [4] | HRT [6] | QC [5] | |
| 3 | HRT [9] | QC [8] | ACI [7] | |
| 3 | 1 | ACI [1] | HRT [3] | QC [2] |
| 2 | HRT [6] | QC [5] | ACI [4] | |
| 3 | QC [8] | ACI [7] | HRT [9] | |
| 4 | 1 | QC [3] | HRT [1] | ACI [2] |
| 2 | HRT [5] | ACI [6] | QC [4] | |
| 3 | ACI [7] | QC [8] | HRT [9] | |
| 5 | 1 | ACI [1] | HRT [3] | QC [2] |
| 2 | QC [5] | ACI [4] | HRT [6] | |
| 3 | HRT [9] | QC [8] | ACI [7] | |
| 6 | 1 | HRT [2] | ACI [3] | QC [1] |
| 2 | ACI [4] | QC [5] | HRT [6] | |
| 3 | QC [9] | HRT [7] | ACI [8] | |
The statistical analysis was conducted to estimate and compare the repeatability of three impactor configurations side-by-side, based on first quantifying the following three principal metrics related to the total mass of albuterol emitted from the inhaler, normalized on a per actuation basis:
Impactor mass (IM),
Ex-actuator mass (Ex-ActM),
Ex-metering valve mass (Ex-MVM).
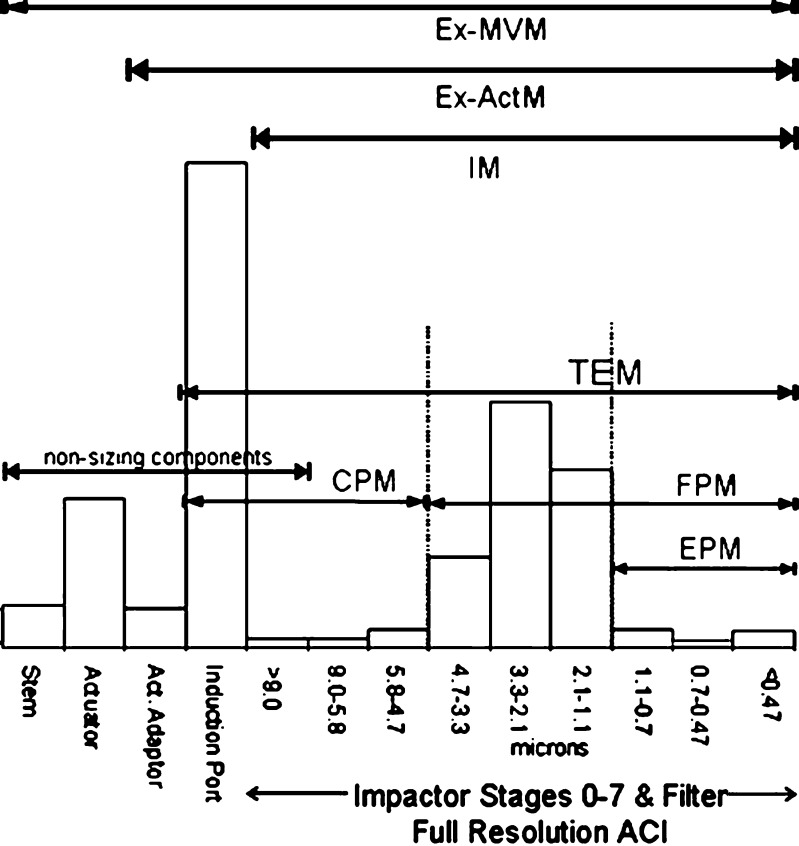
Values of the sub-fractions of the mass/actuation were subsequently established, and the relationship between IM and impactor-sized mass (ISM) was quantified. These sub-fractions are illustrated schematically in Figs. 5 and 6 for the QC and HRT configurations, respectively, using a size scale based on the stages of the full resolution ACI operated at the chosen flow rate.
Fig. 5.
Relationship between measured quantities for the QC and ACI configurations. ACI Andersen 8-stage cascade impactor, APSD Aerodynamic particle size distribution, AUC Area Under the Curve of APSD, ISM Impactor sized mass, i.e., mass entering the impactor excluding non-sizing portions, LPM Large particle mass, MMAD Mass median aerodynamic diameter, QC Quality control, abbreviated impactor for orally inhaled drug product quality control, SPM Small particle mass
Fig. 6.
Relationship between measured quantities for the HRT and ACI configurations. ACI Andersen 8-stage cascade impactor, CPM Coarse particle mass, EPM Extra-fine particle mass, Ex-ActM Mass of active pharmaceutical ingredient ex-mouthpiece actuator, Ex-MVM Mass of active pharmaceutical ingredient ex-inhaler metering valve, FPM Fine particle mass, HRT Human respiratory tract, abbreviated impactor to provide information relating to mass fractions inhalable into the human respiratory tract, IM Impactor mass, i.e., mass entering the impactor including non-sizing portions, ISM Impactor sized mass, i.e., mass entering the impactor excluding non-sizing portions, TEM Total emitted mass
All statistical analyses were performed using SAS Version 9.1, SAS Institute, Cary, North Carolina, USA.
RESULTS
The abbreviated CI configurations (QC and HRT) were each compared to the full impactor configuration (ACI) using mass-based metrics such as IM, Ex-ActM, Ex-MVM, ISM, LPM/SPM ratio, CPM, FPM, and EPM where appropriate. For each mass-based metric, summary statistics (mean, standard deviation, and coefficient of variation (CV)) were computed along with two-sided 95% confidence intervals on the ratio of the abbreviated configuration standard deviation to the full CI configuration standard deviation.
Table II presents the summary statistics for the common principal mass-based IM metric, together with the Ex-ActM and Ex-MVM metrics for both abbreviated (QC and HRT) and full CI configurations. A visual comparison of the means indicates that there is no evidence of a difference between the impactor configuration means within each metric, which suggests that there is no bias between the measures obtained with any of the abbreviated CI configurations relative to the measures obtained with the full CI configuration. Due to the nature of the design, calculations for confidence intervals on mean differences were not easily available. Furthermore, from inspection, rigorous statistical calculations were unnecessary regarding potential bias. Mass recoveries were all within ±15% of label claim.
Table II.
Summary Statistics and 95% Confidence Intervals on Ratio Standard Deviations of Abbreviated to Full Configurations for Metrics Related with Total Mass Per Actuation
| Metric | Impactor configuration | Mean (µg) | Standard deviation (repeatability, µg) | CV (%) | 95% Confidence interval on ratio of standard deviations of abbreviated to full configuration |
|---|---|---|---|---|---|
| IM | ACI | 40.08 | 1.60 | 3.99 | – |
| QC | 40.05 | 2.66 | 6.64 | 0.92; 3.01 | |
| HRT | 40.03 | 2.14 | 5.34 | 0.74; 2.42 | |
| Ex-ActM | ACI | 79.51 | 3.35 | 4.21 | – |
| QC | 79.89 | 3.48 | 4.35 | 0.56; 1.89 | |
| HRT | 80.19 | 3.74 | 4.67 | 0.61; 1.96 | |
| Ex-MVM | ACI | 96.28 | 4.22 | 4.39 | – |
| QC | 96.58 | 3.91 | 4.05 | 0.57; 1.54 | |
| HRT | 96.75 | 3.92 | 4.06 | 0.57; 1.55 |
Inclusion of 1 in confidence interval indicates no statistically significant difference
CV coefficient of variation
The standard deviation and CV estimates for the IM metric obtained with the abbreviated CI configurations appear to be more variable than those obtained for the full CI configurations, whereas the standard deviation and CV estimates of the Ex-ActM and the Ex-MVM are more similar. A formal statistical comparison was performed by assessing the two-sided 95% confidence intervals on the standard deviation ratios. Since the confidence intervals on the standard deviation ratios include 1, the variability of the CI measurements obtained with either abbreviated CI configuration are not statistically different from the variability of the CI measurements obtained with the full CI configuration.
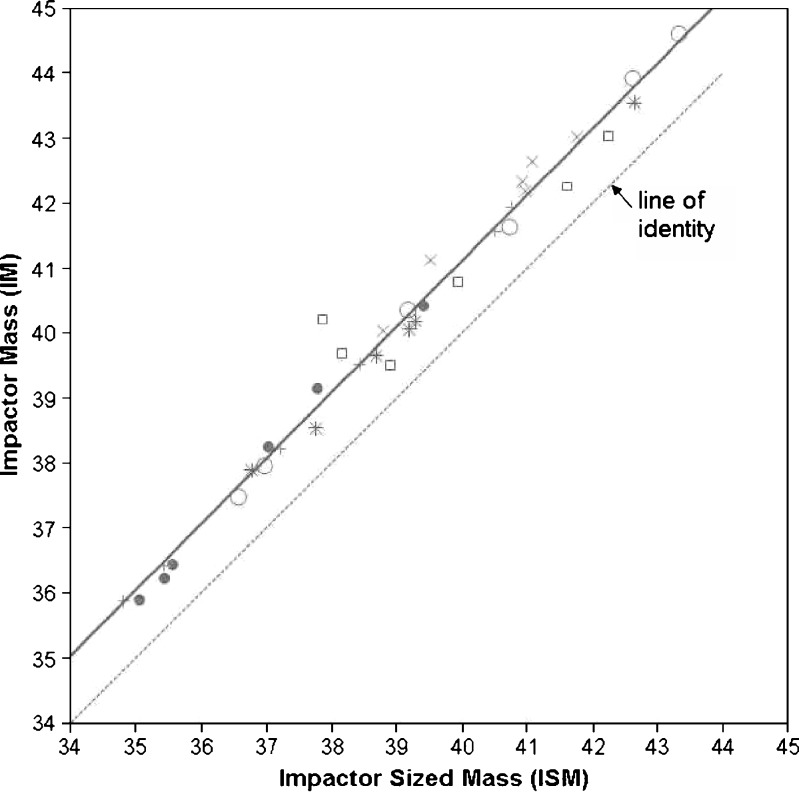
Table III contains the summary statistics for the ISM and LPM/SPM ratio mass-based metrics for the QC abbreviated CI configuration and the full CI configuration. These mass-based metrics could not be determined directly for the HRT CI configuration since this abbreviated configuration does not have an active stage 0 (Fig. 6). However, it is expected that any ISM or LPM/SPM metric precision comparisons of the HRT configuration to the full CI configuration would yield similar conclusions to that of the IM precision comparisons, as a strong linear relationship (R2 = 0.98) exists between the IM and ISM metric as illustrated in Fig. 7. The IM/ISM regression line in comparison to the line of unity displayed in the regression plot indicates that the IM measures are higher than ISM, which is expected since additional mass is collected on stage 0.
Table III.
Summary Statistics and 95% Confidence Intervals on Ratio of Standard Deviations of Abbreviated to Full Configurations for Metrics Related to Inhaler APSD
| Metric | Impactor configuration | Mean | Standard deviation (repeatability) | CV (%) | 95% Confidence interval on ratio of standard deviations of abbreviated to full configuration |
|---|---|---|---|---|---|
| ISM | ACI | 38.97 (μg) | 1.57 | 4.07 | – |
| QC | 38.96 (μg) | 2.68 | 6.87 | 0.93; 3.05 | |
| LPM/SPM | ACI | 2.79 | 0.28 | 9.98 | – |
| QC | 2.69 | 0.35 | 12.83 | 0.68; 2.24 |
Inclusion of 1 in confidence interval indicates no statistically significant difference
CV coefficient of variation
Fig. 7.
Relationship between IM (μg) versus ISM (μg), showing measurements with a different symbol for each of the six inhalers evaluated
As discussed above, the ISM results obtained with the QC impactor relative to the full CI configuration were found to be very similar to the results for IM. A review of the summary statistics indicate that there is no evidence of bias in LPM/SPM ratio metric obtained with the QC impactor relative to the full CI configuration. The standard deviation and CV estimates for the LPM/SPM metric obtained with the abbreviated CI configurations appear to be more variable than those obtained for the full CI configurations. As with the other metrics, the confidence intervals on the standard deviation ratios include 1, indicating that the variability of the LPM/SPM ratios obtained with the QC abbreviated CI configuration are not statistically different from the variability of the LPM/SPM ratios obtained with the full CI configuration.
Table IV contains the summary statistics for the sub-fraction metrics CPM, FPM, and EPM for the HRT and full CI configuration. A comparison of the means shows no evidence of a difference between the impactor configurations for the CPM and FPM sub-fraction; however, there is an obvious difference in the means between the HRT abbreviated CI configuration and the full CI configuration for the EPM sub-fraction. As evidenced by the inclusion of 1 in the confidence intervals on ratio of the standard deviations, the variability in the CPM, FPM, and EPM measurements for the HRT CI configuration is not statistically different from the variability in the full CI configuration sub-fraction metrics. Because the HRT EPM mean is larger than the full CI configuration mean and the standard deviations are comparable, the CV for the EPM measurement is expected to be less for the HRT configuration than for the full CI configuration.
Table IV.
Summary Statistics and 95% Confidence Intervals on Ratio of Standard Deviations of HRT to Full Resolution for Metrics Relating Inhaler APSD
| Metric | Impactor configuration | Mean (µg) | Standard deviation (µg) | CV (%) | 95% Confidence interval on ratio of standard deviations of abbreviated to full configuration |
|---|---|---|---|---|---|
| CPM | ACI | 44.08 | 2.87 | 6.52 | – |
| HRT | 45.17 | 3.00 | 6.64 | 0.57; 1.89 | |
| FPM | ACI | 35.43 | 1.40 | 3.88 | – |
| HRT | 35.00 | 1.74 | 4.97 | 0.69; 2.25 | |
| EPM | ACI | 2.21 | 0.74 | 33.72 | – |
| HRT | 7.93 | 0.99 | 12.44 | 0.73; 2.40 |
Inclusion of 1 in confidence interval indicates no statistically significant difference
CV coefficient of variation
In summary, the results of the statistical comparisons indicate that the precision of the chosen mass-based metrics obtained with either abbreviated configuration were substantially equivalent to the corresponding metrics obtained with the full resolution CI.
DISCUSSION
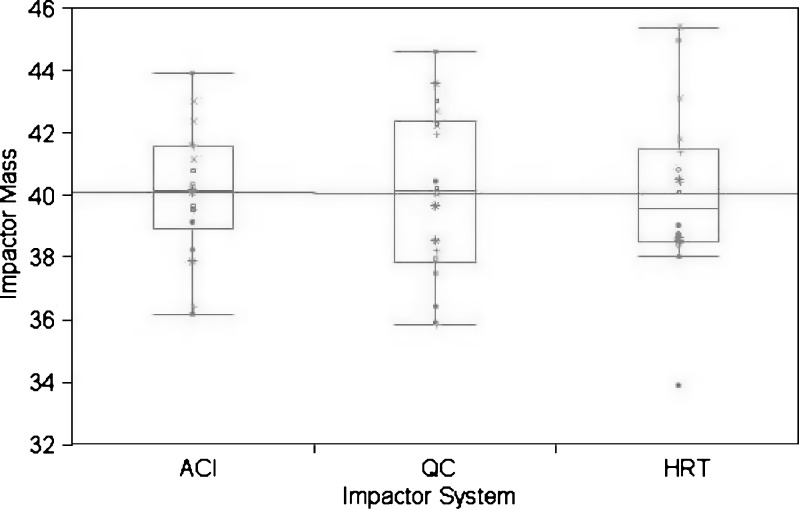
The statistical analysis of the data obtained from the two abbreviated impactor systems presented here indicated that, in general, the precision of either configuration was substantially comparable to that achievable with the full resolution impactor. However, it was observed during the course of the experiment that the abbreviated measurements could be made in approximately one third of the time required to operate the ACI. This suite of measurements also confirmed that the possible confounding effects of inhaler variability (both through-life and from one inhaler to another) had been accounted for, in that there was no discernable pattern in the impactor order for measures of IM (Fig. 8) or with any of the sub-fractions (data not shown). Although there appeared to be slightly more variability in the overall distribution of results from the HRT configuration compared to ACI and QC, this result indicates that there is likely additional variability associated with uncontrolled factors, for instance, the different aerosol dynamics upstream of stage 1 of the QC impactor and that for the full ACI. However, such differences are small compared to the expected inter- and intra-lot variability associated with the drug product.
Fig. 8.
Comparison plots of impactor mass (μg) by inhaler and cascade impactor configuration. ACI Andersen 8-stage non viable cascade impactor, HRT Human respiratory tract, abbreviated impactor to provide information relating to mass fractions inhalable into the human respiratory tract, QC Quality control, abbreviated impactor for orally inhaled drug product quality control
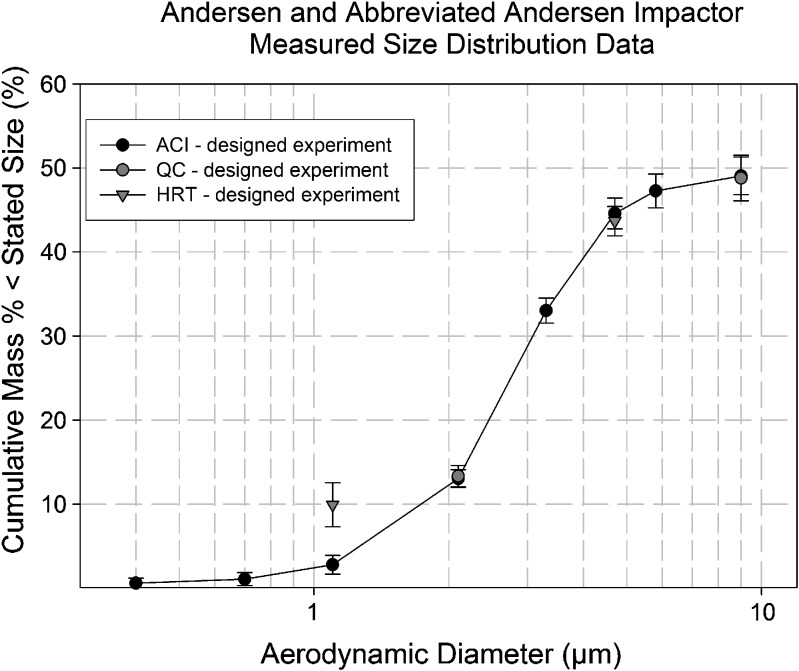
The accuracy of the two abbreviated configurations was comparable with that of the ACI for the determination of the metrics representing total mass per actuation (Table II). However, when the various sub-fractions were compared on a cumulative mass-weighted basis, the extra-fine particle fraction (EPF, i.e., <1.1 µm aerodynamic diameter) measured by the HRT was about 8% higher than expected based on the full size distribution from the ACI (Fig. 9). This behavior corresponded to a 5.7-µg/actuation increase in EPM (Table IV). This bias was not evident with the measures of FPM and CPM for this impactor, nor in the determinations of LPM and SPM with the QC-system, suggesting that the problem was confined to the lowermost stages of the abbreviated HRT configuration where the flow Reynolds number is relatively high for the ACI (292 at 28.3 L/min), and associated with relatively large nozzle diameter-to-collection plate distances greater than 6.0 (14). A follow-on study was therefore undertaken to investigate an alternative cause for these higher than expected values. Results of this follow-on study are reported in a companion paper (Part 2, submitted to AAPS PharmSciTech).
Fig. 9.
Comparative measures of impactor-sized sub-fractions by QC and HRT abbreviated systems with ACI: original measurement set with the HRT-system
The findings from this study clearly support the application of the AIM concept as a means of accelerating the testing of inhalers and related products. However, a larger objective of interest is to validate the use of an AIM-based approach combined with the use of the LPM/SPM ratio to characterize APSD. As noted previously, inhalers used in this study were selected from a short period of manufacture of a single batch to ensure as much homogeneity as possible among samples used in the study to improve the ability to characterize measurement variability. As such, the objective and design of this experiment was not intended to reflect the typical variability of inhalers or establish specifications for inhaler performance. An additional experiment is being planned to complement this study using product samples that better represent the overall variability of typical production performance. The primary objective of this future study will be to evaluate specifically the relationship between the LPM/SPM ratio and MMAD in order to confirm the findings of Tougas et al. (7), and the selection of these product samples should provide a robust evaluation of that relationship.
Other future studies could evaluate the impact and benefits of the AIM-enabled larger sample sizes, such as their ability to improve the quality of QC decisions or their potential to allow quantification of the CI method variability (as separate from the other components of the overall measurement variability).
One of the a priori expectations in the present experiment was that AIM-based methods could be more precise by virtue of eliminating the imprecision arising from stages containing little or no mass. The results presented here demonstrate that the contributors to the overall method precision are more complex and that other effects, possibly altered flow dynamics in the reduced impactors, may have offset the anticipated improvements in precision arising from the above-mentioned cause. Such effects, however, could be investigated and corrected for, and they do not materially alter the value of the AIM-based systems.
Since the AIM concept is not a single impactor design, but rather a general approach, many different configurations of CI and liquid impingers can be adapted for measuring the minimum number of APSD-related metrics to characterize an inhaler-produced aerosol (6). The selection of appropriate stages to create an abbreviated system such as those described here may depend on the particular APSD of the product of interest. It will, therefore, be essential to validate the AIM-based system chosen for a particular product, using the appropriate full resolution CI to provide benchmark data before working with the reduced system on a routine basis. The designed experiment plan developed for this study could be adopted by future users wishing to implement abbreviated impactor testing to provide either QC- or HRT-pertinent size information.
CONCLUSIONS
The presented designed experiment met the goal of assessing the precision of two different abbreviated impactors based on the Andersen concept, relative to the full resolution ACI as the benchmark apparatus. The results also pointed to the need to eliminate particle bounce from the second stage of the HRT-system. Overall substantial agreement was obtained between both abbreviated impactors and the ACI, and therefore, such abbreviated impactors can be substituted for the ACI in certain situations, such as inhaler QC or add-on device testing. Further work of a similar nature is needed to extend this type of measurement method assessment to other abbreviated impactor types and to other test articles reflecting commercial variability.
ACKNOWLEDGMENTS
The authors wish to acknowledge feedback received during the preparation of this manuscript from the other members of the IPAC-RS Cascade Impactor Working Group and from discussions with members of the European Pharmaceutical Aerosol Group (EPAG) Impactor Sub-Team. The helpful comments of the anonymous AAPS PharmSciTech reviewers were also much appreciated.
GLOSSARY
- ACI
Andersen eight-stage non-viable cascade impactor
- AIM
Abbreviated impactor measurement concept
- APSD
Aerodynamic particle size distribution
- CI
Cascade impactor, cascade impaction
- CI WG
The Cascade Impaction Working Group of IPAC-RS
- CPM, CPF
Coarse particle mass, coarse particle fraction
- CV
Coefficient of variation
- EPM, EPF
Extra-fine particle mass, extra-fine particle fraction
- Ex-ActM
Mass of active pharmaceutical ingredient ex-mouthpiece actuator
- Ex-MVM
Mass of active pharmaceutical ingredient ex-inhaler metering valve
- FPM, FPF
Fine particle mass, fine particle fraction
- HFA
Hydrofluoroalkane
- HRT
Human respiratory tract, abbreviated impactor to provide information relating to mass fractions inhalable into the human respiratory tract
- IM
Impactor mass, i.e., mass entering the impactor including non-sizing portions
- IPAC-RS
International Pharmaceutical Aerosol Consortium on Regulation and Science
- ISM
Impactor-sized mass, i.e., mass entering the impactor excluding non-sizing portions
- LPM
Large particle mass
- MMAD
Mass median aerodynamic diameter
- OIP
Orally inhaled product
- pMDI
Pressurized metered dose inhaler
- QC
Quality control, abbreviated impactor for orally inhaled drug product quality control
- SPM
Small particle mass
- TEM
Total emitted mass
- USP/Ph.Eur.
US Pharmacopeia/European Pharmacopeia
REFERENCES
- 1.Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2003;16:341–77. doi: 10.1089/089426803772455622. [DOI] [PubMed] [Google Scholar]
- 2.Bonam M, Christopher D, Cipolla D, Donovan B, Goodwin D, Holmes S, et al. Minimizing variability of cascade impaction measurements in inhalers and nebulizers. AAPS PharmSciTech. 2008;9(2):404–13. doi: 10.1208/s12249-008-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Oort M, Roberts W. Variable flow-variable stage-variable volume strategy for cascade impaction testing of inhalation aerosols. In: Dalby RN, Byron PR, Farr SJ, editors. Respiratory drug delivery—V. Buffalo Grove: Interpharm Press Inc; 1996. pp. 418–21. [Google Scholar]
- 4.Christopher D, Curry P, Doub B, Furnkranz K, Lavery M, Lin K, et al. Considerations for the development and practice of cascade impaction testing including a mass balance failure investigation tree. J Aerosol Med. 2003;16:235–47. doi: 10.1089/089426803769017604. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JP. The role of aerosol measurement for aerodynamic particle size distribution (APSD) in a quality-by-design (QbD) environment. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory drug delivery 2008. River Grove: Davis Healthcare International Publishing LLC; 2008. pp. 779–83. [Google Scholar]
- 6.Mitchell JP. The abbreviated impactor measurement (AIM) concept for aerodynamic particle size distribution (APSD) in a quality-by-design (QbD) environment. Proc. Biennial IPAC-RS Conference, Bethesda, MD, USA. 2008. http://www.ipacrs.com/ipac2008.html. Accessed 10 Sept 2009.
- 7.Tougas TP, Christopher D, Mitchell JP, Strickland H, Wyka B, Van Oort M, et al. Improved quality control metrics for cascade impaction measurements of orally inhaled drug products (OIPs) AAPS PharmSciTech. 2009;10(4):1276–85. doi: 10.1208/s12249-009-9312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JP, Nagel MW, Avvakoumova V, MacKay H, Ali R. The abbreviated impactor measurement (AIM) concept: part 1—influence of particle bounce and re-entrainment—evaluation with a “dry” pressurized metered dose inhaler (pMDI)-based formulation. AAPS PharmSciTech. 2009;10(1):243–51. doi: 10.1208/s12249-009-9202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell JP, Nagel MW, Avvakoumova V, MacKay H, Ali R. The abbreviated impactor measurement (AIM) concept: part 2—influence of evaporation of a volatile component—evaluation with a “droplet producing” pressurized metered dose inhaler (pMDI)-based formulation containing ethanol as co-solvent. AAPS PharmSciTech. 2009;10(1):252–7. doi: 10.1208/s12249-009-9201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.USP33-NF28. Chapter <601> Aerosols, nasal sprays, metered-dose inhalers, and dry powder inhalers. US Pharmacopeial Convention, Rockville, MD, USA. 2009.
- 11.European Pharmacopeia 6.03. Monograph 2.9.18. Preparations for inhalations: aerodynamic assessment of fine particles. European directorate for the quality of medicines (EDQM), Council of Europe, 67075 Strasbourg, France. 2009.
- 12.Dolovich MB, Mitchell JP. Canadian Standards Association standard CAN/CSA/Z264.1-02:2002: a new voluntary standard for spacers and holding chambers used with pressurized metered-dose inhalers. Can Respir J. 2004;11(7):489–95. doi: 10.1155/2004/497946. [DOI] [PubMed] [Google Scholar]
- 13.Trudell Medical International, analytical laboratory procedure (ALP) 025: HPLC drug assay—method for Albuterol, Rev.G, July 2009.
- 14.Marple VA, Olson BA, Miller NC. The role of inertial collectors in evaluating pharmaceutical aerosol delivery systems. J Aerosol Med. 1998;11(S1):139–53. [PubMed] [Google Scholar]