Abstract
Objective
This study examined if pairing a placebo with stimulant medication produces a placebo response that allows children with ADHD to be maintained on a lower dose of stimulant medication. Primary aim was to determine the efficacy, side effects and acceptability of a novel conditioned placebo dose reduction (CPDR) procedure.
Method
Participants included 99 children ages 6 to 12 years with ADHD. After an initial double-blind dose finding to identify optimal dose of mixed amphetamine salts (MAS), subjects were randomly assigned to one of three treatments of eight weeks duration: (a) CPDR condition (50% Reduced Dose/Placebo– RD/P) or (b) a dose reduction only condition (Reduced Dose - RD) or (c) a no reduction condition (Full Dose–FD). The innovative CPDR procedure involved daily pairing of MAS dose with a visually distinctive placebo capsule administered in open label, with full disclosure of placebo use to subjects and parents.
Results
70 children completed the study. There were no differences in subject retention among the three groups. Most subjects in the RD/P group remained stable during the treatment phase, whereas most in the RD group deteriorated. There was no difference in control of ADHD symptoms between the RD/P group and the FD group, and both RD/P and FD groups showed better ADHD control than the RD group. Treatment emergent side effects were lowest in the RD/P group.
Conclusion
Pairing placebos with stimulant medication elicits a placebo response that allows children with ADHD to be effectively treated on 50% of their optimal stimulant dose.
Attention deficit hyperactivity disorder (ADHD) is the most prevalent neurobehavioral disorder in children, with prevalence estimates of 3–12%. Despite clear evidence of the beneficial effects of stimulant therapy in the treatment of ADHD,1,2 there continue to be widespread concerns about over-utilization of stimulant therapy.3,4 Treatment-emergent side effects are common 5,6 and their long-term significance is not fully known.4 Many parents worry about short- and long-term side effects associated with stimulant therapy, and these attitudinal factors contribute to non-adherence, premature stimulant discontinuation, and consequently increasing morbidity. For these reasons, parents and professionals are united in the desire to treat children with the lowest effective doses. 1,7
Strong placebo effects have been shown in clinical trials of treatments for several psychiatric disorders, including depression, anxiety disorders and autism.8,9 Placebo response rates in depression appear to be even higher in pediatric samples than in adult samples.10 Similarly, high placebo response rates have been found in children with ADHD.4,11 Previous clinical trials of stimulants show 30% of children with ADHD are clinical responders to placebo in double-blind trials.2,11,12
There are no previous studies of open-label placebo in children. Brown (1994) proposed the ethical use of open-label placebo as treatment for mild depression in adults.13 That paper included some discussion about the extent to which placebo treatment may be ineffective if both clinician and patient know the placebo is pharmacologically inactive. Only one published study has examined the impact of patient’s knowledge of the placebo’s true nature, suggesting that such knowledge did not preclude the possibility of beneficial response.14
Several studies have suggested that placebo effects may in part represent conditioning phenomena and that learning processes may influence the response to placebo.15–17 In classical (Pavlovian) conditioning, biologically neutral events associated with the administration of pharmacologic agents can become conditioned stimuli capable of producing responses similar to those produced by the active drugs. In behavioral terms, the pharmacological effect of a drug is the unconditioned stimulus. The environmental or behavioral stimuli that are associated with the administration of the drug the bottle, the distinctive taste and appearance of the pill– are the conditioned stimuli. Repeated association of conditioned and unconditioned stimuli eventually enables the conditioned stimuli to elicit a physiological or behavioral response that is similar to the drug response.
The purpose of this proof-of-concept study was to determine if pairing a placebo with stimulant medication produces a placebo response that allows children with ADHD to be treated effectively with a lower dose of stimulant medication. Primary aim was to determine the efficacy, side effects and acceptability of a novel conditioned placebo dose reduction (CPDR) treatment. We hypothesized that (1) stimulant dose reduction plus CPDR will produce a significantly smaller exacerbation of ADHD symptoms than will stimulant dose reduction, and (2) that stimulant dose reduction plus CPDR treatment will produce a reduction in stimulant-related side effects.
METHODS
Sample
The Olson Huff Center is a regional hospital-based multidisciplinary evaluation and treatment center serving children with developmental and behavioral problems in Asheville, NC. The most prevalent diagnosis among children seen is ADHD. The prevalence of common comorbid conditions such as anxiety disorders, depression, and specific learning disability among children with ADHD seen at the Huff Center is similar to general child psychiatric clinics.
Our estimated sample size projection (N = 80) was based on a power analysis and on our previous pilot study.18 Children with a diagnosis of ADHD were recruited by screening the records of current Huff Center patients. Parents were contacted to describe the study and those who expressed interest came with their child to an enrollment visit that included detailed description of the study, informed consent for parents, and child assent. Children who were enrolled following screening, recruitment, and informed consent/assent then proceeded to the diagnostic assessment phase of the study to confirm their ADHD diagnosis, comorbid conditions, and eligibility based on formal assessment of inclusion and exclusion criteria. The research protocol was aproved by Mission Hospitals’ Institutional Review Board.
Inclusion criteria were: (1) between ages 6 and 12 years (grades KG-6), (2) diagnosed ADHD, (3) in residence with same primary caregivers for the last 6 months or longer. Exclusion criteria were: (1) major neurological or medical illness, e.g., epilepsy, cerebral palsy (2) suspected mental retardation or IQ below 80, (3) diagnosis of psychosis, bipolar disorder or autism spectrum disorder (4) history of intolerance to several stimulant medications, and (5) current use of psychotropic medications other than stimulants.
The diagnosis of ADHD was validated for each child using medical examination and the Diagnostic Interview Schedules for Children (DISC) Predictive Scales– parent report version (version 3.0) prior to each child’s entry into the study. Medical examination included careful review of records, history, physical and neurological examination, consistent with recent published clinical guidelines.19 The Vanderbilt Parent and Teacher Rating Scales (described below) were obtained to assist in the diagnostic process and to quantify severity of ADHD symptoms and impairment at the time of the diagnostic assessment. Also, the DISC (described below) was used to identify the presence of comorbid conditions (e.g. oppositional defiant disorder, conduct disorder, depression, anxiety disorders), that were included in post-hoc analyses of child factors related to treatment response.
Ninety nine subjects were enrolled, and 70 completed the study. All subject met DSM-IV criteria for ADHD. Sample demographics are included in Table 1. The sample was representative of the pediatric clinic population from which it was drawn. Most of the children were white, and 68% were male. Average parent educational level was high school graduation. As in most clinical samples of children with ADHD, the sample had a high prevalence of other mental health symptoms based on DISC, including oppositional behavior, depressed mood and anxiety.
TABLE 1.
Subject Disposition, and Demographic and Clinical Description for the Three Groups at Baseline
| Subjects assessed as eligible | 137 | |
| Withdrew before starting study | 38 | |
| Subjects entering Dose Finding | 99 | |
| Withdrew during Dose Finding | 6 | |
| Subjects completing Dose Finding | 93 | |
| Randomized | 93 | |
| Completed | 70 |
| Full Dose (FD) (n = 31) | Reduced Dose (RD) (n = 29) | Reduced Dose/Placebo (RD/P) (n = 33) | |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 9.6 (1.8) | 9.7 (1.9) | 9.6 (1.8) |
| Sex, boys, n (%) | 21 (68) | 20 (69) | 22 (67) |
| Race, white, n (%) | 29 (94) | 27 (93) | 31 (94) |
| Primary diagnosis | |||
| ADHD-C, n (%) | 20 (63) | 18 (62) | 21 (64) |
| ADHD-I, n (%) | 11 (37) | 11 (38) | 12 (36) |
| Comorbid diagnosis* | |||
| ODD, n (%) | 12 (39) | 13 (45) | 11 (33) |
| CD, n (%) | 3 (10) | 2 (7) | 3 (9) |
| DEPR, n (%) | 12 (39) | 10 (34) | 13 (39) |
| GAD, n (%) | 7 (23) | 5 (17) | 7 (21) |
| Discontinued, n | 9 | 6 | 8 |
| Completed, n | 22 | 23 | 25 |
Note: p > .05 for all comparisons. ADHD-C = ADHD (combined type); ADHD-I = ADHD (inattentive type); ODD = oppositional defiant disorder; CD = conduct disorder; DEPR = depression; GAD = generalized anxiety disorder
denotes likelihood of comorbid diagnosis based on screening with DISC– Parent Version
Procedures
First, a one-month dose finding procedure (randomized, double-blind, crossover design than included washout, placebo, low dose, and high dose conditions of one week each) determined the most effective dose of stimulant medication for each participant. ADHD symptoms and medication side effects were measured three times each week using the IOWA Conners parent and teacher rating scales and the Pittsburgh Side Effects Rating Scale (PSERS). Low dose was 0.3 mg/kg/day extended-release mixed amphetamine salts (MAS-XR) and high dose was 0.6 mg/kg/day MAS-XR. The order of treatment conditions was randomized. For the entire group, parent-reported ADHD symptom severity (IOWA-P) was lowest during the high dose week relative to the low dose week and the placebo week (F (2, 172) = 3.06, p = .001). There were no significant differences between the three dose conditions (high, low, placebo) on parent-reported severity of side effects (p > .05); there was a trend for higher reported severity of side effects at high dose conditions. Optimal dose (lowest ADHD symptoms rating and lowest side effects rating) was determined for each participant; for 65% of the participants optimal response was during the high dose week, for 26% of the participants optimal response was during the low dose week, for 3% of the participants optimal response was during the placebo week, and 6% of the participants showed an equivocal pattern of response across the weeks. Six children who experienced treatment-limiting side effects at both doses or who were unable to complete the dose finding phase successfully were excluded from further participation in the study and referred for clinic management.
Next, 93 subjects who completed the dose finding procedure were randomly assigned to one of three open-label groups (see table 1). The groups did not differ significantly in terms of demographic factors (age, gender, maternal education level) or clinical factors (parent or teacher rated severity of ADHD symptoms) at the baseline point prior to randomization for the dose reduction phase (all p’s > 0.05). Twenty three subjects (24.7% of the 93 subjects randomized) did not complete the study (9/31 [29%] in FD group, 6/29 [21%] in RD group, and 8/33 [24%] in RD/P group), with no differences in % of subjects who did not complete among the three groups.
We attempted to interview each of the 29 non-completer’s parents to determine the reasons for withdrawal. Most responded, and some gave more than one reason. Their responses can be grouped into 4 main categories:
Satisfaction with current ADHD treatment, not wanting to change (8 provided this reason)
Behavioral or school problems during the washout week and/or placebo week of Dose Finding (3 provided this reason)
Emergent side effects and/or perceived lack of effectiveness of Adderall (9 provided this reason)
Other reason, no reason given or lost to follow-up (12 subjects)
The control group (n=22) took their full, most effective dose of medication for two months (Full Dose group– FD). The comparison group (n=23) took their most effective dose for one month and then took 50% of that dose for the second month (Reduced Dose group– RD). The experimental group (n=25) had the conditioned placebo dose reduction (CPDR) procedure. They took their most effective dose for the first month plus an additional visually distinctive capsule—the placebo—and then took 50% of that dose for the second month along with the placebo (Reduced Dose plus Placebo group– RD/P). Parent rating scales were obtained three times each week and teacher rating scales twice each week to monitor ADHD symptoms and side effects.
Open-Label Placebo and Developmental Scripts
A deliberate effort to condition a response to placebo was made by pairing placebo with stimulant medication, based on methods described by Suchman and Ader.20 It is important to note that the CPDR procedure used an ethical “open label” (not blind) method of treatment delivery. Placebos were administered with full disclosure, i.e., children and parents were told explicitly at the beginning of the study that the inert capsule was a placebo that contained no active pharmaceutical ingredients. Also, they were told the study was designed to determine if the procedure was effective. In keeping with a proof of concept study, we were neutral regarding the likelihood of success. Positive expectancy was maintained, however, by referring to the placebo both as a placebo and as a Dose Extender. If either the child or parent raised questions about possible mechanisms of placebo effects, we briefly discussed possibilities of mind-body interactions, expectancy and conditioning (described as “a kind of learning”). During the discussion, prompts were given to solicit questions from parents regarding the placebo, and parents’ questions were noted in order to infer their understanding of and attitudes towards placebo use. Children randomized to the RD/P group had an additional discussion of the placebo with the study physician, during which the green and white placebo capsule was shown to the children and explained. To standardize the discussion about the placebo, scripts were developed and pilot tested on children of different ages. The scripts were developmental, i.e., there were different scripts for children of ages 6–7 years, 8–9 years, and 10–12 years. Further details of the qualitative aspects of this research are included elsewhere.21
Measures
Responses to the medications were determined through quantitative analysis of several measures completed by parents (IOWA Conners-Parent Version, Pittsburgh Side Effects Rating Scale) three times each week and measures completed by the child’s teacher (IOWA Conners-Parent Version, Pittsburgh Side Effects Rating Scale) twice each week. The parent-completed measures were not blind and were therefore subject to bias. In addition, an objective measure of attention was obtained on each subject at baseline, at week 4 and at week 8 using a computer task (Conners Continuous Performance Test II).
Diagnostic Interview Schedule for Children–parent version (DISC)
The DISC is a standardized, structured clinical interview to identify psychiatric disorders in children. Parents were used as informants. The DISC screens for DSM-IV based diagnosis of childhood disorders.
Inattention/Overactivity With Aggression (IOWA) Conners Rating scale (parent & teacher versions)
The IOWA Conners scale is a standardized rating scale widely used in treatment studies of ADHD. The overall score for both the Parent and Teacher versions were used as the outcome measure.
NICHQ Vanderbilt Parent and Teacher Assessment Scales
These rating scales have been validated in studies of ADHD. The scale includes all DSM-IV ADHD items and a measure of overall impairment. In addition, the Vanderbilt scales include factors of conduct problems, anxiety and depression, making them especially helpful as part of the diagnostic assessment of study subjects.
Pittsburgh Side Effects Rating Scale (PSERS)
The PSERS is a standardized parent-completed stimulant side effect rating scale used in drug treatment research with children with ADHD. The PSERS measures the presence/absence and severity of 13 potential side effects (tics, tongue movements, skin picking, anxious, sleepy, headache, stomach ache, irritable, cries easily, appetite loss, tremor, nausea, difficulty sleeping).
Conners Continuous Performance Test (CPT)
The CPT is a standard measure of sustained attention performance used in drug treatment studies for children with ADHD. The Conners CPT is a computerized task in which letters are presented on a computer screen, and the subjected is instructed to respond (pressing the space bar) quickly after a letter is presented, unless the letter is an “X”. There are normative data as young as 6 years of age. The primary outcome measures include a series of age-based scores that are related to reaction times, omission errors, commission errors, and variability.
RESULTS
ADHD symptom severity (IOWA)
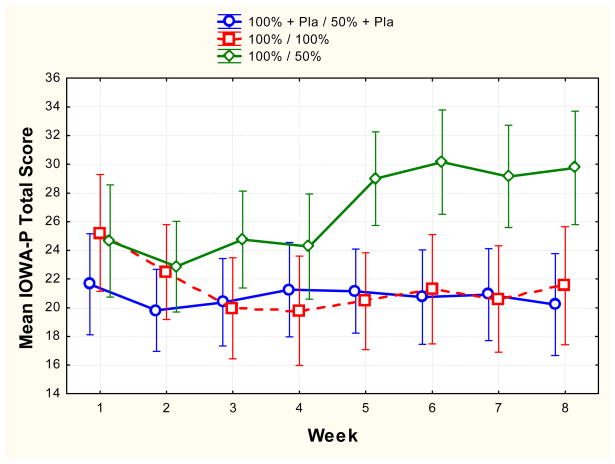
For parent-reported symptom severity (IOWA-P) the RD group showed a pattern of increased symptom severity over time relative to the FD and RD/P groups who showed little change in symptom severity over time (F (14, 166) = 2.89, p = 0.0004). The RD group differed from the other groups at the first through fourth weeks after dose reduction (weeks 5, 6, 7 and 8) (all p’s < .01). The effect size (d) at week 8 for the comparison of the RD/P and the RD groups was 1.27, and for the comparison of the FD and the RD groups was 1.10. The RD/P group showed a pattern of symptom severity over time similar to the FD group at all weeks (all p’s > .10). For teacher-reported severity (IOWA-T), there were no significant differences between the three groups at any time point (all p’s > .10).
Continuous performance test (CPT)
There was a marginally significant decrease in overall attentional performance (CPT T score) from baseline to post-test (week 8) for the FD group relative to the RD group and the RD/P group (F(2, 60) = 2.59, p = .08). The RD/P group showed a pattern of maintained attentional performance (i.e. no change in performance from baseline to post-test at week 8) (p > .10).
Side effects (PSERS)
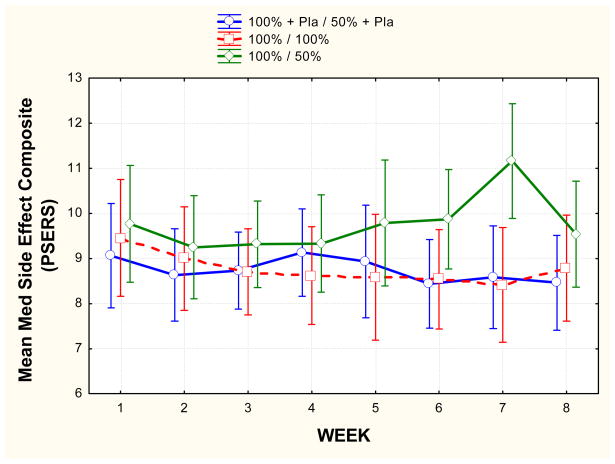
For parent reported medication side effects (PSERS) the RD group showed a pattern of increased reported side effect severity over time relative to the FD and RD/P (F(14, 252) = 2.67, p = .006). The RD group differed from the other groups at the second and third weeks after dose reduction (weeks 6,7). The effect size (d) at week 7 for the comparison of the RD/P and the RD groups was 1.03, and for the comparison of the FD and the RD groups was 0.99. The RD group showed an average increase in reported side effect severity from week 1 to week 7 (p < .001) and the RD/P group showed an average decrease in reported side effect severity over this time period (p < .01).
Based on a comparison of week 1 and week 8 (% change) parent IOWA ratings, most subjects in the RD/P group remained stable during the treatment phase. ADHD symptoms showed a 25% or greater improvement in 36% of subjects in the RD/P group and in 20% of subjects in the FD group during the course of treatment. In contrast, most in the RD group deteriorated (none showed 25% or greater improvement).
CONCLUSIONS
As expected, reducing the dose by 50 % in the RD group led to clinically significant deterioration in ADHD control. In contrast, children with RD/P group maintained effective ADHD symptom control based on parent IOWA ratings during the four-week period after dose reduction. Control of ADHD symptoms was no different in this group than in the FD group. Parents and children were generally accepting of the treatment. The majority of parents reported treatment benefits and expressed supportive attitudes towards the CPDR treatment.21 A majority of the children found the placebo to be useful. An additional provocative finding is that disclosing the true nature of the placebo did not appear to negate the placebo’s effectiveness.
Although poor ADHD control during washout and Dose Finding contributed to discontinuation, treatment acceptability related to the placebo dose reduction was not cited as a reason for discontinuation. Only one subject discontinued because the child did not wish to swallow an additional placebo. Our published data on the qualitative aspects of the study show that the placebo dose reduction treatment had a high level of acceptability to children and their parents.21
A comparison of retention of subjects in recent ADHD clinical trials shows that our rate of attrition (approximately 25%) is similar or lower than most. For example, in a recent trial of extended-release guanfacine, 113 of 324 (35%) did not complete the study.22
Unlike parents, teachers were blind to treatment status of subjects. Teacher ratings failed to show improvement in ADHD symptom control. This may indicate that parent responses were biased by expectancy. However, we had much more difficulty obtaining responses from teachers, limiting our ability to detect real differences. Our results are consistent with Pliszka et al,23 who reported larger placebo effect on parents’ ratings than on teachers’ ratings.
We hypothesized that CPDR would lead to decreased stimulant-related side effects. We found this to be partially true, in that the side effect rate remained low during dose reduction in the RD/P group. In contrast, side effects increased significantly in the RD group. This was a surprising finding, best explained by the global deterioration in these children as their ADHD became poorly controlled. Indeed, some of the items of the PSERS (sad, tearful; stomach ache; tired, sleepy) may be more indicative of uncontrolled ADHD itself than stimulant side effects.
Placebo effects in ADHD clinical trials are usually measured as changes in rating scales of behavior. The effects on objective measures of behavior are not well established. For this reason, we were eager to determine if CPDR would allow children with ADHD to maintain their performance on a CPT. We found that CPT performance in the three groups were not significantly different at baseline or at post-test. There was a trend for the children in the FD group to show deterioration in CPT performance. This was unexpected, but may reflect the fact that research visits were in the mid-afternoon so as not to interfere with school, and subjects were likely to have done their CPTs at a time when they were “coming off” their dose of stimulant. Rebound effects, irritability and poor CPT performance would be more likely to occur in subjects on higher doses of stimulant medication. It is also possible that the higher doses of stimulant needed for behavioral control impaired cognitive performance.
Research in placebo effects suggests several mechanisms.9 Recent evidence implicates neurochemical and neurophysiological changes due to placebo.24, 25 Positive expectancy –the expectation of treatment benefit leading to behavioral change– has been shown to contribute to stimulant effects,26 although studies using balanced placebo designs have shown a lack of expectancy effects among boys with ADHD treated with stimulants.27, 28 Participation in clinical research may itself be therapeutic.29 Changes in caregiver behavior may be an important participation effect that is especially relevant to treatment of children with developmental disorders.30
Our study does not help to answer important questions about mechanisms of placebo effects. We recognized that establishing both potential conditioned effects (by pairing the placebo with 100% stimulant dose) and potential expectancy effects (describing the placebo as a “dose extender”) negated the possibility that we can determine which of these mechanisms contributed to the resultant effects of the CPDR procedure. However, the primary purpose of this study was proof of a novel treatment concept and preliminary evidence of efficacy. Since a demonstration of efficacy must precede examination of mechanism, we designed our procedure accordingly.
One might consider whether merely a discussion with the parent about the potential benefits of a reduced dosage of the medication would have accomplished the same thing as the use of a placebo. Clinical experience suggests otherwise. Efforts to decrease doses of stimulants very often lead to symptom relapse, even when the parent wants to have the child on a low dose. Moreover, the RD group did poorly, with marked deterioration in ADHD control, despite their parents’ desire to have their children treated with lower doses of stimulant. This suggests that the addition of the open-label placebo was a critical element in the effectiveness of the conditioned placebo dose reduction treatment.
As progress is made in understanding mechanisms of placebo effects, we should remain open to ethical innovative uses of placebo effects that complement established therapeutic approaches. Children with ADHD may be a group of patients who could benefit greatly from such novel treatments.
Figure 1.
Mean (and 95% confidence interval) parent-reported severity of ADHD symptoms (IOWA-P) for each group over the 8 week treatment period. Green represents the Reduced Dose (RD) group; red represents the Full Dose (FD) group; blue represents the Reduced Dose plus Placebo (RD/P) group. Note that for the RD and RD/P groups, dose reduction was begun between week 4 and week 5.
Figure 2.
Mean (and 95% confidence interval) parent-reported severity of side effects (PSERS) for each group over the 8 week treatment period. Green represents the Reduced Dose (RD) group; red represents the Full Dose (FD) group; blue represents the Reduced Dose plus Placebo (RD/P) group. Note that for the RD and RD/P groups, dose reduction was begun between week 4 and week 5.
Acknowledgments
The study was funded by the National Institute of Mental Health, Grant number R21 MH068146.
Role of the Funding Source
The NIMH funded this study and had no involvement in any aspects of the study or this paper.
Footnotes
Authors Contributions and Signatures
I declare that I participated in the design and implementation of the study. I had full access to all the data in the study and I had final responsibility for the decision to submit for publication. I am the primary author of the manuscript and I have seen and approved the final version. I have no conflicts of interest to report.
Adrian Sandler
I declare that I participated in the design and implementation of the study. I have seen and approved the final version of the manuscript. I have no conflicts of interest to report.
Corrine Glesne
I declare that I participated in the design of the study and data analysis. I contributed to the manuscript and I have seen and approved the final version. I have no conflicts of interest to report.
James Bodfish
Contributor Information
Adrian D. Sandler, Olson Huff Center, Mission Children’fs Hospital, Asheville NC.
Corrine E. Glesne, Olson Huff Center, Mission Children’fs Hospital, Asheville NC.
James W. Bodfish, Departments of Psychiatry & Pediatrics, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill.
References
- 1.American Academy of Pediatrics. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.MTA Cooperative Group. Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: the multimodal treatment study of children with ADHD. Arch Gen Psychiatry. 1999;56:1088–1096. doi: 10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- 3.Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98:1084–1088. [PubMed] [Google Scholar]
- 4.Whalen CK, Henker B. Stimulant pharmacotherapy for attention-deficit/hyperactivity disorders: an analysis of progress, problems, and prospects. In: Fisher S, Greenberg RP, editors. From Placebo to Panacea: Putting psychiatric drugs to the test. New York, NY: Wiley; 1997. pp. 323–356. [Google Scholar]
- 5.Zametkin AJ, Ernst M. Problems in the management of ADHD. New Engl J Med. 1999;340:40–42. doi: 10.1056/NEJM199901073400107. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with ADHD: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]
- 7.American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 8.Khan A, Kolts RL, Rapaport MH, Krishnan KRR, Brodhead AE, Brown WA. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35:743–749. doi: 10.1017/s0033291704003873. [DOI] [PubMed] [Google Scholar]
- 9.Sandler AD. Placebo effects in developmental disabilities: implications for research and practice. Ment Retard Dev Disabil Res Rev. 2005;11:164–170. doi: 10.1002/mrdd.20065. [DOI] [PubMed] [Google Scholar]
- 10.Birmaher B, Brent DA. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatr. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 11.Swanson JM, McBurnett K, Christian DL, Wigal T. Stimulant medications and the treatment of children with ADHD. In: Ollendick TH, Prinz RJ, editors. Advances in Clinical Child Psychology. New York: Plenum Press; 1995. pp. 265–322. [Google Scholar]
- 12.Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. J Am Acad Child Adolesc Psychiatry. 1997;36:754–763. doi: 10.1097/00004583-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Brown WA. Placebo as a treatment for depression. Neuropsychopharmacology. 1994;10:287–288. doi: 10.1038/npp.1994.53. [DOI] [PubMed] [Google Scholar]
- 14.Park LC, Covi L. Nonblind placebo trial: an exploration of neurotic patients’ responses to placebo when its inert content is disclosed. Arch Gen Psychiatry. 1965;12:336–345. [PubMed] [Google Scholar]
- 15.Wickramasekera I. A conditioned response model of the placebo. Biofeedback and Self-Regulation. 1980;5:5–18. doi: 10.1007/BF00999060. [DOI] [PubMed] [Google Scholar]
- 16.Ader R. The placebo effect: if it’s all in your head, does that mean you only think you feel better? Advances in Mind-Body Medicine. 2000;16:7–11. doi: 10.1054/ambm.2000.0181. [DOI] [PubMed] [Google Scholar]
- 17.Ader R. Much ado about nothing. Advances in Mind-Body Medicine. 2001;17:293–295. [PubMed] [Google Scholar]
- 18.Sandler AD, Bodfish JW. Open-label use of placebos in the treatment of ADHD: A pilot study. Child Care Health Dev. 2008;34:104–110. doi: 10.1111/j.1365-2214.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics. Clinical practice guideline: Diagnosis and evaluation of the child with ADHD. Pediatrics. 2000;105:1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 20.Suchman A, Ader R. Classic conditioning and placebo effects in crossover studies. Clin Pharmacol Ther. 1992;52:372–377. doi: 10.1038/clpt.1992.157. [DOI] [PubMed] [Google Scholar]
- 21.Sandler AD, Glesne CE, Geller G. Children’s and parents’ perspectives on open-label use of placebos in the treatment of ADHD. Child Care Health Dev. 2008;34:111–120. doi: 10.1111/j.1365-2214.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 22.Sallee FR, McGough J, Wigal T, et al. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 23.Pliszka SR, Browne RG, Olvera RL, Wynne SK. A double-blind, placebo-controlled study of Adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:619–626. doi: 10.1097/00004583-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 25.Hunter AM, Leuchter AF, Morgan ML, Cook IA. Changes in brain function (quantitative EEG concordance) during placebo lead-in and treatment outcomes in clinical trials for major depression. Am J Psychiatry. 2006;163:1426–1432. doi: 10.1176/ajp.2006.163.8.1426. [DOI] [PubMed] [Google Scholar]
- 26.Volkow N, Wang G, Ma Y, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelham WE, Hoza B, Pillow DR, et al. Effects of methylphenidate and expectancy on children with ADHD: behavior, academic performance, and attributions in a summer treatment program and regular classroom settings. J Consult Clin Psychol. 2002;70:320–335. [PubMed] [Google Scholar]
- 28.Waschbusch DA, Pelham WE, Waxmonsky J, Johnston C. Are there placebo effects in the medication treatment of children with attention-deficit hyperactivity disorder? J Dev Behav Pediatr. 2009;30:158–168. doi: 10.1097/DBP.0b013e31819f1c15. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz RI, Horwitz SM. Adherence to treatment and health outcomes. Arch Intern Med. 1993;153:1863–1868. [PubMed] [Google Scholar]
- 30.Sandler AD, Bodfish JW. Placebo effects in autism: lessons from secretin. J Dev Behav Pediatr. 2000;21:347–350. doi: 10.1097/00004703-200010000-00005. [DOI] [PubMed] [Google Scholar]