Abstract
Tuberculosis (TB) is a global public health problem and a source of preventable deaths each year, with 8.8 million new cases of TB and 1.6 million deaths worldwide in 2005. Approximately, 10% of infected individuals develop pulmonary or extrapulmonary TB, suggesting that host defense factors influence development of active disease. Toll-like receptor’ (TLR) polymorphisms have been associated with regulation of TLR expression and development of active TB. In the present study, 71 polymorphisms in TLR1, TLR2, TLR4, TLR6, and TLR9 were examined from 474 (295 cases and 179 controls) African-Americans, 381 (237 cases and 144 controls) Caucasians, and from 667 (321 cases and 346 controls) Africans from Guinea-Bissau for association with pulmonary TB using generalized estimating equations and logistic regression. Statistically significant associations were observed across populations at TLR9 and TLR2. The strongest evidence for association came at an insertion (I)/deletion (D) polymorphism (−196 to −174) in TLR2 that associated with TB in both Caucasians (II vs. ID&DD, OR=0.41 [95% CI 0.24–0.68], p=0.0007) and Africans (II vs. ID&DD, OR=0.70 [95% CI 0.51–0.95], p=0.023). Our findings in three independent population samples indicate that variations in TLR2 and TLR9 might play important roles in determining susceptibility to TB.
Keywords: tuberculosis, toll-like receptors, polymorphism, innate immunity
INTRODUCTION
Mycobacterium tuberculosis (M. TB) infects approximately one-third of the human population resulting in 1.6 million deaths worldwide [World Health Organization (WHO) Website 2008; World Health Organization 2004]. Approximately, 10% of individuals infected with M. TB develop active pulmonary disease, suggesting that differences in susceptibility to progression exist (Skamene et al. 1998). The global impact of tuberculosis (TB) occurs despite vaccination programs using Mycobacterium bovis (BCG) and availability of antimycobacterial antibiotics. Genetic susceptibility has long been thought to play a role in determining which infected individuals develop active TB disease.
Multiple lines of evidence support a role for genetics in the development of pulmonary TB. Twin studies have shown that infected identical twins were more likely to develop TB than infected fraternal twin pairs (Comstock 1978). Familial clustering of individuals with pulmonary TB has also been observed, mostly from populations recently introduced to the M. TB pathogen (Greenwood et al. 2000; Sousa et al. 1997). Additionally, both human and mouse studies of mycobacterial infection have identified several potential TB susceptibility or resistance loci including genes involved in toll-like receptor (TLR) signaling (Misch and Hawn, 2008).
TLRs are pattern recognition receptors (PRRs) expressed on macrophages and other leukocytes. They play a fundamental role in phagocytosis and other host defense mechanisms including pattern recognition of microbial pathogens (Flynn et al. 1995; Misch and Hawn 2008). In humans, TLRs consist of ten receptors that are critically important to innate immunity (Akira et al. 2006; Beutler et al. 2006; Misch and Hawn 2008; Takeda et al. 2003). These ten TLRs are classified as members of the IL-1R super-family based on a shared cytoplasmic region known as the TIR (Toll/IL-1R) domain (Misch and Hawn 2008). TLR activation triggers a complex cascade that leads to the induction of a large range of proinflammatory genes (Akira et al. 2006; Beutler et al. 2006). TLRs activate NFkB which is the first line of defense against many pathogens (Bowdish et al. 2009). TLRs play an integral role in the activation of inflammatory cytokine signaling pathways and adaptive immune responses and as a result have become biologically plausible candidate genes in studies of TB susceptibility (Akira et al. 2001). Both human and animal studies of TB have observed that TLRs play a pivotal role in innate immune response to TB. Among the TLRs, TLR1, TLR2, TLR4, TLR6, and TLR9 have been extensively studied in the literature and have been suggested as candidate genes for TB (Akira et al. 2006; Branger et al. 2004; Buwitt-Beckmann et al. 2005–2006; Fremond et al. 2004; Huang et al. 2005; Ma et al. 2007; Morr et al. 2002; Okusawa et al. 2004; Suzuki et al. 2004; Takeshita et al. 2001).
Given the strong functional evidence supporting a genetic component for TLRs in TB susceptibility, in the present study, we sought to comprehensively examine five TLR candidate genes for association with TB using a tag single nucleotide polymorphism (SNPS) or indirect association approach. We examined TB cases and controls from African-Americans and Caucasians from North and South America and Africans from Guinea-Bissau by testing 71 SNPs from TLR1, TLR2, TLR4, TLR6, and TLR9 for association with TB.
METHODS
Study Population
Caucasians and African-American
Participants were ascertained through the North Carolina or South Carolina TB Control Programs, USA, or as patients at the outpatient clinic at F.J. Muñiz Hospital in Buenos Aires, Argentina, between 2002 and 2006. Criteria for inclusion as TB cases were: (a) age 14 years or older and culture-confirmed pulmonary TB, or (b) less than 14 years of age and either culture-confirmed or clinically diagnosed pulmonary TB that included a positive tuberculin skin test plus an infiltrate or hilar adenopathy on chest X-ray. Individuals were eligible to participate if their TB had been diagnosed in the past, or if they were currently receiving TB treatment. All TB cases remained eligible if they also had a diagnosis of extrapulmonary TB. Family members of eligible TB cases, who themselves had a history of TB, were enrolled as part of a multi-case family if review of their records established diagnosis of either pulmonary or extrapulmonary TB. Thus, a small portion of our study subjects enrolled as part of a multi-case-family had extrapulmonary TB only.
The diagnosis of a TB case was confirmed by review of medical records and laboratory reports. Regardless of data source, severity of TB disease was assessed by presence of acid-fast bacilli (AFB) in sputum smears or X-ray evidence of cavitary lesions. When available in the medical record, we documented HIV status for all TB cases. However, participation in this study did not require that the individual authorize review of HIV test results.
Unaffected individuals who were in close contact with cases during the infectious phase of the disease (household contacts such as spouses and partners, and relatives such as parents and siblings) were enrolled as controls. Informed consent was obtained from all subjects or their legal representatives before participation in the study. Human experimentation guidelines of the US Department of Health and Human Services and those of the participating research institutions were followed. The protocol was IRB-approved at Duke University Medical Center, the North and South Carolina Departments of Public Health (USA), Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno” (CEMIC), the F. J. Muñiz Hospital, Buenos Aires, Argentina, and the University of Miami Miller School of Medicine.
Africans
This case-control study was conducted at The Bandim Health Project (BHP), a demographic surveillance site in Bissau, the capital of Guinea-Bissau. BHP has followed this population since 1978. TB incidence in this area is among the highest in the world (470/100,000). The study area has a population of 102,000 and is composed of several ethnic groups including Papel (32%), Manjaco (14%), Mancanha (10%), Balanta (9%), Fulani (13%), Mandinka (7%) and others (15%). Patients included in the study (cases) were residents or long-term guests of Bissau, aged >15 years, newly TB-diagnosed using three sputum examinations for acid-fast bacteria or clinical criteria by the WHO’s definition of active pulmonary TB. No culture of TB was available in Bissau during the study period, as facilities were destroyed during a civil war. Sixty-eight percent (218/321) of the cases were smear-positive. Patients with newly diagnosed TB were enrolled when they started anti-tubercular treatment at local health centers. During the inclusion period from November 2003 to November 2005, 438 TB patients were screened at local health centers: 344 subjects met inclusion criteria and accepted participation, and from these we could obtain 321 DNA samples. There were no exclusion criteria.
Healthy controls were recruited from the study area from May 2005 to November 2005. A random sample of 200 houses was selected from the database of all subjects living in the study area, and houses with a recorded case of TB within the past 2 years were excluded from the sampling. Exclusion criteria for controls included the presence of cough for more than 2 weeks, history of TB and TB in the household within the last 2 years to avoid households with a high-risk of active TB. The composition of the case and control samples was different in terms of sex and ethnicity. These differences are due to the sampling strategy as controls were derived from healthy nuclear families; hence, more healthy married couples were collected, whereas TB patients are more often males. The ethnic differences are due to willingness of healthy subjects to give blood, which was not the same across the ethnic groups, whereas most TB patients accepted to be part of the study regardless of their ethnic background. All subjects were interviewed by field assistants, using a standardized questionnaire on ethnicity, environmental factors, and prior exposure to TB. Permission to perform HIV tests was asked for cases but not for controls, as requiring HIV testing in controls would have negatively influenced participation in the study.
Venous blood samples (4 ml in ethylenediaminetetraacetic acid) were collected from case (N=321) and control (N=347) subjects, and stored in the National Gambian DNA Bank. Ethical approval was granted by the ‘Unidade de Coordenacao de Estudos e Pesquisas em material de Saude’ (Ministry of Health) in Guinea-Bissau. All adults and children’s guardians signed an informed consent for the study prior to enrollment.
Marker selection and genotyping
The 71 SNPs examined were tags selected from African (Yoruba) and Centre d’Etude du Polymorphisme Humain (CEPH) populations from the International HapMap project (http://www.hapmap.org) (The International HapMap Consortium 2003), NCBI build 35 assembly HapMap phase II. Tag SNP selection considered all SNPs with minor allele frequencies (MAF) ≥ 0.05 in the gene region, the boundaries of which were located 2,000 base pairs (bp) 5′ upstream and 3′ downstream of each gene. SNPs in the gene region were grouped into bins of highly correlated SNPs [pairwise correlation coefficient (r2) of 0.80], and a single tag SNP was selected from each bin using the aggressive tagging function (based on 2- and 3-marker haplotypes) in Haploview software. In addition to these tag SNPs, one insertion/deletion polymorphism (−196 to −174 insertion (I)/deletion (D)) in TLR2 that has been previously associated with decreased TLR2 expression was also included in the study (Noguchi et al. 2004).
The TaqMan allelic discrimination assay was used to genotype the 53 SNPs. Assays were obtained from the ABI Assays on Demand or Assays by Design services. Genotyping of the TLR2 insertion deletion polymorphism was performed by PCR with the forward primer 5′-6FAM-CTCGGAGGCAGCGAGAAA-3′ and reverse primer 5′-CTGGGCCGTGCAAAGAAG-3′ (Noguchi et al. 2004). The forward primer was labeled with the fluorescent dye 6-FAM at the 5′ end. The amplified products were analyzed on an ABI 3730xl DNA Analyzer (Applied Biosystems) and fragment analysis was performed with GeneMapper v.4.0 software (Applied Biosystems). 11 of the TLR1 SNPs and 6 of the TLR6 SNPs were genotyped with the Sequenom Mass ARRAY genotyping platform. Supplemental Table 1A has the base pair (bp) locations and the potential function of each gene. All SNPs used in this study had genotyping call rates of 95% or better (mean call rate 98%) and quality control sample match rates of 100%.
Statistical analysis
For the analyses of African-Americans and Caucasians, one TB case and control was selected from each family for tests of Hardy-Weinberg equilibrium (HWE) at each SNP using genetic data analysis (GDA) software (Abecasis and Cookson 2000). For Guinea-Bissau, statistical tests for deviations from HWE were performed by the use of GDA software stratified by affection status. All tests for deviations from HWE were performed by the use of a Fisher’s exact test. Pairwise linkage disequilibrium (LD) between SNPs was calculated using Haploview statistical software according to the Gabriel et al. (2002) algorithm for all populations examined (Gabriel et al. 2002). All analyses were conducted separately in Caucasians, African-Americans, and Africans from Guinea-Bissau.
Genotypic tests of association and analyses of clinical data in our African-American and Caucasian populations were performed using generalized estimating equations (GEE) implemented in SAS (Proc GENMOD) statistical software version 9.1 (SAS Institute, Cary, NC, USA) using the independence correlation matrix. GEE accounts for correlations within families through a robust variance estimator and allows family data to be analyzed with unrelated cases and controls. GEE has been shown to be a valid test of genetic association, gene × gene and gene × environment interactions in family data (Hancock et al. 2007). Additive genotypic models were performed modeling the minor allele as the risk allele (0 vs. 1 vs. 2) and adjusting for age and gender as potential confounders in all analyses. For Caucasians, we also incorporated ascertainment site and an interaction term between SNP and ascertainment site in all models in order to account for potential genetic heterogeneity between the US and Argentina. Samples from Guinea-Bissau were unrelated cases and controls and as a result logistic regression was used for these analyses using STATA 10.0 statistical software (StataCorp 2007) (College Station, TX, USA). In order to assess potential genotype frequency differences between populations, we also performed GEE analyses within case and controls separately using ethnicity as outcome to test for differences for the markers examined.
To account for potential confounding by HIV status, analyses were repeated excluding individuals with TB who were also HIV-positive. All statistical tests are reported at a significance level of α = 0.05 unadjusted for multiple comparisons.
Results
Detailed clinical and demographic information for Guinea-Bissau, African-Americans, and Caucasians has been previously published (Hill et al. 2004; Olesen et al. 2007; Velez et al. 2009). Briefly, the average age (years) was 44.4 for cases and 52.9 for controls in African-Americans, 45.8 and 34.3 for Caucasian US and Argentina cases, 54.7 and 32.4 for Caucasian US and Argentina controls, and 37.2 for cases and 38.12 for controls in Guinea-Bissau. The majority of cases and, in general, a minority of controls were male (African-American 66% of cases and 17% of controls; Caucasian 54% cases and 33% controls; Guinea-Bissau 60% of cases and 50% of controls). Among cases with documented HIV status, 14.2% of African-Americans, 5.7% of Caucasians, and 36% were HIV seropositive. HIV status was not obtained on controls. Examination of allele and genotype frequency differences for cases with and without HIV indicated that there was no evidence for significant differences between the two groups in any of the populations examined; as a result, analyses were performed pooling cases with and without HIV (data not shown).
Among the genes examined 7 SNPs had statistically significant (p ≤ 0.05) associations with TB in either African-Americans or Caucasians (Table 1) in TLR9, TLR2, and TLR4. These include five SNPs in TLR9 significant in African-Americans (rs164637, rs352143, rs5743836, rs352139, and rs352162), one TLR9 SNP in Caucasians (rs5743836), one TLR2 SNP in Caucasians (−196 to −174 I/D), and one TLR4 SNP significant in African-Americans (rs123445353). Among these associations, none had severe departures from HWE (p > 0.01). The deviations in African-American controls were at SNPs rs3804099 (p=0.02) and −196 to −174 I/D (p = 0.04). The deviations in Caucasian controls were at SNPs rs12344353 (p = 0.01). There were no significant deviations from HWE in Africans.
Table 1.
Statistically significant (p≤0.05) genotypic tests of association in African-Americans, Caucasians, and Africans from Guinea-Bissau
| Gene | SNP rs# | Allele frequency (Allele) | p-Valuea | ||||
|---|---|---|---|---|---|---|---|
| African-American | Caucasian | Guinea-Bissau | African-American | Caucasian | Guinea-Bissau | ||
| (a) | |||||||
| TLR9 | rs164637 | 0.01(T) | 0.06(T) | 0.00(T) | 0.011 | 0.883 | – |
| rs352143 | 0.38(G) | 0.18(G) | 0.48(G) | 0.022 | 0.068 | 0.486 | |
| rs5743836 | 0.39(C) | 0.12(C) | 0.45(C) | 0.014 | 0.051 | 0.513 | |
| rs352139 | 0.41(A) | 0.48(A) | 0.37(A) | 0.001 | 0.730 | 0.352 | |
| TLR2 | −196 to −174 I/Db | 0.24(C) | 0.13(C) | 0.30(C) | 0.972 | 0.001 | 0.082 |
| rs893629 | 0.09(A) | 0.01(A) | 0.09(A) | 0.288 | – | 0.006 | |
| rs6535939 | 0.10(T) | 0.01(T) | 0.12(T) | 0.140 | – | 0.014 | |
| rs3804099b | 0.38(T) | 0.36(C) | 0.28(T) | 0.238 | 0.816 | 0.015 | |
| rs7656411 | 0.42(T) | 0.24(G) | 0.30(T) | 0.160 | 0.518 | 0.024 | |
| TLR6 | rs6815827 | 0.13(T) | 0.01(T) | 0.09(T) | 0.517 | – | 0.004 |
| TLR4 | rs12344353b | 0.13(C) | 0.06(C) | 0.14(C) | 0.041 | 0.930 | 0.598 |
| rs5030725 | 0.20(G) | 0.01(G) | 0.22(G) | 0.478 | – | 0.045 | |
| Genes | SNP | Population | Model | OR | 95% CI |
p-Valuea | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| (b) | |||||||
| TLR9 | rs352143 | Caucasian | AA vs. AG vs. GG | 0.64 | 0.40 | 1.03 | 0.068 |
| AA vs. AG&GG | 0.53 | 0.32 | 0.89 | 0.017 | |||
| African-American | AA vs. AG vs. GG | 0.67 | 0.48 | 0.94 | 0.022 | ||
| AA vs. AG&GG | 0.58 | 0.36 | 0.95 | 0.029 | |||
| Guinea-Bissau | AA vs. AG vs. GG | 0.92 | 0.74 | 1.15 | 0.486 | ||
| AA vs. AG&GG | 0.83 | 0.59 | 1.17 | 0.284 | |||
| rs5743836 | Caucasian | TT vs. CT vs. CC | 0.58 | 0.34 | 1.00 | 0.051 | |
| TT vs. CT&CC | 0.50 | 0.28 | 0.87 | 0.015 | |||
| African-American | TT vs. CT vs. CC | 0.63 | 0.43 | 0.91 | 0.014 | ||
| TT vs. CT&CC | 0.54 | 0.32 | 0.92 | 0.024 | |||
| Guinea-Bissau | TT vs. CT vs. CC | 0.93 | 0.75 | 1.16 | 0.513 | ||
| TT vs. CT&CC | 0.86 | 0.62 | 1.20 | 0.370 | |||
| TLR2 | −196 to −174 I/D | Caucasian | II vs. ID vs. DD | 0.45 | 0.28 | 0.74 | 0.001 |
| II vs. ID&DD | 0.41 | 0.24 | 0.68 | 0.0007 | |||
| African-American | II vs. ID vs. DD | 0.99 | 0.62 | 1.59 | 0.972 | ||
| II vs. ID&DD | 1.17 | 0.68 | 2.03 | 0.575 | |||
| Guinea-Bissau | II vs. ID vs. DD | 0.82 | 0.65 | 1.03 | 0.082 | ||
| II vs. ID&DD | 0.70 | 0.51 | 0.95 | 0.023 | |||
In bold are significant (p ≤ 0.05)
– indicates that an analysis could not be performed due to the SNP having a low minor allele frequency or being monomorhpic II double insertion, ID one insertion and one deletion, DD double deletion
p values are presented from GEE analysis of Caucasian and African-American samples and logistic regression analysis of the Guinea-Bissau sample
Deviations from HWE in either African-American or Caucasian controls
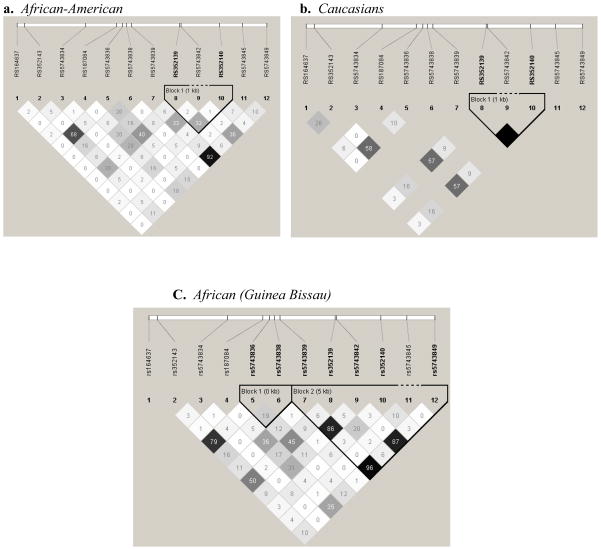
We followed-up all SNPs that demonstrated significant (p ≤ 0.05) to marginal (p ≤ 0.10) levels of association in more than one population with dominant and recessive models. Among these, we observed an inverse association with the dominant model for two TLR9 SNPs in Caucasians (rs352143 AA vs. AG&GG, OR = 0.53 [95% CI 0.32–0.89], p = 0.017; rs5743836 TT vs. CT&CC, OR = 0.50 [95% CI 0.28–0.87], p = 0.015) and at the TLR2 the −196 to −174 I/D in both Caucasians (II vs. ID&DD, OR = 0.41 [95% CI 0.24–0.68], p = 0.0007) and the Guinea-Bissau population (II vs. ID&DD, OR = 0.70 [95% CI 0.51–0.95], p = 0.023) (Table 1b). LD plots (r2)for TLR2 and TLR9, the two genes with strongest associations, are provided in Figs. 1 and 2 and Supplemental Figs. 1 and 2.
Fig. 1.
Linkage disequilibrium structure (r2) for TLR9 in controls. LD plots were generated in Haploview and are presented for: a African-Americans, b Caucasians c Africans (Guinea-Bissau). Within each triangle is presented the pairwise correlation coefficient (r2). Standard color-coding was used for the Haploview LD plots, white, (r2 = 0), shades of gray, (0 < r2 < 1), black, (r2=1). Black squares without numbers indicate complete LD (r2 = 1.00).
Fig. 2.
Linkage disequilibrium structure (r2) for TLR2 in controls. LD plots were generated in Haploview and are presented for: a African-Americans, b Caucasians, c Africans (Guinea-Bissau). Within each triangle is presented the pairwise correlation coefficient (r2). Standard color-coding was used for the Haploview LD plots, white, (r2 = 0), shades of gray, (0 < r2 < 1), black, (r2 = 1). Black squares without numbers indicate complete LD (r2 = 1.00).
Six statistically significant associations were observed in samples from Guinea-Bissau among TLR2 SNPs in moderate LD (rs893629, rs6535939, rs3804099, rs7656411), TLR6 (rs6815827), and TLR4 (rs5030725) (Table 1). There were no statistically significant deviations from HWE in controls for any of the SNPs examined. Of note, we observed a marginally significant association at the −196 to −174 I/D polymorphism (p = 0.082). Given the association observed in Caucasians at this SNP with a dominant model, we further examined this association modeling dominant and recessive effects in Guinea-Bissau. The dominant model (II vs. ID&DD) was statistically significant in Guinea-Bissau with a p = 0.023 and an OR trending in the same direction as in Caucasians (Table 1b). For a complete table of all of the association results for all populations please refer to Supplemental Table 1A–C.
Given that the associations did not overlap between African-Americans and Africans for the associations observed in TLR2 and TLR9 we also tested for genotype frequency differences between these two groups (Supplemental Table 2). We observed several differences at SNPs in both TLR9 and TLR2 in either cases or controls (TLR9 SNPs rs352143, rs5743834, rs187084, rs5743836, rs5743838, and rs352140; TLR2 SNPs −196 to −174 I/D, rs893629, rs1816702, rs6535939, rs3804099, and rs7656411) supporting that the different associations observed for these two groups may be due, in part, to the different allele frequencies of the selected tag SNPs (and thus different tagging efficiency) in these populations.
Discussion
In the present study, we examined 71 markers in six TLR genes for association with TB. We observed statistically significant associations in TLR2, TLR4, TLR6, and TLR9 in at least one population. We observed the strongest evidence for association with TB with TLR2 in Caucasians and Africans, although multiple statistically significant associations were also observed for TLR9 in African-Americans. Although we did not see overlapping associations across African-Americans and Africans. This may be due to slightly different allele frequencies at the chosen tag SNPs between the two populations. These differences could result in differences in the coverage of the untyped variants, leading to disparate association.
We selected these TLR genes because of the strong prior biological evidence supporting their role in TB. Mouse studies have examined TLR2, known to be involved in responses to microbial lipoproteins and peptidoglycans (e.g. Gram-positive bacteria and yeast) and a classically recognized inducer of proinflammatory signaling (Fremond et al. 2004). These studies have observed that mice deficient in myeloid differentiation factor 88 (MyD88 −/−), a critical adapter molecule common to signaling by most TLRs, died within 4 weeks of mycobacterial infection with evidence of massive uncontrolled microbial growth. Vaccination of the MyD88 −/− mice with BCG resulted in activation of a T cell and Th1 response to mycobacterial antigens but this only delayed death. Increased mortality and mycobacterial load in the lungs has also been observed for mice with non-functional TLR4 (Branger et al. 2004). TLR4 is required for recognition of endotoxin of Gram-negative bacteria and has been associated with pulmonary tuberculosis in studies from West Africa (Newport et al. 2004; Takeda et al. 2003). TLR9 is also known to play an important role in the activation of the innate immune system by TLRs, it is the receptor for viral and bacterial CpG-DNA motifs with studies showing that the binding of TLR9 is necessary to drive the Th1 immune response (Huang et al. 2005; Suzuki et al. 2004; Takeshita et al. 2001). TLR1 and TLR6 have both been examined to a lesser extent with regards to their role in TB; however, both form heterodimers with TLR2 to recognize lipoproteins, present on a wide variety of bacteria, fungi, parasites, and viruses (Akira et al. 2006; Buwitt-Beckmann et al. 2005, 2006; Morr et al. 2002; Okusawa et al. 2004). Despite few studies having observed associations with TB in these genes, a recent study by Ma and colleagues observed associations with TLR1 and TLR6 in both African-American and Hispanic populations, suggesting that, either individually or through interaction with TLR2, these genes may influence development of TB (Ma et al. 2007).
Although we did not observe identical results in African-Americans for SNPs in TLR2, the I/D polymorphism −196 to −174 was associated with TB in both Caucasians and Africans from Guinea-Bissau. The TLR2 I/D at −196 to −174 has not previously been associated with TB. Previous studies have observed associations with severity of other inflammatory diseases and TLR2 expression (Noguchi et al. 2004; Wang et al. 2007). In studies examining TLR2 for association with asthma, in vitro reporter construct assays demonstrated that constructs carrying the −196 to −174 deletion had lower luciferase activity in comparison with a construct carrying the wild-type allele. These findings suggest a reduced transcriptional activity associated with this TLR2 deletion (Noguchi et al. 2004). Others observed that the −196 to −174 deletion was present at a significantly higher frequency in ulcerative colitis patients who used steroids relative to controls (Wang et al. 2007). The lower expression of TLR2 correlated with the deletion allele may cause a corresponding increase in a Th1–type response, preventing the development of pulmonary TB in some individuals (reflected in the inverse association with TB susceptibility). To our knowledge, no other study has associated this TLR2 −196 to −174 I/D polymorphism with TB. Further functional studies are necessary to validate these associations.
Despite the interesting findings, we acknowledge our study had limitations. First, cases and controls were significantly different at age at exam and male:female ratios. To account for these differences, both variables were used as covariates in the statistical analyses. Using two different ascertainment centers for collection of the Caucasian dataset, one from the Southeastern US and another from Argentina and including a small number of HIV-positive cases could result in heterogeneity. To account for these effects, we adjusted for ascertainment site in our models and performed sensitivity analysis by omitting HIV-positive individuals from a second analysis. However, HIV status did not influence the significance of our results. We acknowledge that the Guinea-Bissau population was ascertained with slightly different diagnostic criteria, since culture-confirmation and chest X-ray were not available in Guinea-Bissau at the time of the study period. While two positive sputum-smear tests were required for diagnosis of TB in Guinea-Bissau, it is possible that the cases from Guinea-Bissau contain individuals who would not meet criteria used for TB diagnosis in the African-American sample, and this difference could have resulted in the discordant results across the two samples.
A few of the SNPs noted on Table 1 deviated slightly from HWE in controls; however, these deviations were only nominal p > 0.01 and therefore did not suggest significant genotyping error. With regards to the deviation from HWE observed in the −196 to −174 I/D polymorphism in African-American controls, this may be indicative of a protective effect and suggests that more power may be necessary to observe an association in African-Americans. Finally, the number of tests performed may represent another limitation due to possible increase in type I errors. However, we provided three discovery datasets as a way to reduce type I error and to highlight our most interesting findings.
This study implicates TLR2 as a susceptibility gene for TB in Caucasians and Africans from Guinea-Bissau and is also, to our knowledge, one of the largest studies in TB research examining multiple TLR candidate genes and SNPs. Taken together, these results suggest that polymorphisms in TLR2 influence risk for pulmonary TB in Caucasians and Africans, and variation in TLR9 may influence risk in African-Americans. Elucidation of the precise roles these genes play in TB susceptibility depends on isolating functional variants in TLR2 and TLR9 that may explain these variations.
Supplementary Material
Acknowledgments
The work in this manuscript was supported by grant R01 HL068534 from the National Heart, Lung and Blood Institute. C. Hamilton acknowledges support from NIH K24-AI001833. We thank the study participants, without whom this study would have been impossible, the North Carolina TB Control Nurse Consultants (Myra Allen, Dee Foster, Julie Luffman and Elizabeth Zeringue) and county TB nurses who referred subjects to the study. We would also like to thank Martha Fletcher, Elizabeth Levine, Earline Little, and Carol Poszik for assistance in recruiting participants in South Carolina, and Courtney Linton, Regina Carney, and Ann Mosher for recruiting participants in North Carolina. The Guinea Bissau study was funded by the MRC award G0000690 to G. Sirugo, and by Grants from the Danish Medical Research Council, the Danish society of respiratory medicine, the Danish Council of Development Research to C. Wejse and R. Olesen. Integrity of research and reporting: :This submitted manuscript contains experiments that comply with the current laws of the country in which they were performed.
References
- Abecasis GR, Cookson WO. GOLD--graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, Tryggvason K, Gordon S, Russell DG. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branger J, Leemans JC, Florquin S, Weijer S, Speelman P, Van Der PT. Toll-like receptor 4 plays a protective role in pulmonary tuberculosis in mice. Int Immunol. 2004;16:509–516. doi: 10.1093/intimm/dxh052. [DOI] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol. 2005;35:282–289. doi: 10.1002/eji.200424955. [DOI] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. J Biol Chem. 2006;281:9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- Comstock G. Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117:621–624. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Greenwood CMT, Fujiwara M, Boothroyd LJ, Miller MA, Frappier D, Fanning EA, Schurr E, Morgan K. Linkage of tuberculosis to chromosome 2q35 loci, including NRAMP1, in a large aboriginal Canadian family. Am J Hum Genet. 2000;67:405–416. doi: 10.1086/303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Li YJ, Scott WK. Methods for interaction analyses using family-based case-control data: conditional logistic regression versus generalized estimating equations. Genet Epidemiol. 2007;31:883–893. doi: 10.1002/gepi.20249. [DOI] [PubMed] [Google Scholar]
- Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, Donkor SA, Hammond AS, Otu JK, Corrah T, Adegbola RA, McAdam KP. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin Infect Dis. 2004;38:966–973. doi: 10.1086/382362. [DOI] [PubMed] [Google Scholar]
- Huang LY, Ishii KJ, Akira S, Aliberti J, Golding B. Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J Immunol. 2005;175:3964–3970. doi: 10.4049/jimmunol.175.6.3964. [DOI] [PubMed] [Google Scholar]
- Ma X, Liu Y, Gowen BB, Graviss EA, Clark AG, Musser JM. Full-exon resequencing reveals toll-like receptor variants contribute to human susceptibility to tuberculosis disease. PLoS ONE. 2007;2:e1318. doi: 10.1371/journal.pone.0001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- Morr M, Takeuchi O, Akira S, Simon MM, Muhlradt PF. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur J Immunol. 2002;32:3337–3347. doi: 10.1002/1521-4141(200212)32:12<3337::AID-IMMU3337>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Newport MJ, Allen A, Awomoyi AA, Dunstan SJ, McKinney E, Marchant A, Sirugo G. The toll-like receptor 4 Asp299Gly variant: no influence on LPS responsiveness or susceptibility to pulmonary tuberculosis in The Gambia. Tuberculosis (Edinb ) 2004;84:347–352. doi: 10.1016/j.tube.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Noguchi E, Nishimura F, Fukai H, Kim J, Ichikawa K, Shibasaki M, Arinami T. An association study of asthma and total serum immunoglobin E levels for Toll-like receptor polymorphisms in a Japanese population. Clin Exp Allergy. 2004;34:177–183. doi: 10.1111/j.1365-2222.2004.01839.x. [DOI] [PubMed] [Google Scholar]
- Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infect Immun. 2004;72:1657–1665. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M, Aaby P, Rabna P, Worwui A, Chapman H, Diatta M, Adegbola RA, Hill PC, Ostergaard L, Williams SM, Sirugo G. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 2007;8:456–467. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- Skamene E, Schurr E, Gros P. Infection Genomics: Nramp1 as major determinant of natural resistance to intracellular infections. Annu Rev Med. 1998;49:275–287. doi: 10.1146/annurev.med.49.1.275. [DOI] [PubMed] [Google Scholar]
- Sousa AO, Salem JI, Lee FK, Vercosa MC, Cruaud P, Bloom BR, Lagrange PH, Hugo DL. An epidemic of tuberculosis with a high rate of tuberculin anergy among a population previously unexposed to tuberculosis, the Yanomami Indians of the Brazilian Amazon. Proc Natl Acad Sci (USA) 1997;94:13227–13232. doi: 10.1073/pnas.94.24.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 9. 2007. [Google Scholar]
- Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, Gyobu H, Kawarada Y, Kondo S, Akira S, Katoh H, Ikeda H, Nishimura T. Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res. 2004;64:8754–8760. doi: 10.1158/0008-5472.CAN-04-1691. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Leifer CA, Gursel I, Ishii KJ, Takeshita S, Gursel M, Klinman DM. Cutting edge: Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- The International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Velez DR, Hulme WF, Weinberg FB, Levesque M, Abbato E, Estevan R, Patillow SG, Gilbert JR, Hamilton CD, Scott WK. Association of SLC11A1 and pulmonary tuberculosis and interactions with NOS2A/TLR2 in African-Americans and Caucasians. 2009. Submitted in revision edn. [PMC free article] [PubMed] [Google Scholar]
- Wang F, Tahara T, Arisawa T, Shibata T, Nakamura M, Fujita H, Iwata M, Kamiya Y, Nagasaka M, Takahama K, Watanabe M, Hirata I, Nakano H. Genetic polymorphisms of CD14 and Toll-like receptor-2 (TLR2) in patients with ulcerative colitis. J Gastroenterol Hepatol. 2007;22:925–929. doi: 10.1111/j.1440-1746.2007.04909.x. [DOI] [PubMed] [Google Scholar]
- WHO Website. World Health Organization. Programs and projects. Tuberculosis. The Stop TB Strategy. 2008. [Google Scholar]
- World Health Organization. Fact Sheet Number 104. 2004. Tuberculosis. Revised March 2004 edition. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.