SUMMARY
Uptake of the anticancer drug cisplatin is mediated by the copper transporter Ctr1 in cultured cells. Here we show in human ovarian tumors that low levels of Ctr1 mRNA are associated with poor clinical response to platinum-based therapy. Using a mouse model of human cervical cancer, we demonstrate that combined treatment with a copper chelator and cisplatin increases cisplatin-DNA adduct levels in cancerous but not in normal tissues, impairs angiogenesis, and improves therapeutic efficacy. The copper chelator also enhances the killing of cultured human cervical and ovarian cancer cells with cisplatin. Our results identify the copper transporter as a therapeutic target, which can be manipulated with copper chelating drugs to selectively enhance the benefits of platinum-containing chemotherapeutic agents.
INTRODUCTION
Cisplatin is widely used for the treatment of solid tumors. It is a component of first-line treatment for testicular, ovarian, cervical, endometrial, bladder, head and neck, lung, and gastroesophageal cancers (Hussain et al., 2008; Omura et al., 2008; Gallagher et al., 2008; Yang et al., 2008; Lustberg and Edelman, 2007; Fischer and Arcaro, 2008; Cen and Ajani, 2007). It is also used as a second or third-line treatment for prostate and pancreatic cancers, metastatic cancer of the breast, and for melanoma and gliomas (Carrick et al., 2004; Oh et al., 2007; Saif and Kim, 2007; Atallah and Flaherty, 2005; Glioma Meta-Analysis Trialists Group, 2002). The dosage and efficacy of cisplatin, however, are limited by its side effects, the most prominent being nephrotoxicity (Pabla and Dong, 2008).
Cisplatin exerts its cytotoxic effect by forming an intrastrand crosslink on DNA (Jamieson and Lippard, 1999). Studies of cisplatin resistant cell lines have revealed drug uptake as a critical step that governs cisplatin sensitivity in vitro (Hall et al., 2008). A yeast genetic screen for cisplatin-resistant mutants identified the copper transporter CTR1 as a major mediator of cisplatin uptake in yeast and mouse cells (Ishida et al., 2002). Upon exposure to excess copper, Ctr1 protein undergoes endocytosis and degradation (Ooi et al., 1996; Petris et al., 2003). Pretreatment of cells with high copper results in decreased cisplatin uptake and increased resistance to this drug in a CTR1-dependent manner (Ishida et al., 2002), whereas chelating copper with bathocuprione disulphonate has opposite effects (Ishida and Herskowitz, unpublished observations). These results demonstrated that cisplatin uptake can be modulated by copper levels in vitro through Ctr1.
In this study we examined the in vivo roles of Ctr1 and copper in cisplatin uptake and response using a mouse model of human cervical cancer. Cervical cancer is the second most common cause of cancer-related death among women worldwide. Cisplatin has been one of the most active single agents and a component of combined-agent chemotherapy regimens for patients with advanced cervical cancer. The response rates for cisplatin alone range from 13-19%, with an overall survival time of six to nine months; the current combined-agent therapy is only modestly better, with response rates of 27-36 %, with overall survival of eight to ten months (Tao et al., 2008). Cervical cancer is caused by integration and persistent expression of genes of Human Papilloma Viruses (HPV), of which HPV type 16 is detected in a majority of cases of invasive carcinoma (Castellsague, 2008). In the mouse model of cervical carcinoma, K14-HPV16/E2, the HPV16-encoded oncogenes are under the control of the keratin 14 promoter, resulting in targeted oncogene expression in keratin 14-positive squamous epithelial cells, a natural host-cell target for HPV infection in humans (Arbeit et al., 1996). When the estrogen levels of K14-HPV16 females are maintained at a level near their natural peak during estrus with time-released implants of 17ß-estradiol (estrogen, E2), the female mice (referred to as HPV16/E2 mice hereafter) develop progressive cervical intraepithelial neoplasia (CIN) lesions at the transformation zone, the site implicated in the genesis of human cervical cancer (Elson et al., 2000). By six months of age, these CIN lesions progress to invasive squamous cell carcinoma in 90% of the animals. Both male and female K14-HPV16 mice also develop dysplasia of the skin, and skin tumors arise later, with 50% penetrance by 12 months of age (Coussens et al., 1996).
Using this mouse model of human cervical carcinoma, HPV16/E2, we ask whether a copper chelator selectively increases cisplatin uptake and killing of tumor cells without affecting normal organs, thereby increasing the anti-tumoral efficacy of cisplatin. We also analyze human ovarian tumors for expression of the copper transporter Ctr1 to evaluate its association with clinical response to platinum drugs, and assess the capability of the copper chelator to enhance killing of human ovarian and cervical cancer cell lines with cisplatin.
RESULTS
Human Ctr1 mRNA levels in tumors are associated with clinical response to platinum-based chemotherapy in ovarian cancer
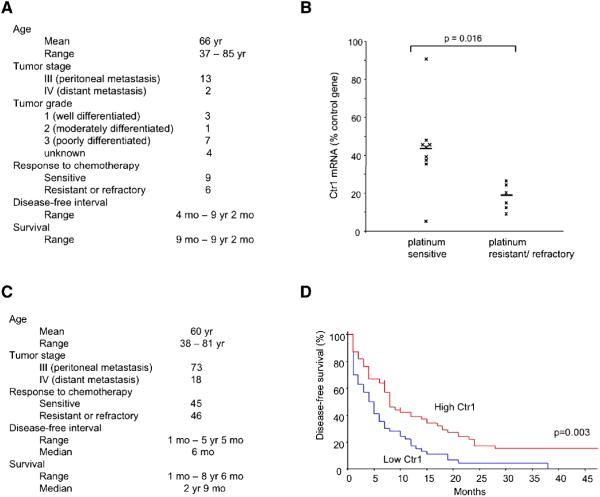
Previous studies using mouse embryonic fibroblasts deleted for the mouse Ctr1 gene demonstrated a correlation between Ctr1 gene dosage and cisplatin sensitivity in vitro: cells that are heterozygous for a knockout allele of mouse Ctr1 were more resistant than wild-type cells to cisplatin, and homozygous mutant cells were more resistant than heterozygous cells (Ishida et al., 2002). These findings raised the hypothesis that Ctr1 mRNA levels correlate with and thus explain differential clinical responses to platinum drugs. To address this possibility we analyzed tumor specimens and the clinical course of 15 ovarian cancer patients. The standard treatment for advanced ovarian cancer is primary cytoreductive surgery followed by chemotherapy that includes a platinum drug. Resistance to platinum-based chemotherapy remains a primary factor in disease recurrence. Figure 1A lists the baseline characteristics of the 15 patients, all with advanced-stage (Stage III or IV), serous epithelial ovarian carcinoma. The tumors were of mixed grade (Figure 1A). Following optimal cytoreductive surgery resulting in residual tumors of 1 cm or less in diameter, the patients received regimens containing either cisplatin or carboplatin, and paclitaxel. Prior clinical studies have revealed equal efficacy for the two platinum compounds (Ozols et al., 2003; du Bois et al., 2003). The mechanisms of resistance are thought to be similar for the two drugs, which share the same putative transporter Ctr1 (Holzer et al., 2006). Of the 15 patients, 9 proved to be sensitive to platinum-based therapy (no evidence of recurrence within 6 months after completion of primary therapy), whereas the remaining 6 were refractory or resistant (progression or recurrence within 6 months of completion of therapy, as indicated by an increase in CA-125 levels, or by CT, ultrasonography, or second-look laparoscopy) (Figure S1A). RNA was prepared from these patients’ tumor samples, and Ctr1 mRNA levels were measured by quantitative RT-PCR. The mean relative Ctr1 mRNA level was substantially higher in platinum sensitive patients than in refractory/resistant patients (43 % vs. 18 % of internal control gene expression, p=0.016) (Figure 1B). Expression levels of the internal control gene human GUS, which encodes β-glucuronidase, were equivalent between the two patient groups (average Ct in sensitive group was 28.2 ± SD 0.8 vs. 28.1 ± SD 1.3 in resistant/refractory group, p=0.69) (Figure S1B). These findings suggest that Ctr1 expression in ovarian tumors is associated with platinum drug response.
Figure 1. Low levels of Ctr1 mRNA in ovarian tumors are associated with poor clinical outcome.
(A) Baseline characteristics of the 15 patients with advanced-stage epithelial ovarian carcinoma, whose tumors were analyzed for relative expression of the Ctr1 copper transporter.
(B) Tumor Ctr1 mRNA levels in platinum sensitive and refractory/resistant ovarian cancer patients. Bars represent mean values. Ctr1 mRNA levels are expressed as a percentage relative to the internal control of human GUS mRNA.
(C) Characteristics of 91 ovarian cancer patients from The Cancer Genome Atlas study.
(D) Kaplan-Meier estimate of disease-free survival for 91 advanced-stage ovarian cancer patients according to Ctr1 expression levels. Patients whose tumors expressed Ctr1 mRNA at levels higher than the median value were classified as “High Ctr1” (red; 45 patients); the remaining patients were labeled “Low Ctr1” (blue; 46 patients). The vertical hash marks (at 7 and 10 months, High Ctr1) represent censored patients who were disease-free at the last follow-up time indicated.
See also Figure S1.
In order to validate these findings in an independent data set, we used clinical and array-based expression data from The Cancer Genome Atlas (TCGA) that are deposited at the Data Coordinating Center for public access (http://cancergenome.nih.gov/). We defined a subset of 91 patients with Stage III or IV serous epithelial ovarian cancer who had undergone a cytoreductive surgery followed by adjuvant chemotherapy consisting of a platinum drug and a taxane (http://tcga-data.nci.nih.gov/tcga/homepage.htm) (Figure 1C). Results for Ctr1 expression were extracted from expression data that were produced using Affymetrix HT Human Genome U133A Array Plate Set (HT_HG-U133A) (http://tcga-data.nci.nih.gov/tcga/dataAccessMatrix.htm). The patients were divided into two groups by the median Ctr1 expression value, resulting in 45 “high Ctr1” (above median) and 46 “low Ctr1” (below median) patients (Figure S1C). High expression of Ctr1 was associated with increased disease-free survival (Figure 1D; log-rank statistic 8.67, hazard ratio 0.53, p=0.003), supporting our hypothesis that Ctr1 levels are prognostic of treatment outcome. These findings suggest that mRNA levels of the human copper transporter Ctr1 in tumors are clinically relevant to the efficacy of platinum therapy.
Ctr1 mRNA levels correlate with cisplatin adduct levels in mouse tissues
To address the in vivo role of Ctr1 in cisplatin uptake, we examined the levels of Ctr1 mRNA and cisplatin-induced DNA adducts in various tissues of HPV16/E2, the mouse model of human cervical cancer. Overall, the levels of cisplatin adducts correlated with Ctr1 mRNA in most organs tested -- skin, lung, liver, pancreas, and uterus (Figure 2). Kidney contained the highest amount of cisplatin adducts and Ctr1 mRNA, which is interesting in light of the prevalence of nephrotoxicity among cisplatin-treated patients. The colon contained fewer adducts relative to Ctr1 mRNA levels. In the duodenum and intestine, Ctr1 protein is localized to the apical surface facing the intestinal lumen and in intracellular compartments (Nose et al., 2006; Kuo et al., 2006). It is possible that such localized Ctr1 in the digestive tract is not involved in the uptake of cisplatin, which is administered intravenously to patients, and in this experiment was supplied intraperitoneally. Notably, the cancerous HPV16/E2 cervix contained more adducts relative to Ctr1 mRNA levels. Expression in the cervix of the internal control gene mouse L19, to which Ctr1 mRNA was normalized, was equivalent to that of other tissues (average tissue L19 Ct 23.5 ± SD 1.1 vs. 23.3 in the cervix) (Figure S2A), indicating that the observed low levels of Ctr1 mRNA in the cervix is not a result of higher internal control gene expression in the tumors. Despite the low Ctr1 mRNA levels, Ctr1 protein was substantially expressed in the cancerous cervix (Figure 3D), suggesting increased protein synthesis and/or reduced protein turnover in this neoplastic tissue. We also measured mRNA levels of the copper exporters, ATP7A and ATP7B, which are involved in exporting cisplatin in vitro (Komatsu et al., 2000; Samimi et al, 2004). Consistent with prior reports (Bull et al., 1993; Tanzi et al., 1993), ATP7B was primarily expressed in the liver (Figure S2B). We were able to detect ATP7A mRNA in all the tissues tested, but did not observe an inverse correlation between ATP7A mRNA levels and tissue platinum levels, further supporting our proposition that the copper transporter Ctr1 is the major determinant for cisplatin accumulation in vivo as well.
Figure 2. Levels of Ctr1 mRNA and cisplatin adducts in various organs of the HPV16/E2 female mice.
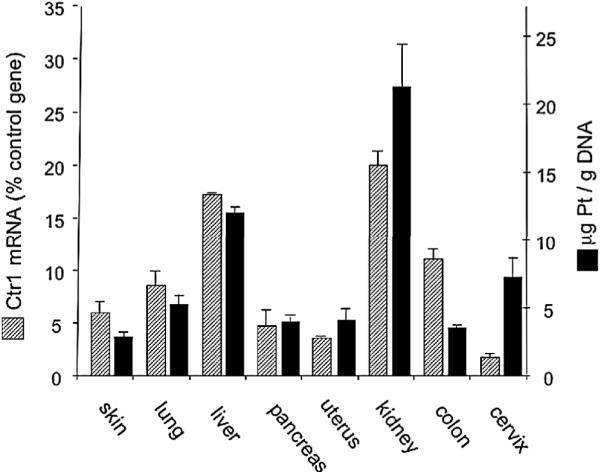
Organs were harvested from three six-month-old HPV16/E2 females and Ctr1 mRNA levels were determined by quantitative RT-PCR (hatched bars). Mouse L19 mRNA levels were used as internal controls. For measurement of cisplatin adducts, genomic DNA was purified from each organ (n=8) two hours after 6 mg/kg cisplatin was injected. Platinum was measured by inductively-coupled plasma mass spectrometry (ICP-MS) and normalized to the amount of DNA (solid bars). Error bars represent the standard error of the mean.
See also Figure S2.
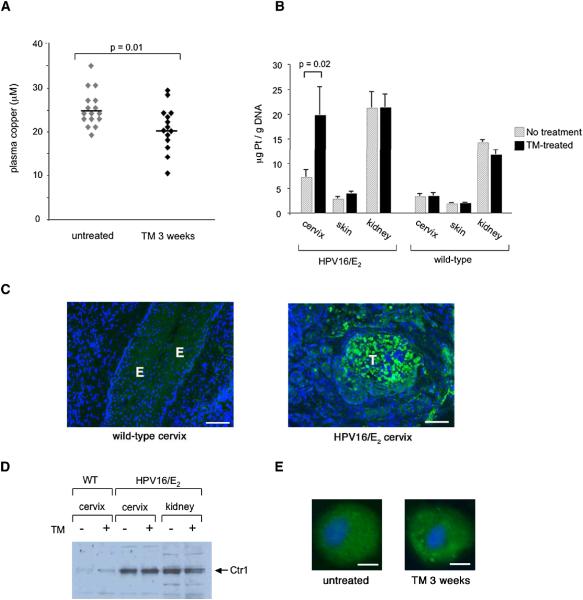
Figure 3. The copper chelator tetrathiomolybdate (TM) enhances cisplatin uptake into the cervix of HPV16/E2 mice.
(A) Plasma copper levels in six-month-old HPV16/E2 females after a three-week daily treatment with 1 mg TM. Bars represent mean values.
(B) Cisplatin adduct levels in the cervix, skin, and kidney of six-month-old HPV16/E2 females (n=12) and wild-type females (n=5) that received either the three-week TM treatment (solid bars) or no treatment (hatched bars). 6 mg/kg cisplatin was injected two hours before organs were harvested. Adduct levels were determined as in Figure 2. Error bars represent the standard error of the mean.
(C) Ctr1 immunohistochemistry reveals positive cells (green) in tumors (T) of the six-month-old HPV16/E2 cervix (right panel) but not in the epithelium (E) of the wild-type cervix (left panel). DAPI staining is shown in blue. The panels shown are representative images of twelve fields in three tissue sections per mouse collected from three mice. Scale bar: 100 μm.
(D) Tissue Ctr1 protein levels are elevated in the neoplastic cervix, but unchanged in the context of TM treatment. Wild-type and HPV16/E2 mice were either treated with 1 mg TM daily for 3 weeks prior to being sacrificed at 6 months of age, or did not receive such treatment. 5 μg of membrane extracts from tissues were pooled from three mice and applied on each well.
(E) Ctr1 protein (green) is predominantly localized in intracellular compartments in cervical carcinoma cells, and is unchanged by TM treatment. HPV16/E2 mice were either treated with 1 mg TM daily for 3 weeks prior to being sacrificed at 6 months of age, or did not receive such treatment. Tissue sections were immunostained with anti-Ctr1(green). Nuclei are stained with DAPI (blue). The images were collected using a 20x objective lens on a fluorescent microscope, and are representative of > 500 cells in 12 sections from 6 tumors. Scale bar: 5 μm.
The copper chelator tetrathiomolybdate increases cisplatin adduct levels in tumors but not normal organs
Having observed an in vivo correlation between levels of the copper transporter Ctr1 and cisplatin adducts in the majority of tissues examined, we next asked whether reducing systemic copper could result in increased cisplatin adducts in tumors. We treated HPV16/E2 mice with the copper chelator tetrathiomolybdate (TM), which is currently granted orphan designation in the United States and Europe for the treatment of Wilson’s disease, an autosomal recessive disorder characterized by excess copper deposition in various organs. TM reduces bioavailable copper levels, primarily by forming a tripartite complex with ingested copper and protein, preventing the absorption of copper in the intestine (Brewer and Merajver, 2002). We determined the maximally tolerated daily dose of TM for the HPV16/E2 female mice to be 1 mg (data not shown). When the mice are subjected to this daily dosage of TM, plasma copper levels start decreasing after three days, and reach a plateau after two weeks. We did not observe any side effects during three weeks of daily TM treatment, at which point the plasma copper levels had decreased by 23% (Figure 3A). This TM dosage resulted in a 2.7-fold increase in cisplatin adduct levels in the cancerous cervix of the HPV16/E2 mice (7.2 μg Pt/ g DNA in control vs. 19.8 μg Pt/ g DNA in TM-treated mice, p=0.02), but not in the wild-type cervix (Figure 3B). Such a difference in the number of adducts is considered significant, since it can result in a thousand-fold differential in cell viability in vitro (Hall et al., 2008). Notably, this TM regimen did not increase adducts in the kidney (Figure 3B), which is the major organ affected by cisplatin toxicity, nor in other non-cancerous organs of the mice. Interestingly, in the absence of TM treatment, the kidneys of HPV16/E2 mice accumulated 50% more platinum adducts than those of wild-type mice (Figure 3B). This increase in the adduct level in the kidneys of HPV16/E2 mice may be due to renal failure from urinary tract obstruction, which is not uncommon both in cervical cancer patients and in this mouse model. TM treatment also produced a marginal 1.4-fold increase in the adduct level in the dysplastic skin of the HPV16/E2 mice (2.8 μg Pt/ g DNA in control vs. 3.9 μg Pt/ g DNA in TM-treated mice, p=0.03), but not in the skin of wild-type mice, suggesting that neoplastic tissues may become more sensitive to changes in copper levels. Notably, we found that Ctr1 protein is highly expressed in tumors of the HPV16/E2 cervix but not in the epithelium of the wild-type cervix, the origin of cervical carcinoma (Figure 3C). We were, however, unable to detect any change in Ctr1 mRNA (data not shown) or protein levels (Figure 3D) in response to TM treatment in the neoplastic HPV16/E2 cervix or in normal tissues. In tumor cells of the HPV16/E2 cervix, Ctr1 protein was predominantly localized in intracellular compartments; Ctr1 remained intracellular in TM-treated HPV16/E2 cervix (Figure 3E). It is possible that the modest change in systemic copper (23% reduction) by TM (Figure 3A) is not sufficient to cause measurable changes in the levels of Ctr1 protein, which is already high in these tumors, or in its localization. However, TM treatment resulted in increased cisplatin uptake in the HPV16/E2 cervix (Figure 3B), demonstrating that even a small reduction in systemic copper levels can increase the transport activity of Ctr1 in tumors. These data indicate that expression of the copper transporter Ctr1 is upregulated in neoplastic tissues compared to cognate normal tissues, and that its transport activity could be further elevated selectively in tumors by reducing systemic copper.
The copper chelator tetrathiomolybdate enhances cisplatin efficacy
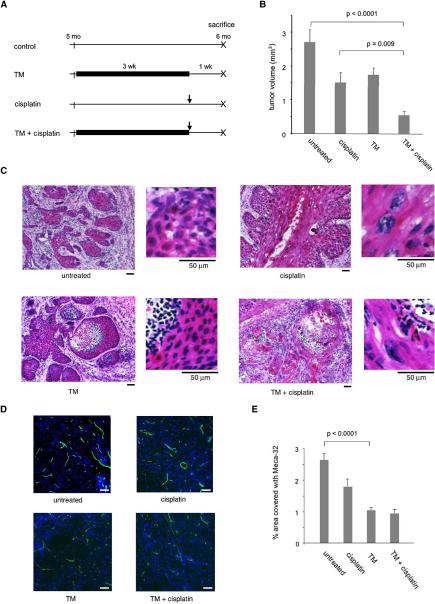
To assess possible therapeutic benefits of using TM to enhance the uptake and consequent cytotoxicity of cisplatin in tumors, cohorts of 14 female HPV16/E2 mice were subjected either to a three-week regimen of oral 1 mg TM daily starting at 22 weeks of age, or to a single intraperitoneal injection of 6 mg/kg cisplatin at 25 weeks of age, or to both (Figure 4A). At 22 weeks, all HPV16/E2 females have high-grade dysplasias (CIN 2/3), and about half have invasive cervical carcinomas. Animals from all treatment arms were sacrificed at 26 weeks of age, when 90% have cervical carcinomas. Tumor volumes were calculated by measuring tumor areas on serial sections. Cisplatin treatment reduced tumor volumes to 55% of untreated cervix (2.7 mm3 in control vs. 1.5 mm3 with cisplatin, p=0.01) (Figure 4B). In the cisplatin-treated cervix, cells had more abundant cytoplasm that appeared keratinized (Figure 4C). Nuclei were enlarged and bizarre in shape, characteristic features of human cervical cancer treated with radiation (Robboy et al., 2002), which causes DNA damage. TM alone also modestly decreased tumor volumes in the cervix, to 65 % of controls (2.7 mm3 in control vs. 1.7 mm3 with TM, p=0.04) (Figure 4B). In the TM-treated cervix, the center of a tumor mass was necrotic and filled with inflammatory cell infiltrates (Figure 4C). The percentage of area covered with meca-32-positive endothelial cells in the TM-treated tumors was 2.6-fold less compared to those from untreated mice (Figures 4D, 4E), consistent with prior observations that TM is antiangiogenic (Brewer and Merajver, 2002). When cisplatin was combined with the TM treatment, tumor volume decreased to 19 % of untreated cervix (2.7 mm3 in control vs. 0.5 mm3 with TM and cisplatin, p<0.0001) (Figure 4B). Microscopically, the cervical carcinoma from the combination arm showed histologic features of the effects seen with both cisplatin and TM monotherapies: enlarged nuclei, necrotic centers, inflammatory cell infiltrates, and reduced vascularity (Figures 4C, 4D). These results demonstrate that TM enhances efficacy of cisplatin synergistically.
Figure 4. Preclinical trials assessing therapeutic effects of the copper chelator tetrathiomolybdate (TM) in combination with cisplatin on de novo cervical carcinomas.
(A) Schematic diagrams of each therapeutic arm. Cohorts of 14 female HPV16/E2 mice were subjected either to a three-week oral administration of 1 mg TM daily starting at five months of age, or to a single intraperitoneal injection of 6 mg/kg cisplatin at five and ¾ months of age, or to both. All the mice were sacrificed at six months of age.
(B) Tumor volumes in the cervix of six-month-old HPV16/E2 mice from each treatment arm. Approximately 30 sections per mouse from cohorts of 14 female HPV16/E2 mice were analyzed as described in Methods. Error bars represent the standard error of the mean.
(C) Representative H&E-stained cervices from each treatment arm, with the treatment indicated below each panel. Cells are shown at a higher magnification on the right. The magnification is scaled by a bar below each panel indicating 50 μm. In the non-treated cancerous cervix (top left), invasive squamous cell carcinomas contain large keratinized cells with hyperchromatic nuclei. In the cisplatin-treated cervix (top right), the cancer cells have more abundant cytoplasm that appears keratinized, with enlarged, bizarre-shaped nuclei. In the TM-treated cervix (bottom left), the center of a tumor mass is necrotic and filled with inflammatory cell infiltrates. The cervix treated with both TM and cisplatin (bottom right) shows histological features of the effects seen with both cisplatin and TM monotherapies: enlarged nuclei, necrotic centers, and inflammatory cell infiltrates. The panels shown are representative of 4 fields in 30 tissue sections collected from cohorts of 14 HPV16/E2 mice.
(D), (E) Vascular density of tumors after treatment. 15 tumor sections from five mice per treatment arm were stained with a pan-endothelial cell antibody Meca-32, followed by FITC-conjugated secondary antibody to visualize tumor endothelial cells. Images were collected from 5-7 fields per section, and vascular density was determined using the Metamorph Angiogenesis Tube Formation program. Data are expressed as the percentage of area covered with Meca-32-positive tube structures (E). Error bars represent the standard error of the mean. Representative images of Meca-32 (green) staining in the cervix of control and TM-treated HPV16/E2 mice are shown in D. Nuclei are stained with DAPI (blue). Scale bar: 50 μm.
The copper chelator tetrathiomolybdate increases cisplatin sensitivity of cancer cells
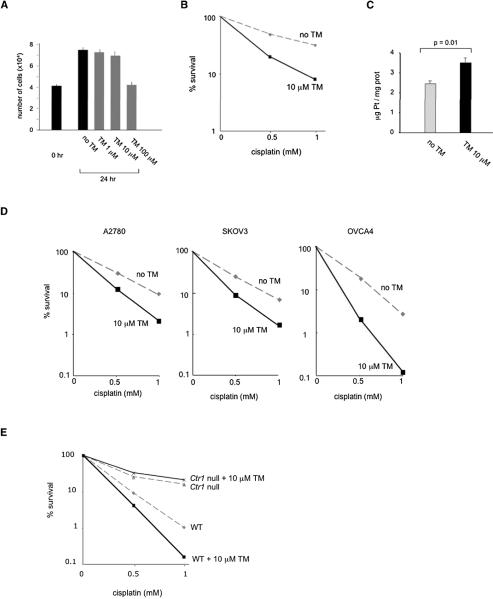
Seeking to separate the antiangiogenic effect of TM on tumor vasculature from its effect on tumor cell uptake of cisplatin, we asked whether TM treatment increases cisplatin sensitivity and uptake in cultures of SiHa cells, which are derived from an HPV16-positive human cervical carcinoma (Friedl et al., 1970). Plasma TM levels in the HPV16/E2 females range from 150 μM to 4 μM, over the period from 3 to 24 hours after oral administration of 1mg TM, respectively (data not shown). We determined that 24-hour incubation with up to 10 μM TM does not inhibit proliferation of SiHa cells (Figure 5A). When SiHa cells were incubated with 10 μM TM for 24 hours and then subjected to a two-hour cisplatin treatment, we observed a two-fold increase in cisplatin sensitivity and a 45 % increase in cisplatin accumulation inside TM-treated cells compared to cells that were not preincubated with TM (Figure 5B, C). We also assessed effectiveness of the combination therapy for ovarian cancer, using three human ovarian cancer cell lines derived from patients’ tumors, A2780, SKOV3, and OVCA4 (Eva et al., 1982; Fogh and Trempe, 1975). These cells had different growth properties: the doubling time was 16 hours for A2780, 24 hours for SKOV3, and 36 hours for OVCA4 (data not shown). We determined that incubation of these cells with 10 μM TM did not have an antiproliferative effect for up to 6 hours in A2780, 12 hours in SKOV3, and 24 hours for OVCA4 (data not shown). When these cells were subsequently treated with cisplatin, they exhibited 1.8 to 2.2-fold increased sensitivity compared to cells that were not preincubated with TM (Figure 5D), demonstrating that TM can increase cisplatin sensitivity of ovarian cancer cells as well. To establish that the effects of TM on enhancing cisplatin-mediated killing were dependent on Ctr1, we compared isogenic mouse embryonic fibroblasts (MEFs) that were either wild-type or deleted for the Ctr1 gene (Lee et al., 2002). The ability of TM to increase cisplatin killing proved to be dependent on Ctr1: in wild-type MEFs, the percentage of cells that survived a two-hour treatment with 1 mM cisplatin was reduced to 0.16% in cultures pretreated with TM, as compared to 1% in control cultures (Figure 5E). In contrast, the percentage of cells surviving cisplatin treatment was not affected by TM treatment in Ctr1-null MEFs (Figure 5D). These results further support the role of the copper chelator TM and the copper transporter Ctr1 in enhancing cisplatin uptake and killing of cancer cells.
Figure 5. Effects of the copper chelator tetrathiomolybdate (TM) on cisplatin sensitivity in cultured mammalian cells.
(A) Effect of TM on cell proliferation in human cervical carcinoma cells. SiHa cells were plated in triplicates and treated with 0, 1, 10, or 100 μM TM for 24 hours. Numbers of cells before and after the treatment are shown. Error bars represent the standard error of the mean.
(B), (C) Effect of TM on cisplatin sensitivity and accumulation in human cervical carcinoma cells. SiHa cells were plated in triplicates and either pretreated with 10 μM TM or mock treated for 24 hours before a two-hour incubation with cisplatin. For cell survival (B), data are expressed as percentage of viable cells compared with control cultures not exposed to cisplatin. For determining cisplatin accumulation (C), cells were lysed and, after a centrifugation, the supernatant was used to determine the platinum content and protein concentration. Cellular platinum readings were normalized to protein concentration. Mean values of the triplicates are shown. Error bars represent the standard error of the mean.
(D) TM increases cisplatin sensitivity in human ovarian cancer cells. Cells were plated in triplicates and either pretreated with 10 μM TM or mock treated for 6 hours (A2780), 12 hours (SKOV3) or 24 hours (OVCA4) before cisplatin treatment. Data are expressed as percentage of viable cells compared with control cultures not exposed to cisplatin.
(E) TM increases cisplatin sensitivity in a Ctr1-dependent manner. Isogenic mouse embryonic fibroblasts that are either wild-type or homozygous for a knockout allele of Ctr1 were either pretreated with 10 μM TM or mock treated for 24 hours before a two-hour incubation with cisplatin. The percentage of viable cells compared with control cultures not exposed to cisplatin is shown.
DISCUSSION
Our studies in a mouse model of HPV16-induced cervical carcinoma demonstrate that the copper chelator tetrathiomolybdate (TM) has a synergistic antitumor effect when combined with cisplatin treatment, by increasing uptake of cisplatin into tumors but not normal tissues while concomitantly inhibiting tumor angiogenesis. We show that cisplatin adduct levels in various mouse organs correlate with mRNA levels of Ctr1, supporting its in vivo involvement in cisplatin uptake. Pretreatment of cultured human cervical and ovarian cancer cells with TM resulted in increased cisplatin sensitivity, encouraging applicability to human cancers. TM-enhanced cisplatin killing was dependent on Ctr1, as mouse cells deleted for the Ctr1 gene were not sensitized to cisplatin by TM, in comparison to isogenic cells with wild-type Ctr1. Notably, low levels of Ctr1 mRNA in human ovarian tumors are associated with decreased disease-free survival after platinum-based therapy in patients, suggesting that Ctr1 may serve as a clinical predictor of response to platinum agents.
Previous genetic experiments with yeast and mouse cells demonstrated that the copper transporter Ctr1 is a major determinant of cisplatin uptake and sensitivity, which could be modulated by copper levels. These in vitro results suggested a strategy to increase cisplatin uptake, but a challenge was to find a clinically feasible method to do so selectively in tumors, without affecting normal organs to avoid untoward toxicities of cisplatin. Surprisingly, we were able to achieve this goal by reducing systemic copper levels with a copper chelator in a mouse model of cervical cancer. We do not fully understand the mechanism by which reduced systemic copper leads to increased cisplatin uptake only in tumors. Copper is essential for a variety of key cellular processes such as respiration, free radical detoxification, and iron uptake (Kim et al., 2008). Cancer cells may have a greater demand for copper than normal cells for proliferation and survival. Indeed, the copper transporter Ctr1 was more highly expressed in cervical carcinoma of the HPV16/E2 mice than in the wild-type cervix. Concordant with our observation is a recent demonstration using micro-beam synchrotron X-ray fluorescence that copper is concentrated in tumor regions of tissue specimens obtained from invasive ductal carcinoma of the breast (Farquharson et al., 2008).
Although pretreatment of mice with the copper chelator TM resulted in increased cisplatin sensitivity and adducts in tumors, we were unable to detect any increase in the levels of Ctr1 mRNA or protein. In vitro, elevated extracellular copper causes endocytosis and degradation of human Ctr1 protein (Petris et al., 2003). We did not, however, observe any change in Ctr1 protein localization in the TM-treated cervix of HPV16/E2 mice. It is possible that copper starvation enhances cisplatin transport activity by changing the conformation of Ctr1, allowing more cisplatin to be transported into cells. Exogenous copper has been shown to induce structural rearrangements in yeast Ctr1 (Sinani et al., 2007). Further biochemical studies will be required to elucidate the mechanism by which copper chelation causes increased cisplatin uptake.
Drug-induced toxicity is a common cause for discontinuation or dose reduction of chemotherapeutic drugs during cancer treatment. Cancers that are deemed “resistant” to a chemotherapeutic drug fail to respond to the dosage tolerated by patients without untoward side effects. These “resistant” cancers might in principle still respond to a higher dosage if toxicities were not manifest. By reducing bioavailable copper with the copper chelator tetrathiomolybdate (TM), we were able to increase cisplatin activity in highly metabolic tumors while comparatively sparing normal organs. TM was developed for the treatment of patients with Wilson’s disease, an autosomal recessive disorder of copper transport that results in excessive accumulation of copper and toxicitiy. Phase II and III trials with Wilson’s disease patients have demonstrated that TM is a fast-acting and well-tolerated drug (Brewer 2009). The major side effect is anemia and leukopenia due to copper depletion, which can be reversed with a drug holiday or dose reduction.
Additionally, the independent anti-angiogenic effects of the copper chelator TM present it as a drug that targets both tumor parenchyma and stroma, by enhancing cisplatin efficacy against the cancer cells whilst inhibiting angiogenesis in the tumor microenvironment. Combination regimens involving copper chelating and platinum-containing drugs may improve the treatment of cervical, ovarian, and other cancers for which cisplatin is currently in use, and for cancers that are treated with carboplatin, whose uptake is also mediated by Ctr1 (Holzer et al., 2006). Such a therapeutic strategy may even prove effective in treating cancers that are inherently resistant to cisplatin or have developed resistance. With the development of a second-generation TM analog that depletes copper more quickly and is more stable (Lowndes et al, 2008), it may be possible to manipulate the activity of Ctr1 even more effectively. The mechanistic principles and results elaborated in this report should motivate discussion of analogous clinical trials in patients with cervical, ovarian, and other platinum-responsive cancers.
EXPERIMENTAL PROCEDURES
Tumor samples
We obtained ovarian tumor RNA samples from the UCSF Ovarian Cancer Tissue / Pathology Bank, which has approval from the UCSF Institutional Review Board to collect ovarian cancer specimens and clinical outcomes data for research. Written informed consent was obtained from all patients contributing specimens to the bank. Fifteen samples were available from women with Stage III/IV serous epithelial ovarian carcinoma who underwent optimal cytoreduction (resulting in residual tumor of 1 cm or less in diameter), received platinum-based therapy, and had clinical outcome data available. Tumor samples were snap frozen in liquid nitrogen at the time of surgery and maintained in a monitored −80 C° freezer. Adjacent sections were used for pathology and to confirm that they contained more that 70% tumor cells. RNA was isolated from snap-frozen tumors using Trizol and its integrity was determined by Agilent 2100 Bioanalyzer. Responses to initial chemotherapy were recorded as either refractory/resistant (if there was progression or recurrence of the disease within 6 months after completion of chemotherapy, as indicated by an increase in CA-125 levels, a positive finding on CT or ultrasonography, or second-look laparoscopy) or sensitive (if there was no such evidence of recurrence within 6 months after completion of chemotherapy).
Determination of mRNA levels using real-time PCR
5 ng of cDNA was used for quantitative RT-PCR to measure mRNA levels of copper transporters in human ovarian tumors and in mouse tissues. The following TaqMan gene expression assay kits (Applied Biosystems) were used: Hs00977268_g1 (human Ctr1), Mm00612987_m1 (mouse Ctr1), Mm00437663_m1 (mouse ATP7A), and Mm00599675_m1 (mouse ATP7B). The human GUS gene, which encodes β-glucuronidase, and the mouse L19 gene were used as internal amplification controls for human and mouse, respectively.
Mouse studies
All animal studies were performed in compliance with the UCSF Guide for the Care and Use of Laboratory Animals, and under approved protocols by the UCSF Institutional Animal Care and Use Committee.
Platinum measurement
After cisplatin treatment, organs were harvested and ~ 30 mg of tissues were digested in 500 μl lysis buffer (50 mM Tris pH 8.0, 20 mM NaCl, 1mM EDTA pH 8.0, 1 % SDS) with 2 mg/ml proteinase K at 55 °C for 16 hours. Digests were treated with 0.2 mg/ml RNase at 37 °C for 1 hour, followed by phenol/chloroform extraction three times. DNA was precipitated with sodium acetate and isopropanol, resuspended in 500 μl H2O, and DNA concentration was measured with a spectrometer. 2 ppb iridium was added to each sample as an internal control for ICP-MS analysis. The platinum reading was normalized to DNA concentration. For measuring cellular platinum, cells were trypsinized, washed with PBS, and lysed with 1% Triton X-100/ 0.1 % SDS on ice for 30 minutes. Lysates were spun at top speed in a microcentrifuge at 4 °C, and the supernatant was used to determine protein concentration (BioRad). An equal volume of nitric oxide was added to the supernatant and digested at 75 °C for 2 hours for ICP-MS analysis. The platinum reading was normalized to protein concentration.
Plasma copper measurements
Plasma was separated from blood using PST tubes with lithium heparin (BD microtainer) and digested at 75 °C for 2 hours with an equal volume of nitric oxide. 100 ppb cobalt was added to each sample as an internal control for ICP-MS analysis.
Immunohistochemistry
OCT-embedded frozen tissues were sectioned at 10 μm thickness. Sections were air dried, acetone treated, and blocked with 5 % BSA and 2.5 % goat serum. Primary antibodies were applied at a dilution of 1:100 for anti-Ctr1 and 1:200 for meca-32. Secondary antibodies were used at 1:500.
Western blotting
Membrane protein extracts were prepared as described (Nose et al., 2006). Protein concentrations were measured using the BioRad Protein DC Assay Kit. 5 μg of membrane extracts from tissues were pooled from three mice and applied on each well. Anti-Ctr1 antibody was used at 1:1000. Peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories) was used as secondary antibodies at 1:10,000. For detection, Amersham ECL Western Blotting Reagents were used.
Measurement of tumor volume
OCT-embedded cervices were serially sectioned at 10 μm thickness and stained with hematoxylin and eosin (H&E) every 100 μm. Areas of squamous cell carcinoma on H&E stained sections were measured using an Axio Imager program. Tumor volume (V) was calculated using the following formula V = 2/3 × A × Z, where A is the maximal tumor area measured on a section and Z is the depth of tumor determined by the number of sections containing tumors.
Tissue culture
SiHa, A2780, SKOV3, and OVCA4 cells were cultured in RPMI medium containing 10 % fetal bovine serum and penicillin/streptomycin. Ctr1 +/+ (wild-type) and −/− mouse embryonic fibroblasts were cultured in DMEM supplemented with 20% fetal bovine serum, 2 mM glutamine, 1x nonessential amino acids, 55μM 2-mercaptoethanol, 50 mg/L uridine, 110 mg/L pyruvate, and penicillin/streptomycin. For determining survival, cells were stained with trypan blue to detect dead cells; unstained cells were counted using a hemocytometer.
Statistics
Wilcoxon analyses were performed to determine two-tailed p values. For Kaplan-Meier survival estimates, p values were determined from a log-rank statistic with one degree of freedom.
SIGNIFICANCE.
Inefficient drug delivery and attendant toxicity represent impediments to successful chemotherapy. We present a strategy to mitigate these limitations for the anticancer drug cisplatin, whose uptake is mediated by the copper transporter Ctr1. We show that therapeutic preconditioning with a copper chelator enhances cisplatin uptake and killing of cancer cells without affecting normal organs in a mouse model of cervical cancer. Concordantly, Ctr1 levels in human ovarian tumors correlate with the efficacy of platinum-based chemotherapy. These results encourage clinical trials aimed to up-regulate Ctr1 in tumors with copper chelators so as to improve the benefits and potentially expand the scope of platinum-based chemotherapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. Nose and D. Thiele (Duke University) for Ctr1 antibody and knockout cell lines, R. Franks (University of California, Santa Cruz) for analytical support with ICP-MS, P. Glenn for the UCSF ovarian tumor database and mRNA, F. Schaufele for technical assistance with microscopy and image analysis, D. Moore for help with statistical analysis on patients, and the UCSF Helen Diller Family Comprehensive Cancer Center Genome Core for quantitative RT-PCR. The UCSF Ovarian Cancer Tissue/ Pathology Core was supported by P01CA64602 (to K.S.M.). We thank members of our laboratories for comments and feedback. This study was initially supported by the NIH grant GM 59256 (to Ira Herskowitz) and subsequently by a Flight Attendant Medical Research Institute Clinical Innovator Award (to D.H. and S.I.), by grants from the NCI (to D.H.), and by an award from the William K. Bowes, Jr, Charitable Foundation (to D.H.). Douglas Hanahan is an American Cancer Society Research Professor.
The results published here are in part based upon data generated by The Cancer Genome Atlas pilot project established by the NCI and NHGRI. Information about TCGA and the investigators and institutions who constitute the TCGA research network can be found at “http://cancergenome.nih.gov”.
This work is dedicated in the memory of our colleague and mentor Ira Herskowitz, who was involved in the inception of this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc. Natl. Acad. Sci, USA. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah E, Flaherty L. Treatment of metastatic malignant melanoma. Curr. Treat. Options Oncol. 2005;6:185–93. doi: 10.1007/s11864-005-0002-5. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Merajver SD. Cancer therapy with tetrathiomolybdate: antiangiogenesis by lowering body copper – a review. Integr. Cancer Ther. 2002;1:327–337. doi: 10.1177/1534735402238185. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. The use of copper-lowering therapy with tetrathiomolybdate in medicine. Expert Opin. Investig. Drugs. 2009;18:89–97. doi: 10.1517/13543780802621859. [DOI] [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Carrick S, Ghersi D, Wilcken N, Simes J. Platinum containing regimens for metastatic breast cancer. Cochrane Database Syst. Rev. 2004;2:1–45. doi: 10.1002/14651858.CD003374.pub2. [DOI] [PubMed] [Google Scholar]
- Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol. Oncol. 2008;110:S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Cen P, Ajani JA. Medical treatment for advanced gastroesophageal adenocarcinoma. Curr. Opin. Gastroenterol. 2007;23:631–635. doi: 10.1097/MOG.0b013e3282f0a933. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Hanahan D, Arbeit JM. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am. J. Pathol. 1996;149:1899–1917. [PMC free article] [PubMed] [Google Scholar]
- du Bois A, Lück HJ, Meier W, Adams HP, Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- Eva A, Robbins KC, Anderson PR, Srinivasan A, Tronick SR, Reddy EP, Ellmore NW, Galen AT, Lautenberger JA, Papas TS, et al. Cellular genes analogous to retroviral onc genes are transcribed in human tumor cells. Nature. 1982;295:116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Elson DA, Riley RR, Lacey A, Thordarson G, Talamantes FJ, Arbeit JM. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 2000;60:1267–1275. [PubMed] [Google Scholar]
- Farquharson MJ, Al-Ebraheem A, Falkenberg G, Leek R, Harris AL, Bradley DA. The distribution of trace elements Ca, Fe, Cu, and Zn and the determination of copper oxidation state in breast tumour tissue using μSRXRF and μXANES. Phys. Med. Biol. 2008;53:3023–3037. doi: 10.1088/0031-9155/53/11/018. [DOI] [PubMed] [Google Scholar]
- Fischer B, Arcaro A. Current status of clinical trials for small cell lung cancer. Rev Recent Clin. Trials. 2008;3:40–61. doi: 10.2174/157488708783330503. [DOI] [PubMed] [Google Scholar]
- Fogh J, Trempe G. New human tumor cell lines. In: Fogh J, editor. Human Tumor Cells In Vitro. Plenum Press; New York and London: 1975. pp. 115–159. [Google Scholar]
- Friedl F, Kimura I, Osato T, Ito Y. Studies on a new human cell line (SiHa) derived from carcinoma of uterus. I. Its establishment and morphology. Proc. Soc. Exp. Biol. Med. 1970;135:543–5. doi: 10.3181/00379727-135-35091a. [DOI] [PubMed] [Google Scholar]
- Gallagher DJ, Milowsky MI, Bajorin DF. Advanced bladder cancer: status of first-line chemotherapy and the search for active agents in the second-line setting. Cancer. 2008;113:1284–1293. doi: 10.1002/cncr.23692. [DOI] [PubMed] [Google Scholar]
- Glioma Meta-Analysis Trialists Group Chemotherapy for high-grade glioma. Cochrane Database Syst Rev. 2002;4:1–23. doi: 10.1002/14651858.CD003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth JD, Grosh WW, Burnett LS, Jones HW, 3rd, Wolff SN, Greco FA. Advanced ovarian cancer: long-term results of treatment with intensive cisplatin-based chemotherapy of brief duration. Ann. Intern. Med. 1988;108:165–170. doi: 10.7326/0003-4819-108-2-165. [DOI] [PubMed] [Google Scholar]
- Hall MD, Okabe M, Shen D-W, Liang X-J, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, an oxaliplatin. Mol. Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Ma YT, Cullen MH. Management of metastatic germ cell tumors. Expert Rev. Anticancer Ther. 2008;8:771–84. doi: 10.1586/14737140.8.5.771. [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- Kim B, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nature Chem. Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T, et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60:1312–1316. [PubMed] [Google Scholar]
- Kuo Y-M, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J. Nutr. 2006;136:21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the Ctr1 high affinity copper transporter. J. Biol. Chem. 2002;277:40253–9. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- Louie KG, Ozols RF, Myers CE, Ostchega Y, Jenkins J, Howser D, Young RC. Long-term results of a cisplatin-containing combination chemotherapy regimen for the treatment of advanced ovarian carcinoma. J. Clin. Oncol. 1986;4:1579–1585. doi: 10.1200/JCO.1986.4.11.1579. [DOI] [PubMed] [Google Scholar]
- Lowndes SA, Adams A, Timms A, Fisher N, Smythe J, Watt SM, Joel S, Donate F, Hayward C, Reich S, et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin. Cancer Res. 2008;14:7526–7534. doi: 10.1158/1078-0432.CCR-08-0315. [DOI] [PubMed] [Google Scholar]
- Lustberg MB, Edelman MJ. Optimal duration of chemotherapy in advanced non-small cell lung cancer. Curr. Treat. Options Oncol. 2007;8:38–46. doi: 10.1007/s11864-007-0020-6. [DOI] [PubMed] [Google Scholar]
- Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Oh WK, Tay MH, Huang J. Is there a role for platinum chemotherapy in the treatment of patients with hormone-refractory prostate cancer? Cancer. 2007;109:477–486. doi: 10.1002/cncr.22439. [DOI] [PubMed] [Google Scholar]
- Omura GA, Gynecologic Oncology Group Progress in gynecologic cancer research: the Gynecologic Oncology Group experience. Semin. Oncol. 2008;35:507–521. doi: 10.1053/j.seminoncol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15:3515–3523. [PMC free article] [PubMed] [Google Scholar]
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer : a Gynecologic Oncology Group study. J. Clin. Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J. Biol. Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Anderson MC, Russell P. Pathology of the female reproductive tract. Churchill Livingstone; 2002. [Google Scholar]
- Saif MW, Kim R. Role of platinum agents in the management of advanced pancreatic cancer. Expert Opin. Pharmacother. 2007;8:2719–27. doi: 10.1517/14656566.8.16.2719. [DOI] [PubMed] [Google Scholar]
- Samimi G, Katano K, Holzer AK, Safaei R, Howell SB. Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol. Pharmacol. 2004;66:25–32. doi: 10.1124/mol.66.1.25. [DOI] [PubMed] [Google Scholar]
- Sinani D, Adle DJ, Kim H, Lee J. Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J. Biol. Chem. 2007;282:26775–26785. doi: 10.1074/jbc.M703973200. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Tao X, Hu W, Ramirez PT, Kavanagh JJ. Chemotherapy for recurrent and metastatic cervical cancer. Gynecol. Oncol. 2008;110:S67–S71. doi: 10.1016/j.ygyno.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Yang ES, Murphy BM, Chung CH, Netterville JL, Burkey BB, Gilbert J, Yarbrough WG, Sinard R, Cmelak AJ. Evolution of clinical trials in head and neck cancer. Crit. Rev. Oncol. Hematol. 2008 Nov 6; doi: 10.1016/j.critrevonc.2008.09.015. 2008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.