Abstract
Background:
A inverse correlation has been found between changes in ionized calcium concentrations and the addition of albumin in vitro, which may explain adverse cardiovascular effects attributed to exogenous albumin in vivo. The purpose of this investigation was to determine the interaction (if any) between exogenous 25% albumin administration (100 ml given over < 30 min) and calcium concentrations in patients, all but one of whom were in an intensive care unit.
Results:
There were no significant differences in the ionized calcium concentrations obtained before, at the end and 6 h after the administration of albumin (1.09 ± 0.23, 1.06 ± 0.22, 1.06 ± 0.21 mmol/l, respectively). Similarly, there were no significant differences in the total calcium concentrations between these same time periods (2.03 ± 0.18, 2.05 ± 0.20, 2.08 ± 0.23 mmol/l, respectively).
Conclusions:
In patients receiving infusions of 25% albumin, it appears that circulating calcium concentrations are well regulated by homeostatic mechanisms. Albumin infusions had no effect on calcium concentrations, although it is possible that temporary changes of questionable clinical importance may have occurred between measurement periods.
Keywords: albumin, albumin administration, calcium concentrations, adverse effects
Introduction
Three forms of calcium circulate in the blood: protein bound (primarily albumin), chelated and ionized. Ionized calcium constitutes 40-50% of total calcium [1,2]. This fraction is the physiologically active form of calcium, which is regulated by homeostasis [3]. Total calcium concentrations measure all three forms and thus may not accurately indicate the amount of ionized calcium in the blood. Changes in protein binding may affect the concentration of unbound, ionized calcium and binding affinity, which is not predictable from one individual to another [4]. Furthermore, binding can be altered in circumstances such as acid-base imbalance and pancreatitis [2]. Theoretically, the addition of exogenous albumin to the bloodstream would increase the number of binding sites for calcium, which could result in a lower ionized calcium concentration. Total calcium concentrations would not be expected to change as a result of this increased binding by albumin, assuming there were no concomitant changes in calcium distribution or elimination.
In a previous study [5] increasing amounts of albumin were added to human sera obtained from placental and cord venous samples in order to detect possible changes in ionized calcium concentrations. Twenty-five per cent albumin was added to each serum sample to achieve incremental concentrations from 0 to 2.0 g/dl. A significant decrease in serum ionized calcium concentrations upon increasing the albumin concentration from 1.0 to 2.0 g/dl was observed. The authors postulated that a decrease in serum ionized calcium concentration in vivo might be corrected by compensatory mechanisms involving parathyroid hormone, calcitriol, and calcitonin. How quickly and to what degree this compensation is effective has not yet been elucidated.
In addition to the in-vitro data, studies involving injured patients [6,7] have found decreases in calcium concentrations that appeared to be associated with albumin administration. The lower ionized calcium concentrations observed in patients receiving supplemental albumin were associated with decreases in left ventricular stroke work index. It was suggested that this decrease in stroke work index might have been at least partly responsible for some of the patients' heart failure and pulmonary edema. It is well established that hypocalcemia is relatively common in critically ill patients, although the incidence has ranged from 12 to 70% in published studies [2,8,9]. Furthermore, albumin infusions are frequently given in the critical care setting as a volume expander [10,11]. Therefore, fluctuations in ionized calcium concentrations due to exogenous albumin administration could place these patients at risk for symptomatic hypocalcemia. Although clinical features of hypocalcemia are often non-specific and may go undetected in milder forms, signs and symptoms of deficiency may include tetany, muscle weakness, parasthesias, adverse cardiovascular effects, bronchospasm, and confusion.
Despite the postulated interaction between albumin and calcium mentioned in published literature, a retrospective analysis of several patients in our intensive care unit did not reveal any changes in ionized calcium concentrations after 25% albumin infusions. This retrospective data collection was possible in some of our pre- and post-liver transplant patients who were having multiple arterial blood gas samples (with assessment of ionized calcium concentrations) drawn while receiving 25% albumin. Given the discrepancies noted between the published literature and patients at our institution, a prospective investigation was conducted to determine whether albumin infusions decreased ionized calcium concentrations in vivo. Because high concentrations of albumin infused as a bolus are more likely to result in a precipitous drop in ionized calcium concentrations, this study only included patients receiving infusions of 25% albumin 100 ml (25 g), over a period of less than 30 min.
Methods and materials
This prospective study was conducted at University Medical Center, a 360-bed tertiary care center located in Southern Arizona. Only English speaking patients who were at least 18 years of age and who received 100 ml 25% albumin (Baxter Healthcare Corporation, Glendale, California, USA) over a period less than 30 min were eligible for enrolment. Because parathyroid disease can cause ionized hypocalcemia, such patients were excluded. Also excluded were patients with acute renal failure, pancreatitis, and marked acid-base imbalances (pH < 7.35 or > 7.45), because these conditions have been associated with protein-calcium binding abnormalities that could affect ionized calcium concentrations. Finally, patients receiving medications that could cause acute changes in calcium or albumin concentrations (eg calcium or phosphate supplements, diuretics, or albumin-containing products) were excluded. Consent was obtained and each patient underwent a protocol previously approved by the institution's Human Subjects Committee.
All patients had indwelling arterial and venous catheters, so potential alterations in calcium concentrations related to the use of tourniquets were not an issue [9]. Because our laboratory performed automated blood chemistry studies, there were recordings for 18 measurements (eg blood urea nitrogen, creatinine, phosphorus, magnesium) in addition to the albumin and total calcium concentrations under direct investigation. From each individual we obtained arterial blood samples at the following three times: within 1h before albumin infusion (baseline), after the end of albumin infusion, and 6h after albumin infusion. At each time, we determined albumin, total calcium, and ionized calcium concentrations. One sample was collected for determination of albumin and total calcium concentrations measured using CX7 (Beckman, LaBrea, California, USA) in our main laboratory. Another sample was collected for determination of the ionized calcium concentration adjusted for pH of 7.4 and measured by ion-selective electrode Synthesis 25 (Instrumentation Laboratory, Lexington, Massachusetts) located in our blood gas laboratory. This institution's standard units and normal adult ranges are as follows: total calcium 2.13–2.65 mmol/l and albumin 3.7–5.0 g/dl. The normal range for ionized calcium at pH 7.4 is 1.12–1.23 mmol/l for adults. A sampling of 25% albumin was analyzed to determine whether the product itself might contain calcium. Calcium was undetectable in the commercially available 25% albumin product (testing methods were unable to detect concentrations < 2 mg/dl).
Information regarding each patient was collected, including age, sex, diagnoses and concomitant disease states, and concurrent medications. Patients were observed and charts were reviewed for evidence of adverse reactions during and shortly after the albumin infusion.
Statistical analysis
The differences in the ionized calcium, total calcium, and albumin concentrations before, at the end of, and 6 h after the albumin infusion were analyzed using repeated measures analysis of variance with significance defined as P < 0.05. Data are expressed as means ± standard deviation, unless otherwise noted.
Results
Nine patients were enroled in the prospective study. All of these patients were receiving the albumin infusions to maintain intravascular volume in the presence of third-spaced fluid accumulation. The albumin was typically infused over 15–20 min (< 30 min was required for study enrolment). It was presumed that a substantial portion of the intravascular expansion would result from fluid shifting caused by the oncotic actions of the 25% albumin solution. Table 1 contains the patient demographics and the patients' primary problems. The majority of enroled patients received the albumin infusions within 5days of major surgery.
Table 1.
Demographics and primary problems of patients in prospective study
| Age | |||
| Pt | (years) | Sex | Primary problem |
| 1 | 62 | Male | Complications of end-stage liver disease secondary to cirrhosis |
| 2 | 56 | Female | Intestinal complications secondary to disseminated malignancy |
| 3 | 84 | Male | Multiple trauma |
| 4 | 37 | Female | Post-liver transplant secondary to hepatitis C |
| 5 | 27 | Female | Post-liver transplant secondary to autoimmune hepatitis |
| 6 | 40 | Male | Complications of end-stage liver disease secondary to hepatitis C |
| 7 | 57 | Male | Multiple trauma and burns from motor vehicle accident |
| 8 | 39 | Male | Post-liver transplant secondary to alcoholic cirrhosis |
| 9 | 62 | Female | Perforated diverticulum with bowel resection and colostomy |
Pt, patient.
There were no significant differences in either the ionized or total calcium concentrations obtained before and after the administration of albumin (Figs 1 and 2). There was no obvious pattern to the changes that did occur in calcium concentrations. The largest decrease in ionized calcium concentrations that occurred in any of the patients was 0.23 mmol/l (1.06 mmol/l before and 0.83 mmol/l at the end of albumin infusion). The 6-h ionized calcium concentration in this patient was 0.96 mmol/l. In contrast, another patient had a baseline ionized calcium concentration of 0.91 mmol/l, which then increased to 0.93 and 0.99 mmol/l at the end of albumin infusion and 6 h after, respectively.
Figure 1.
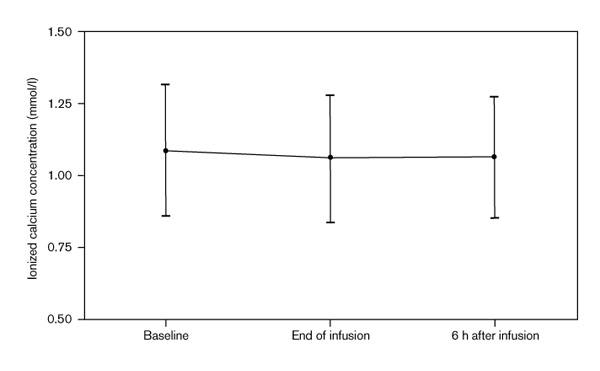
Ionized calcium concentrations before and after albumin infusions.
Figure 2.
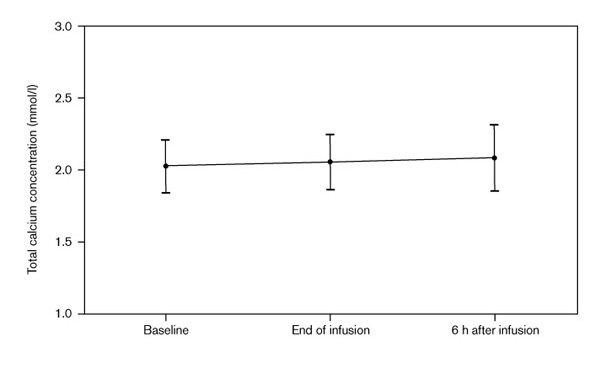
Total calcium concentrations before and after albumin infusions.
There were no changes detected in other parameters that might have influenced calcium concentrations. For example, there were no substantial changes in other laboratory variables such as phosphorus and magnesium. Although all but one of the patients was in the intensive care unit during the study, there were no acute events during the 6-h monitoring period that could potentially have interfered with the calcium measurements.
As expected, the mean albumin concentration did increase from the baseline measurement to the end of and 6 h after administration of the 25% albumin solution (Fig. 3), although the increase was not statistically significant.
Figure 3.
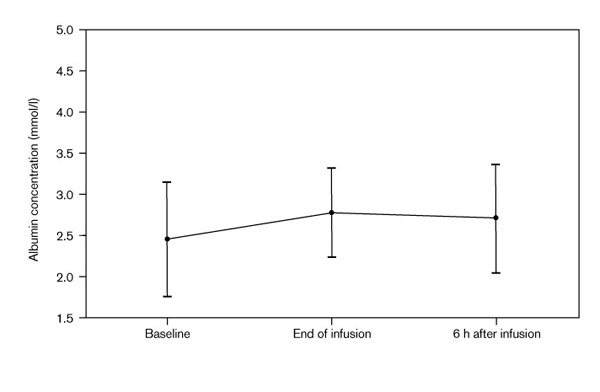
Albumin concentrations before and after albumin infusions.
Discussion
Although the appropriate indications for albumin are controversial, substantial amounts continue to be used, often inappropriately [10]. Using published guidelines, an observational study involving 15 academic medical centers [11] found that 62% of prescriptions for albumin were inappropriate, with an associated cost of US$124939. In addition to efficacy and cost, adverse effects should be considered when discussing therapeutic options.
The primary adverse effects associated with the use of albumin have been extensions of its pharmacologic actions (eg fluid overload resulting in pulmonary edema) [12]. Most of the remaining adverse effects attributed to albumin can be classified by either the involved system (immunologic, coagulation, renal, hepatic, respiratory, cardiovascular) or specific type of problem (metal loading, amino acid alterations). With regard to possible cardiovascular adverse effects, research conducted in the late 1970s and early 1980s on 94 injured patients [6,7] suggested a possible relationship between exogenous albumin administration and negative inotropic effects that were possibly related to alterations in calcium homeostasis. The patients were admitted to an emergency surgery service and were subsequently randomized to receive resuscitation regimens with or without supplemental albumin. The patients randomly allocated to receive supplemental albumin had higher total calcium concentrations (2.15 ± 0.30 mmol/l versus 2.03 ± 0.18 mmol/l; P < 0.025) and lower ionized calcium concentrations (0.91 ± 0.20 mmol/l versus 0.98 ± 0.10 mmol/l; P < 0.025) in the immediate postoperative period. The changes in ionized and total calcium concentrations were moderately correlated (r = 0.4) to changes in left ventricular stroke work index, and the investigators felt that this negative inotropic effect was at least partly responsible for the heart failure and pulmonary edema seen in some of the patients who were supplemented with albumin. Concerns arise as to comparability of the groups in such critically ill patients, however, given possible differences in the resuscitation regimens (eg administration of fresh frozen plasma that contains albumin and citrate, intravenous calcium injections). Also, the homeostatic changes relative to plasma albumin and calcium are complex as demonstrated by decreases in ionized and total calcium concentrations in hypoalbuminemic patients who have undergone cardiopulmonary bypass surgery [13].
Some of the best in-vitro data that support an effect of albumin on ionized calcium concentrations [5] involved the sequential addition of human serum albumin to placental and cord serum samples taken from healthy neonates. As concentrations of albumin added to the serum samples were increased, the ionized calcium concentrations decreased. This negative correlation between the changes in concentrations was significant (P < 0.001, r2 = 0.76) when univariate and multivariate analyses were used. The only other significant changes using multivariate analysis were a negative correlation noted between the change in ionized calcium concentrations compared with baseline ionized calcium concentrations, and a positive correlation noted between the change in ionized calcium concentrations and baseline albumin concentrations. As mentioned by the authors of that study [5], the most important question not answered by this investigation was whether normal in-vivo compensatory mechanisms (ie stimulation of parathyroid hormone secretion, increased production of vitamin D precursors, decreased calcitonin production) would offset the changes induced by albumin.
Our study was unable to find any effects of 25% albumin on either total or ionized calcium concentrations in patients, all but one of whom were in the intensive care unit. It is possible that larger doses of albumin may have an effect on ionized calcium concentrations, although the lack of substantial changes at these concentrations in the present study argues against this. Another study limitation was the relatively small number of enrolled patients and their associated illnesses. It is possible that significant changes in ionized calcium concentrations would be found if more patients were studied, but again the minor fluctuations in concentrations noted in this investigation seem to make this unlikely. We did not exclude patients on the basis of disease states that might affect calcium concentrations, unless the disease state directly affected the body's ability to compensate for hypocalcemia as might occur in patients with parathyroid or renal dysfunction. Despite differing primary diagnoses and secondary problems such as infection that commonly occur in intensive care unit patients, both the total and ionized calcium concentrations were remarkably stable over the measurement period.
One limitation of our study concerns the number of assessment points and the rate of albumin infusion. It was presumed that any effect on ionized calcium would be maximal at the end of the albumin infusion. The ionized calcium concentration was assessed at 6h after albumin infusion to determine whether the concentration was increasing towards normality if a substantial decrease had been found. It is possible, however, that decreases in the ionized calcium concentrations may have occurred during the albumin infusion or between the postinfusion measurement periods. The time period over which the albumin infusions were administered for study inclusion (ie 100 ml given over < 30 min) was based on typical infusion rates observed at the study hospital. This rate of infusion is faster than the 1ml/min rate commonly recommended in medication handbooks for 25% albumin [14]. It is possible that 100 ml infusions of 25% solution given very rapidly (eg < 5 min) might cause clinically important decreases in ionized calcium concentrations. Furthermore, if a more intensive blood sampling strategy had been used in this investigation, it is possible that substantial decreases in ionized calcium concentrations may have been observed. These hypotheses require further investigation. With regard to this study, it is suspected that transient decreases in the ionized calcium concentrations found with more intensive blood sampling would be of questionable clinical importance.
A critically low value for ionized calcium concentrations has been proposed on the basis of an investigation conducted in patients undergoing hepatic transplantation and a review of the literature [15], but the duration of the decrease needed to elicit overt problems is less clear. The authors suggested that the value should not be allowed to fall acutely to more than 0.4 mmol/l below the reference range mean in order to prevent a variety of adverse effects such as tetany, hypotension, and arrhythmias. Studies investigating mortality associated with hypocalcemia [4,9] have had disparate results.
In conclusion, this investigation did not find a significant interaction between 25% albumin administration and calcium concentrations. It is possible that significant decreases in ionized calcium concentrations could occur in populations not studied, and that these decreases could be clinically important in patients predisposed to suboptimal compensatory responses (eg in neonates). Such populations require further study. In adult patients, intensive monitoring of calcium concentrations does not appear warranted during administration of human serum albumin in doses of 25 g or less.
References
- Zaloga GP. Hypocalcemia in critically ill patients. Crit Care Med. 1992;20:251–262. doi: 10.1097/00003246-199202000-00014. [DOI] [PubMed] [Google Scholar]
- Desai TK, Carlson RW, Geheb MA. Prevalence and clinical implications of hypocalcemia in acutely ill patients in a medical intensive care setting. Am J Med. 1988;84:209–214. doi: 10.1016/0002-9343(88)90415-9. [DOI] [PubMed] [Google Scholar]
- McLean FC, Hastings AB. The state of calcium in the fluids of the body. J Biol Chem. 1935;108:285–322. [Google Scholar]
- Koch SM, Mehlhorn U, McKinley BA, Irby SL, Warters RD, Allen SJ. Arterial blood sampling devices influence ionized calcium measurements. Crit Care Med. 1995;23:1825–1828. doi: 10.1097/00003246-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Mimouni A, Mimouni F, Mimouni C, Mou S, Ho M. Effects of albumin on ionized calcium in vitro . Pediatr Emerg Care. 1991;7:149–151. doi: 10.1097/00006565-199106000-00004. [DOI] [PubMed] [Google Scholar]
- Dahn MS, Lucas CE, Ledgerwood AM, Higgins RF. Negative inotropic effect of albumin resuscitation for shock. Surgery. 1979;86:235–241. [PubMed] [Google Scholar]
- Kovalik SG, Ledgerwood AM, Lucas CE, Higgens RF. The cardiac effect of altered calcium homeostasis after albumin resuscitation. J Trauma. 1981;21:275–279. doi: 10.1097/00005373-198104000-00003. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Bredas P, Jankowski S, Kahn RJ. Correction of hypocalcaemia in the critically ill; what is the haemodynamic benifit? Intensive Care Med. 1995;21:838–841. doi: 10.1007/BF01700968. [DOI] [PubMed] [Google Scholar]
- Zaloga GP, Chernow B, Cook D, et al. Assessment of calcium homeostasis in the critically ill surgical patient. Ann Surg. 1985;202:587–594. doi: 10.1097/00000658-198511000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen LC, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus: the University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. . Arch Intern Med. 1995;155:373–379. doi: 10.1001/archinte.155.4.373. [DOI] [PubMed] [Google Scholar]
- Yim JM, Vermeulen LC, Erstad BL, et al. Albumin and nonprotein colloid solution use in US academic health centers. Arch Intern Med. 1995;155:2450–2455. [PubMed] [Google Scholar]
- Gales BJ, Erstad BL. Adverse reactions to human serum albumin. Ann Pharmacother. 1993;27:87–94. doi: 10.1177/106002809302700119. [DOI] [PubMed] [Google Scholar]
- Kaul TK, Bhatnagar NK, Mercer JL. Plasma albumin and calcium levels following cardiopulmonary bypass. Int J Artif Organs. 1989;12:461–465. [PubMed] [Google Scholar]
- Donnelly AJ, Cunningham FE, Baughman VL. Anesthesiology and Critical Care Drug Handbook Ohio: Lexi-Comp Inc; 1999. pp. 34–35.
- Kost GJ, Jammal MA, Ward RE, Safwat AM. Monitoring of ionized calcium during human hepatic transplantation. Am J Clin Pathol. 1986;86:61–70. doi: 10.1093/ajcp/86.1.61. [DOI] [PubMed] [Google Scholar]


