Abstract
Objectives
The objective of this study was to evaluate ternary methacrylate-thiol-ene systems, with varying thiol-ene content and thiol:ene stoichiometry, as dental restorative resin materials. It was hypothesized that an off-stoichiometric thiol-ene component would enhance interactions between the methacrylate and thiol-ene processes to reduce shrinkage stress while maintaining equivalent mechanical properties.
Methods
Polymerization kinetics and functional group conversions were determined by Fourier transform infrared spectroscopy (FTIR). Cured resin mechanical properties were evaluated using a three-point flexural test, carried out with a hydraulic universal test system. Polymerization shrinkage stress was measured with a tensometer coupled with simultaneous real-time conversion monitoring.
Results
The incorporation of thiol-ene mixtures as reactive diluents into conventional dimethacrylate resins previously was shown to combine synergistically advantageous methacrylate mechanical properties with the improved polymerization kinetics and reduced shrinkage stress of thiol-ene systems. In these systems, due to thiol consumption resultant from both the thiol-ene reaction and chain transfer involving the methacrylate polymerization, the optimum thiol:ene stoichiometry deviates from the traditional 1:1 ratio. Increasing the thiol:ene stoichiometry up to 3:1 results in systems with equivalent flexural modulus, 6 – 20 % reduced flexural strength, and 5 – 33 % reduced shrinkage stress relative to 1:1 stoichiometric thiol:ene systems.
Significance
Due to their improved overall functional group conversion, and shrinkage stress reduction while maintaining equivalent flexural modulus, methacrylate-thiol-ene resins, particularly those with excess thiol, beyond the conventional 1:1 thiol:ene molar ratio, yield superior dental restorative materials compared with purely dimethacrylate resins.
Introduction
The vast majority of resin-based composite (RBC) dental restoratives are comprised of dimethacrylate resins. These formulations are functional and have resulted in significant advancements in the field of dentistry. However, there are a number of properties of the resin chemistry that, if improved upon, would increase the performance, longevity and biocompatibility of composite restorations [1-3]. The most significant shortcomings of methacrylate-based resins are high volumetric shrinkage [4], high polymerization stress [5-7] and low functional group conversion [8-10]. Additionally, the residual monomer left in the restoration after curing is extractable and has the potential to leach from the restoration into the body, with undesirable consequences [9,10]. As one example, there is concern that residual monomers may cause allergic reactions and sensitization in patients [11]. Additionally, there is reason to believe that release of the most common reactive diluent, triethylene glycol dimethacrylate (TEGDMA), may also contribute to locally and systemically adverse effects from dental composites [8,12-14].
Upon polymerization, shrinkage stresses transferred to the tooth can cause deformation of the cusp or enamel microcracks [15-17], and stress at the tooth-composite interface may cause adhesive failure, initiation of microleakage and recurrent caries. In addition, significant increases in volumetric shrinkage and shrinkage stress are experienced when the double bond conversion is increased to reduce the leachable monomer [18]. This trade-off between conversion and shrinkage has been an inherent problem with composite restorative materials since their inception. Thus, it is important to develop rapidly curing dental restorative materials with improved monomer conversion and mechanical properties that reduce volumetric shrinkage and shrinkage stress.
The challenge and potential benefits associated with the development of new resin systems can be demonstrated with the recent introduction of the silorane resin-based composite by 3M/ESPE [19]. The methacrylate-free resin matrix relies on cationic ring-opening polymerization of oxirane monomers, which may result in a reduction in polymerization stress [20] primarily due to an inherently lower molar shrinkage value relative to methacrylates. The mechanical properties of the silorane composite are similar to those of dimethacrylate composites [21] and acceptable for use as a dental restorative materials.
Recently, thiol-enes have been investigated as alternatives to dimethacrylate-based RBCs [5,22]. Methacrylate systems polymerize via a radical chain growth polymerization mechanism. In contrast, traditional binary thiol-ene systems utilize ene monomers that alone do not readily engage in chain growth polymerization. The thiol-ene reactions proceed via a radical step growth addition mechanism that comprises the addition of a thiyl radical to an ene functional group, followed by chain transfer to a thiol, thus regenerating the thiyl radical [23-28]. Thiol-enes have advantageous curing kinetics, demonstrating rapid polymerization rates, high overall functional group conversion, and little sensitivity to oxygen inhibition [5,27,29-31]. Most importantly for dental restorative materials, thiol-enes exhibit reduced shrinkage and a delayed gel point conversion [32]. As a result of the delayed gel point, much of the shrinkage occurs prior to gelation, which dramatically reduces the shrinkage stress in the final polymer material [5,22] by allowing flow to accommodate the pre-gel shrinkage. Unfortunately, these previous studies have also shown that traditional binary thiol-ene systems exhibit limited flexural modulus and strength relative to dimethacrylate resins.
Methacrylate-thiol-ene systems exhibit a reaction mechanism that is a combination of both step and chain growth polymerizations [22,33,34]. Additionally, thiyl radicals exhibit an addition rate to methacrylates that is five times greater than the addition rate for allyl ethers [35]. The result of the difference in thiyl radical addition rates is that the early stages of the reaction are dominated by methacrylate homopolymerization and chain transfer and the latter stages of the reaction are dominated by thiol-ene polymerization [34]. The hybrid nature of the polymerization results in reduced shrinkage stress associated with chain transfer delaying gelation and the thiol-ene component remaining as a diluent throughout the initial stages of the reaction [22,34]. Employing thiol-enes as reactive diluents results in systems that combine many of the advantageous properties of both methacrylate and thiol-ene systems, while precluding the use of TEGDMA, which is rather hydrophilic and prone to leaching. The resulting methacrylate-thiol-ene resin systems exhibit equivalent mechanical properties for flexural modulus and strength, equivalent curing rates, increased methacrylate functional group conversion, and reduced shrinkage stress relative to the dimethacrylate control systems [22].
It is well known that for binary thiol-ene step growth polymerizations, the thiol and ene components must be present in a 1:1 stoichiometric ratio of functional groups to achieve complete conversion and maximize polymer mechanical properties [24,27,36,37). Thus, in all previous (meth)acrylate-thiol-ene studies, 1:1 thiol-ene stoichiometries were exclusively investigated [22,32,38-41]. However, in the cases where both (meth)acrylate and ene functional group conversions were resolved in FTIR, lower ene conversions were reported for many of these systems [22,34]. The lower ene conversion in ternary systems results from methacrylate and acrylate functional groups participating in reactions with both thiyl radicals and with methacrylate radicals [24,32,42]. Therefore, in ternary systems once chain transfer to the thiol has occurred, the thiyl radicals are capable or reacting with either the ene or (meth)acrylate species, while the ene functional groups typically only reacts with thiol functionalities. Thus, when a 1:1 thiol:ene mole ratio is utilized in ternary systems, the thiol functional groups become a limiting reagent, resulting in relatively low overall ene functional group conversion. This behavior suggests that a 1:1 thiol-ene ratio would not be optimum for ternary (meth)acrylate-thiol-ene systems and those systems with thiol to ene stoichiometric ratios greater than one would increase the functional group conversions of each component. Additionally, by incorporating more thiol content into the reaction, additional chain transfer will be prevalent, resulting in further delayed gelation and reduced shrinkage stress.
The thiol-ene reaction has numerous inherent advantageous properties for dental restorative materials. One of the primary drawbacks of thiol-ene chemistry is the odor associated with thiol monomers. In the case of the systems being evaluated here, the tetrafunctional thiol monomer has a relatively high molecular weight (488.66 g/mol) and thus a mild odor relative to other lower molecular weight thiol materials. Additionally, when utilized as a reactive diluent, the overall composition contains relatively little thiol monomer further minimizing any potential concerns regarding odor. When further mixed with fillers (70 wt%), methacrylate-thiol-ene composites exhibit no distinguishable odor resultant from the thiol monomer content.
This study further evaluates thiol-enes, particularly in off-stoichiometric molar ratios, as reactive diluents in dimethacrylate resins. Two different ene monomers were utilized, a commercially available triallyl ether (TATATO) and a trinorbornene (TMPTN) that was synthesized as previously reported [43]. Both of these monomers exhibited desirable mechanical properties and low shrinkage stress when evaluated previously in methacrylate-thiol-ene systems [22]. Here, these systems were studied in greater detail by curing with visible light and examining the effects of varying thiol-ene content and stoichiometry. Polymerization kinetics and overall functional group conversion, flexural modulus and strength, and polymerization shrinkage stress were evaluated and compared to control dimethacrylate resins under identical curing conditions. It was hypothesized that increased thiol-ene content and increased stoichiometric ratio of thiol to ene functional groups will result in increased overall functional group conversions and decreased polymerization shrinkage stress, while maintaining flexural properties.
Experimental Section
Materials
The tri-ene monomer triallyl-1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (TATATO) was purchased from Aldrich (Milwaukee, WI). The photoinitiator Irgacure 819 (I819) was donated by Ciba Specialty Chemicals (Tarrytown, NY). The inhibitor aluminum N-nitrosophenylhydroxylamine (Q1301) was donated by Wako Pure Chemicals (Osaka, Japan). 2,2-Bis[4-(2-hydroxy-3-methacryloyloxypropoxy)phenyl]propane (BisGMA), ethoxylated bisphenol-A dimethacrylate (EBPADMA), and triethylene glycol dimethacrylate (TEGDMA) were donated by Esstech Inc. (Essington, PA). Pentaerythritol tetra(3-mercaptopropionate) (PETMP) was donated by Evans Chemetics (Waterloo, NY). All chemicals were used as received. The norbornene monomer trimethylolpropane tri-(norborn-2-ene-5carboxylate) (TMPTN) was synthesized by the previously published procedures [22,43]. Chemical structures of all monomers utilized in this study are shown in Figure 1.
Figure 1.
Chemical structures of monomers utilized in this study.
Methods
Photopolymerization of samples for all analyses was conducted using 0.3 wt% I819 as the photoinitiator and 0.035 wt% Q1301 as inhibitor. Specimens were irradiated with 29 mW/cm2 of light from an EXFO Acticure (Mississauga, Ontario, Canada) with a 400 – 500 nm filter. Irradiation intensity was measured at the sample surface level with an International Light, Inc. Model IL1400A radiometer (Newburyport, MA).
Fourier Transform Infrared Spectroscopy (FTIR)
Kinetic analysis was conducted using a Nicolet 750 Magna FTIR spectrometer (Madison, WI) with a KBr beam splitter and an MCT/A detector. Series scans were recorded at a rate of approximately 2 scans per second until the reaction was complete, as indicated by the functional group absorption peak no longer decreasing. Experiments were conducted in the near infrared region (7000 – 4000 cm-1) with samples placed between glass slides with a 1.0 mm glass spacer. Functional group conversions were monitored utilizing the characteristic =C-H absorption peaks at 6164 cm-1 (methacrylate) and 6132 cm-1 (allyl ether). These methacrylate and allyl ether peaks are overlapped in the near infrared region. Thus, a Gaussian fitting peak deconvolution method was utilized to determine individual functional group conversions. Norbornene functional groups did not exhibit a strong enough absorption in the near infrared region to accurately determine functional group conversion. For each composition, experiments were performed in triplicate.
Flexural Modulus and Strength
Samples measuring 2 × 2 × 25 mm were prepared using Teflon molds and cured under identical conditions as in the FTIR analysis. Polymer flexural strength and modulus were calculated using data obtained from a 3-point flexural test, carried out with a hydraulic universal test system (858 Mini Bionix, MTS Systems Corporation, Eden Prairie, MN, USA) using a span width of 20 mm and a crosshead speed of 1 mm/min. For each material, at least three replicates were evaluated.
Shrinkage stress
Experiments were performed with a tensometer (American Dental Association Health Foundation), which monitors stress development using cantilever beam deflection theory [5]. Simultaneous conversion measurements were facilitated using remote near infrared transmitted through the specimen via fiber optic cables. Specimens were placed between 6 mm diameter glass rods with silane treated ends and measured 1.5 mm in thickness. The curing light was transmitted through the lower glass rod with a measured irradiation intensity of approximately 42 mW/cm2 at the tip of the glass rod. For each composition, experiments were performed in triplicate.
Statistical analysis
The experimental results were analyzed using one-way analysis of variance (ANOVA) for FTIR conversion measurements, shrinkage stress, and flexural modulus testing. Multiple pairwise comparisons were further conducted using Tukey's test or the Tukey-Kramer protocol with a significance level of 0.05.
Results
Polymerization Kinetics and Resin Conversion
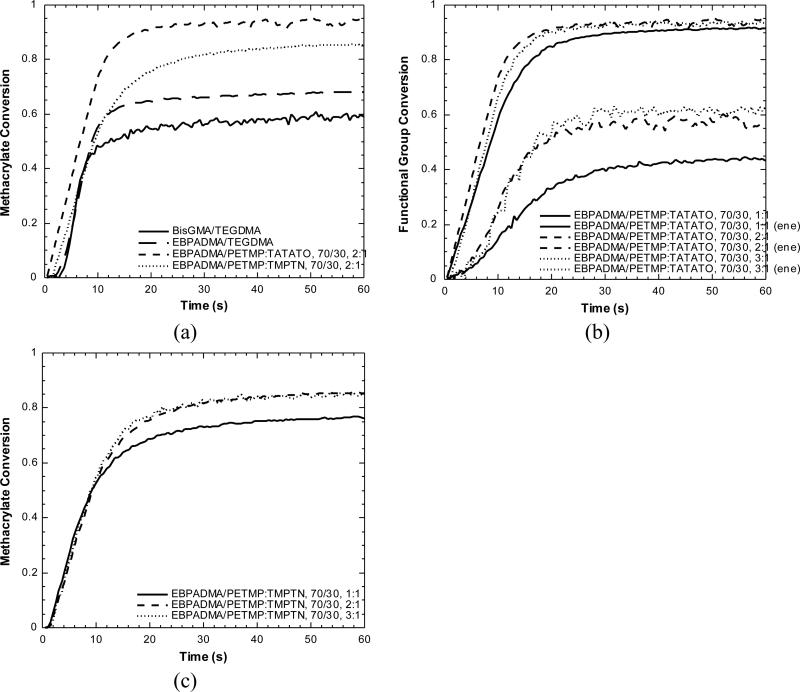
Polymerization kinetics were monitored for the methacrylate-thiol-ene and dimethacrylate control resins under identical curing conditions. Results are shown in Figure 2 and Table 1. The methacrylate-thiol-ene systems all exhibited increased methacrylate conversion relative to the control dimethacrylate systems. For the off-stoichiometric systems containing the same overall weight percent of thiol-ene resin, the ratio of thiol to ene functional groups was 1.5:1, 2:1, or 3:1. As the ratio of thiol to ene functional groups was increased in the EBPADMA/PETMP:TATATO 70/30 system, the ene functional group conversion increased from 45 ± 1 % for the 1:1 system to 61 ± 1 % for the 3:1 system. The ene functional groups polymerize more slowly than the methacrylate functional groups. This behavior is due to the pseudo two-stage reaction between methacrylates and thiol-enes and is consistent with previously observed results for methacrylate-thiol-ene systems [22,32]. Methacrylate functional group conversion also increased slightly from 92 ± 1 % to 95 ± 1 %. In the EBPADMA/PETMP:TATATO 60/40 system, the ene conversion reached 70 ± 1 % for the 2:1 and 81 ± 1 % for the 3:1 system and methacrylate functional group conversion increased to 96 ± 1 % in the 2:1 and 98 ± 1 % in the 3:1 system. The EBPADMA/PETMP:TMPTN also exhibited increased methacrylate functional group conversion as the thiol-ene ratio and content were increased. Norbornene functional group conversions were not resolvable. Kinetic results for norbornene containing resins in Figure 2c demonstrate nearly equivalent polymerization rates for all of the systems that were evaluated as indicated by similar conversion-time lines for each of these functional groups.
Figure 2.
Functional group conversions versus time for (a) BisGMA/TEGDMA, EBPADMA/TEGDMA, EBPADMA/PETMP:TATATO (70/30, 2:1), and EBPADMA/PETMP:TMPTN (70/30, 2:1), (b) EBPADMA/PETMP:TATATO, 70/30, 1:1, 2:1, and 3:1, and (c) EBPADMA/PETMP:TMPTN, 70/30, 1:1, 2:1, and 3:1. All samples contained 0.3 wt% Irgacure 819, 0.035 wt% Q1301, and were irradiated at 29 mW/cm2 with a 400-500 nm filter.
Table 1.
Final conversions for BisGMA/TEGDMA, EBPADMA/TEGDMA, EBPADMA/PETMP:TATATO, and EBPADMA/PETMP:TMPTN systems. All samples contained 0.3 wt% Irgacure 819, 0.035 wt% Q1301, and were irradiated at 29 mW/cm2 with a 400-500 nm filter. Within each column, the letters indicate statistically significant differences (P < 0.05) as determined by a one-way ANOVA and a Tukey post-hoc pair-wise comparison test.
| Formulation | Methacrylate/Thiol:Ene | Thiol:Ene Ratio | Methacrylate Conversion (%) | Ene Conversion (%) |
|---|---|---|---|---|
| BisGMA/TEGDMA | 100/0 | -- | 60 (1)a | -- |
| EBPADMA/TEGDMA | 100/0 | -- | 72 (1)b | -- |
| EBPADMA/PETMP:TATATO | 70/30 | 1:1 | 92 (1)e | 45 (1)a |
| 1.5:1 | 93 (1)e,f | 53 (1)b | ||
| 2:1 | 95 (1)f,g | 60 (1)c | ||
| 3:1 | 94 (1)e,f | 69 (1)d | ||
| EBPADMA/PETMP:TATATO | 60/40 | 2:1 | 97 (1)g,h | 75 (1)e |
| 3:1 | 98 (1)h | 85 (1)f | ||
| EBPADMA/PETMP:TMPTN | 70/30 | 1:1 | 79 (1)c | --- |
| 2:1 | 88 (1)d | --- | ||
| 3:1 | 86 (1)d | --- | ||
| EBPADMA/PETMP:TMPTN | 60/40 | 2:1 | 88 (1)d | --- |
Flexural Modulus and Strength
Results for flexural modulus and strength for methacrylate, methacrylate-thiol, and methacrylate-thiol-ene systems are shown in Table 2. The EBPADMA/PETMP:TATATO system with an initial 1:1 thiol:ene stoichiometric ratio exhibited equivalent flexural modulus and strength relative to the EBPADMA/TEGDMA control resin. Increasing the thiol:ene ratio in the 70/30 EBPADMA/PETMP:TATATO system did not have a statistically significant effect on the flexural modulus and slightly reduced the flexural strength. Increasing the thiol-ene content to 40% (EBPADMA/PETMP:TATATO 60/40) significantly reduced both the flexural modulus and flexural strength for the 2:1 system and resulted in dramatic reductions for the 3:1 system. The EBPADMA/PETMP:TMPTN 70/30 system with 1:1 thiol:ene stoichiometric ratio exhibited statistically equivalent flexural modulus and strength relative to the BisGMA/TEGDMA control resin and improved properties relative to the EBPADMA/TEGDMA control. Increasing the thiol:ene ratio did not have a statistically significant effect on the flexural modulus and slightly reduced the flexural strength. Increasing the thiol-ene content to 40% (EBPADMA/PETMP:TMPTN 60/40, 2:1) resulted in a system with an equivalent flexural modulus with a slightly reduced flexural strength relative to EBPADMA/TEGDMA.
Table 2.
Conversion and flexural modulus/strength data for control dimethacrylate, and methacrylate-thiol-ene systems. All samples contained 0.3 wt% Irgacure 819, 0.035 wt% Q1301, and were irradiated at 29 mW/cm2 with a 400-500 nm filter for 300 seconds. Within each column, the letters indicate statistically significant differences (P < 0.05) as determined by a one-way ANOVA and a Tukey post-hoc pair-wise comparison test.
| Formulation | Methacrylate/Thiol:Ene | Thiol:Ene | Methacrylate Conversion (%) | Flexural Modulus (GPa) | Flexural Strength (MPa) |
|---|---|---|---|---|---|
| BisGMA/TEGDMA | 100/0 | -- | 58 (1)a | 2.0 (0.1)d | 84 (2)f |
| EBPADMA/TEGDMA | 100/0 | -- | 71 (1)b | 1.7 (0.1)c,d | 71 (2)e |
| EBPADMA/PETMP:TATATO | 70/30 | 1:1 | 86 (1)e | 1.8 (0.1)c,d | 71 (3)e |
| 3:2 | f | 1.7 (0.1)c,d | 67 (4)d,e | ||
| 2:1 | 90 (1)f | 1.6 (0.2)c | 62 (4)c,d | ||
| 3:1 | 91 (1)f | 1.6 (0.2)c | 57 (2)c | ||
| EBPADMA/PETMP:TATATO | 60/40 | 2:1 | 94 (1)g | 1.2 (0.3)b | 45 (3)b |
| 3:1 | 97 (1)h | 0.4 (0.1)a | 24 (2)a | ||
| EBPADMA/PETMP:TMPTN | 70/30 | 1:1 | 72 (1)b | 2.0 (0.2)d | 79 (5)f |
| 2:1 | 80 (1)c | 1.7 (0.1)c,d | 69 (1)d,e | ||
| 3:1 | 80 (1)c | 1.9 (0.2)c,d | 62 (2)d | ||
| EBPADMA/PETMP:TMPTN | 60/40 | 2:1 | 83 (1)d | 1.8 (0.1)c,d | 64 (2)d |
Shrinkage Stress
Results for the shrinkage stress values for the range of different ternary formulations are shown in Table 3. The EBPADMA/PETMP:TATATO 70/30 systems all exhibited reduced shrinkage stress as compared to the control resins. As the thiol to ene functional group ratio was increased from 1:1 to 3:1, the shrinkage stress was further reduced from 2.1 ± 0.1 to 1.4 ± 0.1 MPa. The EBPADMA/PETMP:TATATO 60/40 systems exhibited even greater reductions in shrinkage stress than the 70/30 systems. However, these systems displayed significant reductions in flexural modulus and strength (Table 2), which would also serve to limit the shrinkage stress. The EBPADMA/PETMP:TMPTN systems also possessed reduced shrinkage stress relative to the control resins. For the 70/30 systems, the shrinkage stress values ranged from 1.8 ± 0.1 to 1.4 ± 0.1 MPa as the thiol to ene functional group ratio increased from 1:1 to 3:1. The EBPADMA/PETMP:TMPTN 60/40 system with a 2:1 ratio of thiol to norbornene functional groups exhibited the lowest shrinkage stress (for a system without a significant reduction in flexural modulus and strength) at 1.0 ± 0.1 MPa.
Table 3.
Polymerization shrinkage stress and functional group conversion. Samples were irradiated at 42 mW/cm2 as measured through 6 mm glass rods for 300 seconds with a 400-500nm filter. Shrinkage stress development was observed for an additional 300 seconds after irradiation. Within each column, the letters indicate statistically significant differences (P < 0.05) as determined by a one-way ANOVA and a Tukey post-hoc pair-wise comparison test.
| Formulation | Methacrylate/Thiol:Ene | Thiol:Ene Ratio | Methacrylate Conversion (%) | Shrinkage Stress (MPa) |
|---|---|---|---|---|
| BisGMA/TEGDMA | -- | -- | 73 (5)a | 3.3 (0.4)e |
| EBPADMA/TEGDMA | -- | -- | 82 (2)b | 3.8 (0.2)e |
| EBPADMA/PETMP:TATATO | 70/30 | 1:1 | 86 (1)c | 2.1 (0.1)d |
| 3:2 | 94 (1)f,g | 2.0 (0.2)d | ||
| 2:1 | 96 (1)g,h | 1.9 (0.1)d | ||
| 3:1 | 98 (1)h | 1.4 (0.1)b | ||
| EBPADMA/PETMP:TATATO | 60/40 | 2:1 | 97 (1)g,h | 1.3 (0.1)b |
| 3:1 | 99 (1)h | 1.0 (0.1)a | ||
| EBPADMA/PETMP:TMPTN | 70/30 | 1:1 | 87 (1)c,d | 1.8 (0.1)c,d |
| 2:1 | 90 (1)d,e | 1.4 (0.1)b | ||
| 3:1 | 92 (2)e,f | 1.5 (0.1)b,c | ||
| EBPADMA/PETMP:TMPTN | 60/40 | 2:1 | 94 (1)f,g | 1.0 (0.1)a |
Discussion
This work investigated a range of visible light cured methacrylate-thiol-ene systems. Relative to the BisGMA/TEGDMA control resin, methacrylate-thiol-ene systems exhibited equivalent cure speed and up to 24% increased methacrylate functional group conversion for the EBPADMA/PETMP:TATATO system and up to 17% increased methacrylate functional group conversion for the EBPADMA/PETMP:TMPTN system. Increasing the thiol-ene content or thiol-to-ene ratio increased the overall functional group conversions. It is hypothesized that the increased conversion will improve the long-term biocompatibility of these systems as dental restorative materials.
For the methacrylate-thiol-ene systems, increasing the thiol-to-ene stoichiometric ratio in both the systems containing TATATO and TMPTN reduced shrinkage stress without compromising flexural modulus. However, flexural strength was slightly reduced. In the methacrylate-thiol-ene systems, increasing the thiol-ene content from 30 to 40% resulted in further reductions in shrinkage stress. However, in the EBPADMA/PETMP:TATATO system there was also a significant drop in both flexural modulus and flexural strength. In the EBPADMA/PETMP:TMPTN system, increasing the thiol-ene content from 30 to 40% did not significantly impact flexural modulus or strength. Relative to the EBPADMA/TEGDMA control, the EBPADMA/PETMP:TATATO systems exhibited up to 50% reduced shrinkage stress without significant reductions in flexural modulus or strength. In the EBPADMA/PETMP:TMPTN system, up to 74% reduced shrinkage stress was achieved without significantly reducing flexural modulus or strength.
Conclusion
The methacrylate-thiol-ene systems reported in this work exhibited nearly equivalent polymerization rates while achieving higher overall functional group conversions as compared to the dimethacrylate control resins and to ternary thiol-ene-methacrylate resins with stoichiometric ratios of thiol:ene. Additionally, significant reductions in shrinkage stress were achieved while maintaining equivalent flexural modulus and near equivalent flexural strength relative to the controls. The combination of good flexural properties and reduced shrinkage stress in methacrylate-thiol-ene systems will likely result in superior overall dental restorative materials relative to traditional dimethacrylate resin systems.
Acknowledgments
NSF Grant 0626023, NIH/NIDCR Grants DE10959 and DE018233-0142, as well as an NIH Ruth Kirschstein Fellowship (F32DE019072) for K.M. Schreck, and Septodont Confi-Dental Products Division are gratefully acknowledged for funding this work. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi RL, Wiltbank BD, Murchison CF. Cure induced stresses and damage in particulate reinforced polymer matrix composites: a review of the scientific literature. Dent. Mater. 2005;21:43–46. doi: 10.1016/j.dental.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Dauvillier BS, Feilzer AJ, De Gee AJ, Davidson CL. Visco-elastic parameters of dental restorative materials during setting. J. Dent. Res. 2000;79:818–823. doi: 10.1177/00220345000790030601. [DOI] [PubMed] [Google Scholar]
- 3.Yourtee DM, Tong PY, Eick JD, Zhuang WC, Cobb C, Bean TA, Kostoryz EL. In Situ hybridization test for TNF-a: A simplified approach to confirming induction of the cytokine by biomaterials. In Vitro Toxicology. 1997;10:245–251. [Google Scholar]
- 4.Ferracane JL. Developing a more complete understanding of stresses produced in dental composites during polymerization. Dent. Mater. 2005;21:36–42. doi: 10.1016/j.dental.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Investigations of Step-Growth Thiol-Ene Polymerizations for Novel Dental Restoratives. Dent. Mater. 2005;21:1129–1136. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005;21:962–970. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Braga RR, Ferracane JL. Contraction stress related to degree of conversion and reaction kinetics. J. Dent. Res. 2002;81:114–118. [PubMed] [Google Scholar]
- 8.Darmani H, Al-Hiyasat AS. The effects of Bis-GMA and TEGDMA on female mouse fertility. Dent. Mater. 2006;22:353–358. doi: 10.1016/j.dental.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K, Tachino H, Tuchida K, Kubono K. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J. Mater. Sci. - Mater. Med. 2005;16:297–300. doi: 10.1007/s10856-005-0627-8. [DOI] [PubMed] [Google Scholar]
- 10.Pulgar R, Olea-Serrano MF, Novillo-Fertrell A, Rivas A, Pazos P, Pedraza V, Navajas JM, Olea N. Determination of bisphenol A and related aromatic compounds released from Bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000;108:21–27. doi: 10.1289/ehp.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spahl W, Budzikiewicz H, Geurtsen W. Extractable residual monomers from various resin materials — a qualitative study. J. Dent. Res. 1994;73:295. [Google Scholar]
- 12.Hansel C, Leyhausen G, Mai UEH, Geurtsen W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J. Dent. Res. 1998;77:60–67. doi: 10.1177/00220345980770010601. [DOI] [PubMed] [Google Scholar]
- 13.Englemann J, Leyhausen G, Leibfritz D, Geurtsen W. Metabolic effects of dental resin components in vitro detected by NMR spectroscopy. J. Dent. Res. 2001;80:869–875. doi: 10.1177/00220345010800030501. [DOI] [PubMed] [Google Scholar]
- 14.Schweikl H, Schmalz G. Triethylene glycol dimethacrylate induces large deletions in the hprt gene of V79 cells. Mutation Research-Genetic Toxicology and Environmental Mutagenesis. 1999;438:71–78. doi: 10.1016/s1383-5718(98)00164-8. [DOI] [PubMed] [Google Scholar]
- 15.Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J. Dent. Res. 1997;25:435–440. doi: 10.1016/s0300-5712(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 16.Suliman AA, Boyer DB, Lakes RS. Interferometric measurements of cusp deformation of teeth restored with composites. J. Dent. Res. 1993;72:1532–1536. doi: 10.1177/00220345930720111201. [DOI] [PubMed] [Google Scholar]
- 17.Suliman AA, Boyer DB, Lakes RS. Cusp movement in premolars resulting from composite polymerization shrinkage. Dent. Mater. 1993;9:6–10. doi: 10.1016/0109-5641(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Stansbury JW, Bowman CN. Towards the elucidation of shrinkage stress development and relaxation in dental composites. Dent. Mater. 2004;20:979–986. doi: 10.1016/j.dental.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21:68–74. doi: 10.1016/j.dental.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Van Ende A, De Munck J, Mine A, Lambrechts P, Van Meerbeek B. Does a low-shrinking composite induce less stress at the adhesive interface. Dent Mater. 2010;26:215–222. doi: 10.1016/j.dental.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Duarte S, Botta AC, Phark JH, Sadan A. Selected mechanical and physical properties and clinical application of a new low-shrinkage composite restoration. Quintessence International. 2009;40:631–638. [PubMed] [Google Scholar]
- 22.Cramer NB, Couch C, Schreck KM, Carioscia JA, Boulden JE, Stansbury JW, Bowman CN. Investigation of Thiol-Ene and Thiol-Ene-Methacrylate Based Resins as Dental Restorative Materials”. Dent. Mater. 2010;26(1):21–28. doi: 10.1016/j.dental.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobine AF. Radiation Curing in Polymer Science and Technology III. In: Fouassier JD, Rabek JF, editors. Polymerisation Mechanisms. Elsevier Applied Science; London: 1993. p. 219. Chapter 7. [Google Scholar]
- 24.Cramer NB, Bowman CN. Kinetics of thiol-ene and thiol-acrylate photopolymerizations with real-time fourier transform infrared. J. polym. sci., A, Polym. Chem. 2001;39:3311–3319. [Google Scholar]
- 25.Cramer NB, Davies T, O'Brien AK, Bowman CN. Mechanisms and Modeling of a Thiol-ene Photopolymerization. Macromolecules. 2003;36:4631–4636. [Google Scholar]
- 26.Cramer NB, Reddy SK, O'Brien AK, Bowman CN. Thiol-Ene Photopolymerization Mechanism and Rate Limiting Step Changes for Various Vinyl Functional Group Chemistries. Macromolecules. 2003;36:7964–7969. [Google Scholar]
- 27.Hoyle CE, Lee TY, Roper T. Thiol-Enes: Chemistry of the Past with Promise for the Future. J. polym. sci., A, Polym. Chem. 2004;42:5301–5338. [Google Scholar]
- 28.Reddy SK, Cramer NB, Bowman CN. Thiol-Vinyl Mechanisms I: Termination and Propagation Kinetics in Thiol-EnePhotopolymerizations. Macromolecules. 2006;39:3673–3680. [Google Scholar]
- 29.Cramer NB, Scott JP, Bowman CN. Photopolymerization of Thiol-ene Polymers without Photoinitiators. Macromolecules. 2002;35:5361–5365. [Google Scholar]
- 30.Hoyle CE, Cole M, Bachemin M, Kuang W, Kalyanaraman V, Jonsson S. Photoinitiated polymerization of selected thiol-ene systems. Photoinitiated Polymerization. ACS Symposium Series. 2003;847:52–64. [Google Scholar]
- 31.Roper TM, Guymon CA, Jonsson ES, Hoyle CE. Influence of the alkene structure on the mechanism and kinetics of thiol-alkene photopolymerizations with real-time infrared spectroscopy. J. Polym. Sci., A, Polym. Chem. 2004;24:6283–6298. [Google Scholar]
- 32.Chiou BS, Saad AK. Real-Time FTIR and in Situ Rheological Studies on the UV Curing Kinetics of Thiol-ene Polymers. Macromolecules. 1997;30:7322–7328. [Google Scholar]
- 33.Reddy SK, Cramer NB, Bowman CN. Thiol-Vinyl Mechanisms II: Kinetic Modeling of Ternary Thiol-Vinyl Photopolymerizations. Macromolecules. 2006;39:3681–3687. [Google Scholar]
- 34.Lee TY, Smith Z, Reddy SK, Cramer NB, Bowman CN. Thiol-Allyl ether-Methacrylate Ternary Systems. 1. Polymerization Mechanisms. Macromolecules. 2007;40:1466–1472. [Google Scholar]
- 35.Reddy SK, Cramer NB, Kalvaitas M, Lee TY, Bowman CN. Mechanistic Modelling and Network Properties of Ternary Thiol-Vinyl Photopolymerizations. Aust. J. Chem. 2006;59:586–593. [Google Scholar]
- 36.Morgan CR, Magnotta F, Ketley AD. Thiol/Ene Photocurable Polymers. J. Polym. Sci., A, Polym. Chem. 1977;15:627–645. [Google Scholar]
- 37.Jacobine AF, Glaser DM, Grabek PJ, Mancini D, Masterson M, Nakos ST, Rakas MA, Woods JG. Photocrosslinked norbornene thiol copolymers – synthesis, mechanical-properties, and cure studies. J. Appl. Polym. Sci. 1992;45:471–485. [Google Scholar]
- 38.Senyurt AF, Wei HY, Hoyle CE, Piland SG, Gould TE. Ternary Thiol-Ene/Acrylate Photopolymers: Effect of Acrylate Structure on Mechanical Properties. Macromolecules. 2007;40:4901–4909. [Google Scholar]
- 39.Wei HY, Senyurt AF, Jonsson S, Hoyle CE. Photopolymerization of Ternary Thiol-Ene/Acrylate Systems: Film and Network Properties. J. Polym. Sci., A, Polym. Chem. 2007;45:822–829. [Google Scholar]
- 40.Senyurt AF, Hoyle CE, Wei HY, Piland SG, Gould TE. Thermal and mechanical properties of cross-linked photopolymers based on multifunctional thiol-urethane ene monomers. Macromolecules. 2007;40:3174–3182. [Google Scholar]
- 41.Lee TY, Carioscia J, Smith Z, Bowman CN. Thiol-allyl ether-methacrylate ternary systems. Evolution mechanism of polymerization-induced shrinkage stress and mechanical properties. Macromolecules. 2007;40:1473–1479. [Google Scholar]
- 42.Lecamp L, Houllier F, Youssef B, Bunel C. Photoinitiated cross-linking of a thiol-methacrylate system. Polymer. 2001;42:2727–2736. [Google Scholar]
- 43.Carioscia JA, Schneidewind L, O'Brien C, Ely R, Feeser C, Cramer NB, Bowman CN. Thiol-Norbornene Materials: Approaches to Develop High Tg Thiol-Ene Polymers. J. Polym. Sci., A, Polym. Chem. 2007;45:5686–5696. [Google Scholar]