Abstract
Background:
The Portex Soft Seal high-volume, low-pressure cuffed tracheal tube was compared with the Mallinckrodt HiLo, Sheridan Preformed and Portex Profile tracheal tubes for leakage of dye placed in the subglottic space of a pig's trachea which was used in a benchtop mechanical ventilation model and in six isolated pig tracheas.
Results:
There was no leakage, either in the ventilation model or in the isolated tracheas in the Portex Soft Seal group. There was rapid leakage in the ventilation model and in all the isolated tracheas for the Mallinckrodt HiLo, and five out of six isolated tracheas for the Sheridan Preformed and the Portex Profile group.
Conclusions:
This benchtop study suggests that the improved compliance characteristics of the Portex Soft Seal cuff are beneficial in preventing leakage of fluid in these models.
Keywords: aspiration, cuff, intratracheal, intubation, pneumonia, ventilator-associated pneumonia
Introduction
Leakage of infected oropharyngeal secretions occurs past tracheal tube cuffs in critically ill, mechanically ventilated patients. This is the leading cause of tracheobronchial colonization and ventilator-associated pneumonia (VAP) [1].
A tracheal tube with a high-volume, low-pressure (HVLP) cuff does not protect the lower airway from contamination by material leaking from the subglottis [2,3]. This leakage occurs down longitudinal channels caused by folds in the cuff wall material [3,4]. These folds always occur in a HVLP cuff inflated within a trachea, because the diameter of the cuff must be greater than that of the trachea for the intracuff pressure to be equal to the tracheal wall pressure. The Portex Soft Seal cuff (Portex Ltd, Hythe, UK) is made of a more compliant material than are traditional HVLP cuffs. Once inflated within the trachea the improved compliance might lead to the elimination of the folds in the cuff wall for a full circumference, thereby protecting against aspiration.
This study compares the Portex Soft Seal with three standard HVLP cuffed tracheal tubes for leakage of dye past the cuff in a benchtop model of mechanical ventilation, in a rigid cylinder and in isolated pig tracheas with dimensions across the human tracheal diameter range.
Materials and methods
Static pig trachea model
Nine centimetre lengths of six pig tracheas harvested within 24 h of slaughter were chosen to span the range of diameters for the human trachea, for which a size 8 mm internal diameter tube would be used. The range for human tracheal diameter calculated from an autopsy study [5] was 1.4-2.7 cm, and the mean diameter of a female trachea was 1.84 cm. We felt that it would be reasonable to take 1.4-2.5 cm as an estimate for tracheal size appropriate for a size 8 mm internal diameter tube (ie smaller adults are often intubated with 8 mm internal diameter tube and large adults with a size 9mm internal diameter tube). A 2 cm diameter rigid cylinder (20 ml syringe barrel, Plastipak; Becton Dickinson, Drogheda, Ireland) was also used.
The cylinder and all of the tracheas were suspended vertically and were sequentially intubated by one of the investigators, so that the cuff centre was 4 cm below the upper tracheal edge, in a random order (closed envelope technique). The cuff pressure was set at 30 cmH2O with a commercial cuff inflator (Portex). The cuff inflator had been checked against a mercury column to confirm accuracy. The tube was hidden so that the observer was blinded to the tube type used. A stopwatch was started once 3.5 ml blue dyed water had been placed above the cuff. The observer stopped the watch when dye was first observed to drip from the trachea below. If no dye had leaked after 15 min, then this was recorded as no leak.
Dynamic lung/trachea model
The patient model consisted of a model lung that has been described previously (Fig 1) [4], but modified so that instead of a silicone trachea a 9-cm section of pig trachea (anteroposterior diameter 1.6 cm, lateral diameter 1.9 cm, measured using a Vernier caliper) was used. The pig trachea was secured by inserting the tubing 1.5 cm into the lower end of the tracheal lumen and binding it tightly with circumferential elastic bands. The trachea lay at 60° to the horizontal.
Figure 1.
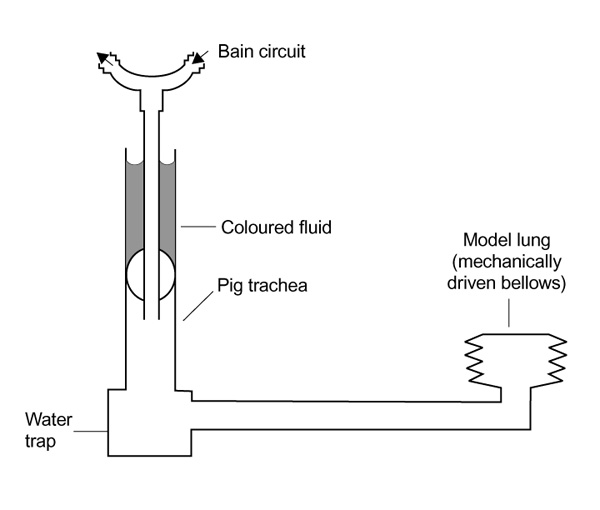
Dynamic lung/trachea model.
The tracheas were intubated in a random order and the upper edge of the cuff lay 3 cm below the upper end of the tracheal segment. The proximal tracheal tube was connected to a Nuffield Penlon ventilator (Penlon Ltd, Abingdon, Oxford, UK) and a Bain breathing system (Intersurgical, Wokingham, UK). The inspiration time was set at 2s and the expiration time at 4s with an inspiration flow rate of 0.375 l/s. The compliance of the lung was adjusted to cause a peak inspiratory pressure of 20 cmH2O.
Blue dyed water (3.5 ml) was instilled above the cuff by one investigator, and an observer blinded to the tube type observed the lower trachea for leakage for 15 min. If no leakage had occurred after 15 min then the cuff was tested by suctioning the trachea for 30s through a 14 CH tracheal suction catheter placed just distal to the tracheal tube tip. The cuffs were also tested for leakage associated with movement of the cuff within the trachea through standardized 2 cm proximal to distal and 45° rotation manoeuvres.
Tubes evaluated
Size 8 mm internal diameter Portex Soft Seal, Portex Profile, Mallinckrodt HiLo (Mallinckrodt Medical GmbH, Hennef/Sieg, Germany) and Sheridan Preformed tubes (Kendall Sheridan, Argyle, New York, USA) were tested.
Results
All cuffs leaked in the rigid 2cm diameter cylinder model (Table 1). There was no leakage in the static and dynamic pig trachea models with the Portex Soft Seal cuff. There was rapid leakage in the dynamic model for the Mallinckrodt HiLo, the Sheridan Preformed and the Portex Profile group. There was leakage in the static model for the Mallinckrodt HiLo, and in five out of six tracheas for the Sheridan Preformed and the Portex Profile group. This difference was statistically significant (P < 0.05, Fisher's exact test).
Table 1.
The effect of cuff type on leakage past the tracheal tube cuff in a static and a dynamic pig trachea model
| Rigid | Isolated | Simulated | Tube | ||
| trachea | pig trachea | Simulated | tracheal | motion in | |
| (2cm) | (n =6) | IPPV | suction | trachea | |
| Mallinckrodt Hilo | Leak | Leak 6/6 | Leak | Leak | Leak |
| Sheridan Preformed | Leak | Leak 5/6 | Leak | Leak | Leak |
| Portex Profile | Leak | Leak 5/6 | Leak | Leak | Leak |
| Portex Soft Seal | Leak | Leak 0/6 | No leak | No leak | No leak |
The six tracheas in the static model had a mean lateral diameter of 1.76 cm (range 1.4-2.45 cm) and a mean anteroposterior diameter of 1.8 cm (range 1.5-2.5 cm).
Discussion
Leakage past the tracheal tube cuff is the major cause of VAP in the critically ill [1]. The acquisition of a VAP involves a number of factors, including altered host defences, colonization with pathogenic bacteria in the aerodigestive tract, and high exposure of lung tissue to these bacteria [6]. Critically ill adults receiving mechanical ventilation have an incidence of VAP between 20 and 60%, and an attributable mortality reported at 27% [7]. A tube that eliminates leakage from the pharynx to the lungs could have an important role in improving the safety of mechanical ventilation. Leakage past an adequately inflated HVLP tracheal tube cuff occurs exclusively along the folds within the cuff wall.
One of the six tracheas did not permit leakage with the Portex Profile and the Sheridan Preformed tube in the static model. This trachea was at the upper end of the tracheal diameter range for a size 8 mm internal diameter cuffed tube (2.5 cm anteroposterior diameter and 2.4 cm lateral diameter). After cuff inflation, if a trachea happens to have a diameter that closely matches that of the cuff then folding of the cuff material may not occur, eliminating the formation of channels, and leakage is prevented. If this occurs in the clinical situation then a higher cuff pressure is likely to be necessary to prevent an air leak.
The increased compliance of the Portex Soft Seal cuff is demonstrated in Figure 2. The mechanism by which the more compliant Portex Soft Seal cuff protects against leakage is the elimination of the folds in the cuff wall for a full circumference of the trachea/cuff contact zone, effectively damming the channels. This does not happen with traditional HVLP cuffs, because they are made of poorly compliant material.
Figure 2.
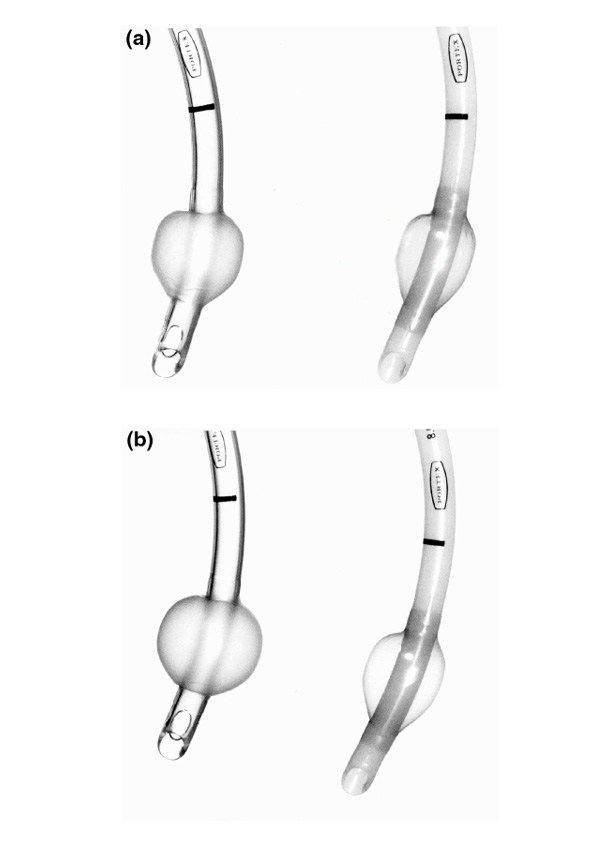
Portex Profile (right) and Soft Seal (left) inflated to (a) 30 cmH2O and (b) 90 cmH2O. Increased distension of the Soft Seal due to increased compliance of the cuff material is seen.
The present study shows that the Portex Soft Seal tracheal tube cuff effectively prevents leakage of subglottic fluid to the lungs in the isolated pig trachea static and dynamic models. Notably, the Soft Seal cuff did not prevent leakage in a rigid cylinder. This was due to the folds in the cuff wall traversing the narrow circumferential compliant protective band. The Soft Seal cuff was effective in the excised pig trachea static and dynamic models, however. This difference might be due to the increased compliance of the trachea compared with that of the rigid model. A compliant trachea would increase the length of the circumferential protective band as the trachea changes its shape to conform to that of the cuff. The excised trachea is likely to be more compliant than the in-situ human trachea, and clinical studies are underway to assess the protective efficiency of the Soft Seal cuff in vivo. The observation period of this study was restricted to 15 min with strict control of cuff pressure. Even if a cuff prevents aspiration at 30 cmH2O intracuff pressure, in clinical practice cuff pressure can frequently fall and a low cuff pressure is associated with an increased risk of VAP [8]. It is of no use to prevent aspiration most of the time, only to allow an intermittent loss of cuff pressure and hence aspiration. Constant pressure inflation devices such as the Cardiff Cuff Controller [9] and more recently the Tracoe cuff inflation unit (Tracoe Gesellschaft für medizinische Bedarfsgegenstände mbH, Neu-Isenburg, Germany) have been designed to maintain intracuff pressure. A cuff that prevents aspiration should perhaps always be used with a constant pressure inflation device to prevent aspiration continuously.
The measurement of inspiratory and expiratory tidal volumes would have offered additional information regarding air leak. In a previous study using a similar model, however, no difference was detectable between the inspiratory and expiratory tidal volume monitors [4]. In clinical practice the detection of aspiration of dye added to enteral feed has been shown to have poor sensitivity [10]. The addition of dye to gastric feed has been shown to have 13% sensitivity relative to a glucose oxidase test strip method [11], but this could be an underestimate because tracheal secretions can contain high glucose concentrations independent of enteral nutrition. Studies using small volumes of dye applied to the tongue have demonstrated low aspiration rates of 20% with HVLP cuffs [12]. If dye is added directly to the subglottic space and aspiration detected bronchoscopically, however, then leakage rates of 100% have been demonstrated [3]. The peak inspiratory pressure was set to 20 cmH2O in the present model. This would be a reasonable pressure to simulate ventilation in many intensive care patients without acute lung injury, but we did not test higher peak pressures that might be more appropriate for patients with decreased lung compliance. An increase in mean tracheal pressures has previously been shown in this model to reduce the rate of cuff leakage, but not to eliminate aspiration [4].
In conclusion, the present study shows that the Portex Soft Seal tracheal tube cuff effectively prevents leakage of subglottic fluid to the lungs in the isolated pig trachea static and dynamic models. The outcome of a clinical randomized controlled trial is required before recommendations can be made.
References
- Craven DE. Prevention of hospital-acquired pneumonia: measuring effect in ounces, pounds, and tons. Ann Intern Med. 1995;122:229–231. doi: 10.7326/0003-4819-122-3-199502010-00013. [DOI] [PubMed] [Google Scholar]
- Pavlin EG, Van Nimwegan D, Hornbein TF. Failure of a high-compliance low-pressure cuff to prevent aspiration. . Anesthesiology. 1975;42:216–219. doi: 10.1097/00000542-197502000-00019. [DOI] [PubMed] [Google Scholar]
- Seegobin RD, Van Hasselt GL. Aspiration beyond endotracheal cuffs. Can Anaesth Soc J. 1986;33:273–279. doi: 10.1007/BF03010737. [DOI] [PubMed] [Google Scholar]
- Young PJ, Rollinson M, Downward G, Henderson S. Leakage of fluid past the tracheal tube cuff in a benchtop model. Br J Anaesth. 1997;78:557–562. doi: 10.1093/bja/78.5.557. [DOI] [PubMed] [Google Scholar]
- Mehta S, Myat HM. The cross-sectional shape and circumference of the human trachea. Ann R Coll Surg Engl. 1984;66:356–358. [PMC free article] [PubMed] [Google Scholar]
- Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoculation. Intens Care Med. 1995;21:365–383. doi: 10.1007/BF01705418. [DOI] [PubMed] [Google Scholar]
- Fagon JY, Chastre J, Hance AJ, et al. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- Rello J, Sonora R, Jubert P, Artigas A, Valles J. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med. 1996;154:111–115. doi: 10.1164/ajrccm.154.1.8680665. [DOI] [PubMed] [Google Scholar]
- Willis BA, Latto IP, Dyson A. Tracheal tube cuff pressure: clinical use of the Cardiff Cuff Controller. Anaesthesia. 1988;43:312–314. [PubMed] [Google Scholar]
- Potts RG, Zaroukian MH, Guerrero PA, Baker CD. Comparison of blue dye visualization and glucose oxidase test strip methods for detecting pulmonary aspiration of enteral feedings in intubated adults. Chest . 1993;103:117–121. doi: 10.1378/chest.103.1.117. [DOI] [PubMed] [Google Scholar]
- Kinsey GC, Murray MJ, Swensen SJ, Miles JM. Glucose content of tracheal aspirates: implications for the detection of tube feeding aspiration. Crit Care Med. 1994;22:1557–1562. [PubMed] [Google Scholar]
- Spray SB, Zuidema GD, Cameron JL. Aspiration pneumonia; incidence of aspiration with endotracheal tubes. Am J Surg. 1976;131:701–703. doi: 10.1016/0002-9610(76)90181-1. [DOI] [PubMed] [Google Scholar]


