Abstract
Purpose: The main purpose of this work was to compare peripheral doses absorbed during stereotactic treatment of a brain lesion delivered using different devices. These data were used to estimate the risk of stochastic effects.
Methods: Treatment plans were created for an anthropomorphic phantom and delivered using a LINAC with stereotactic cones and a multileaf collimator, a CyberKnife® system (before and after a supplemental shielding was applied), a TomoTherapy® system, and a Gamma Knife® unit. For each treatment, 5 Gy were prescribed to the target. Measurements were performed with thermoluminescent dosimeters inserted roughly in the position of the thyroid, sternum, upper lung, lower lung, and gonads.
Results: Mean doses ranged from of 4.1 (Gamma Knife) to 62.8 mGy (LINAC with cones) in the thyroid, from 2.3 (TomoTherapy) to 30 mGy (preshielding CyberKnife) in the sternum, from 1.7 (TomoTherapy) to 20 mGy (preshielding CyberKnife) in the upper part of the lungs, from 0.98 (Gamma Knife) to 15 mGy (preshielding CyberKnife) in the lower part of the lungs, and between 0.3 (Gamma Knife) and 10 mGy (preshielding CyberKnife) in the gonads.
Conclusions: The peripheral dose absorbed in the sites of interest with a 5 Gy fraction is low. Although the risk of adverse side effects calculated for 20 Gy delivered in 5 Gy fractions is negligible, in the interest of optimum patient radioprotection, further studies are needed to determine the weight of each contributor to the peripheral dose.
Keywords: stereotactic radiotherapy (SRT), peripheral dose, multileaf collimator, CyberKnife®, Gamma Knife®
INTRODUCTION
In radiation therapy, a certain portion of the dose is always deposited outside of the radiation field. Estimation of this dose, referred to as the “peripheral dose,” may be important in patients with long life expectancy after treatment, especially when the dose affects healthy anatomical structures with low tolerance to radiation.1, 2, 3
Ionizing radiation’s deleterious effects have been recognized and documented since the discovery of radioactivity. Very high doses received in short times lead to rapid manifestation of functional alterations and organic lesions. Epidemiological evidence of increased risk of developing cancer after long term exposure to total doses higher than 50–100 mSv or acute exposure to doses higher than 10–50 mSv does exist. The effect of absorbing lower doses (<10–50 mSv), however, is still controversial.4 The scarcity of events encountered upon exposure to low-dose radiation requires a very large sample size to demonstrate statistical significance. Indeed, extrapolating results from events of exposure to higher doses and assigning the absorbed dose to an individual pose further difficulties in estimating risk.5 Despite the difficulties, several studies in the literature do underscore the consistency of the models predicting that low doses of radiation can cause a small increase in risk.4, 6, 7
In regard to radiation treatments, a knowledge of the doses to be deposited outside the treatment volume beforehand may allow a risk estimate of detrimental effects and, if possible, precautions to minimize the probability of developing them. To this end, several studies have been carried out with the aim of identifying different components of peripheral dose and the possible actions that can be taken to minimize the risk associated with these peripheral doses.1, 8, 9, 10, 11, 12, 13, 14, 15
Pre-estimation of peripheral dose during a given treatment is difficult because it depends on myriad factors and variables.
The main components of peripheral dose are leakage from LINAC head, scattered radiation from collimators and leaves, and scattered radiation within the patient. The first two contributors depend on the technique employed, as well as the device and its layout. Scattered radiation within the patient, in turn, depends on radiation energy, the distance from the edges of the field, and, to a lesser extent, the field size.
Stereotactic radiotherapy and radiosurgery are able to concentrate accurately the dose within the target, sparing the surrounding normal tissue; they are employed in the treatment of small lesions. The dose concentration within small volumes is achieved through devices that shield an important amount of the radiation produced in the accelerator head. To deliver the same dose to the lesion, stereotactic treatments require a larger number of monitor units (MUs) compared to conventional treatments. This fact leads to an increase in leakage and scattered radiation from the head of the machine and, consequently, to an increase in the whole body dose.
Beams entering and exiting the target contribute to the dose deposition in healthy tissues as well, particularly devices that employ many noncoplanar beams, such as the CyberKnife® (Accuray Inc., Sunnyvale, CA) and Gamma Knife® (Elekta AB, Stockholm, Sweden), which expose a large amount of healthy tissue even considerably far from the target to low doses. The dose contribution from the primary beams entering and exiting the target are considered a part of the peripheral dose.
The purpose of this work was to measure the peripheral dose and the dose to the organs at risk (OARs) absorbed during the treatment of an intracranial lesion.
MATERIALS AND METHODS
Treatment plans
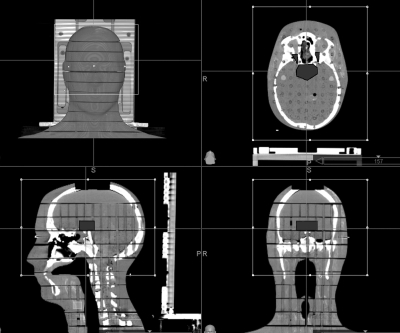
Six treatment plans were created using a male anthropomorphic phantom, Rando® Phantom (Alderson Research Laboratories, INC., Stanford, CA) and delivered with a 6 MV LINAC, a CyberKnife radiosurgery unit (Accuray Inc.), a TomoTherapy® system (TomoTherapy Inc., Madison, WI) and a Gamma Knife unit. The phantom is transected horizontally into 2.5 cm thick slices. To create CyberKnife and TomoTherapy treatment plans, a CT scan of the first ten slices of the phantom (corresponding to the head and neck areas, which is the usual field of view of the CT for an encephalic treatment) was acquired (CT slice thickness: 1.25 mm). To create LINAC and Gamma Knife treatment plans, the same scan was repeated by applying to the head of the phantom a CRW™ localization system (Integra Radionics, Burlington, MA) and a Leksell® stereotactic frame (Elekta AB, Stockholm, Sweden), respectively. The same artificial irregularly shaped brain volume, at the sellar level, was drawn on each CT scan and considered as the target. Maximum target dimensions were 17 mm in the craniocaudal direction, 39 mm in the lateral direction, and 29 mm in the anteroposterior direction, with a volume of 15 cc. Optic nerves and thyroid were drawn on the CT scans too. The thyroid was considered a constraint volume when inverse planning has been used (see Fig. 1) and we disallowed all the beams directly passing through it. For each treatment, 5 Gy were prescribed to the target. The prescription dose was chosen according to the range of linear response of thermoluminescent dosimeters, as indicated by the manufacturer (about 0.2 mGy–10 Gy). The same dose in Gy was given to the peripheral isodose surface, regardless of the percentage values of this surface. The prescription isodose line was different and typical of each delivery technique (70% for LINAC and CyberKnife, 95% for TomoTherapy, and 50% for Gamma Knife). Each treatment was optimized according to the delivery technique to have a target coverage at least 95% and trying to limit optical nerves dose (it was not possible to establish a specific maximum constraint to these organs due to the proximity of the target by these structures because it would have determined an underdosage of the lesion itself).
Figure 1.
Target and critical structures used for planning.
Peripheral doses, dose to the OARs (optic nerves) and the target coverage [the percentage of planning target volume (PTV) covered by the prescribed dose] of each treatment were compared.
Thermoluminescent dosimeters
For measurements, we used thermoluminescent dosimeters LiF TLD 100 (Harshaw, Solon, OH), suitable for insertion inside the phantom, which produced a response independent from the angle of incidence of the radiation and the dose rate.
The TLD crystal’s characterization (measurement of the intrinsic sensitivity factor and calibration) and readings were performed at the Laboratory of Radioprotection of CESNEF (Politecnico of Milan). Each group of TLD crystals was exposed to a 137Cs source of known activity for different time intervals. A correction factor for the energy dependence of the TLDs’ response was applied to derive the dose in the 6 MV photon. Once the exposure values were known for each dosimeter, 20 crystals were selected and used to calculate the sensitivity factor. For all the other crystals, the ratio between the value obtained for each and the average value of the reference dosimeters was used as the sensitivity factor. Five crystals were read for each exposure interval, the average of which, as a function of exposure, helped define the calibration curve. The value of the intrinsic sensitivity factor calculated for each crystal was close to 1 (range: 0.940–1.173).
The readings were performed using a Harshaw 5500 reader (Harshaw, Harshaw Chemical Company, Cleveland, OH). The total uncertainty declared by the Laboratory in the single reading was near 5%. Two nonirradiated dosimeters were used to evaluate the local environmental background.
Preparation of the phantom
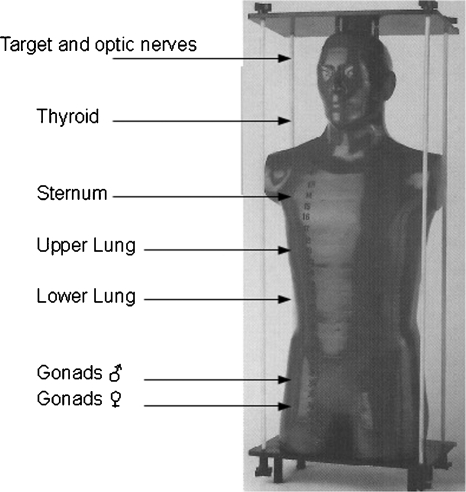
Measurements were performed with dosimeters inserted at various depths inside the phantom. Two TLDs wrapped in aluminum foil were placed at distances between 15.5 and 80.5 cm from the geometric center of the target in the craniocaudal direction, at roughly the locations of the thyroid, the sternum, the upper part of the lung, the lower part of the lung, the ovaries, and the testes. TLDs were also placed within the target and in the position of optic nerves.
The uncertainty in the TLDs positioning was evaluated to be within 2 mm. (see Fig. 2).
Figure 2.
TLDs positions in Rando® Phantom.
LINAC
The linear accelerator used to deliver the treatment was a 6 MV Philips SL 75–5 (Elekta) installed at Foundation IRCCS “Carlo Besta” of Milan.
Two treatments were delivered using noncoplanar arcs. In the first, the beam was collimated with a multileaf collimator (mMLC, 3DLine; leaf projection at the isocenter: 4 mm); in the second, a 25 mm diameter circular collimator (Radionics, Integra Radionics, Burlington, MA) was used. The treatment plans were created with the Ergo treatment planning system (TPS) (3DLine). Four noncoplanar arcs were employed, each covering 90° around the target volume, with the couch positioned at 10°, 70°, 290°, and 350° [International Electrotechnical Commission (IEC) scale). The orientation of the arcs was so chosen that the exiting beams could not pass through the thyroid.
Three isocenters were set up to achieve homogeneous target coverage in the treatment with the cone collimator because of the irregular shape of the lesion.
CyberKnife
CyberKnife system consists of a compact 6 MV linear accelerator mounted on a robotic arm, which allows for the delivery of stereotactic treatments guided by a series of x-ray images. Circular collimators with diameters between 5 and 60 mm can be applied to the LINAC. The robotic arm moves the LINAC with six degrees of freedom, allowing a very high dose conformity.
The CyberKnife system used to deliver the treatment was installed at “Centro Diagnostico Italiano” of Milan. The treatment plan was developed with the TPS Multiplan® (Accuray). The treatment is nonisocentric, and a 20 mm collimator was used. The prescribed dose required 2030 MUs (100 MU output delivers a 100 cGy dose at build up and at 80 cm from the focus). During the treatment, ten localization x rays of the phantom were acquired. Previous measurements reported by Petti et al.2 assessed that the tracking x rays’ contribution to the peripheral dose was negligible. Beams passing through the thyroid were disallowed.
In 2006, the manufacturer of the CyberKnife introduced additional shielding to prevent the radiation leakage that was found to be higher than other systems. This shielding has been installed as standard on all new CyberKnife system since June 2006 and is retrofitted to older systems. Therefore, another treatment plan was generated and delivered, this time using the system with the additional shielding. This plan required 2530 MU to deliver the prescribed dose. No beams were allowed through the thyroid in this case either. It was not possible to use the previous plan because the application of the supplemental shielding had made changes in treatment planning and delivery systems, which required the creation of a new plan.
TomoTherapy
Helical TomoTherapy integrates LINAC and CT technology to deliver intensity-modulated radiotherapy: A 6 MV accelerator mounted on a circular gantry opposite of an array of xenon detectors rotates, while the couch moves through the gantry aperture. The constant emission of the LINAC is modulated with a dynamic multileaf collimator constituted of 64 binary leaves (leaf projection at the isocenter: 6.25 mm). To correct the patient setup, a megavoltage CT can be acquired before each treatment fraction.
The TomoTherapy system used for the irradiation was installed at Foundation IRCCS “S. Raffaele” of Milan. The treatment plan was created with the HT Plans (TomoTherapy). The MUs delivered were 8252. The TomoTherapy unit is calibrated in terms of MU∕min in a reference static geometry (maximum open field, isocenter condition at 85 and 1.5 cm depth). 1 MU∕min corresponds at 1 cGy in that condition.; jaw opening was 2 cm, the modulation factor was 2, and the pitch was 0.2. Directional or complete blocks were not used to avoid a target underdosage. Optimization was, in any case, stressed for optical nerves sparing. (Fig. 1)
Gamma Knife
The Gamma Knife is a radiosurgery system for intracranial lesions only. The apparatus consists of 201 60Co sources distributed on a hemispheric surface so that the beams are focused on a common point. Helmets with circular removable collimators allow for the focusing of the radiation from the sources selected for the treatment.
The Gamma Knife system used to deliver the treatment was a model C installed at Foundation IRCCS S. Raffaele of Milan. The treatment plan was created with the GammaPlan® System (Elekta AB, Stockholm, Sweden). The treatment had 44 isocenters and was delivered with 4 and 8 mm collimator helmets.
Treatment planning parameters are given in Table 1.
Table 1.
Treatment planning parameters. Abbreviations: MUs=monitor units; LINAC=linear accelerator; mMLC=micromultileaf collimator.
| Treatment modality | Prescribed isodose line (%) | Total MUs | Target Coverage (%) |
|---|---|---|---|
| LINAC-mMLC | 70 | 936 | 99 |
| LINAC-cone | 70 | 1953 | 95 |
| CyberKnife preshileding | 70 | 2030 | 95 |
| CyberKnife postshielding | 70 | 2530 | 97.75 |
| TomoTherapy | 95 | 8252 | 95 |
| Gamma Knife | 50 | 15 367a | 100 |
See Sec. 5.
RESULTS
Table 2 shows the doses measured at the locations of interest in the Rando phantom. The positioning is more critical within the primary beam, because of the inhomogeneous dose deposition associated with some irradiation modalities and at peripheral regions of the target because of the steep dose gradient outside the PTV. The doses reported are those from the two TLDs placed in each position. In cases where the measurements were different by more than 7%, both values were reported. It happened only in the target and optic nerves, where the positioning of dosimeters is more critical.
Table 2.
Peripheral dose (mGy) at the locations of interest in the Rando phantom. The doses reported are the average of the readings from two TLDs (uncertainty 5%); in cases where the values were different for more than 7%, both readings were reported. Abbreviations: TLDs=thermoluminescent dosimeters; CC=cranio-caudal; LINAC=linear accelerator; mMLC=micromultileaf collimator; CK=CyberKnife; R=right; nv=nerve; L=left.
| TLDs anatomical position | CC distance from the target (cm) | LINAC-mMLC (mGy) | LINAC-cone (mGy) | CK preshileding (mGy) | CK postshielding (mGy) | TomoTherapy (mGy) | Gamma Knife (mGy) |
|---|---|---|---|---|---|---|---|
| Target ant | 0 | 8200 | 9695∕9199 | 7900 | 6908 | 5322 | 8314∕7587 |
| Target post | 0 | 7500 | 6915∕6234 | 6800 | 7425 | 3588 | 7603∕7427 |
| R optic nv | 0 | 480 | 533∕414 | 870 | 867∕464 | 1772∕1940 | 366∕290 |
| L optic nv | 0 | 870 | 1106∕1119 | 340 | 369 | 2028 | 454∕519 |
| Thyroid | 15.5 | 45.0 | 62.8 | 55.0 | 20.9 | 6.9 | 13.2 |
| Sternum | 30.5 | 10.0 | 18.0 | 27.5 | 9.1 | 2.3 | 4.1 |
| Upper lung | 43.0 | 4.6 | 8.7 | 17.5 | 7.7 | 1.7 | 1.9 |
| Lower lung | 53.0 | 3.0 | 5.8 | 15.0 | 8.3 | 1.4 | 0.98 |
| Ovaries | 75.5 | 1.3 | 2.6 | 10 | 5.8 | 1.6 | 0.36 |
| Testes | 80.5 | 1.1 | 2.2 | 10 | 5.8 | 1.6 | 0.28 |
Optic nerves dose
Optic nerves were the only healthy structures of interest that received a dose from the primary beams for all the delivery technique. Even if the purpose of this study was the measurement of peripheral dose, as a completion, the dose to the optic nerves was measured.
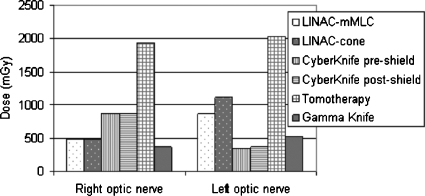
Figure 3 shows doses registered by the TLDs positioned in correspondence to the optic nerves; if the dosimeters at the same location registered different values, the higher one was reported.
Figure 3.
Right and left optic nerve doses measured in each treatment.
The measured values ranged from 340 to 2028 mGy. The lower dose at the right optic nerve was registered in the Gamma Knife treatment; the lower doses at the left optic nerve were measured in the CyberKnife treatments.
The higher doses, both at the right and left optic nerve, were found in the TomoTherapy treatment.
Peripheral dose
Mean doses measured by TLDs in the thyroid position ranged from a minimum of 6.9 mGy in the TomoTherapy treatment to a maximum of 62.8 mGy in the treatment delivered with LINAC equipped with the circular collimator.
Mean doses to the sternum ranged from 2.3 mGy with the TomoTherapy treatment to 27.5 mGy in the treatment delivered with the CyberKnife before the supplemental shielding was applied.
Mean doses to the upper lungs ranged from 1.7 mGy with the TomoTherapy treatment to 17.5 mGy in the treatment delivered with CyberKnife before the supplemental shielding was applied. In the lower lung, values ranged from 0.98 mGy in the Gamma Knife treatment to 15 mGy in the treatment delivered with the CyberKnife before the supplemental shielding was applied.
Mean doses to the gonads, both female and male, ranged from between 0.3 mGy in the Gamma Knife treatment and 10 mGy in the treatment delivered with the CyberKnife before the supplemental shielding was applied.
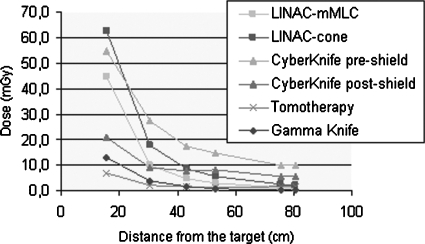
The mean value of the peripheral doses is given in Fig. 4 as a function of the distance from the geometrical center of the target.
Figure 4.
Peripheral dose values measured in each treatment as a function of the craniocaudal distance.
The sharp dose falloff within 20 cm of the target is apparent in TomoTherapy and Gamma Knife treatments and in the one delivered with the CyberKnife after the application of the supplemental shielding on the accelerator head. Beyond 40 cm, doses higher than 10 mGy were measured only in the CyberKnife treatment, before the installation of the supplemental shielding.
DISCUSSION
Dose deposition in the proximity of the target mainly depends on the existence of beams which entered or exited through the OARs, the radiation scattered inside the target itself, and the beams’ directions. LINAC treatments deliver about 500 mGy to the right optic nerve and a dose between about 900 and 1120 mGy to the left optic nerve; CyberKnife treatments delivered about 350 mGy to the left optic nerve and nearly 900 mGy to the right one. It is apparent that the direction of the beam has a greater impact on the dose absorption the closer the OAR is to the target.
In the TomoTherapy treatment, both optic nerve doses were very high. The beam delivery in this technique did not allow for the sparing of the tissues close to the target and at the same craniocaudal position. Similar results have been found during treatment of brain metastases.15
TomoTherapy treatment planning system gives the possibility to block out certain beams, small arc regions to avoid the entrance of the beams either directional or totally. In these simulations we do not use this block option. Our idea is that the maximum optic nerve dose (hence the main dose constraints for optical pathway) could not be much reduced, also if using this planning option, due the optic nerve position which is in direct contact with the target lesion.
CyberKnife shielding and peripheral dose
Only to discriminate the contributions to the peripheral dose in CyberKnife treatments (not for a precise evaluation of peripheral dose), several measurements were made using a phantom consisting of an anthropomorphic head (Accuray phantom) and many solid water slabs (to simulate the rest of the body). These measurements were made with original shielding.
The phantom was irradiated using different-angle shots and using all the available collimators; the measurements were carried out using a Farmer-type ionization chamber positioned roughly in the location of the gonads and the thyroid of a standard-size person. The measurements, made with different collimators, showed that the collimator size does not affect the dose at points of interest. The comparison of the values measured with the larger collimator (60 mm) with the values measured using the “blank” (the “closed collimator”) point out that the contribution of the leakage radiation to the total peripheral dose was more than 95%. In the evaluation of stereotactic peripheral dose, all the beams which might have passed through the thyroid and gonads were eliminated; therefore, we can conclude that the high peripheral dose must be due to the high leakage radiation.
High peripheral doses measured in the CyberKnife treatment found in this study and in few others present in the literature3, 16 have led Accuray to introduce a supplemental shielding.
The treatment was planned and delivered again after the application of the supplemental shielding. The preshielding and postshielding dose values are compared in Table 3. The shielding upgrade in the CyberKnife system led to a reduction in the peripheral dose, which ranged from 74% (at sternum) to 53% (at gonads distances). The doses at the thyroid show that even at this distance, the leakage has an important contribution. Comparison of the two CyberKnife configurations also revealed that the upgrade led to an approximately 60% reduction in the deposition of the peripheral dose, even though the actual MU delivered increased by 1.2 fold (the MU increasing is not correlated with new shielding because the total number of MU is not a possible constraint but it is only a result). The measurements after the linac shielding upgrade demonstrate that the additional shielding decreased the peripheral dose, expressed as a percentage of the delivered MUs, by a maximum of 59% (Refs. 16, 2) or 67% (our study). Both studies showed the dose reduction was greatest for cranial-caudal distances from the geometric center of the target less than 30 cm, and at these distances, the CyberKnife peripheral dose, expressed as a percentage of the delivered MU, is now comparable to that measured for the other treatment modalities in our previous investigation with LINAC. For distances between 30 and 70 cm from the target, the additional shielding reduced the peripheral dose by between 55% and 20% (Refs. 16, 2); in our study by between 56% and 42%.
Table 3.
Comparison between the peripheral dose values measured in the CyberKnife treatments before and after the supplemental shielding was installed.
| Distance from the target (cm) | CyberKnife preshielding dose (mGy) | CyberKnife postshielding dose (mGy) | Dose reduction after shielding application (%) |
|---|---|---|---|
| 30.5 | 27.5 | 9.1 | 66.9 |
| 43 | 17.5 | 7.7 | 56.0 |
| 53 | 15 | 8.3 | 44.7 |
| 75.5 | 10 | 5.8 | 42.0 |
| 80 | 10 | 5.8 | 42.0 |
Since the peripheral dose depends by several factors, and some of them are related to the manufacturing differences between two LINACs and cannot be compensated with just a calibration, we could conclude that our results are consistent with the ones by Petti.2
Comparison among all treatment devices
In the treatments where the beams cover the entire target, the peripheral dose is proportional to the delivered dose, which is linked to the MU. Petti2 showed that in the treatments where the single beams are covering only a portion of the entire target, the peripheral dose is mainly proportional to delivered MU rather than the delivered dose, so dose per MU values at different distances can be used to estimate dose deposition in other similar brain treatments.
The average doses registered by TLDs at distances greater than 30.5 cm from the target were expressed as a percentage of the MUs delivered (Table 4).2
Table 4.
Peripheral dose values as a percentage of the MU (100× dose in cGy∕MU) delivered in each treatment. Abbreviations: MUs=monitor units; LINAC=linear accelerator; mMLC=micromultileaf collimator.
| Distance from the target (cm) | Peripheral dose as percentage of MUs (cGy∕MUs) | |||||
|---|---|---|---|---|---|---|
| LINAC-mMLC (%) | LINAC-cone (%) | CyberKnife preshielding (%) | CyberKnife postshielding (%) | TomoTherapy (%) | Gamma Knife (%) | |
| 30.5 | 0.110 | 0.092 | 0.140 | 0.036 | 0.003 | 0.030 |
| 43 | 0.049 | 0.045 | 0.086 | 0.030 | 0.002 | 0.010 |
| 53 | 0.032 | 0.030 | 0.074 | 0.033 | 0.002 | 0.010 |
| 75.5 | 0.014 | 0.013 | 0.049 | 0.023 | 0.002 | 0.002 |
| 80 | 0.012 | 0.011 | 0.049 | 0.023 | 0.002 | 0.002 |
Usually, an accelerator output is calibrated so that, for example, a 100 MU output would result in the delivery of a 100 cGy dose at a reference point under standardized conditions. An equivalent concept for Gamma Knife can also be defined as dose deposition in a defined irradiation time in the calibration phantom center, under predetermined conditions (detector at isocenter, set collimators size, etc.).2 In this case, the Gamma Knife treatment lasted 60.5 min and the dose rate at the isocenter (at an 8 cm depth, using a 16 mm collimator helmet) was 3.104 Gy∕min. Correcting for the output factors of the collimators used in the treatment, the equivalent Gamma Knife output would be 15 367 MU.
Among the studied techniques, the Gamma Knife and TomoTherapy were associated with the least amount of peripheral dose. In the treatments delivered with LINAC equipped with mMLC or a circular collimator (cone), the peripheral dose per MU did not differ; the mMLC treatment required half the MUs to deliver the same dose with a circular collimator; hence, the absolute values found in the first treatment are half the ones found in the second one.
At the level of the gonads, the CyberKnife peripheral dose before the application of the additional shielding was about three times as high as that measured in the LINAC treatments and 25 times as high as that in the Gamma Knife and TomoTherapy experiments.
Currently, the IEC, responsible for medical equipment standards, is considering possible changes to the leakage requirements in the linear accelerator safety standard in the light of developing treatment techniques that may increase the leakage dose to patients. A number of approaches are possible and are under discussion, depending on the level of concern over leakage radiation.
Estimates of the risk of induction of stochastic effects
The estimate of the risk of induction of events from exposure to low radiation dose can be done using the model proposed by the International Commission of Radiation Protection (ICRP) (Publication 103).17 In fact, the ICRP values, based on the “linear-no-threshold” risk model, and intended to serve as a practical reference in a risk-benefit assessment of a given exposure situation are, however, mired with wide uncertainties. The ICRP itself warns that an a priori estimate of the probability of the induction of stochastic effects using them is not recommended. Nevertheless, models of this type are the only means to estimate the risk of induction of cancer and hereditary effects at low radiation doses.18
The nominal risk of inducing cancer and hereditary effects from ICRP 103 are shown in Table 5.
Table 5.
Nominal risk of induction of cancer and hereditary effects on the whole population per Sv [ICRP 103 (Ref. 17)].
| Tissue | Nominal risk (cases per 10 000 persons per Sv) | ||
|---|---|---|---|
| Total | Fatal | Nonfatal | |
| Thyroid | 33 | 2.3 | 30.7 |
| Lung | 114 | 101.5 | 12.5 |
| Ovaries | 11 | 6.3 | 4.7 |
| Gonads (hereditary) | 20.0 | 16.0 | 4.0 |
The prescription dose in a treatment varies depending on the type and the dimension of the lesion. As an example, we considered a stereotactic treatment of 20 Gy in 5 Gy fractions. The total risk was divided into the fatal and nonfatal components and expressed as cases per 10 000 treated patients. The values are given in Table 6. The ICRP model considers the effective doses as additive, so the fractionation is irrelevant in estimates of risk.
Table 6.
Risk estimates of induction of stochastic effects (induction of cancer and hereditary effects) in each treatment (total dose 20–5 Gy fraction). Abbreviations: LINAC=linear accelerator; mMLC=micromultileaf collimator.
| Nominal risk (cases per 10 000 treated persons) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | LINAC-mMLC | LINAC-cone | CyberKnife preshielding | CyberKnife postshielding | TomoTherapy | Gamma Knife | ||||||||||||
| Total | Fatal | Non- fatal | Total | Fatal | Non- fatal | Total | Fatal | Non- fatal | Total | Fatal | Non- fatal | Total | Fatal | Non- fatal | Total | Fatal | Non- fatal | |
| Thyroid | 5.9 | 0.4 | 5.5 | 8.3 | 0.6 | 7.7 | 7.3 | 0.5 | 6.8 | 2.8 | 0.2 | 2.6 | 0.9 | 0.1 | 0.8 | 1.7 | 0.1 | 1.6 |
| Lung | 1.7 | 1.5 | 0.2 | 3.3 | 3.0 | 0.3 | 7.5 | 6.7 | 0.8 | 3.6 | 3.2 | 0.4 | 0.7 | 0.6 | 0.1 | 0.7 | 0.6 | 0.1 |
| Ovaries | 0.06 | 0.03 | 0.03 | 0.11 | 0.06 | 0.05 | 0.44 | 0.25 | 0.19 | 0.25 | 0.14 | 0.11 | 0.07 | 0.04 | 0.03 | 0.02 | 0.01 | 0.01 |
| Gonads (hereditary) | 0.10 | 0.08 | 0.02 | 0.19 | 0.15 | 0.04 | 0.80 | 0.64 | 0.16 | 0.46 | 0.37 | 0.09 | 0.13 | 0.10 | 0.03 | 0.03 | 0.02 | 0.01 |
It is apparent that the risk of induction of secondary cancers is very small in the organs considered. The higher risk is the development of a tumor (both fatal and nonfatal) within the thyroid after the treatment with the LINAC SL75-5 equipped with cones, which is estimated around 0.08%. Dose absorption in the gonads involves a risk of induction of somatic and hereditary effects that are completely negligible.
The main purpose of this work was the measurement of the peripheral dose associated with different radiotherapy devices in the market and the use of these data to estimate the probability of induction of stochastic effects in organs distant from the target.19 We would like to take this opportunity to emphasize once again that the danger of triggering radiation-induced malignancies increases by orders of magnitude with the proximity to the treated target and the importance of sparing nearby sensitive structures quickly overwhelms the significance of the measurements listed in Table 6.
CONCLUSIONS
The data collected in this study show that the risk relative to the peripheral dose of developing secondary tumors is very small for all of the organs considered. Also, the risk of induction of hereditary effects is negligible; in general, this is true whenever the treatment fields exclude the pelvic region.20
The authors have no actual or potential conflicts of interest. The authors have no financial interest in the instruments presented in this manuscript.
References
- Majali M. and J.Novotny, Jr., “Measurement of the peripheral doses for linac stereotactic radiotherapy,” Radiat. Prot. Dosim. 106(3), 247–252 (2003). [DOI] [PubMed] [Google Scholar]
- Petti P. L. et al. , “Peripheral doses in CyberKnife radiosurgery,” Med. Phys. 33(6), 1770–1779 (2006). 10.1118/1.2198173 [DOI] [PubMed] [Google Scholar]
- Zytkovicz A. et al. , “Peripheral dose in ocular treatments with CyberKnife and Gamma Knife radiosurgery compared to proton radiotherapy,” Phys. Med. Biol. 52, 5957–5971 (2007). 10.1088/0031-9155/52/19/016 [DOI] [PubMed] [Google Scholar]
- Brenner D. J. et al. , “Cancer risk attributable to low doses of ionizing radiation: Assessing what we really know?,” Proc. Natl. Acad. Sci. U.S.A. 100(24), 13761–13766 (2003). 10.1073/pnas.2235592100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoli L.et al. , Radiazioni Ionizzanti a Basse Dosi: Stato dell’Arte Sugli Effetti per la Salute Umana (Istituto Italiano di Medicina Sociale, Roma, 2005). [Google Scholar]
- The National Academies, “Report in brief: Health risks from exposure to low levels of ionizing radiation,” BEIR Report No. BEIR VII, 2005. [PubMed]
- Harrison R. M., “Second cancers following radiotherapy: A suggested common dosimetry framework for therapeutic and concomitant exposures,” Br. J. Radiol. 77, 986–990 (2004). 10.1259/bjr/21023216 [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Upreti R. R., and Deshpande D. D., “Use of peripheral dose data from uniform dynamic multileaf collimation fields to estimate out-of-field organ dose in patients treated employing sliding window intensity-modulated radiotherapy,” Phys. Med. Biol. 51, 2987–2995 (2006). 10.1088/0031-9155/51/11/020 [DOI] [PubMed] [Google Scholar]
- Mutic S., Esthappan J., and Klein E. E., “Peripheral dose distributions for a linear accelerator equipped with a secondary multileaf collimator and universal wedge,” J. Appl. Clin. Med. Phys. 3(4), 302–309 (2002). 10.1120/1.1507921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutic S. and Klein E. E., “A reduction in the AAPM TG-50 reported peripheral dose distributions with tertiary multileaf collimation,” Int. J. Radiat. Oncol., Biol., Phys. 44(4), 947–953 (1999). 10.1016/S0360-3016(99)00092-9 [DOI] [PubMed] [Google Scholar]
- Stovall M. et al. , “AAPM Report No. 50. Fetal dose from radiotherapy with photon beams: Report of AAPM radiation Therapy Committee Task Group 36,” Med. Phys. 22(1), 63–82 (1995). 10.1118/1.597525 [DOI] [PubMed] [Google Scholar]
- Stern R. L., “Peripheral dose from a linear accelerator equipped with multileaf collimation,” Med. Phys. 26(4), 559–563 (1999). 10.1118/1.598557 [DOI] [PubMed] [Google Scholar]
- Majali M., Novotny J., and J.Novotny, Jr., “Computational model for the estimation of the extracranial doses received during Leksell gamma knife model C treatment,” J. Neurosurg. 102, 14–18 (2005). 10.3171/jns.2005.102.s_supplement.0014 [DOI] [PubMed] [Google Scholar]
- Ramsey C. R., “Out-of-field dosimetry measurements for a helical tomotherapy system,” J. Appl. Clin. Med. Phys. 7(3), 1–11 (2006). 10.1120/jacmp.v7i3.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarícano J. A., Yan Y., Shi C., Linskey M., and Ratanatharathorn V., “ Dosimetric comparison of helical tomotherapy and gamma knife stereotactic radiosurgery for single brain metastasis,” Radiat. Oncol. 1, 26 (2006). 10.1186/1748-717X-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C. F. et al. , “Peripheral dose measurement for CyberKnife radiosurgery with upgraded linac shielding,” Med. Phys. 35(4), 1494–1496 (2008). 10.1118/1.2889620 [DOI] [PubMed] [Google Scholar]
- International Commission of Radiation Protection (ICRP), “The 2007 Recommendations of the International Commission on Radiological Protection,” ICRP Publication vol. 37, issue 2–4, pp. 1–332 (2007). [DOI] [PubMed]
- Dörr W. and Herrmann T., “Cancer induction by radiotherapy: Dose dependence and spatial relationship to irradiated volume,” J. Radiol. Prot. 22, A117–A121 (2002). 10.1088/0952-4746/22/3A/321 [DOI] [PubMed] [Google Scholar]
- Lillicrap S. C., Morgan H. M., and Shakeshaft J. T., “Correspondence: X-ray leakage during radiotherapy,” Br. J. Radiol. 73, 793–794 (2000). [DOI] [PubMed] [Google Scholar]
- Mazonakis M. et al. , “Therapeutic external irradiation in women of reproductive age: Risk estimation of hereditary effects,” Br. J. Radiol. 77, 847–850 (2004). 10.1259/bjr/88840344 [DOI] [PubMed] [Google Scholar]