Abstract
Purpose
To determine the in vivo anti-tumor effect of aerosolized Celecoxib (Cxb) in combination with i.v Docetaxel (Doc) and compare the anti-tumor effect with oral Cxb combined with i.v Doc in human orthotopic non-small cell lung cancer (NSCLC) xenograft model.
Materials and Methods
Female Nu/Nu mice were implanted with orthotopic tumors by injecting A549 cells into the lung parenchyma. Seven day after tumor implantation the mice were treated with aerosolized Cxb (30 min exposure/day, 5 mg/ml solution) + i.v Doc (10 mg/kg) and the effect was compared with oral Cxb (150 mg/kg/day) + i.v Doc (10 mg/kg), for 28 days. Small-animal nose only inhalation chamber (CH Technologies, Westwood, NJ) was utilized for aerosol exposure. Therapeutic activity of Cxb (aerosol/oral) + Doc was estimated by differences in lung weight, tumor area and animal body weight. Lung tumor samples isolated from mice were analyzed for (a) PGE2 levels by enzyme immunoassay (EIA) (b) expression of Fas and Factor VIII by immunohistochemistry (c) IL-8 expression using EIA kits and (d) mRNA expression for caspase-3 by Real-Time PCR.
Results
Mice treated with Cxb (aerosol/oral) + Doc showed significant reduction (P < 0.001) in lung weight and tumor area as compared to Cxb or Doc treatments. Cxb (aerosol/oral) + Doc showed increased apoptosis mediated via increased Fas and caspase-3 (P < 0.001) expression as compared to untreated control. Further, the combination treatment showed antiangiogenic effect as demonstrated by reduced expression of Factor VIII, IL-8 (P < 0.001) and PGE2 (P < 0.001) in lung tumors as compared to untreated control. Aerosolized Cxb at a significantly lower therapeutic dose (4.56 mg/kg/day) demonstrated comparable anti-tumor efficacy to orally administered Cxb (150 mg/kg/day).
Conclusion
Cxb was formulated and effectively delivered via aerosolization to treat orthotopic lung tumors in combination with i.v Doc. Cxb when administered by aerosol produced same therapeutic effect as oral Cxb, but at lower therapeutic dose and thus shows promise for the treatment of lung cancer.
Keywords: celecoxib, docetaxel, inhalation, nose-only exposure, orthotopic lung tumor
INTRODUCTION
Lung cancer is the leading cause of cancer death in both men and women in the United States causing 28% of all cancer deaths. Non-small cell lung cancer (NSCLC) patients represent 80% of patients diagnosed with lung cancer. Despite recent advances in chemotherapy, response rates in NSCLC remain < 50% and a third of patients with stage IV disease have a two-year survival rate of < 20% (1). Failure of the current treatment avenues has prompted the need for new drug delivery and therapeutic options for the prevention and treatment of lung cancer. It is significant for the treatment of lung cancer that the therapeutic agent be administered locoregionally and continuously in high enough concentrations in order to eradicate tumors more effectively with minimal systemic side effects (2).
Inhalation drug delivery represents a novel and potential delivery route for the treatment of lung cancer. Several investigators have demonstrated the therapeutic potential of aerosol delivery for lung cancer. Tatsumura and Kagamimori (3) demonstrated that dogs treated with nebulized 5-fluorouracil (5-FU) achieved high concentrations of 5-FU in the trachea, bronchi and lymph nodes and the retention time of inhaled 5-FU was considerably longer in tumor tissues than in other parts. The results showed potent antitumor activity of inhalation therapy with absence of any significant adverse effects. Nebulized liposome formulations of 9-nitrocamptothecin (9-NC) have been studied for the treatment of lung cancer in animal models (4). The aerosolized liposomes with estimated pulmonary deposition of 76.7 μg/kg 9-NC were found to significantly (P < 0.0001) reduce the mean tumor growth when compared to oral treatment (100 μg/kg 9-NC per day) in human lung cancer xenograft growing over the thorax in nude mice. Encapsulation of paclitaxel into lipid vehicles and administration by continuous aerosolization into mice bearing pulmonary renal carcinoma metastases (5) showed that the tumor growth was significantly reduced and survival time was increased when compared to control mice inhaling placebo liposome suspension. The total estimated deposited dose of paclitaxel by liposome aerosol treatment was 5 mg/kg, which is substantially lower than doses used for intravenous administration (> 20 mg/kg). Further improvement in the respiratory deposition of anticancer drugs, camptothecin and paclitaxel, was achieved using 5% CO2 enrichment of air. The increased pulmonary drug concentrations have been attributed to the changed respiratory patterns (6). Recently, the efficacy of aerosol vs. i.p gemcitabine was compared using 0.5 mg/kg dose of gemcitabine given three times weekly for 3.5 weeks (7). The number of metastases in the lung was significantly reduced only in mice receiving aerosol gemcitabine compared to control group (P < 0.05). However, gemcitabine administered i.p was not as effective (P > 0.05 vs. control) as aerosol therapy. Nebulized cyclosporine plus paclitaxel liposomes were demonstrated to be suitable for aerosol treatment of renal cell carcinoma pulmonary metastases in mice (8). Tumor surface areas were significantly smaller in cyclosporine-paclitaxel treated animals when compared to untreated control and paclitaxel or cyclosporine treated animals (P < 0.01).
COX-2 has emerged therapeutic target for the prevention and treatment of various types of cancers (9). COX-2 inhibitors have been found to reduce the tumor growth rate by themselves and exert potent synergistic cytotoxic effect with anticancer drugs in experimental lung cancer models (10). In order to obtain optimum therapeutic outcome of the combined use of cyclooxygenase inhibitors with anticancer drugs, it may be more effective if the cyclooxygenase inhibitor is delivered by inhalation and the anticancer drugs are delivered by their established route of administration. This assumes to be a more viable approach due to the non-specific deposition with undesirable toxicity following inhalation delivery of anticancer agents (8).
Previous investigations in our lab have shown the potential of inhalation delivery of COX-2 inhibitors for the enhancement of cytotoxic activity of drugs used in the treatment of lung cancer. For this purpose, aerosolized nimesulide in combination with doxorubicin was used as a model for proof-of-principal of this concept (11). Nimesulide-MDI (40 shots) in combination with doxorubicin (0.01 μg/ml) showed a cell kill of more than 60%, which was significantly higher (P < 0.01) than individual treatments. TUNEL staining showed apoptosis in over 30% of A549 cells treated with aerosolized nimesulide and doxorubicin combination vs. negligible as seen in cells treated individually with nimesulide and doxorubicin. Further, Celecoxib (Cxb) was formulated as a metered dose inhaler formulation (12) and had a medication delivery of 231.3 μg/shot, MMAD 1.4 μm and GSD 1.9 and respirable fraction of 50.7%. Aerosolized Cxb was found to significantly enhance the in vitro cytotoxicity and apoptotic response of Docetaxel (Doc) against A549 and H460 cells which was mediated via increased expression of PPAR-γ and p-53 as well as activation of caspase pathway. These findings showed promise and prompted in vivo evaluation of aerosolized Cxb for the treatment of NSCLC. The in vivo evaluation of aerosol Cxb generates more interest and significance due to the recent reports of Cxb inducing heart attacks and strokes in the Phase III cancer studies (13). We hypothesized that targeted delivery of Cxb to the lungs via aerosol may yield a higher drug concentration in the lungs and offer a novel therapeutic option for the use of Cxb in lung cancer. Further, in the present study, Doc is used as chemotherapeutic agent. It is the first and only chemotherapeutic agent approved by the FDA for treating both newly diagnosed NSCLC, in combination with cisplatin, and previously treated advanced NSCLC, as a single agent. Doc is also reported to improve survival and decrease in cancer-related symptoms in patients with NSCLC who had failed platinum-based first-line chemotherapy (14). Recent reports have demonstrated the efficacy of Doc in combination with irinotecan and Cxb in patients with NSCLC (Phase I studies) (15).
The objectives of this study are: (a) To study the potential inhalation delivery of Cxb in orthotopic NSCLC xenograft model. (b) To determine the in vivo anti-tumor effect of aerosolized Cxb in combination with i.v administration of Doc and compare the anti-tumor effect with orally administered Cxb combined with i.v Doc (c) To study the underlying mechanisms involved in the cytotoxicity of the combination treatment.
MATERIALS AND METHODS
Materials
Docetaxel and Celecoxib were provided as generous gifts from Sanofi-Aventis (Collegeville, PA) and Pfizer (Skokie, IL), respectively. All tissue culture chemicals were obtained from Sigma Chemical Company (St. Louis, MO). Vitamin E TPGS was procured from Eastman chemical company (Kingsport, TN). All other chemicals were of reagent grade. The human lung tumor cell line A549 was obtained from American Type Culture Collection (Rockville, MD). A549 cells were grown in F12K medium supplemented with 10% fetal bovine serum. All the tissue culture media contained penicillin (50,000 U/ml), streptomycin (0.1 mg/ml) and neomycin (0.2 mg/ml). The tumor cells were grown in standard tissue culture conditions, passaged at 80–90% confluence and cytotoxicity experiments were performed between 2–20 passages.
Nu/Nu mice and BALBc mice were purchased from Harlan Inc. (Indianapolis, IN) and housed in standard cages with food and water provided ad libitum. All experiments were performed with the approval of the Institutional Animal Care and Use Committee, Florida A&M University.
Orthotopic Tumor Implantation (16,17)
Female Nu/Nu mice were anesthetized and a 5 mm skin incision was made to the left chest, ~5 mm from scapula. On observing left lung motion through the pleura, a 28-gauge needle attached to a 0.1 ml Hamilton syringe was directly inserted through the sixth intercostal space into the lung to a depth of 3 mm. A549 tumor cells (1 million per mice) suspended in PBS (pH 7.4) were injected into the lung parenchyma. Wounds were closed with a surgical skin clip. A pilot study showed that all the nude mice develop lung tumors at 1–2 weeks after intrapulmonary injection of tumor cells.
Aerosol Drug Delivery
Aqueous formulation of Cxb suitable for nebulization was prepared by partly dissolving Cxb (50 mg) in 0.5 ml ethanol and 0.5 ml PEG 400. The mixture was finally mixed with 500 mg of molten Vitamin E TPGS and slowly dispersed into 8.5 ml distilled water to achieve a solution with final concentration of 5 mg/ml. The solution was freshly prepared and aerosols were generated by nebulization at room temperature with a PARI LC STAR jet nebulizer using dry compressed air at a flow rate of 4.5 l/min. Female Nu/Nu mice (22 ± 2 g) were constrained in the cone ended plastic animal holders which are designed in such a way that only the nose of the mice were exposed to the aerosol cloud. Each tube was tapered at one end to approximately fit the shape of the animal’s head and the diameter of the cylindrical portion of the cone was such that the animal could not turn in the cone. Small animal exposure inhalation chamber (CH Technologies, Westwood, NJ) consisting of 12 ports located peripherally around a central delivery plenum was utilized for aerosol exposure. The downstream side of the chamber was connected to the jet nebulizer that produced aerosol. The nose-only exposure system allows a fresh supply of the test atmosphere to each animal, independent from other animals. A schematic illustration of nose-only aerosol exposure system is shown in Fig. 1. The estimated total deposited amount of inhaled Cxb (D) for the ambient air was calculated by the following formula: D = C × V × DI × T; (8) where C (concentration of Cxb in aerosol volume) = Amount of Cxb collected in each port of inhalation chamber (5.7 mg)/Total volume of air withdrawn in 30 min (30 min × 4.5 liters/number of ports 12 = 11.25 l) = 507.5 μg/l, V = volume of air inspired by the animal during 1 min [for mice, V = 1.0 l-min/kg], DI = estimated deposition index [fraction of inhaled dose deposited throughout the respiratory tract (for mice DI = 0.3)] (7,8); T = Duration of treatment in min (T = 30 min). Under these experimental conditions the estimated total deposited dose of Cxb during 30-min treatment was 4.56 mg/kg/day.
Fig. 1.
Schematic illustration of Cxb aerosol generation and nose-only exposure to mice.
Aerosol Characterization
To determine aerosol concentrations, measured volumes of aerosol were drawn through filters, which were subsequently were analyzed for Cxb by a UV-visible method (λ = 250 nm). To determine particle size, aerosol was drawn through Mercer-type cascade impactor (In Tox, Albuquerque, NM) equipped with filters on each stage and a backup filter. The individual filters were analyzed for Cxb and the MMAD and GSD were calculated from the data using Battelle software.
Inhalation/Oral Delivery of Cxb with i.v Doc in Mice with Orthotopic Lung Tumors
Seven days after tumor implantation, the animals were randomly divided into following groups to receive Cxb and Doc formulations. One group of mice was left untreated, the second group received aerosolized Cxb + i.v. Doc (n = 10), the third group received oral Cxb + i.v. Doc (n = 10), the fourth group received only i.v. Doc (n = 10), the fifth group received only aerosolized Cxb (n = 10) while the sixth group received only oral Cxb (n = 10). Treatment of mice with aerosol (30 min exposure/day, 5 mg/ml aqueous Vitamin E TPGS solution) was performed as described before up to 28 days. Cxb was administered orally (150 mg/kg/day) as a 2% w/v solution in ethanol: PEG 400: water (22.2:66.6: 11.2, v/v). Doc infusion concentrate (20 mg in 0.5 ml of polysorbate 80) was diluted in 1.5 ml of 13% ethanol which was further diluted with sterile saline to a final concentration of 2.5 mg/ml was given intravenously (10 mg/kg, on day 14, 18 and 22) (18). Therapeutic activity of Cxb (aerosol/oral) + Doc treatment was estimated by differences in lung weight, tumor area and animal body weight. The tumor dimensions were measured using a linear caliper and the tumor volume was calculated using the equation V (mm3) = a × b2/2, where a is the largest diameter and b is the smallest diameter.
Immunohistochemistry of Orthotopic Lung Tumor Tissues
Tissues were formalin fixed, paraffin embedded and 5 μm sections were prepared for control and all other treatments. Peroxidase method was used for the immunostaining of Fas and Factor VIII. Briefly, the 5 μm sections were cleared in xylene and hydrated in different grades of alcohol. Next, the endogenous peroxidase activity was quenched by incubating the slides in 3% H2O2 solution. Further, the slides were washed again three times with PBS and were incubated with primary antibodies Fas and Factor VIII for overnight at 4°C. Location of the primary antibodies was achieved by the application of horseradish peroxidase-conjugated secondary antibodies for 30 min at room temperature and diamino benzidine substrate. The slides were counterstained with hematoxylin. Specific staining for each protein was categorized as either positive or negative based on the presence of brown colored staining. Clear staining of the cytoplasm and cell membrane was the criterion for a positive reaction. All the immunostaining slides were observed under an Olympus BX40 light microscope equipped with computer controlled digital camera and imaging software.
Caspase 3 Gene Expression in Orthotopic Lung Tumor Tissues
The lung tumor tissues collected at the end of the study were processed to isolate total RNA using the RNeasy Protect Mini kit (Qiagen Inc., Valencia, CA). Total RNA was quantified and 100 ng of the RNA was reverse transcribed using TaqMan reverse transcription reagents at 48°C for 30 min. The cDNA was then amplified using TaqMan Universal PCR master mix and Assay on Demand primer probe sets for caspase-3 and internal control 18s RNA in a Real-time PCR system 7300 (Applied Biosystems, Foster City, CA). Data was collected at the end of 40 cycles and analyzed using the RQ Study software v1.3 (Applied Biosystems, Foster City, CA).
IL-8 (CXC8) Levels in Orthotopic Lung Tumor Tissues
Lung tumor tissues, from control and different treatments were cut into small pieces and homogenized in PBS. The homogenate was centrifuged at top speed for 10 min to sediment the tissue fragments. Next, the lysis buffer (25 mM HEPES, Triton-X 0.1%, NaCl 300 mM, b-glycerophosphate 20 mM, MgCl2 1.5 mM, EDTA 0.2 mM, DTT 25 mM) with protease inhibitors (sodium orthovanadate 4 mM, sodium fluoride 400 mM, benzamidine 20 mM, Leupeptin 2 mg/ml, Aprotinine 4 mg/ml, PMSF 500 mM) were added. Samples were vortexed, incubated on ice for 30 min, centrifuged again and the supernatant was stored at −80°C until further analysis.
The lung tissue samples were subjected to quantitative analysis using EIA kits for IL-8 (Pierce Endogen, Rockford, IL) according to the manufacturer’s protocol. Each sandwich EIA kit used a polyclonal antibody to bind the target protein in the sample. After a short incubation, the excess sample was washed out and a secondary antibody labeled with the enzyme horseradish peroxidase was added. The labeled antibody was attached to the specific protein of interest and captured on the plate. Next, the excess-labeled antibody was washed out and the substrate was added. The substrate reacted with the labeled antibody bound to the sample protein and the color was read at 450 nm with correction at 550 nm in a microquant plate reader (Biotek Instruments Inc, Winooski, VT).
Determination of PGE2 Levels in Orthotopic Lung Tumor Tissues
PGE2 content in tumor samples were determined using the method suggested by Trifan et al. (19) and analyzed using a colorimetric enzyme immunoassay method. Briefly, the tumor tissues were homogenized in prostaglandin extraction buffer [70% ethanol and 30% of 0.1 M sodium phosphate (pH 4.0)] and incubated on wet ice for 30 min. The samples were centrifuged and the supernatant was collected. A known volume of supernatant (about 250 μl) was dried under nitrogen and resuspended in assay buffer and was analyzed as per manufacturer’s recommendations (Assay Designs, Ann Arbor, MI).
Statistical Analysis
Differences in lung weight and tumor volume between various treatment groups were analyzed by non-parametric Mann-Whitney Test. One-way ANOVA followed by Tukey’s Multiple Comparison Test was performed to determine the significance of difference in the expression of caspase-3, IL-8 and PGE2. The statistical analysis was performed using GraphPad PRISM version 2.0 software (San Diego, CA).
RESULTS
Aerosol Characteristics
Cxb aqueous formulation suitable for nebulization was developed at a concentration of 5 mg/ml (concentration of Cxb in aerosol volume for 30 min exposure = 507.5 μg/l). The MMAD and GSD values for aerosolized Cxb solution were 1.68 μm and 1.36, respectively. Aerosol particles with these characteristics are well suited for pulmonary deposition throughout the respiratory zone (7). Using this aerosol, the estimated total deposited dose of Cxb during the 30-min treatment period was found to be 4.56 mg/kg/day as described in Materials and Methods.
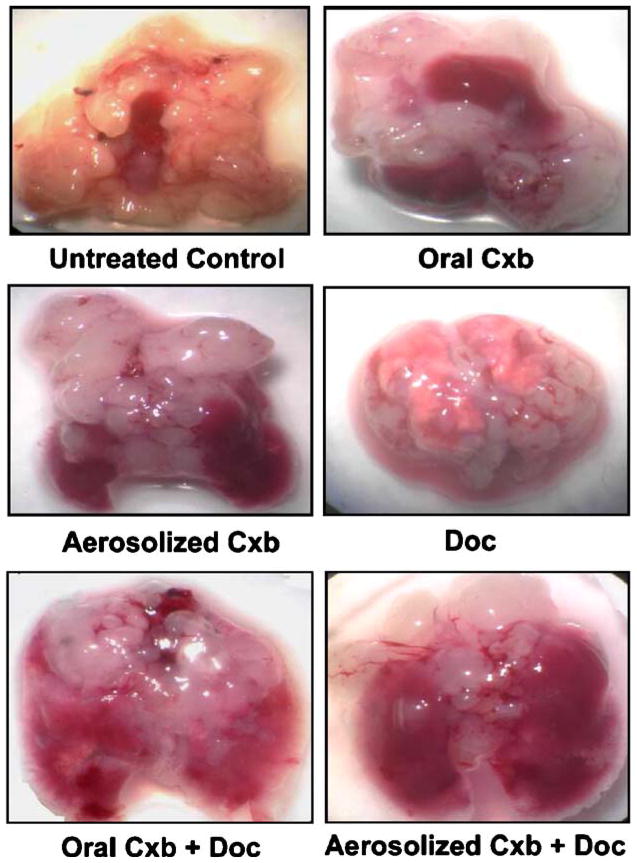
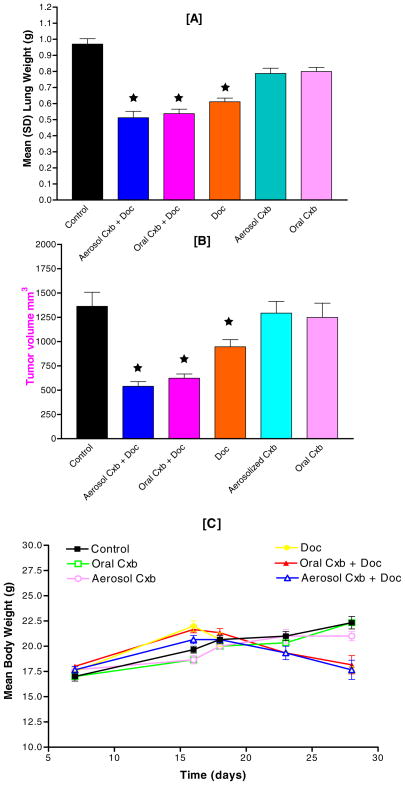
Anti-Tumor Effect of Cxb (aerosol/oral) + Doc Combination against A549 Orthotopic Tumors
Initial pilot studies showed that nude mice implanted with 1 million A549 cells develop fairly uniform tumors in 1–2 weeks. After 7 days of tumor inoculation the average lung weight and tumor volume were 250 ± 18.45 mg and 200 ± 24.34 mm3, respectively. Treatment was given for 28 days, starting 7 days after tumor inoculation for a total of 21 days. Figure 2 shows the photo micrographic view of orthotopic lung tumors at the end of study schedule. As evident, Cxb (aerosol/oral) + Doc treatments significantly inhibited the growth of A549 orthotopic tumors in the lungs. Treatment response in terms of mean lung weights is shown in Fig. 3A. Mice receiving Cxb (aerosol/oral) + Doc & Doc alone had significantly lower lung weight (P < 0.001) than untreated animals. The average weight of mouse lung is 150–200 mg. At the end of 28 days, there was almost a five-fold increase in the lung weight in untreated animals (approximately 1.0 g) due to orthotopic lung tumors. Treatment groups, Cxb (aerosol/oral) + Doc and Doc alone, however showed 2–3 fold increase in lung weight. In comparison, Fig. 3B shows the tumor volume-time data profile of Cxb (aerosol/oral) + Doc, Doc alone and Cxb (aerosol/oral) alone as compared to untreated control. It is evident from Fig. 3B that in the A549 tumors, the combination of Cxb (aerosol/oral) + Doc produced a greater antitumor effect as compared to Cxb (aerosol/oral) or Doc treatments. At 28 days post tumor implantation, the tumor volumes were found to be 1,362.4 ± 322.87, 1,248.36 ± 326.82, 1,292.38 ± 268.38, 945.78 ± 164.73, 622.64 ± 96.19 and 539.66 ± 107.28 mm3 (expressed as mean ± SD) in untreated, Cxb oral, Cxb aerosol, Doc, Cxb oral + Doc and Cxb aerosol + Doc treated mice, respectively. These results indicate that there was 61 and 54% reduction in the tumor volume following Cxb aerosol + Doc and Cxb oral + Doc treatments, respectively, as compared to control. Figure 3C shows the body weight of mice during 28-day treatment schedule with various combinations of Cxb and Doc treatments. Cxb (aerosol/oral) + Doc and Doc alone treated mice weighed significantly (P < 0.05) less than untreated control.
Fig. 2.
Effect of Cxb (aerosol/oral) and Doc treatments on A549 orthotopic lung tumors. Nude mice with orthotopic tumors received various treatments for 28 days starting at day 7 following tumor inoculation. Representative lungs with tumors are shown from untreated mice and various treatment groups.
Fig. 3.
Cxb (aerosol/oral) and Doc reduced tumor growth when administered separately or together. A549 cells at 1 × 106 cells/mouse were implanted into the lung parenchyma of Nu/Nu mice. Seven days after tumor implantation, mice were divided into various groups and received treatments upto 28 days. Therapeutic activity of treatments was estimated by differences in (A) lung weight (B) tumor volume and (C) animal body weights. ★ P < 0.001 vs. control.
Effect of the Combination of Cxb (Aerosol/Oral) with Doc on Fas Expression
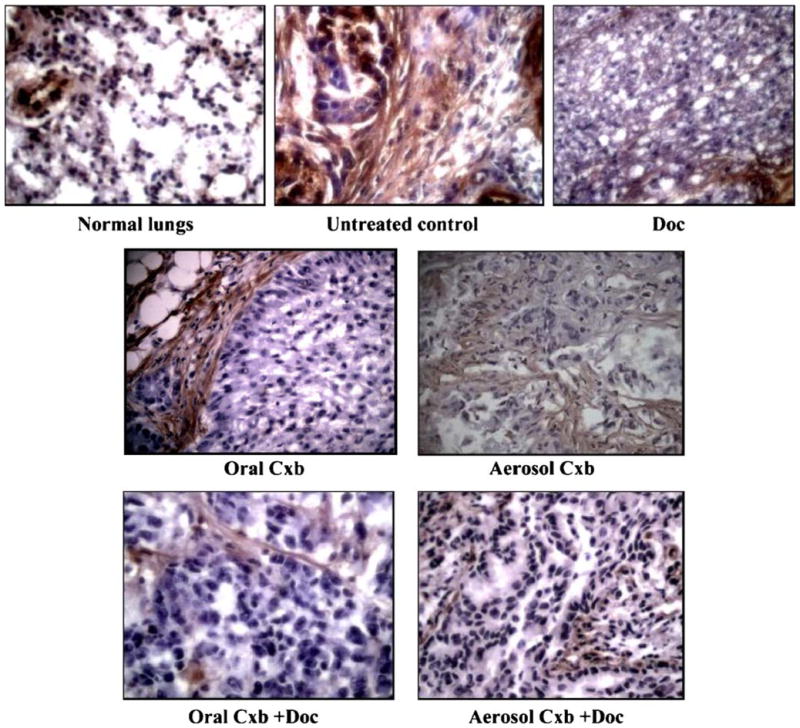
Fas expression was examined in A549 orthotopic xenograft tumors treated with various combinations of Cxb and Doc. Fas expression (brown-stained areas) was negligible in untreated tumors whereas it was up regulated in animals treated with Cxb (aerosol/oral) + Doc and Doc alone (Fig. 4). This upregulation of the Fas expression was parallel to the expression in normal lungs.
Fig. 4.
Fas expression in A549 lung tumors in mice following treatment with Cxb (aerosol/oral) and Doc. Lungs were resected from mice at the end of treatment, fixed in 10% formalin buffer and paraffin embedded. Sections were prepared and stained as described in Materials and Methods section. Anti-Fas immunoreactivity is shown as the brown stained areas. Counterstaining was done by hematoxylin. Original magnification ×40.
Induction of Apoptosis in A549 Orthotopic Tumors by the Combination of Cxb (Aerosol/Oral) with Doc
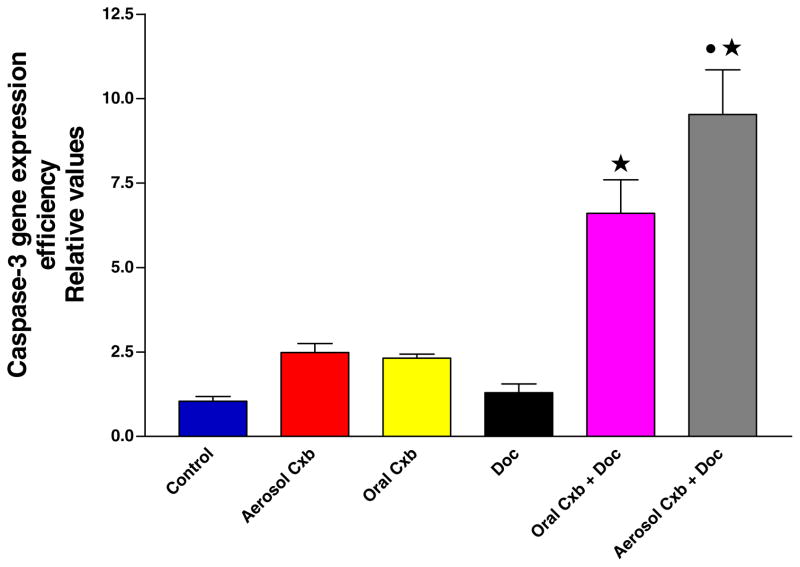
It is evident from Fig. 5 that the expression of caspase-3 in A549 tumors treated with aerosol/oral Cxb + Doc was significantly increased in comparison to untreated control (P < 0.001). However, Cxb or Doc alone did not show any statistically significant difference than untreated control (P > 0.05). Further, the caspase-3 expression in A549 tumors treated with aerosol Cxb + Doc was significantly higher in comparison to oral Cxb + Doc treatment (P < 0.01). Thus aerosol Cxb with Doc showed enhanced apoptotic response than oral Cxb with Doc treatment.
Fig. 5.
Caspase-3 gene expression as measured by enzyme immunoassay in tumors harvested at 28 days from untreated control and various treatment groups. Statistical significance of the difference in caspase-3 with respect to control: ★ P < 0.001; • P < 0.01 vs. Oral Cxb + Doc; Data presented are means ± SE (n = 6).
Cxb (Aerosol/Oral) + Doc Combination Inhibits Angiogenesis in Orthotopic A549 Xenografts
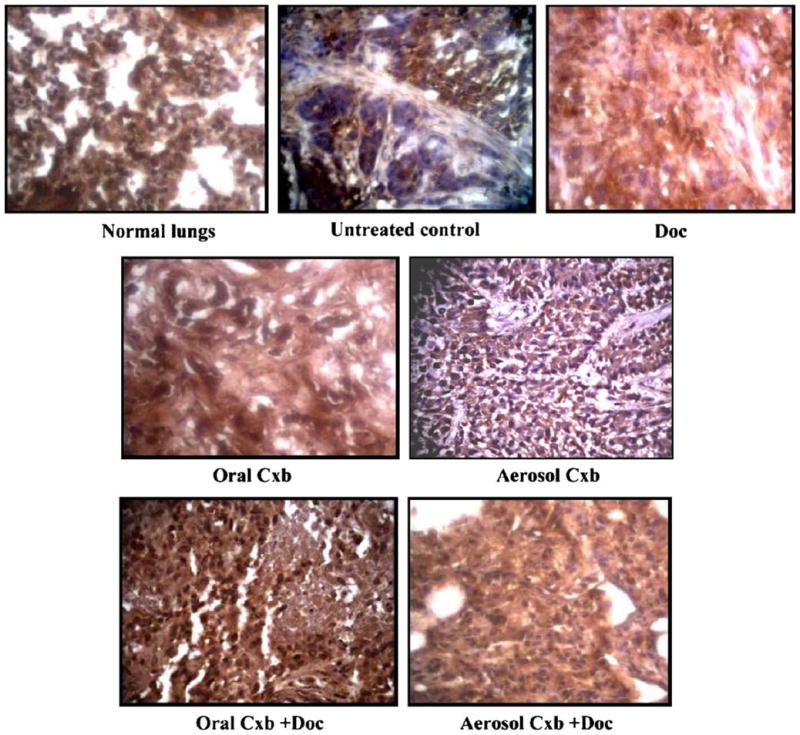
To determine whether Cxb-Doc combination exhibits antiangiogenic potential contributing to tumor growth inhibition, we evaluated the expression of Factor VIII in orthotopic A549 tumor tissue sections. Figure 6 shows the results of immunohistochemical analysis of tumors with Factor VIII antibody. There was significant increase in the expression of Factor VIII in tumor tissues harvested from untreated mice. Treatment with Cxb (aerosol/oral) + Doc showed a marked decrease in the expression of Factor VIII indicative of antiangiogenic potential of the combination treatment. It is also evident from Fig. 6 that Doc alone also showed a marked decrease in Factor VIII expression, whereas Cxb (aerosol/oral) did not produce any significant decrease in Factor VIII expression.
Fig. 6.
Factor VIII expression in A549 lung tumors in mice following Cxb (aerosol/oral) treatment with Doc. Lungs were resected from mice at the end of treatment, fixed in 10% formalin buffer and paraffin embedded. Sections were prepared and stained as described in Materials and Methods section.
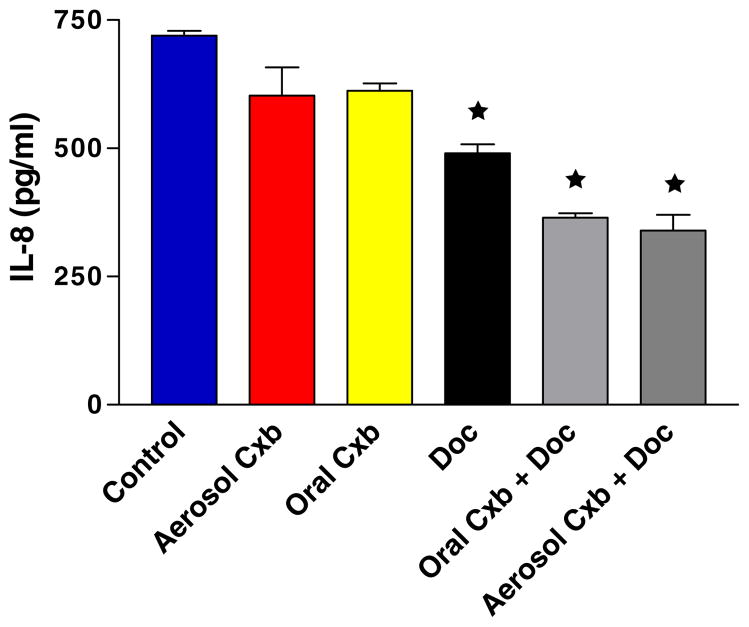
The antiangiogenic potential was further supported by the significant down regulation of IL-8, which mediates angiogenic activities by stimulating the proliferation and migration of endothelial cells. As shown in Fig. 7, the IL-8 levels were significantly (P < 0.01) down regulated due to treatment with Cxb aerosol + Doc (339.50 ± 43.17 pg/ml), Cxb oral + Doc (364.80 ± 11.98 pg/ml) and Doc (490.33 ± 24.29 pg/ml) as compared to untreated control (719.74 ± 13.45 pg/ml).
Fig. 7.
IL-8 (CXC8) levels in tumors harvested at 28 days from untreated control and various treatment groups. Statistical significance of the difference in IL-8 with respect to control: ★ P < 0.01. Data presented are means ± SE (n = 6).
Prostaglandin E2 Measurements
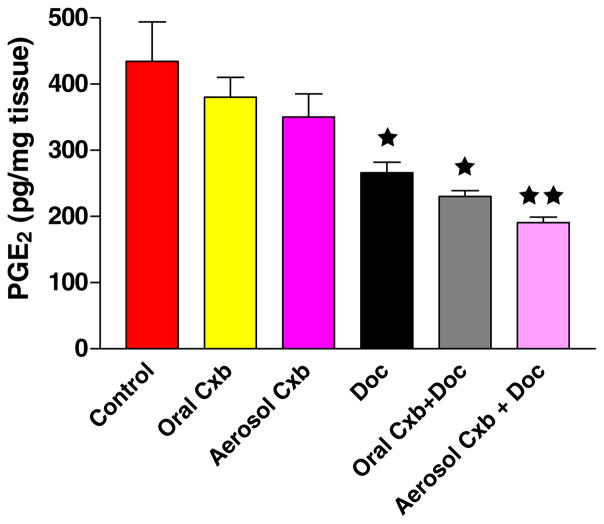
The production of PGE2 after various treatments was determined in A549 orthotopic xenograft tumors (Fig. 8). The tumors obtained from untreated control mice expressed very high levels (434.51 ± 84.17 pg/mg) of PGE2. In comparison, the levels of PGE2 in tumors obtained from mice treated with the combination of Cxb (aerosol/oral) + Doc and Doc alone were significantly reduced to 190.50 ± 12.02 (P < 0.001), 230.00 ± 12.72 (P < 0.001) and 266.00 ± 22.62 (P < 0.01) pg/mg, respectively. This indicates that intratumor PGE2 levels were reduced by 56.15, 47.06 and 38.70% in mice treated with Cxb (aerosol/oral) + Doc and Doc alone, respectively. Cxb (aerosol/oral) alone did not show any significant effect in reducing the PGE2 production.
Fig. 8.
PGE2 levels as measured by enzyme immunoassay in tumors harvested at 28 days from untreated control and various treatment groups. Statistical significance of the difference in PGE2 with respect to control: ★★ P < 0.001; ★ P < 0.01. Data presented are means ± SE (n = 6).
DISCUSSION
One of the major limitations in the treatment of lung cancer is the inability to deliver adequate concentrations of the drug to the tumor without any major systemic side effects. Lungs are not the main organs for drug deposition with the conventional routes of drug delivery. Promising results have been obtained when the aerosol/inhalation route was used to treat lung tumors in several animal studies and clinical trials (20–23). A major problem with the administration of aerosol to animals is the contribution of systemic dosage of particles deposited in the respiratory tract above the lungs. It is difficult to deliver aerosol to mouse lungs without its deposition in the nose and gastrointestinal tract. The smaller tidal volumes of mice also result in lower aerosol deposition compared to humans. Deposition of aerosols in mice depends upon particle size, delivery device and formulation parameters. In mice, about 30% of inhaled particles within the diameters of 1–2 μm deposit in the respiratory tract but less than 10% penetrate to the lung parenchyma (24). This amount varies with the duration of treatment and method of aerosol exposure. Different types of inhalation chambers are available for aerosol exposure. In whole-body exposure chambers, aerosol is delivered into a box where mice are allowed to inhale with their body immersed in aerosol cloud. This results in large deposition of drug all over the animal’s body leading to ingestion by mouth and unwanted toxic responses. Animals also tend to avoid aerosol exposure by covering their noses with fur. In the present study, nose-only inhalation exposure chamber was used for aerosol exposure. The procedure and system is simple, does not require anaesthetization and a large number of animals can be exposed to aerosol at once. Knight et al. (4) previously demonstrated the efficacy of 9-nitrocamptothecin liposome aerosol delivered via nose only method in mice bearing human lung cancer xenografts. Their results showed that the mean tumor growth rate in the aerosol treatment group was seven times slower than in untreated animals (57.9 vs. 441 mm3) at the end of 28-day treatment schedule. Isotretinoin administered as aerosol by nose-only exposure system demonstrated effective chemopreventive properties even at low doses against urethane induced lung cancer in A/J mice (25). The calculated daily pulmonary dose of isotretinoin following nose-only exposure varied from 0.034–12.4 mg/kg per 45-min exposure. In comparison, orally administered isotretinoin at a much higher dose (>40 mg/kg daily) failed to prevent lung cancer in A/J mice. Our results demonstrate potent anti-cancer efficacy of Cxb aerosol administered by nose-only exposure system. The MMAD and GSD of Cxb aerosol generated by PARI LC Star Jet nebulizer in our studies were found to be 1.68 μm and 1.36, respectively. Aerosol particles with these characteristics are well suited for pulmonary deposition throughout the respiratory zone (7). In the nose only inhalation chamber, aerosol particles were collected from positions where mice inhale and therefore the data represents the particle size distribution of Cxb aerosol actually inhaled by mice. Previous studies using gemcitabine aerosol (7) (MMAD = 0.8 μm and GSD = 2.1), cyclosporine aerosol (8) (MMAD = 1.6 μm and GSD = 1.9) and paclitaxel aerosol (8) (MMAD = 2.2 μm and GSD = 1.9) have demonstrated potent anticancer activities with estimated deposited dose of inhaled drug ranging from 0.5–10 mg/kg/day. As described above, the estimated deposited dose of Cxb in lungs in our studies was 4.56 mg/kg/day and is within the limits demonstrated in previous studies using other aerosolized drugs. Raabe et al. (26) studied quantitative deposition of aerosols of different sizes (0.05 to 3.0 μm) in the respiratory airways in rats. Accordingly the mean percentage deposition of aerosol particles with aerodynamic diameter 2.09 μm was found to be 17.9 ± 4.5% in the Naso-pharynx region. In the present study Cxb aerosol administered to mice had MMAD of 1.68 μm. Thus we anticipate naso-pharynx deposition close of 17% with Cxb aerosol employed in this study.
Aerosolization of poorly water-soluble drugs via nebulization and the consequent aerosol exposure to mice pose difficulties in the proof-of-concept studies such as the current study. Dahl et al. (25) used ethanolic solutions of 13-cis Retinoic acid for chemoprevention in A/J mice which showed reductions of tumor multiplicity ranging from 56 to 80% (P < 0.005). In the present study, attempts were made to formulate Cxb as an aqueous formulation using ethoxylated derivative of Vitamin E viz TPGS, suitable for inhalation delivery with a nebulizer. Vitamin E TPGS is available commercially and has been used to enhance solubility and permeability of oral formulations (27,28). Previously, corticosteroids have been formulated as aqueous solutions using Vitamin E TPGS, which were administered either nasally or by inhalation. The use of Vitamin E TPGS in corticosteroid formulations was found particularly advantageous due its ability to solubilize corticosteroids and form stable micellar solutions suitable to be administered by inhalation (29). Somavarapu et al. (30) demonstrated the utility of Vitamin E TPGS as a matrix material blended with poly (caprolactone) for nasal immunisation with diphtheria toxoid. Similar attempt has been made in the present study to solubilize Cxb and to form a stable micellar solution upon dilution in a aqueous phase that can be administered by inhalation.
Recent reports from clinical trials have shown that Cxb induces heart attacks and strokes in the Phase III cancer studies (13). This is primarily attributed to the high oral dose of Cxb (400 mg, twice daily) employed in these studies. Data from our present study has showed that targeted delivery of Cxb by inhalation produced significant therapeutic activity at a very low therapeutic dose. The estimated deposited dose of aerosol Cxb was calculated to be 4.56 mg/kg per exposure period of 30 min. Under similar conditions, other researchers have shown the estimated total deposited dose of inhaled paclitaxel (5), cyclosporine (8) and gemcitabine (7) to be 7.8, 6.1 and 0.5 mg/kg, respectively. As described above, the combination of aerosolized Cxb (4.56 mg/kg/day) + i.v Doc (10 mg/kg) produced potent inhibition of the orthotopic lung tumor growth as compared to the other treatments. Superior efficacy of aerosol delivery as compared to conventional systemic delivery has been demonstrated by other researchers as well. Jia et al. (31) demonstrated that intranasal instillation of gemcitabine at a dose lower than the typical intravenous dose was more effective at inhibiting osteosarcoma lung metastases than systemic gemcitabine. Koshkina et al. (5) showed in their studies with camptothecin that biodistribution and pharmacokinetics of aerosolized drug were different than systemic administration in favor of pulmonary administration. Knight et al. (4) also demonstrated that nude mice with subcutaneous xenograft of human cancer did not respond to oral treatment with liposomal 9-nitrocamptothecin while the same dosage given by aerosol was highly effective. It is primarily reasoned that during aerosol treatment the inhaled drug is processed through the lungs before it gets into systemic circulation, whereas the drug administered systemically is first diluted in the blood before it reaches the lung. Further, direct lung delivery is not likely to produce any major cardiovascular problems primarily because very low amount of Cxb (as compared to oral administration) is required to be deposited in the lungs for anticancer effect. In general, the plasma concentrations following aerosol delivery are found to be less than 1/10th of maximum plasma concentrations after i.v administration (32). Further studies are however warranted to understand the cardiovascular safety of inhaled Cxb.
In the present study, we examined the effect of combination treatment of aerosol Cxb + i.v. Doc against oral Cxb + i.v. Doc, in vitro and in vivo. The intrabronchial tumor cell implantation in nude mice is demonstrated to resemble natural progression pattern of human lung cancer (33) and is therefore considered for in vivo testing of aerosolized Cxb with i.v Doc combination. Our previous research reports have demonstrated that Cxb interacts synergistically and exhibits a dose-dependent potentiation of Doc activity against A549 and H460 cells as measured by Combination index (CI) values following isobologramic analysis (34). The method takes into consideration the potency of each drug and shape of their dose-effect curves to quantitate the synergism or antagonism at different concentrations and at different effect levels (50 or 70% inhibition). The CI values at different concentrations of Cxb and Doc ranged from 0.4 to 1.1 indicative of synergistic to additive interaction. In a recent study, Nakata et al. (35) demonstrated that Cxb enhanced response of A431 human tumor xenograft in nude mice to the chemotherapeutic agent Doc by an enhancement factor (EF) of 2.07. Cxb also enhanced tumor response when added to the Doc plus radiation treatment (EF = 2.13). The EF is calculated by dividing the TCD50 value (tumor control dose 50, defined as the dose yielding tumor control in 50% of animals) of single treatment group by TCD50 value of combined treatment group.
In the current study, the combination of oral Cxb (150 mg/kg/day) with i.v Doc (10 mg/kg) showed slightly less inhibition of tumor growth as compared to the aerosol Cxb + i.v Doc combination. However, the oral Cxb with i.v Doc showed greater anti-tumor effect as compared to oral Cxb, aerosol Cxb and i.v Doc alone treatments even in an orthotopic lung tumor model employed in the current study. These results are in agreement with our previous data using the same combination in s.c A549 xenografted tumor model (18). In both the studies, we used oral Cxb at 150 mg/kg/day, which is below its maximum dose (250 mg/kg/day) employed by Zweifel et al. (36) and Davis et al. (37) in human head and neck squamous cell carcinoma, Col 26 murine carcinoma and HT-29 human colon carcinoma xenograft models. Our previous report also acknowledged that oral Cxb employed at 150 mg/kg/day or higher dose was effective in inhibiting lung tumors in combination with Doc (18). In a separate study (unpublished data) following single oral dose in a solution, Cxb (150 mg/kg) administered to BALBc mice, reached maximum pulmonary deposition of 8.4 ± 1.24 mg/kg following 1 h of administration. Similarly, Cxb aerosol (30 min exposure, 5 mg/ml Vitamin E TPGS solution) exposure to BALBc mice produced maximum pulmonary deposition of 3.57 ± 0.84 mg/kg following 1 h of starting aerosol exposure. Briefly, after sacrificing the animals 1 h after oral administration/aerosol exposure, lung tissue samples were removed and the right and left lobes of the lungs were quickly frozen in liquid nitrogen and stored at −20°C, before analysis. Cxb lung content was estimated following tissue homogenization on a mini-bead beater (Wig-L. Bug, Model 3110B, Crescent Dental MFR. Co., Lyons, IL). The tissue samples were homogenized with methanol, and the tissue homogenate was centrifuged at 16,000 rpm for 5 min at 5°C. The supernatant fraction was evaporated and reconstituted in the mobile phase, sonicated in the water bath and centrifuged at 1,000 rpm for 10 min. Supernatant fractions were analyzed by HPLC. Acetonitrile: water–acetic acid (60:40) with 0.05% glacial acetic acid was used as mobile phase and pumped at a flow rate of 1 ml/min. A 10 cm × 4.6 mm ID C18 analytical column packed with 5-μm reversed-phase particles and a Novo-Pak C8 guard column was used for chromatographic separation. The calculated amount of Cxb deposition following 1 h of exposure was close to the predicted value (4.56 mg/kg/exposure). However such estimations are compounded by factors such as efficiency of drug extraction and contribution from blood flow in the lungs when the animal is sacrificed. Similar reasonable agreement between the experimental deposition and mathematical data was demonstrated by Nadithe et al. (38) for 99m Tc-labeled human serum albumin aerosol deposition in mouse lungs. However, further studies are being conducted in our laboratory to fully characterize the pulmonary kinetics of inhaled Cxb.
Efficiency of oral/aerosol Cxb alone and in combination with Doc was demonstrated in the present study against A549 orthotopic lung tumors. Several different models have been developed to study human lung cancer. Various techniques (intrathoracic, intrapleural and intrabronchial) have been used to introduce tumor cells into the lung parenchyma. However, the histological characteristics of orthotopic tumor models are found to be consistent with the clinical tumor from which the cell lines are derived (39,40). Furthermore, when the cells are implanted intrabronchially, tumor is predominantly grown in the lung parenchyma in contrast to intrathoracically implanted tumors that are frequently located in the chest wall or pleural space. In view of these potential advantages, we considered the intrabronchial orthotopic model for in vivo testing of aerosolized Cxb. Further, the procedure is safe with low procedure related mortality (33).
We have previously reported the molecular mechanisms associated with the antiproliferative effect of the combination of Cxb with Doc in s.c. A549 tumors (18) and the combination of aerosolized Cxb with Doc in vitro in lung cancer cell lines (12). We observed that the combination of Cxb with Doc significantly improves the apoptotic response, inhibits angiogenesis and alters the expression of various proteins involved in the prostaglandins pathway (cPLA2 and mPGES) without altering the expression of COX-1 and COX-2. In the present study, we evaluated the role of fatty acid synthase (Fas) and FAS ligand (FasL) death receptor pathway in the increased anti-tumor effect of aerosolized Cxb with Doc. This pathway has been implicated in the apoptosis induced by Doc (41) and other chemotherapy-induced apoptosis in leukemia and several solid tumors (42). FasL is constitutively expressed in the lung tissue, hence over expression of Fas causes the activation of death receptor pathway leading to apoptosis. As shown in Fig. 4, Fas expression was significantly increased in lungs from mice treated with aerosolized/oral Cxb with Doc. Recently, Fas pathway has been shown to contribute to the therapeutic effect of aerosol gemcitabine (7). Along with the increase in Fas expression we also looked at caspase-3 expression. Caspases prove to be one of the key mechanisms in the induction of apoptosis, as they are involved in the downstream of several apoptotic pathways. It is known that procaspase 8 leads to caspase-8 formation, which can trigger caspase-3 release leading to apoptosis. Our earlier in vitro studies using aerosol Cxb demonstrated induction of apoptosis and decrease in pro-caspase 3 (12). In the current study as well we found significant increase in caspase-3 gene expression as a result of Cxb (aerosol/oral) + Doc treatments (Fig. 5). Moreover, aerosolized Cxb + Doc significantly enhanced caspase-3 expression as compared to oral Cxb + Doc and that too at a much lower therapeutic dose of Cxb. Therefore our results suggest that the enhanced anti-tumor effect of the combination of aerosolized Cxb with Doc is mediated via apoptosis. Ming et al. (43) demonstrated similar involvement of caspase-3 activation in apoptosis assessed in HepG2 cell treated with 20 μM troglitazone. Studies with malignant astrocytes and human liver cancer showed that PPAR-γ activation leads to increase in caspase 3 activity resulting in induction of apoptosis.
Another important rate limiting step in tumor development is angiogenesis. Avascular tumors are limited in size by the diffusion distance of oxygen, nutrients, and cellular waste through the interstitium. Although tumors often utilize the existing vasculature, a factor acting as a switch for the angiogenic surge is required for the tumor propagation, invasion and metastasis (44). We demonstrated in our study that in the lungs of mice treated with Cxb (aerosol/oral) and Doc, there was reduced expression of Factor VIII compared to untreated control (Fig. 6). Factor VIII is an endothelial cell marker and decrease in its expression can be correlated with reduction in blood vessel density, leading to reduced angiogenesis. There are varieties of factors that modulate angiogenesis. Amongst these are a family of CXC chemokines such as IL-8 (CXCL8), ENA78 (CXCL5), and Gro-α (CXCL1). Previous studies have established the involvement of CXC chemokines in human NSCLC (45). Hence in our study, with the decrease in Factor VIII expression, we looked at the IL-8 levels. We found that the Cxb (aerosol/oral) and Doc treatments significantly reduced IL-8 levels (Fig. 7) confirming that the combination treatments showed antiangiogenic property contributing to reduced tumor growth. We have previously demonstrated the inhibition of vascular endothelial growth factor (VEGF) expression in the combined treatment of Cxb with Doc in s.c A549 tumors (18). Based on this and the current study, it may be said that the combined treatment of Cxb with Doc results in reduced expression of both VEGF and Factor VIII.
The induction of COX-2 and its associated production of PGE2 from arachidonic acid are thought to play a role in the initiation and maintenance of cancer cell survival and growth (46). Phase II clinical data on the use of Cxb along with Doc in lung cancer patients indicated that PGE2 levels were significantly reduced in lung tumor biopsies (47). Earlier in vitro studies in our laboratory, using A549 and H460 cells, also indicated that Cxb and Doc combination significantly inhibited PGE2 levels compared to untreated controls (12). In our present in vivo study using the aerosol and oral Cxb in combination with Doc, we looked for lung tumor PGE2 levels as an indicator of the role of PGE2 in mediating the angiogenesis in lung tumors. Consistent with our previous observations of reduced intratumor PGE2 levels using COX-2 inhibitors like nimesulide and Cxb (11,12), we found that PGE2 levels in the lungs were significantly lowered as a result of Cxb (aerosol/oral) and Doc treatments (Fig. 8). Our results indicate that the combination of aerosol/oral Cxb with Doc produces significant anti-tumor effect, which is mediated via reduced PGE2 and IL-8 levels, leading to reduced angiogenesis (as indicated by reduced Factor VIII expression) and increased apoptosis mediated via Fas pathway.
CONCLUSIONS
These studies indicate the potential of inhalation delivery of Cxb for the treatment of lung cancer. Aerosolized Cxb at a much lower therapeutic dose than oral Cxb in combination with Doc showed significant inhibition of tumor progression, which was mediated via increased apoptosis and reduced angiogenesis. Although the development of a proper inhalation device in lung cancer patients is essential, further preclinical studies are needed to evaluate and explore the advantages associated with inhalation delivery of Cxb.
Acknowledgments
The authors acknowledge the financial support provided by RCMI award, G12RR03020-11 from NIH.
ABBREVIATIONS
- COX-2
cyclooxygenase-2
- Cxb
Celecoxib
- Doc
Docetaxel
- GSD
geometric standard deviation
- MMAD
mass median aerodynamic diameter
- NSCLC
non-small cell lung cancer
- PGE2
prostaglandin E2
- Vitamin E TPGS
tocopheryl polyethylene glycol 1000 succinate
References
- 1.Tian Y, Klegerman ME, Hickey ME, Hickey AJ. Evaluation of microparticles containing doxorubicin suitable for aerosol delivery to the lungs, PDA. J Pharm Sci Technol. 2004;58:266–275. [PubMed] [Google Scholar]
- 2.Zou Y, Fu H, Ghosh S, Farquhar D, Klostergaard J. Antitumor activity of hydrophilic paclitaxel copolymer prodrug using locoregional delivery in human orthotopic non-small cell lung cancer xenograft models. Clin Cancer Res. 2004;10:7382–7391. doi: 10.1158/1078-0432.CCR-04-0334. [DOI] [PubMed] [Google Scholar]
- 3.Tatsumura T, Kagamimori S. Further study of nebulization chemoctherapy, a new chemotherapeutic method in the treatment of lung carcinomas: fundamental and clinical. Br J Cancer. 1993;68:1146–1149. doi: 10.1038/bjc.1993.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight V, Koshkina NV, Waldrep JC, Giovanells B, Gilbert GE. Anticancer effect of 9-nitrocamptothecin liposome aerosol on human cancer xenograft in nude mice. Cancer Chemother Pharmacol. 1999;44:177–186. doi: 10.1007/s002800050965. [DOI] [PubMed] [Google Scholar]
- 5.Koshkina NV, Waldrep JC, Roberts LE, Melton S, Knight V. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin Cancer Res. 2001;7:3258–3262. [PubMed] [Google Scholar]
- 6.Koshkina NV, Knight V, Gilbert GE, Golunski E, Roberts LE, Waldrep JC. Improved respiratory delivery of the anticancer drugs, camptothecin and paclitaxel, with 5% CO2 enriched air: pharmacokinetic studies. Cancer Chemother Pharmacol. 2001;47:451–456. doi: 10.1007/s002800000230. [DOI] [PubMed] [Google Scholar]
- 7.Koshkina NV, Kleinerman ES. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer. 2005;116(3):458–463. doi: 10.1002/ijc.21011. [DOI] [PubMed] [Google Scholar]
- 8.Koshkina NV, Giovanells B, Roberts LE, Gilbert GE, Knight V. Cyclosporin A Aerosol improves the anticancer effect of Paclitaxel aerosol in mice. J Aerosol Med. 2004;17:7–14. doi: 10.1089/089426804322994415. [DOI] [PubMed] [Google Scholar]
- 9.Leahy M, Ornbrg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 10.Hida T, Kozaki KI, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahasi T. Cyclo-oxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006–2011. [PubMed] [Google Scholar]
- 11.Haynes A, Shaik MS, Chatterjee A, Singh M. Evaluation of an aerosolized selective COX-2 inhibitor as a potentiator of doxorubicin in a non-small-cell lung cancer cell line. Pharm Res. 2003;20(9):1485–1495. doi: 10.1023/a:1025774630993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes A, Shaik MS, Chatterjee A, Singh M. Formulation and evaluation of aerosolized celecoxib for the treatment of lung cancer. Pharm Res. 2005;22(3):427–439. doi: 10.1007/s11095-004-1881-z. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Flower R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. New Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 14.Haura EB. Treatment of advanced non-small cell lung cancer: a review of current randomized clinical trials and examination of emerging therapies. Cancer Control. 2001;8(4):326–336. doi: 10.1177/107327480100800404. [DOI] [PubMed] [Google Scholar]
- 15.Argiris A, Kut V, Luong L, Avram MJ. Phase I and pharmacokinetic study of docetaxel, irinotecan, and celecoxib in patients with advanced non-small cell lung cancer. Invest New Drugs. 2005;3:49–59. doi: 10.1007/s10637-005-3259-4. [DOI] [PubMed] [Google Scholar]
- 16.Onn A, Isobe T, Wu W, Itasaka S, Shintani T, Shibuya K, Kenji Y, O’Reilly M, Fidler IJ, Herbst R. Epidermal growth factor receptor tyrosine kinase inhibitor does not improve paclitaxel effect in an orthotopic mouse model of lung cancer. Clin Cancer Res. 2004;10:8613–8619. doi: 10.1158/1078-0432.CCR-04-1241. [DOI] [PubMed] [Google Scholar]
- 17.Luh HH, Che T. An orthotopic model of human osteosarcoma growth and spontaneous pulmonary metastasis. Clin Exp Metastasis. 2005;22(4):319–329. doi: 10.1007/s10585-005-0365-9. [DOI] [PubMed] [Google Scholar]
- 18.Shaik MS, Chatterjee A, Jackson T, Singh M. Enhancement of anti-tumor activity of docetaxel by celecoxib in lung tumors. Int J Cancer. 2006 January 15;118(2):396–404. doi: 10.1002/ijc.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trifan OC, Durham WF, Salazar VS, Horton J, Levine BD, Zweifel BS, Davis TW, Masferrer JL. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 2002;62:5778–5784. [PubMed] [Google Scholar]
- 20.Kohlhaufl M, Haussinger K, Stanzel F, Markus A, Tritschler J, Muhlhofer A, Morresi-Hauf A, Golly I, Scheuch G, Jany BH, Biesalski HK. Inhalation of aerosolized vitamin a: reversibility of metaplasia and dysplasia of human respiratory epithelia—a prospective pilot study. Eur J Med Res. 2002;7:72–78. [PubMed] [Google Scholar]
- 21.Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Lung deposition of hydrofluroalkane-134a Beclomethasone Is greater than that of chloroflurocarbon fluticasone and chloroflurocarbon beclomethasone: a crossover study in healthy volunteers. Chest. 2002;122:510–516. doi: 10.1378/chest.122.2.510. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Chen HT, Roa W, Finlay WH. Farnesol for aerosol inhalation: nebulization and activity against human lung cancer cells. J Pharm Pharmaceut Sci. 2003;6:95–100. [PubMed] [Google Scholar]
- 23.Wattenberg LW, Wiedmann TS, Estensen RD. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997;57:5489–5492. [PubMed] [Google Scholar]
- 24.Sharma S, White D, Imondi AR, Placke ME, Vali DM, Kris MG. Development of inhalational agents for oncologic use. J Clin Oncol. 2001;19:1839–1847. doi: 10.1200/JCO.2001.19.6.1839. [DOI] [PubMed] [Google Scholar]
- 25.Dahl AR, Grossi IM, Houchens DP, Scovell LJ, Placke ME, Imondi AR, Stoner GD, Deluca LM, Wang D, Mulshine JL. Inhaled isotretinoin (13-cis Retinoic acid) is an effective lung cancer chemopreventive agent in A/J mice at low doses: a pilot study. Clin Cancer Res. 2000;6:3015–3024. [PubMed] [Google Scholar]
- 26.Raabe OG, Bayati MA, Teafue SV, Rasolt A. Regional deposition of inhaled monodisperse coarse and fine aerosol particles in small laboratory animals. Ann Occup Hyg. 1988;32(Suppl 1):53–63. [Google Scholar]
- 27.Varma MV, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur J Pharm Sci. 2005 July–August;25(4–5):445–453. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Feng SS. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006 January;27(2):262–270. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 29.Saidi Z, Boris K. Aqueous composition containing corticosteroids for nasal and pulmonary delivery. 6,241,969. US patent. 2001
- 30.Somavarapu S, Pandit S, Gradasi G, Bandera M, Ravichandran E, Alpar OH. Effect of vitamin E TPGS on immune response to nasally delivered diphtheria toxoid loaded poly(caprolactone) microparticles. Int J Pharm. 2005 July;298(2):344–347. doi: 10.1016/j.ijpharm.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–506. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 32.Hershey AE, Kurzman ID, Forrest LJ, Bohling CA, Stonerook M, Placke ME, Imondi AR, Vail DM. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: Proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res. 1999;5:2653–2659. [PubMed] [Google Scholar]
- 33.Gagnadoux F, Urban T. Aerosol delivery of chemotherapy in an orthotopic model of lung cancer. Eur Respir J. 2005;26:1–5. doi: 10.1183/09031936.05.00017305. [DOI] [PubMed] [Google Scholar]
- 34.Fulzele SV, Shaik MS, Chatterjee A, Singh M. Anticancer effect of celecoxib and aerosolized docetaxel against human non-small cell lung cancer cell line, A549. J Pharm Pharmacol. 2006;58(3):327–336. doi: 10.1211/jpp.58.3.0006. [DOI] [PubMed] [Google Scholar]
- 35.Nakata E, Mason KA, Hunter N, Hussain A, Raju U, Liao A, Lang KK. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol Biol Phys. 2004;58(2):369–375. doi: 10.1016/j.ijrobp.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 36.Zweifel BS, Davis TW, Ornberg RL, Masferrer JL. Direct evidence for a rolw of cyclooxygenase-2 derived prostaglandin E2 in human head and neck xenograft tumors. Cancer Res. 2002;62:6706–6711. [PubMed] [Google Scholar]
- 37.Davis TW, O’Neal JM, Pagel MD, Zweifel BS, Mehta PP, Heuvelman DM, Masferrer JL. Synergy between celecoxib and radiotherapy results from inhibition of cyclo-oxygenase-2 derived prostaglandin E2, a survival factor for tumor and associated vasculature. Cancer Res. 2004;64:279–285. doi: 10.1158/0008-5472.can-03-1168. [DOI] [PubMed] [Google Scholar]
- 38.Nadithe V, Rahmatalla M, Finlay WH, Mercer JR, Samuel J. Evaluation of nose-only aerosol inhalation chamber and comparison of experimental results with mathematical simulation of aerosol deposition in mouse lungs. J Pharm Sci. 2003;92(5):1066–1076. doi: 10.1002/jps.10379. [DOI] [PubMed] [Google Scholar]
- 39.McLemore TL, Liu MC, Blank PC. Novel intra-pulmonary model for orthotopic propogation of human lung cancers in athymic mice. Cancer Res. 1987;47:5132–5140. [PubMed] [Google Scholar]
- 40.McLemore TL, Eggleston JC, Shoemaker RH. Comparison of intrapulmonary, percutaneous intrathorasic and subcutaneous models for the propogation of human pulmonary and non-pulmonary cell lines in athymic nude mice. Cancer Res. 1988;48:2880–2886. [PubMed] [Google Scholar]
- 41.Yoo GH, Piechocki MP, Ensley JF, Nguyen T, Oliver J, Meng H, Kewson D, Shibuya TY, Lonardo F, Tainsky MS. Docetaxel induced gene expression patterns in head and neck aquamous cell carcinoma using cDNA microarray and power blot. Clin Cancer Res. 2002;8(12):3910–3921. [PubMed] [Google Scholar]
- 42.Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT. Sensitization of cancer cells treated with cytotoxic drugs to fas-mediated cytotoxicity. J Natl Cancer Inst. 1997 June 4;89(11):783–789. doi: 10.1093/jnci/89.11.783. [DOI] [PubMed] [Google Scholar]
- 43.Ming Y, Deng H, Zhao JM, Dong D, Tan X. PPAR-gamma pathway activation results in apoptosis and COX-2 inhibition in HepG2 cells. World J Gastroenterol. 2003;9(6):1220–1226. doi: 10.3748/wjg.v9.i6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keshamouni VG, Arenberg DA, Reddy RC, Newstead MJ, Anthwal S, Standiford TJ. PPAR-gamma activation inhibits angiogenesis by blocking ELR + CXC chemokine production in non-small cell lung cancer. Neoplasia. 2005 May;7(3):294–301. doi: 10.1593/neo.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White ES, Strieter RM, Arenberg DA. Chemokines as therapeutic targets in non-small cell lung cancer. Curr Med Chem Anti-Canc Agents. 2002 May;2(3):403–417. doi: 10.2174/1568011024606406. [DOI] [PubMed] [Google Scholar]
- 46.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 47.Csiki I, Dang T, Gonzalez A, Sandler A, Carbone D, Choy H, Campbell N, Garcia B, Morrow J, Johnson DH. Cyclooxygenase-2 (COX-2) inhibition docetaxel (Txt) in recurrent nonsmall cell lung cancer (NSCLC): preliminary results of a Phase II trail (THO-0054) Proc Am Soc Clin Oncol. 2002;21:A1187. [Google Scholar]