Abstract
Chronic manganese (Mn) exposure produces a neurological syndrome with psychiatric, cognitive and parkinsonian features. Gene expression studies in the frontal cortex of Cynomolgus macaques exposed to different doses of Mn showed gene expression changes associated with cell cycle regulation, DNA repair, apoptosis, ubiquitin-proteasome system, protein folding, cholesterol homeostasis, axonal/vesicular transport and inflammation. Amyloid-beta (A-beta) precursor-like protein 1 (APLP1), a member of the amyloid precursor family, was the most highly up-regulated gene. Immunohistochemistry confirmed increased APLP1 expression and revealed the presence of A-beta diffuse plaques. Cortical neurons and white matter fibers from Mn-exposed animals exhibited accumulation of silver grains indicative of on-going degeneration. Cortical neurons also expressed nuclear hypertrophy, intracytoplasmic vacuoles, and apoptotis stigmata. The levels of p53 were increased in neurons and glial cells in Mn-exposed tissue. Analysis of another amyloidogenic protein, -synuclein, also exhibited aggregation in the gray and white matter from Mn-exposed animals. In summary, chronic Mn exposure in non-human primates produces a cellular stress response leading to neurodegenerative changes, diffuse A-beta plaques and -synuclein aggregation in the frontal cortex. These changes may help explain the cognitive and working memory deficits expressed by these animals.
Keywords: Manganese, frontal cortex, neurodegeneration, Alzheimer's Disease, APLP1, neurotoxicity
It is now well recognized that chronic manganese (Mn) exposure produces a neurological syndrome with psychiatric, cognitive and parkinsonian features. Until recently, the great majority of studies examine the neurological consequences of chronic Mn exposure were focused on Mn effects on the basal ganglia due to its effects on motor function. During the last decade, a number of human studies have implicated Mn-induced deficits in attention and cognitive impairment that may persist long after exposure (Josephs et al., 2005; Bouchard et al., 2007; Bowler et al., 2006, 2007; Klos et al., 2007). An association between environmental exposure to Mn and deficits in measure of intellectual function has been described in children (Takser et al., 2003; Wasserman et al., 2006). It has been suggested that Mn effects on the frontal cortex and subcortical structures may be associated with these cognitive and executive function deficits (Josephs et al., 2005; Bowler et al., 2006a).
Based on these observations in Mn-exposed humans, we performed gene array analysis of frontal cortex tissue from Mn-exposed non-human primates (Guilarte et al., 2008). The array consisted of 9600 features that corresponded to 6700 unique genes. The results showed that 61 genes were significantly up-regulated (Z score > 1.5) and 4 genes were significantly down-regulated (Z score < 1.5). Grouping of these genes by biological categories indicated that chronic Mn exposure affected genes associated with: 1) cholesterol metabolism/transport, 2) axonal/vesicular transport, 3) inflammation/immune response, 4) cell cycle regulation/DNA repair/DNA biosynthesis, and 5) proteasome/protein folding/protein turnover. However, the most highly up-regulated gene was Amyloid- Precursor Protein 1 (APLP1), a member of the Amyloid Precursor Protein (APP) family associated with Alzheimer's disease. Immunohistochemistry confirmed increased APLP1 expression and revealed the presence of amyloid-β diffuse plaques (Guilarte et al., 2008). Further analysis of the Mn-induced gene changes indicated that many of the genes were activated by the transcription factor p53 or their protein product interacted with p53 to change its function. Consistent with a role of p53 in the gene expression changes noted, p53 protein levels were increased in frontal cortex neurons and glial cells (Guilarte et al., 2008).
The observation that Mn-exposed animal exhibited diffuse amyloid-β plaques was new and unexpected since these were young adult animals (6-8 years at the end of the study) that should not have any evidence of plaque formation at this age. This is important because aged monkeys (in their 20s) do begin to express diffuse amyloid- plaques as a function of the normal aging process (Kimura et al., 2003, 2005). Therefore, it appears that chronic Mn exposure may accelerate some of these putative pathological processes. To investigate whether the effect of chronic Mn-exposure was specific to amyloid-β, we also performed immunohistochemistry in the same animals for another amyloidogenic protein, α-synuclein. Our results indicated that animals exposed to Mn exhibit α-synuclein positive aggregates similar to the stages of Lewy bodies and Lewy neurites in the frontal cortex (data not shown). α-synuclein positive aggregates were present in neurons and glial cells in the grey and adjacent white matter. Therefore, it appears that chronic Mn exposure may produces changes in the aggregation of amyloid-β and α-synuclein.
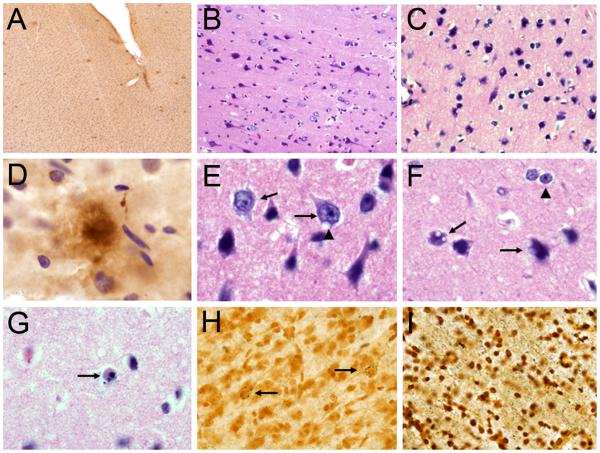
From a neuropathological perspective, we found that in the frontal cortex of these Mn-exposed animals, cortical neurons and white matter fibers exhibited accumulation of silver grains indicative of on-going degeneration. [See Figure 1 for examples for neuropathological changes observed in the frontal cortex of Mn-exposed animals]. Cortical neurons also expressed nuclear hypertrophy, intracytoplasmic vacuoles, and apoptotis stigmata. Activation of astrocytes was present in both the grey and white matter and we found numerous Alzheimer's type II astrocytes (Guilarte et al., 2008). In summary, this study showed that chronic Mn exposure in non-human primates produces a cellular stress response leading to neurodegenerative changes, and diffuse amyloid-β plaques in the frontal cortex.
Figure 1.
Neuropathology in the frontal cortex of manganese-exposed non-human primates. A) Low magnification (4×) image of diffuse β-amyloid plaques (6E10 immunohistochemistry) in the frontal cortex of a Mn-exposed animal. B) H&E staining of frontal cortex tissue from a Mn-exposed animal shows numerousneurons with hypertrophic nuclei. C) H&E staining of frontal cortex tissue from a Mn-exposed animal shows numerous neurons expressing intracytoplasmic vacuoles. D) High magnificantion (100×) image of a diffuse β-amyloid plaque (6E10) immunohistochemistry as shown in panel A. E) High magnification (100×) image of neurons with hypertrophic nuclei (arrows) as shown in panel B. One of the neurons also has an intracytoplasmic vacuole (arrow head). F) High magnification (100×) image of neurons with intracytoplasmic vacuoles (arrows) as in panel C and Alzheimer's type II astrocytes (arrow head). G) High magnification image (100×) of an apoptotic cell in the frontal cortex of a Mn-exposed animals (H&E staining). H) Silver staining of frontal cortex gray matter in a Mn-exposed animal. Arrows indicate neurons accumulating silver grains indicative of a neurodegenerative process. I) Silver staining of frontal white gray matter in a Mn-exposed animal. See extensive silver grain accumulation in white matter fibers.
The gene expression changes and neuropathology in the frontal cortex of Mn-exposed animals suggests that neurological function mediated by the frontal cortex is affected in these animals. Behavioral examination of the Mn-exposed animals provided evidence of cognitive and working memory deficits (Schneider et al., 2006; 2009). These studies are novel in that they provide underlying cellular and molecular changes in the frontal cortex of non-human primates that expressed behavioral changes similar to those documented in humans occupationally exposed to Mn.
A central player in the proposed mechanism of Mn-induced neurodegeneration in the frontal cortex is the transcription factor p53. This is consistent with the fact that several of the genes whose expression was changed in Mn-exposed frontal cortex are documented to be p53 target genes or their protein product interacts with p53 to alter its function (Guilarte et al., 2008). p53 is a transcription factor known to regulate several major cellular functions including gene transcription, DNA synthesis and repair, cell cycle regulation, senescence and apoptosis (Culmsee and Mattson, 2005). These are some of the same biological categories whose genes were found affected in the frontal cortex of Mn-exposed animals (See Table 1 in Guilarte et al., 2008). Activation of p53 activity is known to occur following insults that produce genotoxic or oxidative stress (Morrison and Kinoshita, 2000; Culmsee and Mattson, 2005). Manganese produces oxidative stress in cell culture systems (Hirata, 2002; HaMai and Bondy, 2004; Latchoumycandane et al., 2005). Thus, p53 activation may play a central role in the gene expression changes documented in the frontal cortex of Mn-exposed non-human primates. The involvement of p53 activation in the toxic effects of Mn in the frontal cortex is suggested by p53 immunoreactivity in cells with apoptosis stigmata (Guilarte et al., 2008). Consistent with this observation, non-transcriptional p53-induced apoptosis mediated by p53 localization in the cytoplasm or in the mitochondria has been documented (Moll et al., 2005). Increased p53 reactivity was also present in the cytoplasm of neurons with hypertrophic nuclei and in the nucleus and processes of glial cells in the cortex of Mn-exposed animals (Guilarte et al., 2008). This pattern of p53 subcellular localization in neuronal and glial cells has been described in the brain of patients with neurodegenerative diseases including AD (de la Monte et al., 1997). Therefore, increased p53 protein expression is commonly associated with neuronal injury and cell death in neurodegenerative diseases and we now show it to be involved in Mn-induced neurodegeneration. These studies provide new information on Mn-induced effects in the frontal cortex, a brain region that has not been previously associated with Mn neurotoxicity. Unlike the globus pallidus, a brain region that accumulates the highest concentration of Mn in the brain, the frontal cortex accumulates much lower Mn concentrations. Therefore, our data suggests that Mn-induced neurodegeneration is not only defined by the degree of Mn accumulation but also by the intrinsic vulnerability of a particular brain region to Mn neurotoxicity.
Acknowledgements
This work was supported by NIEHS grant # ES010975 to TRG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouchard M, Mergler D, Baldwin M, Panisset M, Bowler R, Roels HA. Neurobehavioral functioning after cessation of manganese exposure: a follow-up after 14 years. Am J Ind Med. 2007;50:831–840. doi: 10.1002/ajim.20407. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: Neurological and neuropsychological sequelae. Neurotoxicology. 2006a;27:327–332. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL. Dose-effect relationship between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007;64:167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Sohn YK, Wands JR. Correlates of p53- and Fas (CD95)-mediated apoptosis in Alzheimer's disease. J Neurol Sci. 1997;152:73–83. doi: 10.1016/s0022-510x(97)00131-7. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- Hirata Y. Manganese-induced apoptosis in PC12 cells. Neurotoxicol Teratol. 2002;24:639–653. doi: 10.1016/s0892-0362(02)00215-5. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowel CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tanemura K, Nakamura S-I, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes in Alzheimer's disease-associated proteins in cynomolgus monkey brains. Biochem Biophys Res Comm. 2003;310:303–311. doi: 10.1016/j.bbrc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Kimura N, Yanagisawa K, Terao K, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes of intracellular A in cynomolgus monkey brains. Neuropath. Appl Neurobiol. 2005;31:170–180. doi: 10.1111/j.1365-2990.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Kinoshita Y. The role of p53 in neuronal cell death. Cell Death Diff. 2000;7:868–879. doi: 10.1038/sj.cdd.4400741. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR. Effects of chronic manganese exposure on working memory in non-human primates. Brain Res. 2009;1258:86–95. doi: 10.1016/j.brainres.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ashan H, Levy D, Factor-Litvak P, Kline J, vanGene A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Persp. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]