Abstract
Introduction:
Platelet hyperreactivity associates with cardiovascular events in humans. Studies in mice and humans suggest that prostaglandin E2 (PGE2) regulates platelet activation. In mice, activation of the PGE2 receptor subtype 3 (EP3) promotes thrombosis, but the significance of EP3 in humans is less well understood.
Objectives:
To characterize the regulation of thromboxane-dependent human platelet activation by PGE2.
Patients/Methods:
Platelets collected from nineteen healthy adults were studied using an agonist of the thromboxane receptor (U46,619), PGE2, and selective agonists and/or antagonists of the EP receptor subtypes. Platelet activation was assayed by (1) optical aggregometry, (2) measurement of dense granule release, and (3) single-platelet counting.
Results:
Healthy volunteers demonstrated significant interindividual variation in platelet response to PGE2. PGE2 completely inhibited U46,619-induced platelet aggregation and ATP release in 26% of subjects; the remaining 74% had partial or no response to PGE2. Antagonism of EP4 abolished the inhibitory effect of PGE2. In all volunteers, a selective EP2 agonist inhibited U46,619-induced aggregation. Furthermore, the selective EP3 antagonist DG-041 converted all PGE2 nonresponders to full responders.
Conclusions:
There is significant interindividual variation of platelet response to PGE2 in humans. The balance between EP2, EP3, and EP4 activation determines its net effect. PGE2 can prevent thromboxane-induced platelet aggregation in an EP4-dependent manner. EP3 antagonism converts platelets of nonresponders to a PGE2-responsive phenotype. These data suggest that therapeutic targeting of EP pathways may have cardiovascular benefit by decreasing platelet reactivity.
Keywords: EP receptors, Platelet aggregation, Platelet reactivity, Prostaglandin E2, Thromboxane
Platelet hyperreactivity contributes to the pathophysiology of occlusive thrombi that cause cardiovascular events. Patients with increased platelet reactivity are at increased risk for complications after percutaneous coronary interventions (PCI) and cardiovascular mortality.[1-5] Endothelium-derived mediators, including several prostaglandins, influence blood flow and coagulation, in part by modulating platelet reactivity. Prostacyclin (PGI2), the most studied of these prostanoids, inhibits platelet aggregation and adhesion.[6-8] The endothelium of the human microvasculature produces PGE2,[9] and in healthy humans, both PGI2 and PGE2 modulate the coronary response to sympathetic stimulation[10], suggesting that PGE2 contributes to vascular physiology and thrombosis.
Four G-protein-coupled receptors mediate the actions of PGE2: EP1, EP2, EP3 (multiple splice variants), and EP4. In mice, low concentrations (e.g., 100 nM) of PGE2 potentiate platelet aggregation by activating EP3, whereas high concentrations (e.g., 0.6 mM) inhibit aggregation by stimulating the prostacyclin receptor (IP).[11] EP3−/− mice have increased bleeding times and decreased susceptibility to thromboembolism when challenged with intravenous or periadventitial delivery of arachidonic acid.[12, 13] Furthermore, local delivery of arachidonic acid to arterial walls stimulates production of PGE2 in mice, and plaque-produced PGE2 can activate platelet EP3, facilitating arterial thrombosis.[14] These animal data have driven the ongoing clinical development of an EP3 antagonist (DG-041) as an antiplatelet agent.[15, 16]
In humans, the role of PGE2 has been well studied in the context of plaque rupture, but its effect on platelet reactivity is not well understood. In atherosclerotic plaque, macrophages primarily produce PGE2.[17, 18] Studies in the early 1970s noted that the effect of PGE2 on platelets was either inhibitory or stimulatory depending on its concentration and the species of animal examined.[19-22] Bruno et al. reported that low doses of PGE2 did not potentiate aggregation induced by either ADP or collagen, but higher (supraphysiological) doses (>1 μM) inhibited aggregation.[19] Others have observed potentiation of ADP- or collagen-induced aggregation at low doses (10-100 nM PGE2) and inhibition at high doses (10 μM PGE2), similar to the data from mice.[23, 24] Inferences from animal data suggest that these effects are mediated by EP3 and IP, respectively, but there are few studies in humans that characterize the mechanisms of PGE2 action on platelet function, especially with regard to modulation of thromboxane-mediated events. Accordingly, we investigated the effects of PGE2 on thromboxane-mediated human platelet activation using PGE2 and selective agonists and antagonists of EP subtypes.
Materials and Methods
Materials
U46,619, PGE2, butaprost free acid, sulprostone, and PGE1-OH were obtained from Cayman Chemical (Ann Arbor, MI). DG-041 was synthesized by the Vanderbilt Institute of Chemical Biology Synthesis Core. MF-191 was kindly provided by Merck Frosst Canada Ltd. (Kirkland, Quebec). CHRONO-LUME® was purchased from Chrono-Log Corporation (Havertown, PA). PGE2 stock (2 mg/ml in EtOH; 5.67 mM) was kept at −20°C and serially diluted in PBS to 100X working concentrations.
Human Subjects and Blood Collection
This study was approved by the Vanderbilt University Institutional Review Board. Nineteen healthy adult volunteers (11 men, 8 women) participated after providing written informed consent. All denied taking any medications within the preceding 14 days. Blood was drawn using a 19-gauge butterfly cannula; the first 3 ml was discarded before drawing blood into a polypropylene syringe containing 3.2% sodium citrate (final dilution 1:9). Blood was centrifuged at 190 × g for 10 minutes at room temperature and the platelet-rich plasma (PRP) was transferred to a polypropylene container. The residual blood was centrifuged at 2000 × g for 10 minutes and the platelet-poor plasma (PPP) was transferred to a separate polypropylene container. The platelet concentration of PRP was determined using a Z1 Dual Threshold particle counter (Beckman-Coulter) and was adjusted to 2.5×108 platelets/ml with autologous PPP.
Platelet Aggregation
Measurements of platelet aggregation in PRP were made using light transmission aggregometry using a Chrono-Log lumi-aggregometer (Model 460VS). The aggregometer was calibrated for each sample to read 0-10% light transmission for PRP and 90-100% light transmission for PPP. Aliquots of PRP were placed in siliconized glass cuvettes containing teflon-coated stir bars and incubated for 2 minutes at 37°C. When measurement of concomitant ATP release was desired, CHRONOLUME® reagent was added per the manufacturer's protocol and incubated for an additional 2 minutes. Compounds of interest and aggregation-inducing agonists were added to the sample after initiation of recording. Samples were stirred at 1200 rpm throughout. Maximum percentage aggregation (0-100 %) and ATP release were calculated using Aggrolink software (Chrono-Log). Experiments were completed within 2.5 hours of phlebotomy.
Single-Platelet Counting
In contrast to optical aggregometry, which measures macroaggregation, single-platelet counting measures microaggregation. After incubating PRP with compounds of interest for 6 minutes at 37 °C, stirred throughout at 1200 rpm as above, a 50 μl aliquot was removed for single-platelet counting. This aliquot was added to 5 μl of 10 X fixation buffer (0.1% formaldehyde in 3 mM sodium EDTA) and incubated at room temperature for at least 30 min. The fixed platelets were counted within one hour using a Z1 Dual Threshold particle counter (Beckman-Coulter) with a 50 μm aperture and monitoring the volumes between 3 and 30 fl.
Preparation of EP Agonists/Antagonists
All EP agonists/antagonists were freshly diluted in PBS to avoid vehicle-mediated effects on platelet aggregation (tested in parallel aggregation reactions).
Statistics
Normal distribution was assessed using Shapiro-Wilk test. Data are presented as mean ± SEM. Differences between two groups were analyzed using the Student's t-test for normally distributed data and using the Mann-Whitney test when a normal distribution was not assumed. Differences between more than two groups were analyzed by repeated measures ANOVA (or Friedman test) when samples from individual volunteers were subjected to several treatments in a single experiment; post hoc comparisons were made using Newman-Keuls (or Dunn's) multiple comparison test. P < 0.05 was considered statistically significant.
Results
Effect of PGE2 on Platelet Activation
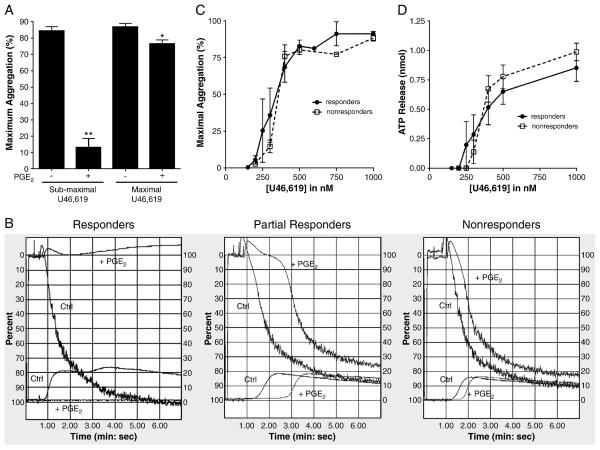
We activated platelets in PRP with the thromboxane receptor agonist U46,619. For each of the 19 subjects studied, we determined the dose-response curve for U46,619 with respect to platelet aggregation (maximum chart deflection within 6 minutes). We consistently observed maximal aggregation responses with 1 μM U46619 (“maximal” dose) and “submaximal” aggregation responses with 300-500 nM U46,619. Most individuals had minimal, if any, platelet aggregation induced by < 300 nM U46,619.
We investigated the effects of PGE2 on platelets with three measures of platelet activation in citrated PRP: (1) macroaggregation using optical aggregometry, (2) release of dense granules, and (3) single-platelet counting. In 5 of 19 (26%) individuals, 100 nM PGE2 inhibited ~85 % of U46,619-induced platelet aggregation at doses of U46,619 that produced submaximal responses in the absence of PGE2 (Fig. 1A). We refer to these individuals as “PGE2 responders”; a typical aggregation curve is shown in figure 1B (left panel). All subjects were tested on more than one day, and each individual's response to PGE2 was consistent. Pre-incubation with the IP antagonist CAY10441 (1 μM) or 100 μM aspirin did not affect this inhibition of U46,619-induced aggregation by PGE2 (data not shown). Furthermore, the inhibitory effect of PGE2 depended on the dose of the platelet agonist: in these same individuals, 100 nM PGE2 inhibited only 12 % of U46,619-induced platelet aggregation when a maximal dose of U46,619 was used (Fig. 1A); increasing the dose of PGE2 to 5 μM produced similar results (data not shown).
Figure 1.
(A) Effect of preincubation with 100 nM PGE2 before U46,619-induced aggregation among PGE2 responders. PGE2 significantly inhibited aggregation in this subgroup of healthy volunteers (** P < 0.001 for submaximal U46,619, n=5; * P < 0.005 for maximal U46,619, n=3; paired t-test). (B) Representative aggregation traces from a PGE2 responder, partial responder, and nonresponder. Aggregation was induced with submaximal concentrations of U46,619. PGE2 (100nM) was added 30 seconds before U46,619. Upper curves: aggregation. Lower curves: ATP release. (C) Dose-response relationships for U46,619-induced aggregation and (D) ATP release in human PRP for both responders and nonresponders showing no difference in the dose-response to U46,619 between these groups. Bars represent mean ± SEM.
In 14 of 19 (74%) individuals, 100 nM PGE2 did not significantly attenuate the magnitude of U46,619-induced platelet aggregation regardless of the dose of U46,619, even when we increased the dose of PGE2 to 5 μM. In 6 of these 14 individuals, PGE2 slowed the rate of aggregation, but the maximal aggregation achieved was similar to that when PGE2 was not present (Figure 1B, middle panel). In the remaining 8 of these 14 individuals, PGE2 seemed to have no effect on the rate or magnitude of platelet aggregation (Fig. 1B, right panel). Throughout the current report, we refer collectively to these 14 individuals as “PGE2 nonresponders,” recognizing that the “partial responders” appear to have an intermediate phenotype that requires further exploration.
Because the inhibitory effect of PGE2 among responders is dependent on the concentration of U46,619, we investigated whether differential sensitivity to U46,619 may explain the interindividual variation in response to PGE2. The U46,619 dose-response curves were similar between PGE2 responders and nonresponders (Fig. 1C and 1D), making this an unlikely explanation for our observations.
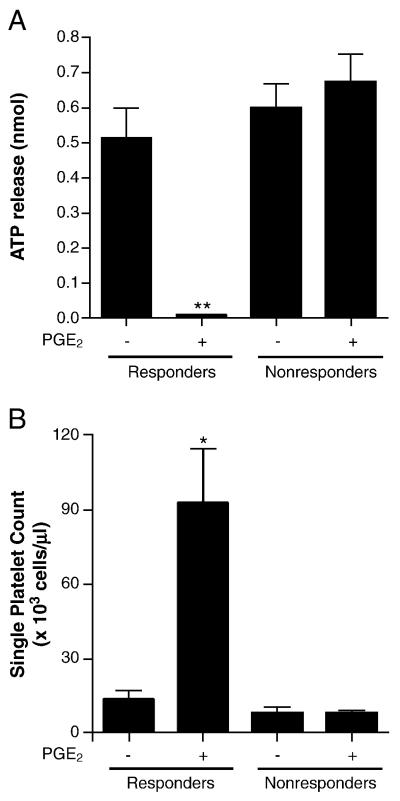
Because platelet aggregation is a relatively insensitive measure of platelet activation, we employed more sensitive techniques to investigate whether PGE2 had effects on the platelets of individuals deemed “nonresponders” by optical aggregometry. Measurement of dense granule release, another marker of platelet activation, corroborated the results obtained from optical aggregation (Fig. 2A). Specifically, 100 nM PGE2 completely suppressed U46,619-induced dense granule release in the PGE2 responders when we used submaximal (but not maximal) doses of agonist. Furthermore, we did not detect an effect of PGE2 on dense granule release among the PGE2 nonresponders.
Figure 2.
Pre-incubation with PGE2 (100 nm) inhibited platelet dense granule release (A) and increased the number of single platelets remaining after the addition of agonist (B) among PGE2 responders but not among PGE2 nonresponders. (** P<0.005, n=5; *P < 0.05, n=4; paired t-test).
Changes in light transmission during optical aggregometry require the macroaggregation of platelets. In contrast, formation of microaggregates in response to an agonist may be missed by optical aggregometry. To determine whether PGE2 may have a biological effect on the formation of microaggregates, especially among the apparent nonresponders, we counted single platelets after incubating with a submaximal dose of U46,619 in the presence and absence of PGE2 (Fig. 2B). Using this measure of platelet activation, we did not detect an effect of PGE2 on U46,619-induced platelet activation among PGE2 nonresponders. In contrast, among PGE2 responders, we did count significantly more single platelets after U46,619-induced activation in the presence of PGE2; this both confirmed the inhibitory effect of PGE2 in this population and served as a positive control for our single-platelet count method.
Because of the concordance observed between optical aggregometry, dense granule release, and single-platelet counting, we chose to use optical aggregometry as our primary measure for the subsequent investigations of the specific contributions of each EP subtype.
Balance of EP3 and EP4 Determines Inhibition of Platelet Aggregation by PGE2
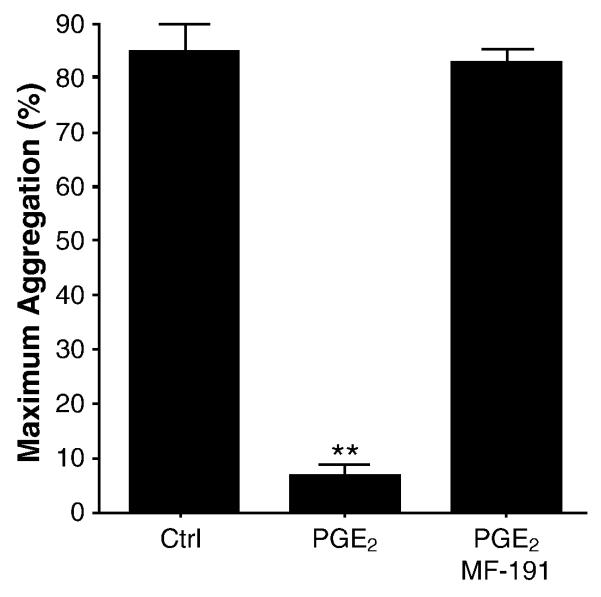
Because the PGE2 receptor subtypes 2 and 4 (EP2, EP4) are Gs-coupled receptors that increase cAMP[25], we hypothesized that PGE2-mediated inhibition of U46,619-induced platelet aggregation may involve one, or both, of these receptors. MF-191 is a selective EP4 antagonist (500-fold selective for EP4 compared with EP1-3, DP, CRTH2, TP, IP, FP) that exhibits an IC50 of 4.5 nM in cell-based cAMP accumulation assays (Merck Frosst; personal communication). In PGE2 responders, preincubation with 50 nM MF-191 before the addition of PGE2 completely abrogated the inhibition of U46,619-induced platelet aggregation and ATP release by PGE2 (Figure 3). MF-191 did not affect platelet aggregation in the absence of U46,619 or PGE2 (data not shown). These data suggest the inhibition of U46,619-induced platelet aggregation by PGE2 requires EP4.
Figure 3.
Pretreatment with the EP4 antagonist MF-191 (50nM) blocked the inhibitory effect of PGE2 among responders (n=4). Aggregation was induced with submaximal concentrations of U46,619. Bars represent mean ± SEM. (P<0.0001 by repeated measures ANOVA; ** P<0.001, compared with all other conditions by Newman-Keuls multiple comparison test.)
The ultimate effect of PGE2 on human platelet aggregation seems to depend on the balance of proaggregatory and antiaggregatory stimuli generated by agonism of the EP subtypes. EP3 activation has been shown to potentiate platelet aggregation. We confirmed that sulprostone potentiated aggregation and dense granule release induced by subthreshold concentrations of U46,619 in a dose-dependent fashion in our population (Supporting Figure S1). As expected, sulprostone did not stimulate platelet aggregation in the absence of U46,619 (data not shown).
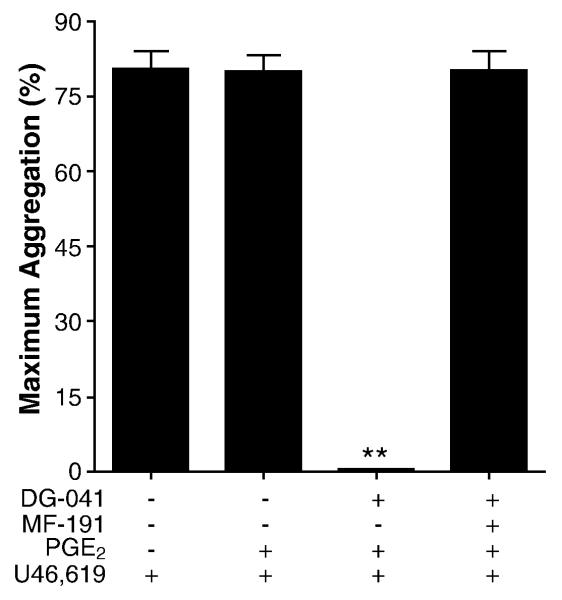
All volunteers responded similarly to sulprostone regardless of their response to PGE2, leading to the hypothesis that EP3-mediated potentiation of aggregation outweighed any inhibitory signals in nonresponders. Consistent with this hypothesis, 100 nM PGE2 completely inhibited U46,619-induced aggregation in nonresponders when their platelets were pretreated with the specific EP3 antagonist DG-041 (1 μM); this inhibition required EP4 as shown by co-preincubation with the EP4 antagonist MF-191 and DG-041 (Figure 4). DG-041 had no effect on U46,619-induced aggregation in the absence of PGE2 (data not shown). These data demonstrate the role of EP4 in mediating the effect of PGE2 and suggest that the balance of EP3 and EP4 signaling determines the net response of human platelets to PGE2 when the thromboxane receptor is stimulated.
Figure 4.
EP3 antagonist converted nonresponders to a responsive phenotype that requires EP4 (n = 6). Human PRP from PGE2 nonresponders was pretreated with the EP3 antagonist DG-041 (1 μM) and/or the EP4 antagonist MF-191 (50 nM) before the addition of PGE2 (100 nM) and the subsequent induction of aggregation with submaximal concentrations of U46,619. (P<0.0001 by repeated measures ANOVA; ** P < 0.001 compared with all other conditions by Newman-Keuls multiple comparison test.)
As activation of EP1 increases intracellular calcium, we expected EP1 agonism to potentiate, not inhibit, platelet aggregation. Because an adequately selective EP1 agonist is not available, we combined an EP3 antagonist (1 μM DG-041) with an EP1/EP3 agonist (0.5 μM 17-phenyl-trinor-PGE2). This pretreatment did not potentiate aggregation induced by a subthreshold concentration of U46,619. As a positive control, the EP1/EP3 agonist alone did potentiate aggregation in the absence of DG-041 (data not shown).
EP3 Negates EP2-mediated Inhibition of Platelet Aggregation
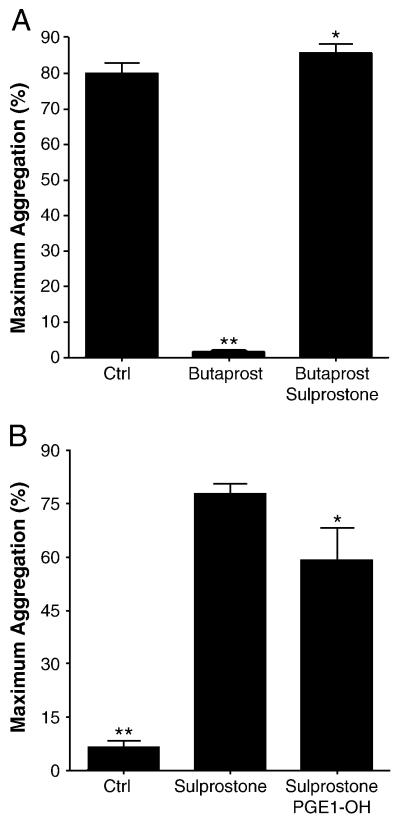
Because the ultimate effect of PGE2 depends on the net effect of several receptors, we used combinations of selective EP agonists to pharmacologically characterize platelet responses in the presence of submaximal concentrations of U46,619. For each individual, we constructed dose-response curves for each EP agonist in isolation and selected the lowest doses required to achieve a measurable response (Supporting Figures S1, S2, and S3). Preincubation with butaprost free acid, an EP2 agonist, completely inhibited U46,619-induced platelet aggregation (N=8), regardless of the individual's PGE2 responsiveness. The addition of sulprostone to the preincubation mixture completely prevented this inhibition (Figure 5A). These data not only support the presence of functional EP2 on human platelets but also demonstrate that the net effect of combined EP2 (antiaggregatory) and EP3 (proaggregatory) activation may favor potentiation of aggregation.
Figure 5.
(A) The EP2 agonist butaprost free acid (~250 nM) inhibited aggregation of human PRP induced by submaximal concentrations of U46,619. Co-incubation with the EP3 agonist sulprostone (~25nM) masked this effect. (P<0.0001 by repeated measures ANOVA, n = 8; ** P<0.001, compared with other conditions, * P>0.05 compared with control, by Newman-Keuls multiple comparison test.) (B) Sulprostone potentiated aggregation induced by subthreshold concentrations of U46,619 (~200 nM). Co-incubation with the EP4 agonist PGE1-OH partially attenuated this potentiation. (P<0.0001 by repeated measures ANOVA, n = 9; ** P < 0.001 compared with other conditions, * P < 0.05 compared with sulprostone by Newman-Keuls multiple comparison test.)
EP4 Modestly Inhibits EP3-mediated Potentiation of Aggregation
We hypothesized that EP4 may inhibit platelet aggregation, in part, by attenuating EP3-mediated potentiation of aggregation. The addition of up to 8 μM PGE1-OH, an EP4 agonist, significantly inhibited EP3-mediated potentiation of U46,619-induced aggregation, but the effect was modest compared with the inhibitory effect of PGE2 (Figure 5B). The response of individual subjects to PGE1-OH alone did not correlate with the individual's PGE2 responsiveness. These data suggest that both EP2 and EP4 are required for a net inhibitory effect of PGE2 on human platelet aggregation; antagonism of EP4 is sufficient to block this response.
Discussion
The role of PGE2 in the progression of atherosclerotic vascular disease and platelet function has been studied for more than 35 years, but much remains unknown. In mice, PGE2 is prothrombotic and EP3 mediates this effect[12-14], rationalizing the development of an EP3 antagonist as a new antiplatelet agent. In humans, the effects of PGE2 on platelet reactivity are less consistent; significant interindividual differences have been described but surprisingly little is known about the mechanisms and significance of these variations. Further understanding of the mechanisms underlying this interindividual variability of platelet response to PGE2 will better inform our knowledge of atherothrombotic risk.
In contrast to the potentiation effect on platelet aggregation that is most often reported, we found that nanomolar concentrations of PGE2 completely inhibit platelet aggregation induced by the thromboxane receptor agonist U46,619 in 26% of individuals (responders). Interestingly, PGE2 only inhibits aggregation induced by submaximal concentrations of U46,619; PGE2 modestly affects aggregation induced by concentrations of U46,619 that produce a maximal response. In 24% of individuals, PGE2 affected the timing but not the final magnitude of aggregation (partial responders), and in the remaining 50%, PGE2 did not have detectable effects (nonresponders). These phenotypes were subject-specific and reproducible, with some subjects studied on more than five separate occasions. This interindividual variability raises the question of whether “PGE2 responsiveness” defines subgroups of individuals with varying platelet reactivity.
In mice, EP3 mediates PGE2-induced potentiation of platelet aggregation.[12] We used the EP3 agonist sulprostone to test whether stimulation of EP3 could potentiate aggregation induced by subthreshold concentrations of U46,619 in humans. As previously reported in both mice and humans,[15] we observed that sulprostone potentiated aggregation in all volunteers tested, regardless of their PGE2-response phenotype. This suggests that, in humans, the net effect of PGE2 is not due to the sole activation of EP3 but is dependent on simultaneous activation of other EP receptor subtypes.
Previous RT-PCR and Southern blot analyses suggested very low expression of EP2 on both mouse and human platelets.[13, 26] However, since platelets are enucleate, the existence of functional platelet proteins should not be assumed based solely on the presence or absence of their mRNA. We used butaprost free acid, a selective EP2 agonist, to pharmacologically probe platelet EP2. In all subjects, butaprost completely inhibited platelet aggregation induced by submaximal concentrations of U46,619, and the potency of butaprost did not associate with PGE2 responsiveness. Preincubation with the EP4 antagonist MF-191 did not antagonize this effect, supporting the selectivity of both butaprost for EP2 and MF-191 for EP4. Inhibition was not observed when platelets were pretreated with both sulprostone and butaprost, however, indicating that EP3 activation may outweigh the inhibitory effect of EP2. These data support the presence of functional EP2 on human platelets and suggest that interindividual differences at EP2 alone are unlikely to explain the variability in PGE2 response.
We evaluated the responder cohort and found that selective antagonism of EP4 completely abrogates the PGE2-mediated inhibition of U46,619-induced platelet aggregation in these individuals. Interestingly, in these same subjects, stimulation of EP4 with the agonist PGE1-OH is insufficient to inhibit U46,619-induced aggregation to a similar degree, even at high concentrations (Supporting Figure S3). This suggests that either PGE1-OH lacks sufficient potency or activation of EP4 alone is required, but not sufficient, to replicate the PGE2-responsive phenotype.
Because EP2 and EP4 decreased platelet reactivity in all volunteers tested, we hypothesized that PGE2 would inhibit U46,619-mediated aggregation in nonresponders in the presence of an EP3 antagonist. We tested this hypothesis in our cohort of nonresponders and found that preincubation with the EP3 antagonist DG-041 converted their platelets to the responsive phenotype. This effect was dependent on EP4 signaling, similar to the PGE2 responders, suggesting that the inhibitory effect of PGE2 is normally overwhelmed by a predominant EP3-mediated response among nonresponders.
It is commonly cited that low concentrations (typically < 1μM) of PGE2 potentiate platelet aggregation and higher (supraphysiological) concentrations inhibit aggregation by activating another receptor.[11-13, 19, 21, 24, 27-31] Although this biphasic effect seems consistent in mice, the earliest studies of PGE2 and platelet reactivity describe effects that are variable depending on species, agonist, and concentration of PGE2.[19-22] In humans, the effects of nanomolar concentrations of PGE2 have been controversial, with some studies demonstrating significant potentiation and others showing none. Notably, the majority of these studies presented detailed analyses of relatively few subjects, potentially underestimating the heterogeneity of the population. Although some of the heterogeneity in the early literature could derive from variability in platelet handling, PGE2 preparations, and other systematic differences between laboratories, investigators recognized that the PGE2 “effect varies from donor to donor.”[29] Furthermore, one of the larger studies, which involved 30 subjects, also identified significant interindividual variation in PGE2 effect.[27] Gresele et al. observed potentiation of arachidonic acid (AA)-induced aggregation only in the 4 subjects whose platelet aggregation was not inhibited by a thromboxane synthase inhibitor. In contrast, PGE2 did not potentiate aggregation among the 26 subjects who they found to be “thromboxane synthase responders.”[27] These studies support our observation of interindividual variability in the effect of PGE2 on platelet reactivity and indicate the need to better understand the underlying biology.
We observed that the concentration of agonist affects platelet response to PGE2. The physiological significance of this dependence on agonist concentration is speculative, but a teleological view suggests that PGE2 may decrease reactivity of circulating platelets at the periphery of a thrombus (where agonist concentration is submaximal) but may not have an effect on thrombosis at the site of a vascular lesion (where agonist concentration is maximal); this hypothesis is consistent with the observation that the EP3 antagonist DG-041 does not increase bleeding time.[15, 16] Similarly, the production of nanomolar concentrations of PGE2 in plaques [32] could limit extension of an associated thrombus at the periphery. This dose-dependent effect could explain some of the controversial data in humans.
Although our study is too small to accurately determine the proportion of the population that may respond to PGE2, the observation that 26% of our healthy volunteers responded to PGE2 suggests that this may be a common phenotype. This may have clinical importance, especially since our data suggest that the PGE2-responsive phenotype may associate with low platelet reactivity, which correlates with a lower risk of recurrent cardiovascular events.[3-5] Whether this phenotype confers a lower risk of cardiovascular events remains to be determined.
We observed that EP2 and EP4 signaling are required for PGE2 to decrease platelet reactivity but the underlying mechanisms of interindividual variability require further exploration. Genetic variation, in the genes encoding either the EP receptors or the downstream signaling proteins, is one possibility. EP4 presents particularly complex possibilities: EP4 signal transduction can occur not only by stimulating adenylate cyclase leading to increases in intracellular cAMP, but also by activation of phosphatidylinositol 3-kinase-mediated pathways;[25] other proteins, such as the recently described EP4 receptor-associated protein (EPRAP) could also play a role.[33] Platelet inhibition by EP2 is consistent with its known coupling to Gs and stimulation of adenylate cyclase;[25] detailed study of variability in signal transduction and its correlation with PGE2 phenotype is a subject of ongoing investigation in our laboratory.
This study has several limitations. First, EP agonists and antagonists are selective but not completely specific, allowing for the possibility of off-target effects; therefore, we employed the lowest concentrations possible of the most selective agents available to maximize the probability that the targeted EP receptor was responsible for the observed effects. Moreover, when possible, we used antagonists of other receptor subtypes to further assess the specificity of the studied pharmacologic agent. Second, the experiments described only used the agonist U46,619. This agonist was chosen because of the major role that thromboxane plays in the modulation of physiological and pathological thrombosis as well as the paucity of literature describing the effects of PGE2 on thromboxane-induced aggregation. Third, as mentioned previously, the clinical significance of the PGE2-responsive phenotype has not been addressed; this is a major aim of ongoing investigation in our laboratory.
In conclusion, our data show that functional EP2, EP3, and EP4 are present in human platelets and that the net effect of PGE2 on platelet aggregation results from the balance between these different receptors. Nanomolar concentrations of PGE2 completely prevent thromboxane-induced platelet aggregation in a quarter of the population in an EP4-dependent manner. In the rest of the population, pharmacological antagonism of EP3 leads to PGE2 having an EP4-dependent inhibitory effect similar to PGE2 responders. These results are concordant with the published data for the EP3 antagonist DG-041 in humans and provide a hypothetical molecular mechanism for studying the interindividual variability in PGE2 modulation of platelet reactivity. Future studies will address whether this interindividual variability may contribute to variation in cardiovascular risk.
Supplementary Material
Acknowledgement
The project described was supported by Award Numbers 5P50HL081009, 5T32GM07569-32 (JPS), DK37097, and 5T32GM07569 (EVH) from the NIH. The authors would like to thank John A. Oates for his constructive advice, Merck-Frosst Canada for their generous gift of MF-191, and the VICB Chemical Synthesis Core for their assistance in the synthesis of DG-041.
Abbreviations
- PGE2
prostaglandin E2
- PCI
percutaneous coronary interventions
- EP
PGE2 receptor
- IP
prostacyclin receptor
- PGI2
Prostacyclin
- PRP
platelet-rich plasma
- PPP
platelet-poor plasma
- TP
thromboxane receptor
- RT-PCR
reverse transcription-polymerization chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work has been carried out at Vanderbilt University.
Disclosure of Conflict of Interests
The authors have no conflicts of interest.
Supplementary data
Additional Supplementary data may be found in the online version of this article:
References
- 1.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N. Engl. J. Med. 1992 Jan 23;326(4):242–50. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N. Engl. J. Med. 1992 Jan 30;326(5):310–8. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 3.Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Holoch PA, Sobel BE, Schneider DJ. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation. 2001 Jul 10;104(2):181–6. doi: 10.1161/01.cir.104.2.181. [DOI] [PubMed] [Google Scholar]
- 4.Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Sobel BE, Schneider DJ. Usefulness of platelet reactivity before percutaneous coronary intervention in determining cardiac risk one year later. Am. J. Cardiol. 2003 Apr 1;91(7):876–8. doi: 10.1016/s0002-9149(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 5.Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N. Engl. J. Med. 1990 May 31;322(22):1549–54. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 6.Czervionke RL, Smith JB, Fry GL, Hoak JC, Haycraft DL. Inhibition of prostacyclin by treatment of endothelium with aspirin. Correlation with platelet adherence. J. Clin. Invest. 1979 May;63(5):1089–92. doi: 10.1172/JCI109379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus AJ, Weksler BB, Jaffe EA, Broekman MJ. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J. Clin. Invest. 1980 Nov;66(5):979–86. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc. Natl. Acad. Sci. U S A. 1977 Sep;74(9):3922–6. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charo IF, Shak S, Karasek MA, Davison PM, Goldstein IM. Prostaglandin I2 is not a major metabolite of arachidonic acid in cultured endothelial cells from human foreskin microvessels. J. Clin. Invest. 1984 Sep;74(3):914–9. doi: 10.1172/JCI111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neri Serneri GG, Gensini GF, Abbate R, Castellani S, Bonechi F, Dagianti A, Arata L, Fedele F, Iacoboni C, Prisco D. Physiologic role of coronary PGI2 and PGE2 in modulating coronary vascular response to sympathetic stimulation. Am. Heart J. 1990 Apr;119(4):848–54. doi: 10.1016/s0002-8703(05)80322-9. [DOI] [PubMed] [Google Scholar]
- 11.Andersen NH, Eggerman TL, Harker LA, Wilson CH, De B. On the multiplicity of platelet prostaglandin receptors. I. Evaluation of competitive antagonism by aggregometry. Prostaglandins. 1980 May;19(5):711–35. doi: 10.1016/0090-6980(80)90170-7. [DOI] [PubMed] [Google Scholar]
- 12.Fabre JE, Nguyen M, Athirakul K, Coggins K, McNeish JD, Austin S, Parise LK, FitzGerald GA, Coffman TM, Koller BH. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J. Clin. Invest. 2001 Mar;107(5):603–10. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma H, Hara A, Xiao CY, Okada Y, Takahata O, Nakaya K, Sugimoto Y, Ichikawa A, Narumiya S, Ushikubi F. Increased bleeding tendency and decreased susceptibility to thromboembolism in mice lacking the prostaglandin E receptor subtype EP(3) Circulation. 2001 Sep 4;104(10):1176–80. doi: 10.1161/hc3601.094003. [DOI] [PubMed] [Google Scholar]
- 14.Gross S, Tilly P, Hentsch D, Vonesch JL, Fabre JE. Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J. Exp. Med. 2007 Feb 19;204(2):311–20. doi: 10.1084/jem.20061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heptinstall S, Espinosa DI, Manolopoulos P, Glenn JR, White AE, Johnson A, Dovlatova N, Fox SC, May JA, Hermann D, Magnusson O, Stefansson K, Hartman D, Gurney M. DG-041 inhibits the EP3 prostanoid receptor--a new target for inhibition of platelet function in atherothrombotic disease. Platelets. 2008 Dec;19(8):605–13. doi: 10.1080/09537100802351073. [DOI] [PubMed] [Google Scholar]
- 16.Singh J, Zeller W, Zhou N, Hategen G, Mishra R, Polozov A, Yu P, Onua E, Zhang J, Zembower D, Kiselyov A, Ramirez JL, Sigthorsson G, Bjornsson JM, Thorsteinsdottir M, Andresson T, Bjarnadottir M, Magnusson O, Fabre JE, Stefansson K, Gurney ME. Antagonists of the EP(3) Receptor for Prostaglandin E(2) Are Novel Antiplatelet Agents That Do Not Prolong Bleeding. ACS Chem. Biol. 2009 Feb 5; doi: 10.1021/cb8002094. [DOI] [PubMed] [Google Scholar]
- 17.Belton OA, Duffy A, Toomey S, Fitzgerald DJ. Cyclooxygenase isoforms and platelet vessel wall interactions in the apolipoprotein E knockout mouse model of atherosclerosis. Circulation. 2003 Dec 16;108(24):3017–23. doi: 10.1161/01.CIR.0000104565.78013.AD. [DOI] [PubMed] [Google Scholar]
- 18.Cipollone F, Prontera C, Pini B, Marini M, Fazia M, De Cesare D, Iezzi A, Ucchino S, Boccoli G, Saba V, Chiarelli F, Cuccurullo F, Mezzetti A. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation. 2001 Aug 21;104(8):921–7. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- 19.Bruno JJ, Taylor LA, Droller MJ. Effects of prostaglandin E2 on human platelet adenyl cyclase and aggregation. Nature. 1974 Oct 25;251(5477):721–3. doi: 10.1038/251721a0. [DOI] [PubMed] [Google Scholar]
- 20.Kloeze J. Influence of prostaglandins on platelet adhesiveness and platelet aggregation. In: Bergström S, Samuelsson B, editors. Prostaglandins. Proceedings of the Second Nobel Symposium; Stockholm. June 1966; Stockholm, New York, London: Almqvist & Wiksell; Interscience Publishers; 1967. pp. 241–52. [Google Scholar]
- 21.MacIntyre DE, Gordon JL. Calcium-dependent stimulation of platelet aggregation by PGE. Nature. 1975 Nov 27;258(5533):337–9. doi: 10.1038/258337a0. [DOI] [PubMed] [Google Scholar]
- 22.Shio H, Ramwell PW, Jessup SJ. Prostaglandin E2: effects on aggregation, shape change and cyclic AMP of rat platelets. Prostaglandins. 1972 Jan;1(1):29–36. doi: 10.1016/0090-6980(72)90063-9. [DOI] [PubMed] [Google Scholar]
- 23.Gray SJ, Heptinstall S. The effects of PGE2 and CL 115,347, an antihypertensive PGE2 analogue, on human blood platelet behaviour and vascular contractility. Eur. J. Pharmacol. 1985 Aug 15;114(2):129–37. doi: 10.1016/0014-2999(85)90620-x. [DOI] [PubMed] [Google Scholar]
- 24.Gray SJ, Heptinstall S. Interactions between prostaglandin E2 and inhibitors of platelet aggregation which act through cyclic AMP. Eur. J. Pharmacol. 1991 Feb 26;194(1):63–70. doi: 10.1016/0014-2999(91)90124-9. [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007 Apr 20;282(16):11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 26.Paul BZ, Ashby B, Sheth SB. Distribution of prostaglandin IP and EP receptor subtypes and isoforms in platelets and human umbilical artery smooth muscle cells. Br. J. Haematol. 1998 Sep;102(5):1204–11. doi: 10.1046/j.1365-2141.1998.00910.x. [DOI] [PubMed] [Google Scholar]
- 27.Gresele P, Blockmans D, Deckmyn H, Vermylen J. Adenylate cyclase activation determines the effect of thromboxane synthase inhibitors on platelet aggregation in vitro. Comparison of platelets from responders and nonresponders. J. Pharmacol. Exp. Ther. 1988 Jul;246(1):301–7. [PubMed] [Google Scholar]
- 28.Matthews JS, Jones RL. Potentiation of aggregation and inhibition of adenylate cyclase in human platelets by prostaglandin E analogues. Br. J. Pharmacol. 1993 Feb;108(2):363–9. doi: 10.1111/j.1476-5381.1993.tb12810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salzman EW, Kensler PC, Levine L. Cyclic 3′,5′-adenosine monophosphate in human blood platelets. IV. Regulatory role of cyclic amp in platelet function. Ann. N. Y. Acad. Sci. 1972 Oct 27;201:61–71. doi: 10.1111/j.1749-6632.1972.tb16287.x. [DOI] [PubMed] [Google Scholar]
- 30.Tynan SS, Andersen NH, Wills MT, Harker LA, Hanson SR. On the multiplicity of platelet prostaglandin receptors. II. The use of N-0164 for distinguishing the loci of action for PGI2, PGD2, PGE2 and hydantoin analogs. Prostaglandins. 1984 May;27(5):683–96. doi: 10.1016/0090-6980(84)90007-8. [DOI] [PubMed] [Google Scholar]
- 31.Vezza R, Roberti R, Nenci GG, Gresele P. Prostaglandin E2 potentiates platelet aggregation by priming protein kinase C. Blood. 1993 Nov 1;82(9):2704–13. [PubMed] [Google Scholar]
- 32.Holmes DR, Wester W, Thompson RW, Reilly JM. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. Journal of Vascular Surgery. 1997 May;25(5):810–5. doi: 10.1016/s0741-5214(97)70210-6. [DOI] [PubMed] [Google Scholar]
- 33.Takayama K, Sukhova GK, Chin MT, Libby P. A novel prostaglandin E receptor 4-associated protein participates in antiinflammatory signaling. Circ. Res. 2006 Mar 3;98(4):499–504. doi: 10.1161/01.RES.0000204451.88147.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.