Abstract
The status of the neuroendocrine reproductive axis differs dramatically between early development, puberty, and various stages of adulthood, and also differs in several critical ways between the sexes, including its earlier pubertal activation in females than males and the presence of neural circuitry that generates preovulatory hormone surges in females but not males. The reproductive axis is controlled by various hormonal and neural pathways that converge upon forebrain gonadotropin-releasing hormone (GnRH) neurons, and many of the critical age and sex differences in the reproductive axis likely reflect differences in the “upstream” circuits and factors that regulate the GnRH system. Recently, the neural kisspeptin system has been implicated as an important regulator of GnRH neurons. Here I discuss the evidence supporting a critical role of kisspeptin signaling at different stages of life, including early postnatal and pubertal development, as well as in adulthood, focusing primarily on information gleaned from mammalian studies. I also evaluate key aspects of sexual differentiation and development of the brain as it relates to the Kiss1 system, with special emphasis on rodents. In addition to discussing recent advances in the field of kisspeptin biology, this paper will highlight a number of unanswered questions and future challenges for kisspeptin investigators, and will stress the importance of studying the kisspeptin system in both males and females, as well as in multiple species.
Keywords: kisspeptin, Kiss1, GPR54, Kiss1R, sexual differentiation, sex differences, development, puberty, sexual maturation, brain, hypothalamus, hormone
1. Introduction
Physiology and behavior often vary between different developmental stages of an animal’s life, not to mention between animals of opposite sexes. This is especially true for the mammalian neuroendocrine reproductive axis, the status of which differs dramatically between early development, puberty, and various stages of adulthood. The neuroendocrine reproductive axis also differs between the sexes in several dramatic ways, including its earlier activation in females than males during pubertal maturation, the presence of neural circuitry that generates preovulatory hormone surges in females but not males, and the display of various sexually dimorphic reproductive behaviors. In adulthood, the reproductive axis is controlled by various hormonal and neural pathways that converge upon forebrain gonadotropin-releasing hormone (GnRH) neurons. GnRH neurons direct the activation of the rest of the reproductive axis by stimulating the pituitary to synthesize and secrete gonadotropin hormones [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] which activate the gonads. Many of the critical age and sex differences in the control of the reproductive axis are thought to reflect key differences in the “upstream” neural circuits and factors that regulate the GnRH system.
In the past decade, the newly-discovered kisspeptin system has been implicated as an important regulator of GnRH neurons, both in development and adulthood. In this review, I discuss the recent evidence obtained during the “Kisspeptin Era” of reproduction research that supports a critical role of kisspeptin signaling in the control of the reproductive axis at different stages of life, including postnatal and pubertal development, as well as in adulthood. I also review key aspects of sexual differentiation and development of the brain as it relates to the kisspeptin system. The role of kisspeptin signaling in adulthood has already been extensively reviewed and I will therefore focus more on the latest findings connecting the kisspeptin system to sexual differentiation and development, including the period of pubertal maturation. In addition, though kisspeptin has now been identified in a wide range of vertebrate species and taxa, this present discussion will focus primarily on mammals, with special emphasis placed on rodents. Kisspeptin’s role in other vertebrate classes has been reviewed in recent articles by (Biran et al. 2008; Elizur 2009; Oakley et al. 2009).
2. The Link between the Kiss1 System and Adulthood Fertility
2.1 Discovery and localization of Kiss1 and the kisspeptin receptor
The human KiSS1 gene was first discovered in 1996 as a metastisis-suppressor in human cell lines (Lee et al. 1996; Lee and Welch 1997). However, its critical role in reproduction would not surface for another 7 years. In 1999, the gene for an orphan G-protein-coupled receptor, termed GPR54, was first identified in rodents and discovered in humans soon thereafter (Lee et al. 1999; Kotani et al. 2001; Muir et al. 2001). In 2001, several groups reported that the protein product of the Kiss1 gene, kisspeptin, is a high-affinity ligand for GPR54 (Kotani et al. 2001; Muir et al. 2001; Ohtaki et al. 2001). Because of this, GPR54 has recently been renamed Kiss1R (i.e., the kisspeptin receptor).
The Kiss1 gene encodes a 145 amino acid protein which, in primates, is processed to produce a 54 amino acid peptide called kisspeptin, which, like several other neuropeptides, possesses a distinct structural RF-amide motif (Arg-Phe-NH2) in its C-terminal region. (Note that in rodents, the mature kisspeptin product is 52 amino acids with a RY C-terminal). In a wide range of mammals, including rats, mice, hamsters, sheep, non-human primates, and humans, Kiss1 mRNA has been detected by either in situ hybridization or RT-PCR in two discrete regions of the hypothalamus, the anteroventral periventricular nucleus—periventricular nucleus continuum (AVPV/PeN) and the arcuate nucleus (ARC; the homologue of the primate infundibular nucleus) (Gottsch et al. 2004; Shahab et al. 2005; Smith et al. 2005; Kauffman et al. 2007; Rometo et al. 2007; Shibata et al. 2007; Smith 2009). Immunohistochemistry studies have identified kisspeptin protein immunoreactivity in these same two hypothalamic regions in several species (Adachi et al. 2007; Decourt et al. 2008; Ramaswamy et al. 2008; Smith et al. 2008; Clarkson et al. 2009; Ohkura et al. 2009). In some cases, mild kisspeptin immunoreactivity has been reported in the dorsomedial nucleus (DMN); however, Kiss1 mRNA has never been detected in this region using several techniques, raising the possibility that the kisspeptin-immunoreactivity in the DMN reflects non-specific binding of some kisspeptin antibodies to other RF-amide peptides [reviewed in (Mikkelsen and Simonneaux 2009; Oakley et al. 2009)]. Interestingly, the Kiss1 gene is also expressed in several peripheral tissues, most notably, the placenta, ovary, testis, pituitary, pancreas, and adipose tissue (Kotani et al. 2001; Ohtaki et al. 2001; Terao et al. 2004; Castellano et al. 2006; Torricelli et al. 2008). However, little is currently known regarding kisspeptin’s role outside the brain, and the present review will focus on the roles of neural kisspeptin. Like Kiss1, the Kiss1R gene is expressed in both peripheral tissues (placenta, pancreas, kidney, testis, and pituitary) and the brain, most notably the hypothalamus, preoptic area, hippocampus, habenula, and amygdala. At present, there is not a good antibody available for detection of Kiss1R protein, precluding localization of the receptor at the protein level.
2.2 Stimulation of the adult reproductive axis by kisspeptin
Although Kiss1 and Kiss1R were discovered in 1996 and 1999, respectively, the role of the Kiss1 system in regulating reproduction was not realized until 2003 when three groups independently reported that humans and mice with mutations in the Kiss1R gene display striking deficits in reproductive function, including impaired sexual maturation, low levels of sex steroids and gonadotropins, acyclicity, and infertility (de Roux et al. 2003; Funes et al. 2003; Seminara et al. 2003). These initial findings were soon echoed by other similar reports in Kiss1R knockout (KO) mice (Messager et al. 2005; Kauffman et al. 2007; Lapatto et al. 2007), and also extended to mice lacking a functional Kiss1 gene (d'Anglemont de Tassigny et al. 2007; Lapatto et al. 2007; Clarkson et al. 2008). The initial findings in humans suggested that kisspeptin signaling, via Kiss1R, was essential for proper pubertal development and reproductive function. This conjecture was tested and extended in a flurry of scientific activity in the years that followed. It is now clear that hypothalamic kisspeptin directly activates GnRH neurons to stimulate the reproductive axis. The evidence for this is summarized as follows:
In mice, rats, hamsters, sheep, primates (including humans) and other mammals, kisspeptin treatment potently stimulates LH and FSH secretion (Dhillo et al. 2005; Kinoshita et al. 2005; Messager et al. 2005; Navarro et al. 2005; Navarro et al. 2005; Shahab et al. 2005; Greives et al. 2007; Kauffman et al. 2007).
The stimulatory effect of kisspeptin on gonadotropin secretion is prevented with co-treatment of GnRH receptor antagonists (Gottsch et al. 2004; Irwig et al. 2004; Shahab et al. 2005), suggesting that kisspeptin activates the reproductive axis at the level of GnRH neurons.
Kisspeptin treatment induces Fos expression in GnRH neurons (Irwig et al. 2004; Kauffman et al. 2007) and evokes prolonged firing of action potentials in GnRH neurons of mouse brain explants (Han et al. 2005; Pielecka-Fortuna et al. 2008).
Kisspeptin stimulates the in situ release of GnRH from rodent hypothalamic explants and in vivo release of GnRH into the ovine portal system (Messager et al. 2005; d'Anglemont de Tassigny et al. 2008).
Kisspeptin fibers appear to make appositions with GnRH neuron somata or axons (Clarkson and Herbison 2006; Decourt et al. 2008; Ramaswamy et al. 2008).
GnRH neurons express Kiss1R (Irwig et al. 2004; Han et al. 2005; Messager et al. 2005), suggesting that kisspeptin directly stimulates GnRH cells.
Collectively, these findings demonstrate that kisspeptin-Kiss1R signaling in the brain is both necessary and sufficient for promoting normal adulthood GnRH secretion and maintaining fertility. Of note, kisspeptin administration fails to stimulate LH secretion or induce Fos in GnRH neurons in Kiss1R KO mice as it does in wildtype (WT) mice (Kauffman et al. 2007), suggesting that kisspeptin’s effects on the GnRH axis are mediated specifically by Kiss1R (and not another yet-to-be-identified receptor).
2.3 Regulation of adult Kiss1 neurons by gonadal steroids and circadian signals
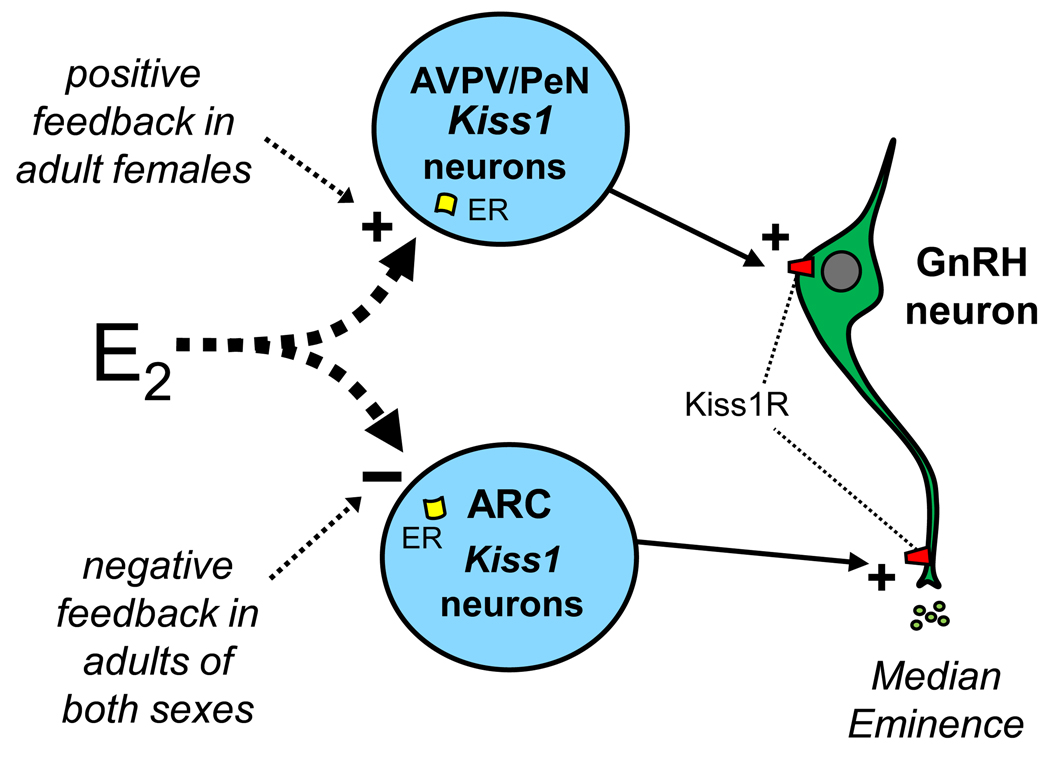
The secretion of GnRH in adulthood is regulated by feedback of gonadal sex steroids [i.e., testosterone (T) and estradiol (E2)], but the neuroanatomical and molecular mechanisms underlying this regulation are not entirely known. GnRH neurons do not express estrogen receptor α (ERα) or the androgen receptor (AR), the receptor subtypes thought to mediate steroidal feedback, suggesting that other sex steroid-sensitive neurons upstream of GnRH neurons receive and transmit steroid feedback signals to the reproductive axis. Recent evidence has implicated hypothalamic Kiss1 neurons as these upstream steroid-sensitive neurons. In adult rodents, sheep, and primates, Kiss1 gene expression in the brain is strongly regulated by E2 and T. Interestingly, in rodents, the effects of gonadal steroids on Kiss1 expression are region-specific: in the ARC, E2 or T inhibits the expression of Kiss1, whereas in the AVPV/PeN these steroids increase Kiss1 gene expression (Fig. 1) (Smith et al. 2005; Smith et al. 2005; Kauffman et al. 2007). Conversely, in the absence of adulthood gonadal steroids (such as in gonadectomized animals), Kiss1 levels are increased in the ARC and decreased in the AVPV/PeN (Smith et al. 2005; Smith et al. 2005; Kauffman et al. 2007; Kauffman et al. 2009). The regulatory effects of sex steroids on Kiss1 expression are likely direct, as most adult Kiss1 neurons express estrogen receptor-α (ERα), and many express androgen receptor (AR) and ERβ (Smith et al. 2005; Smith et al. 2005; Smith et al. 2006; Clarkson et al. 2008). A recent study has suggested that the ability of E2 to inhibit Kiss1 expression in the ARC and stimulate Kiss1 in the AVPV/PeN reflects different modes of intracellular E2 signaling in each region, with “classical” E2 signaling working in AVPV/PeN Kiss1 neurons and “non-classical” E2 signaling existing in ARC Kiss1 cells (Gottsch et al. 2009).
Fig. 1.
Kiss1 neurons project to and stimulate GnRH neurons, acting at either GnRH cell bodies (AVPV/PeN neurons) or GnRH fiber terminals in the median eminence (ARC neurons). Kiss1 neurons express sex steroid receptors and are differentially regulated by gonadal steroids. E2’s stimulation of Kiss1 expression in the AVPV/PeN and inhibition of Kiss1 in the ARC appears to underlie steroid-mediated positive and negative feedback, respectively. ER = estrogen receptors. Kiss1R = kisspeptin receptor.
The differential effects of E2 and T on Kiss1 neurons in the ARC and AVPV/PeN may reflect kisspeptin’s involvement in gonadal steroid-mediated negative and positive feedback control of reproduction (Fig. 1). In sheep, rodents, and primates, the ARC (or its primate homolog, the infundibular nucleus) comprises the neural elements that mediate gonadal steroid negative feedback regulation of GnRH (Ferin et al. 1974; Smith and Davidson 1974; Scott et al. 1997), and Kiss1 neurons in the ARC likely provide the cellular mechanism orchestrating this phenomenon. This conjecture is based on the already discussed facts that ARC neurons project to GnRH cells, ARC Kiss1 cells express ERα and AR, and gonadal steroids dramatically inhibit ARC Kiss1 expression. Moreover, the ability of gonadectomy to stimulate the reproductive axis is not observed in gonadectomized Kiss1R KO mice (Dungan et al. 2007), suggesting that the stimulation of GnRH neurons after removal of sex steroids is normally induced by kisspeptin signaling. In support of this, gonadectomy concurrently stimulates both ARC Kiss1 expression and LH secretion, and the post-gonadectomy increase in LH secretion is blocked by a selective kisspeptin antagonist (Roseweir et al. 2009).
In female rodents, the AVPV/PeN mediates the positive feedback effects of E2 on GnRH secretion, thereby triggering the preovulatory LH surge (Herbison 2008). Kiss1 neurons in the AVPV/PeN appear to comprise the cellular conduit for this positive feedback process. Supporting this contention, AVPV/PeN Kiss1 neurons express ERα (Smith et al. 2005; Smith et al. 2005; Smith et al. 2006) and directly innervate GnRH cell bodies (Wintermantel et al. 2006) (Fig. 1). Moreover, Kiss1 expression in the AVPV/PeN is increased, along with Fos in Kiss1 cells, during the preovulatory LH surge (Smith et al. 2006; Robertson et al. 2009). Furthermore, central infusion of kisspeptin antiserum or antagonist blocks the preovulatory LH surge (Kinoshita et al. 2005; Pineda et al. 2010), and Kiss1 KO mice lacking kisspeptin cannot generate an LH surge in response to E2 treatment (Clarkson et al. 2008). Lastly, the preovulatory LH surge is known to be gated by a circadian clock in the suprachiasmatic nucleus (de la Iglesia and Schwartz 2006), and we recently determined that Kiss1 neurons in the AVPV/PeN of E2-treated females display a significant circadian pattern of gene expression and neuronal activation, in direct synchrony with the circadian timing of LH secretion (Robertson et al. 2009). Interestingly, we found that the circadian activation of AVPV/PeN Kiss1 neurons was dependent on the presence of E2 (Robertson et al. 2009), suggesting that E2 may regulate the LH surge by both increasing kisspeptin production and promoting circadian activation of kisspeptin cells. Collectively, these findings indicate that Kiss1 neurons in the AVPV/PeN are stimulated by both E2 and circadian signals, thereby generating the precisely-timed preovulatory LH surge in adult female rodents. Note that the positive feedback event in sheep, and perhaps other non-rodent species, may be mediated by Kiss1 neurons in the ARC rather than the AVPV/PeN (or preoptic region), a possibility which is an active topic of research in several labs (reviewed in (Kauffman et al. 2007; Smith 2009).
In addition to being differentially regulated by gonadal steroids, the ARC and AVPV/PeN Kiss1 populations differ in several other ways. First, the two Kiss1 populations appear to stimulate different parts of the GnRH neuron: AVPV/PeN Kiss1 neurons project to and stimulate GnRH cell bodies, whereas evidence now hints that ARC Kiss1 neurons project to GnRH fiber terminals in the median eminence (Fig. 1) (Wintermantel et al. 2006; d'Anglemont de Tassigny et al. 2008; Ramaswamy et al. 2008). Second, as discussed below, Kiss1 expression in the adult AVPV/PeN is sexually differentiated whereas that in the ARC is not (Kauffman et al. 2007). Third, the cellular phenotype of Kiss1 neurons differs between region: ARC Kiss1 neurons co-express the neuropeptides dynorphin and neurokinin B, whereas AVPV/PeN neurons do not; however, Kiss1 neurons in the AVPV/PeN express galanin, and in some species, tyrosine hydroxylase (Kauffman 2009; Navarro et al. 2009; Oakley et al. 2009). Thus, it is important to keep in mind that Kiss1 neurons in the AVPV/PeN and ARC exhibit critical physiological and anatomical differences which may underlie different biological functions of the two populations.
3. Sexual Differentiation and the Kiss1 System: A Bidirectional Relationship
3.1 Mechanisms of sexual differentiation of the brain and behavior
Mammals, including humans, exhibit sex differences in numerous anatomical, physiological, and behavioral traits, ranging from complex copulatory, sociosexual, and parental behaviors to endocrine secretion patterns. In addition to sex differences in the physiology and behavior of normal animals, there are numerous health disorders and diseases in humans which present with dissimilar frequency between the sexes, including depression (2.5X more common in women), autism (4X more common in boys), idiopathic hypogonadotropic hypogonadism (5X more common in men), or precious puberty (10X more common in girls) (Wing 1981; Weissman et al. 1984; Cesario and Hughes 2007; Fechner et al. 2008). Presumably, many of the sex differences in physiology and behavior reflect underlying sex differences in brain circuitries and neural mechanisms. Indeed, the central nervous system is anatomically and physiologically differentiated between the sexes (13, 66, 92), including numerous documented sex differences in diverse brain regions ranging from the cerebral cortex to the hypothalamus to the amygdala (see reviews by (Cooke et al. 1998; Simerly 1998; de Vries and Sodersten 2009; Forger 2009). The nature of sex differences in the brain are diverse and region- and trait-specific; for example, neural sex differences range from differences in synapse morphology to neuron number to specific gene expression levels. Moreover, neural sex differences do not all favor the same one sex over the other, and hence, many traits are male-biased whereas others are female-biased. For example, the medial preoptic nucleus of rats contains more neurons in males than females, whereas the AVPV is typically larger and possesses more tyrosine hydroxylase-expressing neurons in females than in males. Note that while numerous sex differences in brain structures and cellular phenotypes have been identified, in many cases, the specific overlaying behavior or physiology that these neural sex difference control has remained a mystery (discussed in (Shah et al. 2004; de Vries and Sodersten 2009).
There are several ways that sex differences in the brain can develop, including sex chromosome gene-dependent mechanisms and gonadal sex steroid-dependent mechanisms (Arnold et al. 2003). To date, most sex differences in the brain have been attributed to sex differences in the actions of gonadal steroid secretions during key stages of development (the so-called “organizational hypothesis”), whereas only a handful of sexually-dimorphic traits in mammals have been attributed to differences in sex chromosome genes between males and females (De Vries et al. 2002; Gatewood et al. 2006; Park et al. 2008). Whether the small number of sexually-dimorphic traits exhibiting this sex chromosome gene-dependent mode of induction reflects less formal attention by investigators or simply a less common biological mechanism remains to be determined.
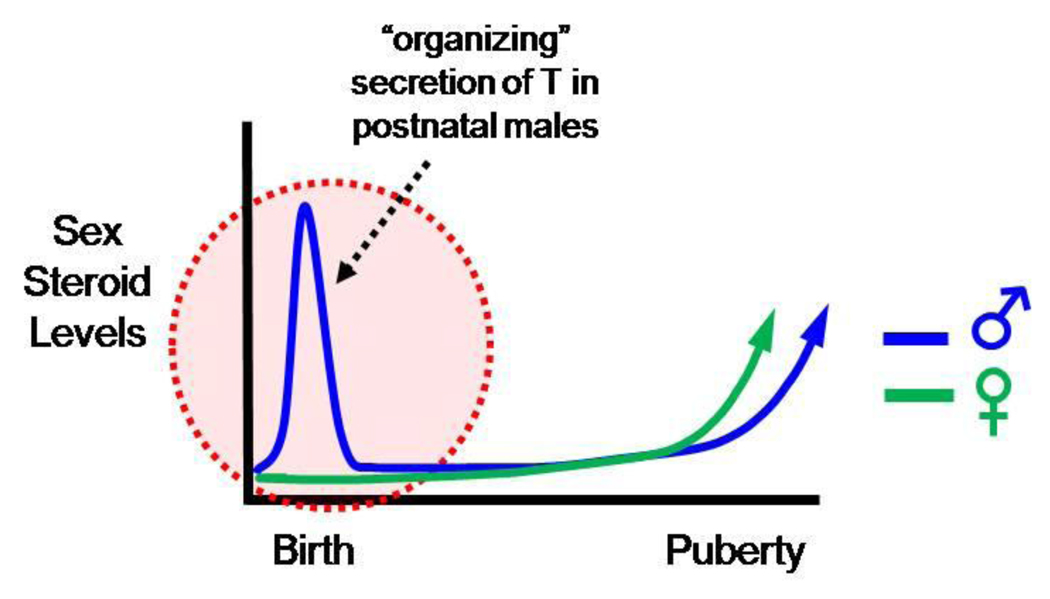
The central tenet of the “organizational hypothesis” is that the brain is initially bipotential and develops to be male-like or female-like under the direction of the sex steroid environment during the perinatal “critical period” (Phoenix et al. 1959). This critical period may be before birth, around birth, or soon after birth, depending on the species. During the critical period, the acute secretion of T in males, but not females, causes the brain to change its developmental fate, structure, and function, thereby differentiating to be masculinized (and defeminized) (Fig. 2) (Simerly 1998; Simerly 2002). Perinatal T secreted by males directs the brain’s sexual differentiation via activation of either AR or ER pathways (the latter, after its aromatization to E2 in select neural target tissues). In contrast to males, females do not normally secrete significant levels of circulating gonadal steroids during the perinatal critical period (Fig 2); the lack of high circulating sex steroids in perinatal females results in their brains differentiating to be feminized (and demasculinized). In support of this model, acute sex steroid treatment to postnatal females can induce the development of a male-like brain, and castration of newborn male rodents causes their brains to differentiate to be female-like in adulthood. Studies in rodents have determined that the critical period lasts approximately a week (10 days in rats) after birth, after which additional modulation of the sex steroid environment no longer alters the developmental fate of most sexually dimorphic traits [however, recent evidence suggests that some brain traits may also be influenced by sex steroid signaling at the critical stage of puberty (reviewed in (Schulz and Sisk 2006; Schulz et al. 2009)].
Fig. 2.
Sex-specific pattern of gonadal steroid secretion in early development in rodents. In perinatal males, but not females, there is an acute surge of testosterone (T) which organizes the development of the brain to be male-like. In females, the absence of circulating postnatal sex steroids results in sexually-dimorphic brain circuits developing to be female-like.
3.2 Sexual differentiation of AVPV/PeN Kiss1 neurons
In rodents and sheep, the ability of adult females, but not adult males, to display an E2-induced preovulatory LH surge (i.e., “positive feedback”) is sexually differentiated (Karsch and Foster 1975; Corbier 1985). In normal male rodents, exposure to T or its estrogenic metabolites during early postnatal life (or during prenatal life in male sheep) permanently alters the circuitry in the developing brain, preventing these animals from being able to generate a GnRH/LH surge in adulthood in response to exogenous E2 treatment. In contrast, in the absence of circulating postnatal gonadal steroids (i.e., normal females), the neural mechanisms necessary for generating the LH surge fully develop. In support of this model, female rodents treated acutely with T or E2 at birth fail to develop the LH surge-generating circuitry (Barraclough 1961; Gogan et al. 1980). Conversely, newborn male rodents that are castrated during the postnatal critical window are able to generate an E2-induced LH surge as adults, just like normal adult females (Gogan et al. 1980; Gogan et al. 1981; Corbier 1985). The identity of the specific sexually-dimorphic neural populations that govern the LH surge has remained elusive. Studies in the 1980’s implicated the AVPV/PeN as being a critical part of the LH surge generating mechanism. Estrogen receptors, including ERα, are expressed in some AVPV/PeN neurons, and lesions of the AVPV/PeN block spontaneous and steroid-induced preovulatory surges (reviewed in (Herbison 2008). Moreover, several aspects of the AVPV/PeN are sexually dimorphic, with females possessing more neurons overall than males, as well as greater numbers of neurons containing tyrosine hydroxylase (TH; i.e., dopaminergic cells) and GABA/glutamate (Simerly et al. 1985; Simerly et al. 1997; Petersen et al. 2003; Ottem et al. 2004). However, the actual involvement of any of these sexually-dimorphic AVPV/PeN systems in the LH surge has been equivocal.
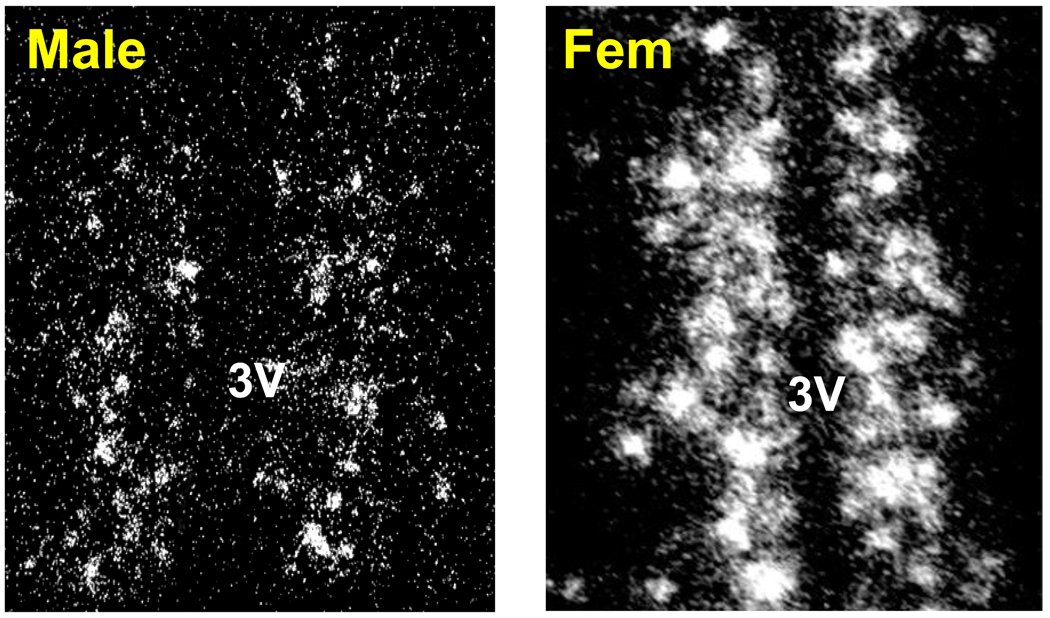
Given the critical role of AVPV/PeN Kiss1 neurons in directing the LH surge phenomenon (discussed earlier), we recently tested the possibility that the sex difference in the LH surge reflects sexual differentiation of the Kiss1 system in the AVPV/PeN. We found that, like TH neurons, Kiss1 neurons in the rat AVPV/PeN are sexually differentiated, with adult females possessing many more Kiss1 cells than males (Kauffman et al. 2007). More specifically, using in situ hybridization we determined that the number of Kiss1 mRNA-expressing neurons in the AVPV/PeN of adult female rats is as much as 25 times greater than in adult males (Kauffman et al. 2007). Similar sex differences in kisspeptin protein levels in the AVPV/PeN, as determined by immunohistochemistry, were reported in intact adult mice (Clarkson and Herbison 2006). However, in that study, the level of circulating adult sex steroids was not controlled between the sexes, and it therefore was unclear if adult females have more kisspeptin-ir neurons in the AVPV/PeN simply because they have higher sex steroids levels (circulating sex steroids stimulate Kiss1 gene expression in the AVPV/PeN, as discussed earlier). To assess whether sex differences in AVPV/PeN Kiss1 neurons are attributable to sex differences in circulating levels of T or E2 in adulthood, we measured Kiss1 expression in adult gonadectomized male and female rats receiving identical sex steroid treatments (i.e., with or without E2 implants). Even with equivalent sex steroid levels between the sexes, females had higher Kiss1 expression in the AVPV/PeN than did males (Kauffman et al. 2007); thus, adult females possess more Kiss1 cells in this region than males, regardless of the circulating adult sex steroid milieu. This finding has since been replicated for Kiss1 mRNA levels in mice (A.S. Kauffman, unpublished observations; Fig. 3), kisspeptin protein levels in mice (Gonzalez-Martinez et al. 2008), and both kisspeptin protein and Kiss1 mRNA levels in rats (Homma et al. 2009). Given the importance of kisspeptin in promoting the LH surge, the sex difference in AVPV/PeN Kiss1 expression in adulthood may account for the sex-specific ability of female rodents, but not males, to produce an LH surge. That is, adult females have high Kiss1 expression in the AVPV/PeN and display an LH surge, whereas adult males have few Kiss1 cells in the AVPV/PeN and cannot generate an LH surge.
Fig. 3.
Sex-specific pattern of Kiss1 gene expression in the AVPV/PeN of adult male and female mice that were gonadectomized and treated with similar levels of E2. As in rats, adult female mice have significantly more Kiss1 neurons in this region than adult males. 3V = third ventricle.
How does the sex difference in Kiss1 neurons in the AVPV/PeN develop? Like most sex differences in the brain, the sex difference in Kiss1 neurons appears to be organized early in perinatal development by the actions of sex hormones. This conclusion is based on the initial finding that female rats treated postnatally with a single injection of androgen (to mimic acute androgen secretion in postnatal males) possess very few Kiss1 neurons in the AVPV/PeN as adults, similar to adult males (Kauffman et al. 2007). Furthermore, newborn male rats castrated on the day of birth (to remove circulating androgens) display elevated female-like Kiss1 levels in adulthood (Homma et al. 2009), indicating that the AVPV/PeN Kiss1 system is sexually-differentiated under the influence of postnatal gonadal steroids. The developmental effects of postnatal sex steroids on the differentiation of Kiss1 neurons in the AVPV/PeN could be mediated by either AR- or ER-dependent pathways. Some recent evidence suggests that at least ER-dependent pathways may be involved. Kisspeptin immunoreactivity in the AVPV/PeN is reduced in transgenic female mice that were perinatally exposed to E2 due to an absence in alpha-fetoprotein, which normally binds E2 during development and prevents it from acting in the brain (Gonzalez-Martinez et al. 2008); these same females were incapable of mounting an LH surge in response to sex steroid treatment in adulthood. Thus, sexual differentiation of the AVPV/PeN kisspeptin system can be influenced by the actions of perinatal E2 signaling. In support of this, female rats treated with a single E2 injection during the postnatal critical period display male-like levels of Kiss1 and kisspeptin in the AVPV/PeN in adulthood (Homma et al. 2009). However, whether or not developmental AR signaling can also influence the Kiss1 sex difference has not been assessed, nor has the specific postnatal ER subtype pathway been identified.
Whereas postnatal E2 can organize the trajectory of Kiss1 sexual differentiation and E2 in adulthood transiently increases Kiss1 gene expression, recent evidence suggests that E2 also acts between the postnatal period and adulthood to promote proper Kiss1 development in the AVPV/PeN. Female mice lacking E2 either permanently or during just the peripubertal period (PND 22–30) express very low levels of kisspeptin-ir in the AVPV/PeN as adults (Clarkson et al. 2009; Gill et al. 2009), even when given supplemental E2 in adulthood (Bakker et al. 2009; Gill et al. 2009). Thus, E2 appears to be necessary during peripubertal life for maintaining normal female-like Kiss1 levels in the AVPV/PeN, corroborating recent findings that sex steroids can act during or before puberty to regulate development of certain neuronal populations (Schulz et al. 2004; Sisk and Zehr 2005; Zehr et al. 2006; Ahmed et al. 2008). The critical peripubertal period(s) when E2 is important for normal AVPV/PeN Kiss1 development in females requires more investigation, as do the mechanisms by which peripubertal E2 influences AVPV/PeN Kiss1 development.
As mentioned above, the AVP/PeN also contains a sexually differentiated population of TH-positive (i.e., dopaminergic) neurons (Simerly et al. 1985; Simerly and Swanson 1987), which, like the Kiss1 population, is greater in adult females than adult males. This raises the question, are the sexually differentiated populations of Kiss1 and TH cells in the AVPV/PeN the same neurons or separate sexually dimorphic systems? Interestingly, in female rats, only a small percentage of AVPV/PeN neurons coexpress both TH and Kiss1 mRNA, and the few neurons that do coexpress both genes contain only low levels of TH mRNA (Kauffman et al. 2007). Thus, although there is a slight overlap in the anatomical distribution of these two neuronal populations and a low degree of co-labeling for some Kiss1 neurons, the sexually-dimorphic Kiss1 neurons and TH-expressing cells in the AVPV/PeN appear to represent two separate, sexually-differentiated populations, at least in rats. In contrast, preliminary data suggests that, in mice, many Kiss1 neurons in the AVPV/PeN also to coexpress TH mRNA (R.A. Steiner, personal communication), though this preliminary finding is awaiting further confirmation. Unlike kisspeptin, the precise role, if any, of AVPV/PeN dopamine in the LH surge mechanism is ambiguous.
3.3 Species-specific sex differences in Kiss1 neurons in the ARC of adult animals
In contrast to the well-studied and well-characterized sexually dimorphic systems of the AVPV/PeN, the ARC has received much less attention regarding sexual differentiation. This may be because the ARC, unlike the AVPV/PeN and other sexually-dimorphic regions, does not appear to exhibit noticeable sex differences in overall nucleus size or total number of neurons. However, using more specific analyses, several studies have reported sex differences in the rodent ARC. For example, both the number of synapses and the morphology of astroglia in the ARC differs between male and female rodents, as does the pattern of axonal wiring of neurokinin B/dynorphin neurons and the expression of the growth hormone-releasing hormone gene (Mong et al. 1996; Mong et al. 1999; Nurhidayat et al. 2001; Mong and McCarthy 2002; Ciofi et al. 2006).
Based on the above findings, the ARC should be considered a sexually dimorphic nucleus. However, in contrast to the AVPV/PeN, the ARC of adult rodents displays no sex differences in either the total number of Kiss1 neurons or the amount of Kiss1 mRNA per cell (Kauffman et al. 2007). The lack of sex difference in ARC Kiss1 expression is not altered by circulating sex steroids: adult male and female rats display similar high levels of Kiss1 expression in the ARC following gonadectomy and similar reduced Kiss1 expression after sex steroid replacement (Kauffman et al. 2007; Homma et al. 2009). Likewise, in adult rats, kisspeptin proteins levels in the ARC, as measured by immunohistochemistry, are similar between males and females under a variety of hormone conditions (Homma et al. 2009). We recently extended these findings in rats to adult mice, in which the level of Kiss1 expression in the ARC is equivalent between the sexes, regardless of adulthood sex steroid milieu (Kauffman et al. 2009). It has been proposed that Kiss1 cells in the ARC of both males and females provide tonic stimulatory input to GnRH neurons and relay negative feedback effects of sex steroids to the GnRH axis (reviewed in (Popa et al. 2008; Oakley et al. 2009). Because tonic GnRH secretion and negative feedback occur to a similar degree in both sexes (unlike positive feedback), it is not entirely surprising that there is a lack of sex differences in ARC Kiss1 neurons, at least in adult rodents. However, as discussed later, the ARC Kiss1 system of rodents may not always be similar between the sexes, as recent evidence suggests a sex difference in the regulation of ARC Kiss1 neurons in prepubertal animals (Kauffman et al. 2009).
Interestingly, recent analysis of Kiss1 expression in the ARC of non-rodent species has yielded a different picture with regards to sexual differentiation. In particular, emerging evidence suggests that male and female sheep contain different numbers of Kiss1 neurons in the ARC, raising the possibility of an important sex difference in the ovine Kiss1 system. Specifically, intact adult ewes have more than twice as many Kiss1 neurons in the ARC than intact adult rams (Cheng et al. 2009). Unfortunately, the level of sex steroids was not controlled in this study, and thus, direct comparison of ARC Kiss1 levels in gonadectomized ewes and rams (with or without equivalent sex steroid treatment) is still needed. Interestingly, prenatal androgen treatment, which masculinizes a number of traits in female sheep, did not reverse the sex difference in ARC kisspeptin cells (despite an effect on ARC dynorphin and neurokinin B expression in the same ewes) (Cheng et al. 2009). Thus, unlike the Kiss1 system in the rodent AVPV/PeN, the Kiss1 system of the ovine ARC may not be sexually differentiated by perinatal sex steroids. One caveat, however, is that prenatal androgen in sheep was only given for a defined 30 day period, and it remains possible that androgen treatment at other prenatal (or postnatal) periods might alter the sexual differentiation of ovine Kiss1 neurons in the ARC.
The reason for the species difference in sexual dimorphism in the ARC Kiss1 system has not been definitively tested, but may relate to species differences in the neural substrates mediating sex steroid feedback. In rodents, positive feedback of E2 is mediated by the AVPV/PeN, correlating with a sex difference in Kiss1 in this region. In contrast, in sheep, the preoptic area (analogous to the AVPV/PeN) does not appear to be critical for positive feedback. Rather, the ovine ARC has been implicated in mediating the sexually dimorphic preovulatory LH surge (Smith 2009), correlating nicely with the presence of a Kiss1 sex difference in this region in sheep. In addition, negative feedback effects of progesterone are sexually dimorphic in sheep, and have also been suggested to occur in the ARC (Goodman 1996; Goodman et al. 2004; Foradori et al. 2005). Thus, the ovine sex difference in ARC Kiss1 neurons, which express both progesterone and estrogen receptors, may relate to sex differences in both positive and negative feedback in sheep. Additional studies are required to determine whether or not the ARC Kiss1 system of other species (such as monkeys and humans) displays sex differences in adulthood.
3.4. Kisspeptin signaling influences the sexual differentiation process
Interestingly, in addition to being sexually differentiated itself, the Kiss1 system of rodents is also essential for promoting the developmental mechanisms that underlie the sexual differentiation process. That is, as will be discussed, kisspeptin signaling appears to be involved in the early developmental processes that control proper sexual differentiation of multiple sexually dimorphic traits. Thus, the relationship between sexual differentiation and kisspeptin is bidirectional: kisspeptin signaling promotes the sexual differentiation process, and sexual differentiation alters kisspeptin neuron development in select brain regions.
As discussed earlier, sexual differentiation of the male brain (and the behaviors it controls) typically occurs during critical developmental windows during which the presence of T (or its metabolite E2) organizes sexually dimorphic neural populations to to be male-like. In the absence of circulating perinatal sex steroids, as in normal developing females, the brain develops to be female-like. It is not entirely known how perinatal sex steroid secretion is regulated, or why only males exhibit high circulating levels of sex steroids at this time. In collaboration with the Rissman and Steiner labs, I tested whether kisspeptin signaling, which is required for GnRH-mediated sex steroid secretion in adulthood, is also involved in promoting postnatal T secretion in newborn male rodents (Kauffman et al. 2007). If so, we speculated that sexually-dimorphic traits should be female-like in adult males that lacked kisspeptin signaling in early development. To address this possibility, we tested whether sexual differentiation is impaired in male Kiss1R KO mice (which are unable to signal with kisspeptin). In rodents, sociosexual olfactory preference is sexually differentiated in early development by postnatal sex steroids (Bakker 2003; Bodo and Rissman 2007; Baum 2009). We found that adult Kiss1R KO males fail to display typical male-like olfactory preference behavior, despite chronic testosterone treatment in adulthood. Rather, adult Kiss1R KO males behaved similarly to wildtype female mice when choosing a conspecific partner (Kauffman et al. 2007). This finding suggests an essential role for kisspeptin-Kiss1R signaling in either the development and/or display of this sexually dimorphic olfactory preference behavior. However, because several other sexually dimorphic traits were also female-like in Kiss1R KO males (see below), it is most likely that sexual differentiation of the neural circuits underlying olfactory behavior was impaired, rather than a deficit in adulthood activation of the behavior.
As described earlier, both tyrosine hydroxylase (TH) and Kiss1 neurons in the AVPV/PeN are sexually-differentiated by postnatal sex steroids, with adult females normally having more TH and Kiss1 neurons in the AVPV/PeN than adult males. Similar to olfactory preference behavior, the sexual differentiation of both of these hypothalamic systems was impaired in Kiss1R KO male mice, with these males displaying female-like numbers of Kiss1 and TH-immunoreactive neurons in the AVPV/PeN in adulthood (Kauffman et al. 2007). Similarly, absent Kiss1R signaling resulted in the impaired sexual differentiation of the motor neurons of the spinal nucleus of the bulbocavernosus (SNB), a well-characterized sexually dimorphic trait in the spinal cord of rodents (Breedlove and Arnold 1980; Breedlove and Arnold 1981). In adult Kiss1R KO males, the number of SNB motoneurons was significantly lower than that of wildtype males and similar to that of wildtype females (Kauffman et al. 2007). Collectively, the similar findings in preference behavior, TH and Kiss1 neuroanatomy, and spinal cord motoneuron number suggest that kisspeptin-Kiss1R signaling is essential for proper sexual differentiation in normal males.
Although adult Kiss1R KO animals of both sexes normally have absent sex steroid levels, the lack of normal male-like phenotypes in sexually-dimorphic traits in Kiss1R KO males was not due to deficient T in adulthood, because all mice were treated with T in adulthood prior to testing (Kauffman et al. 2007). Rather, the female-like phenotypes in adult Kiss1R KO males are likely attributable to impairments in the sexual differentiation process in early development. However, the exact mechanism of kisspeptin-Kiss1R signaling in the postnatal sexual differentiation process is unclear. Given the established role of kisspeptin in stimulating the GnRH axis in adulthood, kisspeptin-Kiss1R signaling may also be required in males during postnatal life for GnRH-mediated T secretion. Whether T levels are actually diminished in Kiss1R KO males during the postnatal critical period remains to be determined, and is currently under investigation in our lab. Another possibility is that postnatal T production in newborn males depends on peripheral kisspeptin-Kiss1R signaling occurring directly in the testes, independent of kisspeptin’s regulation of GnRH secretion in the brain. Both Kiss1 and Kiss1R are expressed in the testis, at least in adulthood (Kotani et al. 2001; Ohtaki et al. 2001); whether or not Kiss1 or Kiss1R are actually present in the testis during the critical perinatal period remains to be determined, as would the mechanism of how Kiss1R activation in the testis would affect T secretion. The role of the Kiss1 system in perinatal development and sexual differentiation in species other than mice has not yet been studied. However, a human infant with a non-functional Kiss1R gene was recently reported to possess a micropenis and cryptorchidism at birth (Semple et al. 2005), suggesting reduced androgen levels during perinatal development in this particular individual.
4. Kisspeptin Signaling and Pubertal Development
4.1 Overview of puberty and sexual maturation
Puberty is not a single event but rather a dynamic process encompassing a number of important developmental changes whereby an individual first attains the capacity to be fertile. Puberty therefore represents the critical transition between childhood (juvenile state) and adulthood. In fact, the term puberty is derived from the Latin work pubertas, meaning adulthood. During puberty, secondary sex characteristics appear, the adolescent physical growth spurt occurs, and reproductive competence is achieved (reviewed in (Grumbach 2002; Pinyerd and Zipf 2005). In addition, critical psychological changes occur during puberty (the term adolescence is often used to refer to the period of psychological and social transition between childhood and adulthood, rather than the biological changes of sexual maturation denoted as puberty) (Sisk and Foster 2004; Pinyerd and Zipf 2005).
The onset of puberty is generally defined as the activation (or in some species, a re-activation) of the previously-dormant neuroendocrine reproductive axis (Grumbach 2002; Ojeda and Skinner 2006; Plant and Witchel 2006). Thus, puberty onset is reflected by increased GnRH secretion, which activates the rest of the reproductive axis, thereby stimulating gametogenesis, estrous/menstrual cyclicity, ovulation, development of secondary sex characteristics, and contributing to physical growth. Despite the prevailing dogma that puberty is governed by enhanced GnRH secretion, the specific neural and molecular mechanisms that trigger GnRH secretion in order to initiate puberty remain one of the enigmas of modern science, as does the temporal mechanism that times when puberty actually occurs (Richter 2006). Moreover, in virtually all mammals studied, puberty onset is dramatically different between the sexes, usually earlier in females (though in sheep, for example, males enter puberty first). However, the mechanism(s) underlying this key sex difference in sexual maturation, as well as the adaptive significance of early puberty in one sex versus the other, remains a mystery. Lastly, decades of research have highlighted species differences in the neural and hormonal mechanisms that regulate pubertal activation, precluding simple comparisons and generalizations between even closely-related species. However, in all species, activation of GnRH neurons remains a consistent pubertal hallmark, and any species differences occur “upstream” of GnRH secretion at afferent regulatory signals.
4.2 The kisspeptin system is necessary and sufficient for normal puberty
In mammals, including humans, activation of GnRH neurons and downstream reproductive hormone secretion is the key event representing the onset of puberty. Although the specific neural and molecular mechanisms that trigger GnRH secretion at puberty remain unknown, kisspeptin signaling has recently been implicated in the pubertal process in a number of mammalian species. In fact, the involvement of kisspeptin in regulating the neuroendocrine reproductive axis was first brought to light based on clinical studies of individuals who had impaired or absent sexual maturation. In 2003, Seminara and colleagues and de Roux et al. independently reported that puberty was dramatically impaired in humans with spontaneous mutations in the Kiss1R gene (de Roux et al. 2003; Seminara et al. 2003). These findings occurred in two separate consanguineous families and were caused by different base pair deletions/substitutions, with the common result that the Kiss1R protein was not functional in either case. An additional study followed in 2005 similarly detailing an individual with pubertal impairment in the face of a disabling Kiss1R mutation (Semple et al. 2005). A recent report estimated that the prevalence of Kiss1R mutations in humans encompasses approximately 5% of normosmic idiopathic hypogonadotropic hypogonadism (IHH) cases and about 2% of total IHH cases (Bianco and Kaiser 2009). Interestingly, at present, there are no reports of delayed or absent puberty attributed to mutations in the human KiSS1 gene.
In support of the initial findings in humans, the necessity of intact kisspeptin signaling for puberty onset has since been extended to other mammalian species, primarily mice, in which transgenic knockout technology has allowed for the creation of Kiss1R KO mice. Seminara and colleagues first showed that Kiss1R KO mice do not progress through puberty, and have severe deficits in adulthood reproductive function (Seminara et al. 2003). This finding has been replicated and extended by other labs. In all cases, Kiss1R KO mice are sexually immature, with small gonads, absent spermatogenesis, impaired ovulation, low sex steroid and gonadotropin levels, and absent or impaired estrous cyclicity (Funes et al. 2003; Messager et al. 2005; Dungan et al. 2007; Kauffman et al. 2007; Lapatto et al. 2007). Similarly, there have been several recent reports of impaired sexual maturation and reproductive deficiencies in mice lacking a functional Kiss1 gene (d'Anglemont de Tassigny et al. 2007; Lapatto et al. 2007). Of note, Kiss1R KO mice have normal hypothalamic distribution and content of GnRH (Messager et al. 2005), suggesting that targeted deletion of Kiss1R does not significantly affect GnRH neuron migration or synthesis. In fact, some GnRH secretagogues, such as galanin-like peptide, are able to promote GnRH and LH secretion in Kiss1R KO mice (Dungan et al. 2006), further evidencing that the GnRH system is functional in these mice, other than the fact that their GnRH neurons cannot respond to kisspeptin signals.
In addition to disabling mutations in the Kiss1 system that prevent puberty onset, a recent clinical study found that a case of precocious puberty was linked to an activating mutation of the Kiss1R gene (Teles et al. 2008). In this particular case, a single base pair substitution in the Kiss1R gene resulted in prolonged intracellular signaling by the receptor in response to kisspeptin binding, thereby resulting in hyperstimulation of the reproductive axis. Animal studies have mimicked this effect by experimentally treating prepubertal rodents or monkeys with exogenous kisspeptin. In these prepubertal animals, kisspeptin treatment was found to initiate various aspects of precocious puberty (such as enhanced LH secretion or early vaginal opening, a common marker of puberty in rodents) (Navarro et al. 2004; Shahab et al. 2005). Thus, kisspeptin signaling is not only necessary for initiating puberty onset, but it is also sufficient for triggering various indices of puberty. However, while prepubertal kisspeptin treatments undoubtedly stimulated GnRH secretion before it is normally activated, it remains unclear if these treatments actually altered the developmental mechanisms that normally time and trigger puberty. Indeed, it is unclear if kisspeptin signaling represents a key component of the puberty-triggering mechanism (including, but not limited to, the pubertal “clock”) or simply a downstream effector of the puberty mechanism elsewhere in the brain. Future studies are necessary to tease this issue apart.
4.3 Changes in the neural Kiss1 system during puberty
The findings discussed in the previous section indicate that kisspeptin signaling is critical for pubertal maturation. To date, it has been all-but-assumed that the location of kisspeptin signaling during puberty is in the brain, especially since kisspeptin’s effects on the reproductive axis appear to be at the level of GnRH neurons. Although it was determined in 2004 that exogenous kisspeptin treatments can induce indices of puberty onset, it was not until 2008 that endogenous secretion of kisspeptin was actually found to increase during puberty. Working with hypothalamic explants from female monkeys, Keen and colleagues elegantly showed that neural kisspeptin secretion, as measured with radioimmunoassay, was elevated in pubertal compared to juvenile monkeys (Keen et al. 2008). Moreover, kisspeptin secretion was shown to be pulsatile and to pulse in synchrony with GnRH pulses. Thus, Keen et al. speculated that increased kisspeptin pulsatility drives increased GnRH pulsatility in the pubertal monkey. Similar studies in other species have not yet been published. Despite the important contribution of this pubertal monkey study to our understanding of kisspeptin’s role in the pubertal process, the neuroanatomical source of the pubertal kisspeptin secretion was not determined. Thus, it remains unknown whether the kisspeptin pulses were coming from ARC Kiss1 neurons, preoptic Kiss1 neurons, or both (or neither).
To elucidate the neuroanatomical specificity of the pubertal kisspeptin signal, several studies have measured Kiss1 mRNA or kisspeptin protein in the brains of rodents and monkeys at different ages. In initial studies, total Kiss1 mRNA levels in the entire rat and primate hypothalamus, as measured with RT-PCR, were mounds to be higher in adults compared to juveniles (Navarro et al. 2004; Shahab et al. 2005), suggesting enhanced kisspeptin synthesis during puberty; however, developmental changes in Kiss1 expression specifically within discrete neural populations (AVPV/PeN and ARC) were not assessed. Thus, while it is clear that Kiss1 expression increases from the juvenile period to adulthood, the location of this increase remained unknown. Subsequently, several studies in rodents aimed at assessing developmental changes in Kiss1 expression and/or kisspeptin protein immunoreactivity specifically in the AVPV/PeN and the ARC (Han et al. 2005; Clarkson and Herbison 2006; Takase et al. 2009). Unfortunately, the findings have not been entirely consistent, and conflicting results may reflect species or sex differences, dissimilarity in the specific ages that animals were studied, or technical differences. Looking at male mice, Han et al. (2005) found that Kiss1mRNA levels, as measured with in situ hybridization, were higher in adult than juvenile males, specifically in the AVPV/PeN. However, because AVPV/PeN Kiss1 gene expression is stimulated by sex steroids, it is not clear if the higher Kiss1 expression in adults simply reflects higher circulating sex steroid levels at this age. In contrast to the AVPV/PeN, Han et al., (2005) found no significant differences between adult and juvenile males in Kiss1 levels in the ARC. Clarkson and Herbison (2006) reported similar developmental increases in kisspeptin-immunoreactivity in the AVPV/PeN in both male and female mice (higher in adults compared to juvenile and prepubertal animals). Again, whether these increases in kisspeptin levels merely reflect increasing levels of sex steroids (which upregulate kisspeptin production in the AVPV/PeN) was not determined. Unfortunately, kisspeptin immunoreactivity in the murine ARC was not quantified in either sex, owing to the difficulty of identifying discrete neuron cell bodies which were obscured by dense kisspeptin-immunoreactivity fibers in this region. Thus, it is unknown if kisspeptin protein in the murine ARC fails to show a developmental change as reported by Han et al. (2005) for Kiss1 mRNA levels in this nucleus.
Most recently, Takase et al (2009) have addressed this issue in developing female rats. In this study, Kiss1 mRNA levels were higher in adulthood than in prepubertal animals in both the ARC and AVPV/PeN (as measured by RT-PCR), as was kisspeptin protein-immunoreactivity. This finding in female rats contradicts that in male mice in which the ARC Kiss1 system does not appear to change with puberty (Han et al. 2005). To determine if sex steroids influence the increases in Kiss1 levels in rats, Takase et al (2009) treated separate cohorts of female rats with constant low E2 levels at each age and measured Kiss1 mRNA levels several days later. As in intact animals, Kiss1 was higher in both the ARC and AVPV/PeN in adults than in prepubertal females, even when sex steroid levels were equal between age groups. Thus, at least in female rats, Kiss1/kisspeptin increases with puberty in both the ARC and AVPV/PeN, and this increase is independent of changes in circulating sex steroids. Whether this is the case for male rats, or males and females of other species, remains to be determined.
In mice, the number of kisspeptin-containing fibers that appose GnRH neurons was reported to increase at puberty (Clarkson and Herbison 2006). This suggests that pubertal maturation may also include the completion of developmental circuitry coupling Kiss1 and GnRH neurons. Note, however, that higher sex steroids in adulthood would result in elevated kisspeptin synthesis in the AVPV/PeN, which could cause more kisspeptin-immunoreactivity to be present. Thus, it is possible that the degree of innervation of GnRH neurons by kisspeptin neurons is not different before and after puberty, but that the technical ability to visualize these kisspeptin axonal fibers is enhanced in adulthood when more kisspeptin is being produced. This possibility notwithstanding, the prospect that puberty includes an increase in kisspeptin fiber targeting of GnRH neurons is intriguing and deserves more investigation. Whether or not sex differences might occur in this facet of kisspeptin development also remains to be seen.
In addition to Kiss1, changes in the kisspeptin receptor, Kiss1R, may also be involved in pubertal maturation, though this has received less attention. Low doses of kisspeptin treatment are less effective at stimulating gonadotropin secretion and GnRH neuronal firing activity in juvenile than adult rodents (Han et al. 2005; Castellano et al. 2006), suggesting that kisspeptin has a reduced ability to activate the GnRH system before puberty. This may reflect lower levels of Kiss1R before puberty. In support of this, in rats of both sexes and female monkeys, but not male mice, hypothalamic Kiss1R expression in is higher in adulthood than in juveniles (Navarro et al. 2004; Han et al. 2005; Shahab et al. 2005). However, in most cases, Kiss1R was only measured well before or well after puberty, not during the pubertal transition. Navarro et al. (2004) measured Kiss1R levels on one day during the actual pubertal period and found that total hypothalamic Kiss1R expression was higher on that one day than in both juvenile and adult rats. Importantly, the elevated Kiss1R levels occurred earlier in female than male rats, corresponding with earlier puberty onset in the former. Thus, increases in hypothalamic Kiss1R may comprise a critical aspect of the pubertal process, though more work on this subject is needed. In addition, it is possible that the ability of the Kiss1R protein to signal within GnRH neurons changes with puberty, a prospect that could be independent of changes in Kiss1R mRNA levels.
4.4 Sex differences in the steroid-independent regulation of prepubertal Kiss1 neurons
Although the precise mechanisms underlying puberty onset remain unknown, the regulation of pubertal maturation is thought to include a complex mixture of gonadal steroid-dependent and steroid-independent mechanisms acting at the level of the brain (Ebling 2005; Ojeda and Skinner 2006; Plant and Witchel 2006; Herbison 2007). For example, in rodents, negative feedback inhibition of reproductive neural circuits by low levels of gonadal steroids is a primary mechanism for suppressing the reproductive axis before puberty (Ojeda and Skinner 2006). One component of pubertal maturation includes a developmental decrease in the sensitivity of key reproductive circuits to gonadal steroid negative feedback, thereby allowing enhanced activation of the GnRH axis. However, in rats, this developmental shift in gonadal steroid feedback sensitivity typically occurs on the day of vaginal opening; since vaginal opening is a “downstream” event in the pubertal process, it is assumed that puberty onset has already occurred by the time of the change in gonadal steroid sensitivity. Thus, additional, non-gonadal factors have been implicated. This is especially true in primates, in which gonadectomy during the juvenile phase does not result in elevated gonadotropin secretion (even though gonadal steroid feedback has been completely removed). Rather, gonadotropin secretion in agonadal males and females stays low until just before the time of normal puberty onset, when there is a large increase in gonadotropin output (Plant and Witchel 2006). These findings in primates indicate that there is steroid-independent regulation of the developing reproductive axis. Puberty onset may therefore reflect removal of inhibitory input into reproductive circuits, or conversely, enhancement of stimulatory input, either of which might occur independent of changes in gonadal steroid. A good amount of work during the 1990’s focused on possible steroid-independent inhibitory factors and their role in puberty onset, including neuropeptide Y and GABA. However, the exact role of these neural factors in puberty still remains unclear. In addition, whether sex differences in the onset of puberty (earlier in females than males) reflect sex differences in putative steroid-independent factors remains untested.
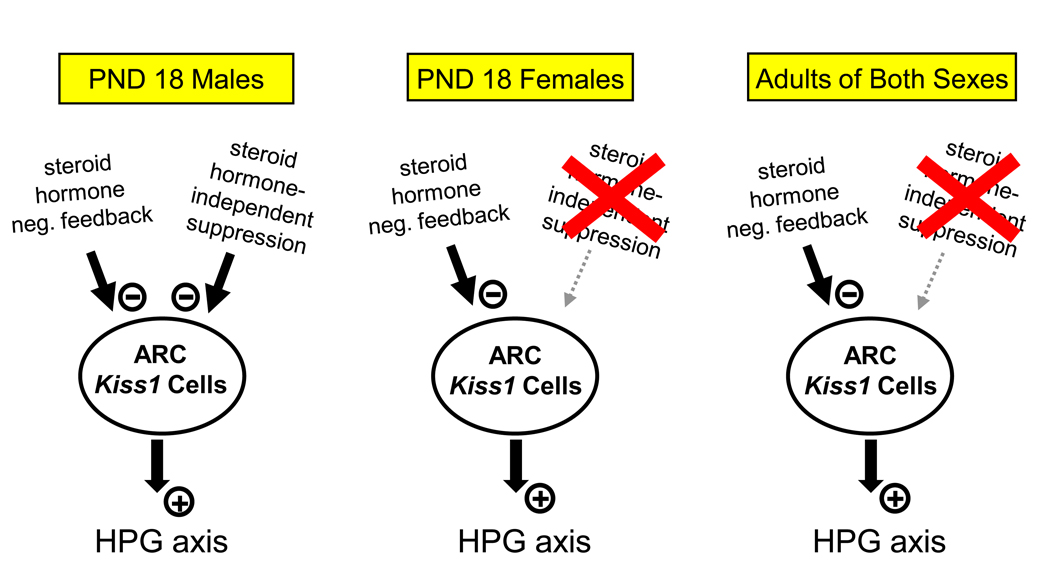
Because kisspeptin is thought to play an important role in puberty, I recently examined whether Kiss1 neurons in the prepubertal rodent brain are regulated by the gonadal steroid-dependent and/or steroid-independent factors known to govern puberty onset (Kauffman et al. 2009). I postulated that if pubertal activation of reproductive circuits—including Kiss1 neurons—is predominantly regulated by changes in sensitivity to gonadal steroid negative feedback, then removal of gonadal steroids before puberty should induce a precocious activation of both Kiss1 neurons and gonadotropin secretion. However, if the prepubertal reproductive axis is also suppressed by gonadal steroid-independent inhibition, then removal of gonadal steroid feedback before puberty may not induce early activation of either Kiss1 circuits or LH secretion because non-gonadal, central restraining mechanisms are still present. Moreover, I hypothesized that earlier puberty onset in females compared to males may reflect sex differences in the timing of the gating of peripubertal Kiss1 circuits, such that there is greater (or longer-lasting) inhibition of Kiss1 circuitry in prepubertal males compared to prepubertal females. Using mice, I found that in prepubertal females, LH secretion and Kiss1 levels in the ARC increased dramatically 2 and 4 days after ovariectomy (Kauffman et al. 2009). This finding suggests that there is virtually no gonadal steroid-independent restraint on reproductive circuits in prepubertal females at this age (postnatal day (PND) 16–18). Thus, the prepubertal female reproductive axis appears to be kept quiescent predominantly by gonadal steroid negative feedback (at least at ages PND 16–18). In contrast, in prepubertal males of the same ages, neither LH levels nor the ARC Kiss1 expression increased 2 or 4 days following castration (Kauffman et al. 2009). This outcome indicates that gonadal steroid-independent mechanisms contribute significant suppression on reproductive circuits (including Kiss1 neurons) in prepubertal male mice, at least at the ages tested. Furthermore, adult mice of both sexes exhibited increases in both LH levels and ARC Kiss1 expression 4 days following gonadectomy (Kauffman et al. 2009), highlighting that sex differences in the steroid-independent regulation of ARC Kiss1 neurons and LH secretion are manifest only during peripubertal development. These findings demonstrate that the regulation of reproductive status during prepubertal development is sexually dimorphic, with males, but not females, exhibiting gonadal steroid-independent suppression of Kiss1 and LH levels (Fig. 4). This Kiss1 sex difference may relate to known sex differences in pubertal maturation in mammals, including humans (boys usually mature later than girls), though additional experimentation is needed to support this idea.
Fig. 4.
The reproductive circuits of prepubertal (PND 16–18) male mice are suppressed by both gonadal steroids and gonadal steroid-independent factors. In contrast, reproductive circuits in prepubertal females and adults of both sexes are predominantly suppressed by gonadal steroids. Gonadectomy (removing hormone negative feedback) therefore increases ARC Kiss1 levels in adults and prepubertal females, but not prepubertal males (who still have gonad-independent restraint mechanisms present). It is unknown if this sex difference relates to sex differences in pubertal maturation.
Interestingly, whereas castrated prepubertal males do not show elevated LH secretion or ARC Kiss1 levels after 2 or 4 days (on PND 16–18), these prepubertally castrated males do have increased ARC Kiss1 and LH levels when measured later in adulthood (Kauffman et al. 2009). Notably, these increases in Kiss1 expression occurred in the absence of any developmental changes in gonadal steroids (since the mice were castrated on PND 14). Thus, sometime between PND 18 and adulthood, there is a key developmental change in non-gonadal regulation of male reproductive circuits (including Kiss1 neurons in the ARC). The identity of the gonadal steroid-independent factor(s) is unknown, as is the specific time in peripubertal development when this non-gonadal suppression is lifted. These are currently hot topics of study in our lab. Regardless, this finding supports that of Takase et al (2009) who reported developmental increases in Kiss1 expression in rats that were independent of changes in circulating sex steroid levels.
Unlike Kiss1 expression in the ARC, Kiss1 levels in the AVPV/PeN were robustly decreased (by approximately 50%) in prepubertal female mice that displayed precocious LH secretion following ovariectomy (Kauffman et al. 2009). It is therefore unlikely that AVPV/PeN Kiss1 neurons were responsible for driving the elevated gonadotropin secretion in these prepubertal PND 18 females since these AVPV/PeN neurons were making less kisspeptin at this time. Rather, the ARC Kiss1 population, which was activated at this time, could be promoting the elevated LH secretion. However, whether or not pubertal activation of the reproductive axis (which normally occurs a week after PND 18) similarly involves regulation of Kiss1 neurons located in the ARC, rather than the AVPV/PeN, is unclear. The fact that Kiss1 levels in rodents increase during puberty in both the AVPV/PeN and ARC suggests that either site (or both) may be involved. Recently, Clarkson et al. (2009) proposed an AVPV/PeN-specific model to explain puberty onset based on the observation that ovariectomized prepubertal mice fail to display vaginal opening (i.e., “puberty”) or elevated kisspeptin protein levels in the AVPV/PeN later in early adulthood. They postulated that E2 during the prepubertal period stimulates Kiss1 neurons in the AVPV/PeN, thereby activating GnRH secretion. Such GnRH secretion subsequently drives additional downstream E2 production, producing a “feed-forward” activational loop of the kisspeptin-GnRH axis (Clarkson et al. 2009). However, it is not clear how this model would explain puberty in male rodents (especially rats), which have only a few Kiss1 neurons in the AVPV/PeN, even after high E2 treatment. Moreover, it is important to note that in the adult female rodent, Kiss1 mRNA and kisspeptin protein levels in the AVPV/PeN are highly E2-dependent and decrease markedly in the absence of E2 (Smith et al. 2005; Smith et al. 2006; Adachi et al. 2007; Kauffman et al. 2007). Because the ovariectomized juvenile mice were never replaced with E2 later in adulthood (Clarkson et al. 2009), it is possible that the low kisspeptin levels observed in the adult AVPV/PeN reflects absent circulating E2 at this time. Despite this caveat, it is certainly possible, and even likely, that the AVPV/PeN Kiss1 system contributes to the enhanced drive to GnRH neurons during puberty. If so, higher Kiss1 levels in the AVPV/PeN in females than males may contribute in some manner to sex differences in pubertal timing. Regardless, this would not exclude an important pubertal role for other neural circuits as well, including ARC Kiss1 neurons.
5. Summary and Perspectives
In the past decade, investigations in a number of mammalian species, primarily rodents, sheep, and monkeys, have yielded abundant information about the role of kisspeptin signaling in the developmental regulation of the reproductive axis, as well as information regarding the development of Kiss1 populations themselves. To this end, we now know that Kiss1 neurons in the AVPV/PeN are sexually differentiated under the direction of sex steroids early in postnatal development, and that this sexually-dimorphic population of Kiss1 neurons comprises a key part of the neural mechanism underlying the GnRH/LH surge that occurs in adult females. Moreover, the Kiss1 neurons in the adult AVPV/PeN are also under the influence of circadian regulatory signals, which may provide the mechanism by which the LH surge is timed to occur at a specific time of day. Intriguingly, it also appears that developmental kisspeptin-Kiss1R signaling may itself be critical for the process of sexual differentiation of the brain and behavior, perhaps via the regulation of postnatal T secretion. The Kiss1 system is also critical for pubertal maturation, and kisspeptin signaling arising from either the ARC or AVPV/PeN (or both sites) may comprise a key element of the mechanism triggering puberty onset. If so, then sex differences in the Kiss1 system, or the “upstream” systems that regulate Kiss1 neurons, may contribute to differences in puberty onset between males and females. Despite these advances in the field of kisspeptin biology, many questions and challenges remain. Several important goals are to 1) assess how and when sex steroids act in the brain to alter the development of Kiss1 neurons in the AVPV/PeN, as well as identify the key sex steroid receptor pathways involved; 2) elucidate the presence or absence of sexual differentiation of Kiss1 neurons in the ARC of species besides mice, rats and sheep, and define the functional role of any ARC Kiss1 sex differences; 3) assess whether sex differences in Kiss1 neurons persist in species in which the LH surge is not sexually dimorphic, such as certain primates; 4) understand the role, if any, of kisspeptin-Kiss1R signaling in regulating the perinatal T surge that occurs in developing males; 5) identify the processes by which pubertal activation of Kiss1 neurons is timed and triggered. In addition to these issues, it will be important for future studies to study kisspeptin biology in both males and females, as well as in multiple species, as discussions presented herein have evidenced critical inconsistencies in some of the kisspeptin data available between sexes and species.
Acknowledgments
Dr. Kauffman’s research is supported by NICHD grant R00 HD056157.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11(9):995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol. 2003;15(6):615–621. doi: 10.1046/j.1365-2826.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- Bakker J, Pierman S, Gonzalez-Martinez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.11.005. (In Press) [DOI] [PubMed] [Google Scholar]
- Barraclough CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Horm Behav. 2009;55(5):579–588. doi: 10.1016/j.yhbeh.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco SD, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5(10):569–576. doi: 10.1038/nrendo.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran J, Ben-Dor S, Levavi-Sivan B. Molecular identification and functional characterization of the kisspeptin/kisspeptin receptor system in lower vertebrates. Biol Reprod. 2008;79(4):776–786. doi: 10.1095/biolreprod.107.066266. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25(7):2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210(4469):564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225(2):297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147(10):4852–4862. doi: 10.1210/en.2006-0117. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, et al. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol. 2006;257–258:75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs. 2007;36(3):263–274. doi: 10.1111/j.1552-6909.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in the sheep. Endocrinology. 2009 doi: 10.1210/en.2009-0541. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141(4):1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150(7):3214–3220. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, de Tassigny XD, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurons in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19(4):323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Corbier P. Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology. 1985;116(1):142–147. doi: 10.1210/endo-116-1-142. [DOI] [PubMed] [Google Scholar]
- D'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104(25):10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147(3):1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22(20):9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Sodersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55(5):589–596. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus Interactions with GnRH neuronal system. J Chem Neuroanat. 2008;36(3–4):131–137. doi: 10.1016/j.jchemneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Dungan H, Gottsch ML, Byquist A, Hohmann J, Clifton DK, et al. GnRH secretagogues stimulate luteinizing hormone secretion in GPR54 knockout mice; Society for Neuroscience Annual Meeting; Atlanta, GA. 2006. Program #658.10. [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27(44):12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129(6):675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- Elizur A. The KiSS1/GPR54 system in fish. Peptides. 2009;30(1):164–170. doi: 10.1016/j.peptides.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Fechner A, Fong S, McGovern P. A review of Kallmann syndrome: genetics, pathophysiology, and clinical management. Obstet Gynecol Surv. 2008;63(3):189–194. doi: 10.1097/OGX.0b013e3181641278. [DOI] [PubMed] [Google Scholar]
- Ferin M, Carmel PW, Zimmerman EA, Warren M, Perez R, et al. Location of intrahypothalamic estrogen-responsive sites influencing LH secretion in the female Rhesus monkey. Endocrinology. 1974;95(4):1059–1068. doi: 10.1210/endo-95-4-1059. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol. 2009;21(4):393–399. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, et al. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26(8):2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Wang O, Majzoub J, Kaiser U. Altered feedback response to estradiol in GnRH-deficient hpg mice emphasizes differential regulation of AVPV and ARC kisspeptin neurons. P2-268. The Annual Meeting of the Endocrine Society; Washington D.C.. 2009. [Google Scholar]
- Gogan F, Beattie IA, Hery M, Laplante E, Kordon D. Effect of neonatal administration of steroids or gonadectomy upon oestradiol-induced luteinizing hormone release in rats of both sexes. J Endocrinol. 1980;85(1):69–74. doi: 10.1677/joe.0.0850069. [DOI] [PubMed] [Google Scholar]
- Gogan F, Slama A, Bizzini-Koutznetzova B, Dray F, Kordon C. Importance of perinatal testosterone in sexual differentiation in the male rat. J Endocrinol. 1981;91(1):75–79. doi: 10.1677/joe.0.0910075. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology. 2008;149(5):2333–2340. doi: 10.1210/en.2007-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL. Neural systems mediating the negative feedback actions of estradiol and progesterone in the ewe. Acta Neurobiol Exp (Wars) 1996;56(3):727–741. doi: 10.55782/ane-1996-1178. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, et al. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148(3):1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57 Suppl 2:2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Genetics of puberty. Horm Res. 2007;68 Suppl 5:75–79. doi: 10.1159/000110583. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57(2):277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, et al. Significance of Neonatal Testicular Sex Steroids to Defeminize Anteroventral Periventricular Kisspeptin Neurons and the GnRH/LH Surge System in Male Rats. Biol Reprod. 2009 doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Foster DL. Sexual differentiation of the mechanism controlling the preovulatory discharge of luteinizing hormone in sheep. Endocrinology. 1975;97(2):373–379. doi: 10.1210/endo-97-2-373. [DOI] [PubMed] [Google Scholar]
- Kauffman AS. Sexual differentiation and the Kiss1 system: Hormonal and developmental considerations. Peptides. 2009;30(1):83–93. doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30(10):504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex Differences in the Regulation of Kiss1/NKB Neurons in Juvenile Mice: Implications for the Timing of Puberty. Am J Physiol: Endocrinol and Metab. 2009;297:1212–1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27(33):8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54ȡ/− mice. Endocrinology. 2007;148(10):4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446(1):103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57(12):2384–2387. [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30(1):26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19(4):1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm Behav. 1996;30(4):553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res. 2002;139(2):151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10):4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146(4):1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146(1):156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561(Pt 2):379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, et al. Regulation of GnRH Secretion by Kiss1/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurhidayat, Tsukamoto Y, Sasaki F. Role of the gonads in sex differentiation of growth hormone-releasing hormone and somatostatin neurons in the mouse hypothalamus during postnatal development. Brain Res. 2001;890(1):154–161. doi: 10.1016/s0006-8993(00)03159-0. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, et al. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821. doi: 10.1111/j.1365-2826.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK. Knobil and Niell's Physiology of Reproduction. J. D. Neill Elsevier; 2006. Puberty in the rat; pp. 2061–2126. [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24(37):8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Burns-Cusato M, Dominguez-Salazar E, Riggan A, Shetty S, et al. Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain Behav. 2008;7(6):609–617. doi: 10.1111/j.1601-183X.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69(6):1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, et al. Critical Roles of Kisspeptins in Female Puberty and Preovulatory Gonadotropin Surges as Revealed by a Novel Antagonist. Endocrinology. 2010 doi: 10.1210/en.2009-0803. (In Press) [DOI] [PubMed] [Google Scholar]
- Pinyerd B, Zipf WB. Puberty-timing is everything! J Pediatr Nurs. 2005;20(2):75–82. doi: 10.1016/j.pedn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SM. Knobil and Neill's Physiology of Reproduction. J. D. Neill Elsevier; 2006. Puberty in non-human primates and humans; pp. 2177–2230. [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149(9):4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter LM. Studying adolescence. Science. 2006;312(5782):1902–1905. doi: 10.1126/science.1127489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian Regulation of Kiss1 Neurons: Implications for Timing the Preovulatory GnRH/LH Surge. Endocrinology. 2009;150:3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, et al. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45(4):242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Scott CJ, Kuehl DE, Ferreira SA, Jackson GL. Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology. 1997;138(9):3686–3694. doi: 10.1210/endo.138.9.5401. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, et al. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90(3):1849–1855. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, et al. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, et al. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2007;19(6):432–438. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92(2):195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400(1):11–34. doi: 10.1016/0006-8993(87)90649-4. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40(6):501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94(25):14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smith ER, Davidson JM. Location of feedback receptors: effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinology. 1974;95(6):1566–1573. doi: 10.1210/endo-95-6-1566. [DOI] [PubMed] [Google Scholar]
- Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: Comparative aspects. Peptides. 2009;30(1):94–102. doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, et al. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol. 2009;21(6):527–537. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, et al. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004;1678(2–3):102–110. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Torricelli M, Galleri L, Voltolini C, Biliotti G, Florio P, et al. Changes of placental Kiss-1 mRNA expression and maternal/cord kisspeptin levels at preterm delivery. Reprod Sci. 2008;15(8):779–784. doi: 10.1177/1933719108322442. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Leaf PJ, Holzer CE, 3rd, Myers JK, Tischler GL. The epidemiology of depression. An update on sex differences in rates. J Affect Disord. 1984;7(3–4):179–188. doi: 10.1016/0165-0327(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Wing L. Sex ratios in early childhood autism and related conditions. Psychiatry Res. 1981;5(2):129–137. doi: 10.1016/0165-1781(81)90043-3. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66(6):578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]