Abstract
Oxygen is not only an obviously important substrate, but it is also a regulatory signal that controls expression of a specific genetic program. Crucial mediator of the adaptive response of cells to hypoxia is the family of Hypoxia-Inducible Transcription Factors (HIFṣ. The fetal growth plate, which is an avascular structure of mesenchymal origin, has a unique out-in gradient of oxygenation. HIF-1α is necessary for chondrogenesis in vivo by controlling a complex homeostatic response that allows chondrocytes to survive and differentiate in a hypoxic environment. Moreover, HIFs are also essential in osteogenesis and joint development. This brief Perspective summarizes the critical role of HIFs in endochondral bone development.
Introduction
In recent years, it has become increasingly clear that oxygen is not only an obviously important substrate, but it is also a regulatory signal that controls expression of a specific genetic program. Reduced availability of oxygen, or hypoxia, activates a transcriptional response that has an important role both in pathological conditions, such as ischemia or tumorigenesis, and in normal development [1, 2].
Hypoxia, i.e. reduced availability of oxygen, is not an absolute but rather a relative concept. During development, all embryonic tissues experience a low oxygen tension before the circulatory system forms. This physiological hypoxia is essential for the vascularization of the placenta and of the embryo [3]. Once heart and blood vessels have formed and are functional, gradients of oxygenation are still present in the developing embryo [3]. The fetal growth plate is an outstanding example of how low availability of oxygen can activate a non-redundant genetic program with an essential homeostatic role during development. Hypoxia is also involved in the homeostasis of adult tissues that are physiologically hypoxic, like the articular joints. In this review, we will summarize the current knowledge of how the genetic program activated by hypoxia controls fetal growth plate development, osteoblast activity, and joint formation and homeostasis.
Hypoxia, HIFs and the others
Mammalian cells have developed sensors of low oxygen tension that trigger a complex, but elegant homeostatic response, which includes regulation of glucose utilization, iron transport, hematopoiesis, angiogenesis, cell survival and apoptosis [4]. The best characterized transcription factors involved in the cellular adaptation to hypoxia are the Hypoxia Inducible Transcription Factors (HIFs) [5].
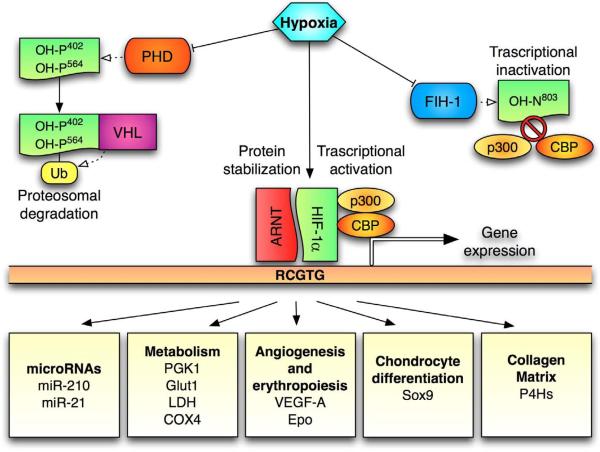
HIF-1 is a heterodimer of two proteins, HIF-1 and HIF-1β; HIF-1β is constitutively expressed, whereas HIF-1α is the hypoxic –responsive component of the complex. When oxygen drops below 5%, HIF-1α translocates to the nucleus, heterodimerizes with the β subunit, and initiates its transcriptional program (Figure 1). Stability of HIF-1α protein is hypoxia-sensitive, through oxygen-dependent hydroxylation of specific residues within its amino acid sequence. In particular, a family of HIF prolyl 4-hydroxylases is responsible for the hydroxylation of two proline residues (P402 and P564) in the oxygen dependent degradation domain (ODDD) of HIF-1 [6]. The E3 ubiquitin ligase Von Hippel-Lindau (VHL) binds to the hydroxylated HIF-1 and targets it to the proteasome for degradation [7, 8]. In hypoxic conditions, hydroxylation of HIF-1α does not occur, and the α subunit is free to migrate to the nucleus, bind its β counterpart, and thus act as a transcription factor along with other co-factors. An additional level of regulation mediated by cellular oxygen levels is given by the factor inhibiting HIF (FIH-1) [9], an asparagine hydroxylase that hydroxylates the N803 residue in the C-terminal transactivation domain of HIF-1α [10, 11]. In hypoxic conditions N803 is not hydroxylated by FIH-1; therefore, HIF-1α can interact with its transcriptional coactivators, p300 and CBP (CREB binding protein), and hence further promote transcription of genes that have hypoxia responsive enhancer elements (HRE) in their promoter region [12, 13]. Most of the genes regulated by hypoxia in a HIF-1 dependent fashion contain HRE sites in their promoter, and are activated through the mechanism described above. Interestingly, HIF-1 can interact with both the transcriptional coactivator histone acetyltransferase p300, and with histone deacetylases, like HDAC7 [14]. These findings might appear paradoxical at first glance, though it is not surprising that different target genes may require different cofactors.
Figure 1. Diagram of the HIF-1α pathway.
In normoxia prolyl-hydroxylases (PHDs) hydroxylates prolyns 402 and 564 in the O2 –dependent degradation domain (ODDD) of HIF-1α, leading to its proteosomal degradation mediated by the von Hippel-Lindau (VHL) ubiquitine ligase. Also the factor inhibiting HIF (FIH) can hydroxylate HIF-1α on asparagine 803 in its carboxy-terminal transactivation domain (C-TAD), leading to transcriptional inactivation. When HIF is stabilized in normoxia, a plethora of genes that contain hypoxia responsive elements (HRE, 5'-RCGTG-3') are expressed. Among the hypoxia-induced genes there are microRNAs, genes involved in metabolism, angiogenesis, erythropoiesis, chondrocyte differentiation and collagen matrix.
Chromatin remodeling proteins are not only co-factors but also targets of HIF-1 action. Histone demethylases such as Jumonji-domain containing proteins (JMJs) are regulated by hypoxia in a HIF-1 dependent manner. JMJs are a class of enzymes that demethylate histone arginine and lysine residues through an oxidative reaction requiring the cofactors Fe(II) and alpha-ketoglutarate [15, 16]. Recent findings have unveiled a role for JMJD1A in the upregulation of adrenomedullin and growth and differentiation factor 15 (GDF15) in hypoxia, effects which could ultimately positively modulate tumor growth [17].
Non-canonical modes of action of HIF-1α, leading to down-regulation or repression of gene expression without direct interaction of HIF-1α with HRE elements, have also been proposed. The “HIF-1α -c-Myc” axis (reviewed in [18, 19]) is a very interesting and intriguing example of HIF-1α mediated, HRE-independent gene regulation. In particular, it has been reported that the PAS-B domain of HIF-1α displaces c-Myc from specific promoter targets, and this results in significant changes of expression of c-Myc-dependent genes. An example of the dynamic interaction between HIF-1α and c-Myc is the upregulation of the cell cycle checkpoint gene p21 in hypoxia [20]. p21 is encoded by a HRE deficient gene, and its expression is normally suppressed by c-Myc; however, in hypoxic conditions p21 mRNA and protein levels increase due to the HIF-1α dependent displacement of c-Myc from its promoter region, which relieves the genetic repression [20].
Stimuli other than hypoxia also cause HIF-1α to accumulate in normoxic cells, but the molecular mechanisms are not yet fully understood [21].
Notably, null mice lacking HIF-1α die at very early stage of embryonic development, a finding which further proves the critical role of HIF-1 in development [22]. Other two HIF-α isoforms have been recently identified, HIF-2α and HIF-3α. The HIF-2α isoform is regulated by oxygen with a modality very similar to HIF-1α. However, the two alpha subunits differ in their biological function. HIF-2α and HIF-1α have common targets as well as specific ones [23–26]. Moreover, they show different tissue localization: HIF-1α is ubiquitous, whereas HIF-2α can be found mainly in blood vessels, lung, kidney, interstitial cells, liver and neural crest [27]. Lastly, in some genetic backgrounds mice lacking HIF-2α survive postnatally [28], where, as aforementioned, lack of HIF-1α causes early embryonic lethality. Differently from HIF-1α and HIF-2α, the biological role of the HIF-3α isoform is still largely unknown, though it has been proposed that this protein could have a dominant negative function, since it lacks the transactivation domains [29].
It is important to highlight that the hypoxic response is very complex, and it goes beyond stabilization and increased transcriptional activity of HIFs [30]. Along these lines, it is interesting to note that the genetic program activated by hypoxia also includes regulation of specific microRNAs. MicroRNAs are believed to play an important role in the down-regulation of genes in response to stress, and recently they have been described as active regulators of the HIF-mediated hypoxic response [31–33]. Even if microRNAs represent only 1% to 2% of the eukaryotic transcripts, they finely tune gene expression by regulating 30% of the coding mRNAs [34, 35]. miRNAs are small non-coding RNAs of 19–24 nucleotides long, transcribed by RNA polymerase II and processed by Drosha and Dicer, a class of RNase III enzymes; they act as posttranscriptional repressors, by pairing to the 3' untranslated region of the target mRNA and degrading it or inhibiting its translation. In recent years their importance in many pathologies, like cancer, as well as in development has been unveiled [36, 37]. Striking correlations have been found between microRNA expression and hypoxia [32], particularly in regard to miR-210 [38–40] and miR-21 [41]. Not surprisingly, microRNAs that specifically target HIF-1α expression, like the miR-17-92 cluster and miR-199a, have also been identified [42, 43].
HIFs and endochondral bone development
Bone can form through two different mechanisms, intramembranous and endochondral. While the flat bones of the skull develop from mesenchymal cells that directly differentiate into osteoblasts (intramembranous bone formation), the other skeletal bones are derived from the replacement of a chondrocyte anlage by bone, according to a very well-defined temporal and spatial pattern [44–47]. This latter process is called endochondral bone development [44–47], andan increasing body of evidence has highlighted the essential role of the genetic program turned on by hypoxia in regulating it [48]. Chondrocytes in the fetal growth plate synthesize collagen type II, are highly proliferative, and while they divide they also pile up to form a columnar layer (Figure 2). The most distal cells of the columnar layer stop proliferating, exit the cell cycle, and differentiate into hypertrophic chondrocytes, which produce collagen type X and mineralize their surrounding matrix (Figure 2). Programmed death of the hypertrophic chondrocytes allows blood vessels invasion, and replacement of cartilage by bone, the so-called primary spongiosa (Figure 2). The fetal growth plate is a unique mesenchymal tissue, since it is avascular for most of its length, though it does require the angiogenic switch in order to be replaced by bone. Consistent with its avascularity, the fetal growth plate contains hypoxic regions. As shown by immunohistochemistry for the EF5, a bio-reductive marker of hypoxia, the more hypoxic chondrocytes are found in the round proliferative layer, especially near the joint space, in the center of the columnar layer and the upper part of the hypertrophic zone, whereas the late hypertrophic chondrocytes at the border with the primary spongiosa are not hypoxic [49] (Figure 2). This specific spatial distribution of hypoxic areas is consistent with both the avascularity of cartilage and, conversely, the extensive vascularization of the surrounding soft tissue and of the primary spongiosa.
Figure 2. Schematic representation of the fetal growth plate.
Mesenchymal cells differentiate into round proliferative chondrocytes and when they divide are piled up in a layer of columnar proliferative chondrocytes. Both layers produce collagen type II. The latest chondrocytes are the hypertrophic ones that express collagen type X and mineralize their matrix. When hypertrophic chondrocytes undergo apoptosis, the space is invaded by blood vessels and colonized by osteoblasts, which produce the primordial trabecular bone, also called primary spongiosa. The most hypoxic areas in the growth plate are shown in red.
In the last few years, numerous studies have been conducted in the attempt to understand how the genetic program controlled by hypoxia modulates endochondral bone formation. The use of genetically modified mice turned out to be a successful and informative strategy in eviscerating the role of HIFs in growth plate and bone development. In vitro studies with primary chondrocytes or chondrogenic cell lines maintained in hypoxia, and/or in which the HIF pathway had been genetically or pharmacologically modified, further helped in dissecting out the complex interactome controlled by hypoxia in the developing bone. Taken together, an essential and non-redundant role of the HIFs in endochondral bone development has emerged. It has yet to be established, though, whether HIFs are not only necessary but also sufficient for cartilage formation, at least in specific settings in vivo. Notably, other genes, such as CCN2/CTGF and PTEN [50, 51], have been shown to cooperate with HIF-1 in cartilage development, contributing to form a network of actions that is destined to expand in the future.
HIF-1 α and chondrocyte survival
Conditional deletion of HIF-1α in limb bud mesenchyme or in chondrocytes, achieved by the use of the Cre-loxP strategy, has demonstrated that HIF-1α is necessary for chondrogenesis in vivo by turning on a complex homeostatic response that allows chondrocytes to survive and differentiate in a hypoxic environment.
Lack of HIF-1α causes massive cell death of the inner chondrocyte layer in the developing growth plate [49, 52]. Numerous molecular mechanisms could be invoked as downstream mediators of HIF-1α survival function. Consistent with its hypoxic status, the fetal growth plate lives on anerobic glycolysis [49]. Notably, in HIF-1α null growth plates, level of phosphoglycerate kinase (PGK-1), a key enzyme in anaerobic glycolysis and a classical downstream target of HIF-1α, are extremely low, which suggests that anerobic glycolysis could be affected in mutant chondrocytes [49].
In addition to metabolism, impaired up-regulation of vascular endothelial growth factor (VEGFA), another well-characterized downstream target of HIF-1α, could be responsible, at least in part, for the massive cell death phenotype observed in HIF-1α null growth plates. VEGF-A mRNA is expressed not only in late hypertrophic chondrocytes, where it is important for replacement of cartilage by bone [53–55], but also, although to a lesser extent, in the center of the proliferative layer and in the upper hypertrophic zone, i.e in the hypoxic regions of the growth plate [56]. Notably, conditional knockout of VEGF-A in chondrocytes causes cell death in the center of mutant growth plates, indicating that VEGF-A is critical for chondrocyte survival [53]. This cell death is similar to the phenotype observed in chondrocytes lacking HIF-1α, which suggests that HIF-1α and VEGF-A could be part of a common pathway that supports chondrocyte survival in endochondral bone development. However, it is intriguing that VEGF-A mRNA is up –regulated in the viable chondrocytes surrounding the area of cell death in HIF-1α null growth plates, raising questions about contribution of this growth factor to the overall HIF-1α phenotype [49]. Moreover, both receptors for VEGF-A, VEGFR2 and VEGFR1, are not expressed in the fetal growth plate [53], whereas the co-receptors neuropilin-1&2 and VEGFR3, which does not bind VEGF-A [57], are [53]. Taken altogether, these findings imply that either VEGF-A produced by hypoxic chondrocytes diffuses out of the growth plate and controls angiogenesis in the surrounding soft tissue, or that this growth factor acts locally in chondrocytes through an autocrine/intracrine mechanism that does not require either VEGFR1 or VEGFR2. Additional studies are needed to reach a better understanding of these apparent paradoxes. More in general, it is intriguing that the proliferative layer of the fetal growth plate, despite expressing VEGF-A, is avascular, and that up-regulation of VEGF-A expression, as it occurs in mice lacking VHL in chondrocytes or overexpressing one of the VEGF isoforms (VEGF164), does not cause ectopic vessel formation [56, 58–60]. To date, we do not have a definitive explanation for this additional paradox, though slow remodeling of the extracellular matrix could be responsible for reduced availability of the active form of VEGF-A, and, therefore, for the intrinsic resistance of cartilaginous matrix to be invaded by blood vessels [55, 61, 62]. Among the survival pathways controlled by HIF in chondrocytes, the role autophagy is still ambiguous. It has been recently shown that autophagy is constitutively on in growth platechondrocytes. Interestingly, hypoxia may trigger autophagy, with mechanisms that may or may not involve the transcription factor HIF-1α [63–66]. Recently, in vitro evidence in support of a role for autophagy in chondrocyte survival has been provided [67]. Moreover, a relation between HIF-1α and increased accumulation of autophagic proteins such as Beclin1 has been documented, at least in vitro [68]. Intriguingly, HIF-2 which is also expressed in chondrocytes, has been reported to be a suppressor of autophagy in vitro [69].
Taken together, it is possible that one of the mechanisms adopted by HIF-1α to allow survival of chondrocytes in a hypoxic environment could indeed involve modulation of the autophagic process.
HIF-1 α and chondrocyte proliferation
HIF-1α also modulates chondrocyte proliferation. In fetal growth plates deficient in HIF-1α, the proliferation rate of viable chondrocytes is strikingly increased [49]. Conversely, chondrocyte proliferation rate is markedly reduced in mice that lack VHL in chondrocytes, which is concomitant with an increase of the cyclin-dependent kinase inhibitor p57 mRNA [56]. These latter findings are consistent with the notion that hypoxia leads to cell cycle arrest [70], at least in part through up-regulation of HIF-1α transcriptional activity and inhibition of c-MYC activity, as reported above [20]. Overexpression of HIF-1α in a model of fibrosarcoma derived by fibroblasts lacking VHL, also shows diminished proliferation rate due to an increase in the cyclin-dependent kinase inhibitors p21 and p27 [71]. Slow proliferation in VHL null chondrocytes and fibroblasts is paradoxical in light of the very well-known role of VHL as tumor suppressor, and more studies are necessary to reach a better understanding of these apparent incongruent findings.
HIF-1 α and chondrocyte differentiation
HIF-1α is not required for the formation of precartilaginous condensation [52, 72], but has a non-redundant and critical role in the differentiation of mesenchymal cells into chondrocytes. Lack of HIF-1α in limb bud mesenchyme causes a remarkable delay in cartilage formation [52, 72]. Further studies are required in order to understand whether hypoxia per se is needed for differentiation of mesenchymal cells into chondrocytes in vivo, or whether the critical role of HIF-1α in early chondrogenesis is essentially homeostatic.
In any event, the involvement of HIF-1α in cell differentiation is tissue-specific, because HIF-1α maintains stem cells in an undifferentiated state [23, 73–77], inhibits differentiation of mesenchymal cells into osteoblasts, adipocytes and myocytes [23, 78–80], yet stimulates differentiation of trophoblastic cells and dopaminergic neurons and chondrocytes [23, 81–83]. HIF-1 may positively modulate chondrogenesis by up-regulating expression of Sox9 [52, 84], a master regulator of chondrogenesis [47, 85–87]. In mouse bone marrow stromal (ST2) cells, in particular, hypoxia increases nuclear accumulation of HIF-1α and Sox9 transcription [84]. Similar findings have been reported in limb bud micromass cultures [52], but have not been confirmed in primary chondrocytes or in ex-vivo metatarsal explants [72].
HIF-1 α and the cartilaginous matrix
Recent experimental evidence has indicated that regulation of post-translational modification of collagens, with hydroxylation of collagen prolines in particular, could be one of the modalities by which HIF-1α regulates chondrocyte survival and differentiation. Prolyl-4-hydroxylases I and II (P4HaI and P4HaII) are the enzymes responsible for generating 4-hydroxyprolines in the collagens; these are essential for the formation of triple-helical collagens [88]. P4Has have much lower Km forO2 than the PHDs, which trigger HIF-1α degradation (20 vs. 250, respectively)[88]. This indicates that P4Has require a minimal amount of O2 for proper function, i.e. they still function enzymatically at low O2 levels. The alpha subunits of P4HaI and P4HaII are targets of hypoxia in chondrocytes and other cell types in a HIF-1α -dependent fashion. Proper accumulation of extracellular matrix is not only essential for organ development, but also promotes cell differentiation and survival through specific cell-matrix interactions [89]. HIF-1α may thus operate as a survival and differentiation factor in chondrocytes, improving the efficiency of post-translational modifications of collagen type II and, in so doing, promoting the formation of a proper extracellular matrix.
The positive effect of HIF-1α on matrix accumulation in chondrocytes is consistent with the role of hypoxia in promoting fibrosis in pathological conditions. Moreover, it has been reported that lysyl oxidase, which is responsible for the formation of cross-links between collagen molecules, is induced by hypoxia and is essential for metastasis of highly malignant and hypoxic tumors [90, 91].
HIF-1α, chondrocytes and microRNAs
Not surprisingly, microRNAs have been recently involved in chondrocyte differentiation [92–94]. In particular, mouse genetics has demonstrated that Dicer is necessary for chondrocyte proliferation and for overall skeletal development [95]. Notably, mir-199a, has been independently implicated in both chondrogenesis and hypoxic response. Mir-199a down regulates HIF-1α and, inhibits early chondrogenesis by targeting Smad1 [43, 92, 93]. To this end, it is unknown whether mir-199a regulates chondrogenesis also by modifying HIF-1α activity.
HIFs and osteogenesis
As hypertrophic chondrocytes in the developmental growth plate undergo apoptosis, blood vessels invade the hypertrophic cartilage and the primary ossification center begins to form. A tight coupling between osteogenesis and angiogenesis is therefore essential for bone formation, and the genetic program regulated by hypoxia considerably impacts this mechanism. Notably, whereas cartilage is an avascular and hypoxic mesenchymal tissue [48, 49, 58, 72, 96], bone is highly vascularized, though the bone marrow is relatively hypoxic when compared to other adult organs (see below) [23].
Osteoblasts express HIF proteins and, not surprisingly, hypoxia stimulates VEGF-A mRNA production in this cell type [97]. If the HIF pathway is manipulated in osteoblasts, bone is deeply affected, both in volume and in vascular architecture [98, 99]. The conditional upregulation of HIF proteins in osteoblasts, obtained by the deletion of VHL in osteocalcin expressing cells (ΔVHL), leads to an increase in VEGF-A protein. Strikingly, bone mass in the long bones of ΔVHL mice is augmented and this is mainly due to an increase in osteoblast number and in bone formation rate, and not to an impairment of osteoclast number or activity [98]. Conversely, a considerable decrease of both bone volume and VEGF-A expression is observed if HIF-1α or HIF-2α are disrupted in osteoblasts (ΔHIF-1α and ΔHIF-2α) [98, 99]. Consistent with the VEGF-A data, a striking correlation between degree of ossification and vascularization, both in the ΔVHL and ΔHIFs has been identified, which imply that changes in bone volume and architecture observed in these mutant mice could be indeed secondary, at least in part, to changes in vasculature. The pericytic mesenchymal stem cells- like cells (MSCs) could indeed represent the physiological link between osteogenesis and angiogenesis. These cells reside in the bone marrow vascular niche, have osteogenic potential and may account for the increased osteoblast number in highly vascularized ossification centers [100, 101].
In bone repair, angiogenesis and osteogenesis coupling also plays a pivotal role. At fracture sites, inflammatory signals and mechanical stimuli, along with hypoxia, are the key factors that stimulate the physiological processes involved in bone repair. Angiogenesis in this setting is essential and, if it is delayed, chondrocytes and not osteoblasts will constitute the new tissue [102]. An interesting model to study bone repair is distraction osteogenesis (DO). In DO a close temporal and spatial relationship between angiogenesis and osteogenesis is established, with an external fixation device that gradually applies a mechanical separation across the fracture [103]. Interestingly, in DO all the expression of the three VEGF isoforms and both VEGFR-1 and VEGFR-2 mRNAs are enhanced, and if VEGFR-1 and VEGFR-2 are depleted, there is a considerable decrease in blood vessel number and, remarkably, in bone repair [104]. Notably, lack of VHL considerably accelerates bone repair in a DO model, and this is parallel to a significant increase in blood vessels [105].
Despite all these experimental evidence that invoke a critical role for angiogenesis in the increased bone volume observed when the HIF-1/VEGF pathway is up regulated, it is likely that cell autonomous mechanisms are as important. In support of this alternative mechanism, osteoblast lacking HIF-1α show impaired proliferation in vitro, whereas lack of HIF-2α does not affect osteoblast function in vitro [99]. Moreover, osteoblasts isolated from a mutant mouse expressing only the VEGF120 isoform do not properly differentiate in vitro [106]. Lastly, overexpression of the isoform VEGF165 in osteoblasts results in an increase of bone mass, and this effect appears to be secondary to activation of beta catenin in osteoblasts [58]. Curiously, intermittent hypoxia inhibits Runx2, the master transcription factor of osteoblastogenesis [107], in osteoblasts in vitro [78], a paradox requiring further investigation. And talking about paradoxes, whereas endochondral bone is clearly affected by lack of VHL or HIFs, intramembranous bone is definitively less so [98].
HIFs and joints
The synovial joint is an avascular tissue both in development and in adult life [108]. It experiences a high degree of hypoxia and the HIF proteins and VEGFA are highly expressed at this level [72]. Moreover, hypoxia has been involved in the pathogenesis of osteoarthritis (OA) [109].
In agreement with these findings, mice lacking HIF-1α in limb bud mesenchyme delays joint development with yet unknown mechanisms [72]. Notably, hypoxia upregulates matrix accumulation in Sox9-dependent fashion in articular surface chondrocytes in vitro [110, 111]. The relevance of this finding for articular cartilage surface homeostasis in vivo needs to be established.
Conclusions
In this brief Perspective, we have highlighted the critical role of hypoxia and HIFs in chondrocyte survival and differentiation, in bone development and in joint formation and homeostasis. It will be now important to identify the molecular mechanisms that mediate the complex and multifaceted action of this family of transcription factors in each of these processes. The identification of such mechanisms may significantly expand our understanding of both cellular adaptation to hypoxia and cartilage formation, bone modeling and remodeling and articular surface homeostasis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–94. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–73. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- [3].Fryer BH, Simon MC. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–8. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- [4].Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol. 2006;91:249–86. doi: 10.1016/j.pbiomolbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [5].Semenza G, Wang G. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoitein gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- [7].Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara J, Lane W, Kaelin W. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: imlications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- [8].Jaakkola P, Mole D, Tian Y, Wilson M, Gielbert J, Gakell S, Kriegsheim A, Heberstreit H, Mukherji M, Schofield C, Maxwell P, Ratcliffe P. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- [9].Mahon P, Hirota K, Semenza G. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxiainducible factor. Genes Dev. 2002;16:1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- [12].Chandel NS, Simon MC. Hypoxia-inducible factor: roles in development, physiology, and disease. Cell Death Differ. 2008;15:619–20. doi: 10.1038/cdd.2008.11. [DOI] [PubMed] [Google Scholar]
- [13].Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–34. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem. 2004;279:41966–74. doi: 10.1074/jbc.M406320200. [DOI] [PubMed] [Google Scholar]
- [15].Wellmann S, Bettkober M, Zelmer A, Seeger K, Faigle M, Eltzschig HK, Buhrer C. Hypoxia upregulates the histone demethylase JMJD1A via HIF-1. Biochem Biophys Res Commun. 2008;372:892–7. doi: 10.1016/j.bbrc.2008.05.150. [DOI] [PubMed] [Google Scholar]
- [16].Pollard PJ, Loenarz C, Mole DR, McDonough MA, Gleadle JM, Schofield CJ, Ratcliffe PJ. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1alpha. Biochem J. 2008;416:387–94. doi: 10.1042/BJ20081238. [DOI] [PubMed] [Google Scholar]
- [17].Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth. Mol Cell Biol. 30:344–53. doi: 10.1128/MCB.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang LE. Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ. 2008;15:672–7. doi: 10.1038/sj.cdd.4402302. [DOI] [PubMed] [Google Scholar]
- [19].Koshiji M, Huang LE. Dynamic balancing of the dual nature of HIF-1alpha for cell survival. Cell Cycle. 2004;3:853–4. doi: 10.4161/cc.3.7.990. [DOI] [PubMed] [Google Scholar]
- [20].Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–56. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zelzer E, levy Y, Kahana C, Shilo B, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor 1 alpha. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor −1alpha. Cardiovascular Res. 2003;60:569–79. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- [23].Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–96. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxiainducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–86. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299–306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- [27].Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. Faseb J. 2003;17:271–3. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- [28].Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–40. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- [29].Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277:32405–8. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- [30].Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- [31].Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–31. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- [35].Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- [36].Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279–89. doi: 10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]
- [37].Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–8. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- [39].Mathew LK, Simon MC. mir-210: a sensor for hypoxic stress during tumorigenesis. Mol Cell. 2009;35:737–8. doi: 10.1016/j.molcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756–68. doi: 10.4161/cc.8.17.9387. [DOI] [PubMed] [Google Scholar]
- [41].Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–25. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, Takahashi T. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008;68:5540–5. doi: 10.1158/0008-5472.CAN-07-6460. [DOI] [PubMed] [Google Scholar]
- [43].Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–86. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Karsenty G. The complexities of skeletal biology. Nature. 2003;423:316–8. doi: 10.1038/nature01654. [DOI] [PubMed] [Google Scholar]
- [45].Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328:658–65. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- [46].Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- [47].Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–12. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- [48].Provot S, Schipani E. Fetal growth plate: a developmental model of cellular adaptation to hypoxia. Ann N Y Acad Sci. 2007;1117:26–39. doi: 10.1196/annals.1402.076. [DOI] [PubMed] [Google Scholar]
- [49].Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–76. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nishida T, Kondo S, Maeda A, Kubota S, Lyons KM, Takigawa M. CCN family 2/connective tissue growth factor (CCN2/CTGF) regulates the expression of Vegf through Hif-1alpha expression in a chondrocytic cell line, HCS-2/8, under hypoxic condition. Bone. 2009;44:24–31. doi: 10.1016/j.bone.2008.08.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yang G, Sun Q, Teng Y, Li F, Weng T, Yang X. PTEN deficiency causes dyschondroplasia in mice by enhanced hypoxia-inducible factor 1alpha signaling and endoplasmic reticulum stress. Development. 2008;135:3587–97. doi: 10.1242/dev.028118. [DOI] [PubMed] [Google Scholar]
- [52].Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–28. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- [53].Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–71. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- [54].Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- [55].Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pfander D, Kobayashi T, Knight MC, Zelzer E, Chan DA, Olsen BR, Giaccia AJ, Johnson RS, Haase VH, Schipani E. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development. 2004;131:2497–508. doi: 10.1242/dev.01138. [DOI] [PubMed] [Google Scholar]
- [57].Roskoski R., Jr. VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375:287–91. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- [58].Maes C, Goossens S, Bartunkova S, Drogat B, Coenegrachts L, Stockmans I, Moermans K, Nyabi O, Haigh K, Naessens M, Haenebalcke L, Tuckermann JP, Tjwa M, Carmeliet P, Mandic V, David JP, Behrens A, Nagy A, Carmeliet G, Haigh JJ. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J. 29:424–41. doi: 10.1038/emboj.2009.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Eshkar-Oren I, Viukov SV, Salameh S, Krief S, Oh CD, Akiyama H, Gerber HP, Ferrara N, Zelzer E. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009;136:1263–72. doi: 10.1242/dev.034199. [DOI] [PubMed] [Google Scholar]
- [60].Takimoto A, Nishizaki Y, Hiraki Y, Shukunami C. Differential actions of VEGF-A isoforms on perichondrial angiogenesis during endochondral bone formation. Dev Biol. 2009;332:196–211. doi: 10.1016/j.ydbio.2009.05.552. [DOI] [PubMed] [Google Scholar]
- [61].Iruela-Arispe ML, Carpizo D, Luque A. ADAMTS1: a matrix metalloprotease with angioinhibitory properties. Ann N Y Acad Sci. 2003;995:183–90. doi: 10.1111/j.1749-6632.2003.tb03221.x. [DOI] [PubMed] [Google Scholar]
- [62].Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–81. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- [64].Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [65].Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–42. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Srinivas V, Shapiro I. Chondrocytes embedded in the epipheseal growth plates of long bones undergo autophagy prior to the induction of osteogenesis. Autophagy. 2006;3:215–216. doi: 10.4161/auto.2649. [DOI] [PubMed] [Google Scholar]
- [68].Bohensky J, Shapiro I, Leshinsky S, Terkhorn S, Adams C, Srinvas V. HIF-1 regulation of chondrocyte apoptosis: induction of the autophagic pathway. Autophagy. 2007;3:207–214. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- [69].Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, Srinivas V. Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009;60:1406–15. doi: 10.1002/art.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Goda N, Ryan H, Khadivi B, McNulty W, Rickert R, Johnson R. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23:359–69. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mack FA, Patel JH, Biju MP, Haase VH, Simon MC. Decreased growth of Vhl−/−fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol Cell Biol. 2005;25:4565–78. doi: 10.1128/MCB.25.11.4565-4578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–64. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jeong C, Lee H, Cha J, Kim J, Kim K, Kim J, Yoon D, Kim K. Hypoxia-inducible factor −1alpha inhibits self-renewal of mouse embryonic stem cells in vitro via negative regulation of the leukemia inhibitory factor-STAT3 pathway. J Biol Chem. 2007;282:13672–9. doi: 10.1074/jbc.M700534200. [DOI] [PubMed] [Google Scholar]
- [74].Lin Q, Lee Y, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–83. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- [75].Sainson R, Harris A. Hypoxia-regulated differentiation: let's step it up a Notch. Trends Mol Med. 2006;12:141–3. doi: 10.1016/j.molmed.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [76].Gustafsson M, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas J, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [77].Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S. Hypoxia alters gene expression in human neuroblastoma cells towards an immature and neural crest-like phenotype. Proc Natl Acad Sci. 2002;99:7021–6. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Salim A, Nacamuli R, Morgan E, Giaccia A, Longaker M. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–16. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- [79].Yun Z, Lin Q, Giaccia A. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005;25:3040–55. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yun Z, Maecker H, Johnson R, Giaccia A. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC-1/Stra13 a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- [81].Dahl KC, Fryer B, Mack F, Compernolle V, Maltepe E, Adelman D, Carmeliet P, Simon M. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–91. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–6. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–83. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–22. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- [85].Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B, Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–90. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- [86].Huang W, Zhou X, Lefebvre V, de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–58. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes & Dev. 2002;16:2813–28. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hirsila M, Koivunen P, Gunzler V, Kivirikko K, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–80. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- [89].Egerbacher M, Haeusler G. Integrins in growth plate cartilage. Pediatr Endocrinol Rev. 2003;1:2–8. [PubMed] [Google Scholar]
- [90].Erler J, Bennewith K, Nicolau M, Dornhofer N, Kong C, Le Q, Chi J, Jeffrey S, Giaccia A. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- [91].Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Suomi S, Taipaleenmaki H, Seppanen A, Ripatti T, Vaananen K, Hentunen T, Saamanen AM, Laitala-Leinonen T. MicroRNAs Regulate Osteogenesis and Chondrogenesis of Mouse Bone Marrow Stromal Cells. Gene Regul Syst Bio. 2008;2:177–91. doi: 10.4137/grsb.s662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lin EA, Kong L, Bai XH, Luan Y, Liu CJ. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem. 2009;284:11326–35. doi: 10.1074/jbc.M807709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ohgawara T, Kubota S, Kawaki H, Kondo S, Eguchi T, Kurio N, Aoyama E, Sasaki A, Takigawa M. Regulation of chondrocytic phenotype by micro RNA 18a: involvement of Ccn2/Ctgf as a major target gene. FEBS Lett. 2009;583:1006–10. doi: 10.1016/j.febslet.2009.02.025. [DOI] [PubMed] [Google Scholar]
- [95].Kobayashi T, Lu J, Cobb BS, Rodda SJ, McMahon AP, Schipani E, Merkenschlager M, Kronenberg HM. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc Natl Acad Sci U S A. 2008;105:1949–54. doi: 10.1073/pnas.0707900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schipani E. Hypoxia and HIF-1 alpha in chondrogenesis. Semin Cell Dev Biol. 2005;16:539–46. doi: 10.1016/j.semcdb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [97].Steinbrech DS, Mehrara BJ, Saadeh PB, Chin G, Dudziak ME, Gerrets RP, Gittes GK, Longaker MT. Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plast Reconstr Surg. 1999;104:738–47. doi: 10.1097/00006534-199909030-00019. [DOI] [PubMed] [Google Scholar]
- [98].Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–26. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shomento SH, Wan C, Cao X, Faugere MC, Bouxsein ML, Clemens TL, Riddle RC. Hypoxia-inducible factors 1alpha and 2alpha exert both distinct and overlapping functions in long bone development. J Cell Biochem. 2009;109:196–204. doi: 10.1002/jcb.22396. [DOI] [PubMed] [Google Scholar]
- [100].Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- [101].Maes C, Kobayashi T, Kronenberg HM. A novel transgenic mouse model to study the osteoblast lineage in vivo. Ann N Y Acad Sci. 2007;1116:149–64. doi: 10.1196/annals.1402.060. [DOI] [PubMed] [Google Scholar]
- [102].Choi IH, Ahn JH, Chung CY, Cho TJ. Vascular proliferation and blood supply during distraction osteogenesis: a scanning electron microscopic observation. J Orthop Res. 2000;18:698–705. doi: 10.1002/jor.1100180504. [DOI] [PubMed] [Google Scholar]
- [103].Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990:8–26. [PubMed] [Google Scholar]
- [104].Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/JBMR.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL. Activation of the hypoxiainducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105:686–91. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, D'Amore PA, Olsen BR. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- [107].Ducy P, Zhang R, Geoffroy V, Ridall A, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- [108].Gibson JS, Milner PI, White R, Fairfax TP, Wilkins RJ. Oxygen and reactive oxygen species in articular cartilage: modulators of ionic homeostasis. Pflugers Arch. 2008;455:563–73. doi: 10.1007/s00424-007-0310-7. [DOI] [PubMed] [Google Scholar]
- [109].Coimbra IB, Jimenez SA, Hawkins DF, Piera-Velazquez S, Stokes DG. Hypoxia inducible factor-1 alpha expression in human normal and osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2004;12:336–45. doi: 10.1016/j.joca.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [110].Lafont JE, Talma S, Hopfgarten C, Murphy CL. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J Biol Chem. 2008;283:4778–86. doi: 10.1074/jbc.M707729200. [DOI] [PubMed] [Google Scholar]
- [111].Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]