Abstract
Introduction
Although invasive intraductal papillary mucinous neoplasms (IPMN) of the pancreas is thought to be more indolent than sporadic pancreatic adenocarcinoma (PAC), the natural history remains poorly defined. We compare survival and identify prognostic factors following resection for invasive IPMN vs. stage-matched PAC.
Methods
The Surveillance, Epidemiology, and End Results (SEER) database (1991-2005) was utilized to identify 729 patients with invasive IPMN and 8082 patients with PAC who underwent surgical resection.
Results
Patients with resected invasive IPMN experienced improved overall survival when compared to resected PAC (median survival 21mos vs. 14mos, p<0.001). Stratification by nodal status demonstrated no difference in survival among node positive patients, however, median survival of resected, node negative, invasive IPMN was significantly improved compared to node negative PAC (34mos vs. 18mos, p <0.001). On multivariate analysis PAC histology was an adverse predictor of overall survival (HR 1.31, 95% CI 1.15-1.50) compared to invasive IPMN. For patients with invasive IPMN, positive lymph nodes (HR 1.98, 95% CI 1.50- 2.60), high tumor grade (HR 1.74, 95% CI 1.31- 2.31), tumor size >2cm (HR 1.50, 95% CI 1.04- 2.19), and age >66 years (HR 1.33, 95% CI 1.03- 1.73) were adverse predictors of survival.
Conclusions
Although node negative invasive IPMN shows improved survival following resection compared to node negative PAC, the natural history of node positive invasive IPMN mimics that of node positive PAC. We also identify adverse predictors of survival in invasive IPMN to guide discussions regarding use of adjuvant therapies and prognosis following resection of invasive IPMN.
Keywords: IPMN, Pancreatic Cancer, Stage-matched, Survival, Outcomes
Introduction
Increasing use of cross-sectional imaging and standardization of nomenclature has meant that intraductal papillary mucinous neoplasm (IPMN) of the pancreas is now a well established clinical and pathological entity.1, 2 In fact, it is estimated that IPMNs currently account for up to 20% of resected pancreatic neoplasms.3-5 The World Health Organization defines IPMN as a mucin-producing pancreatic neoplasm characterized by tall, columnar epithelium that arises either in the main pancreatic duct (main duct IPMN), its major branches (branch duct IPMN), or both (mixed).1, 2 IPMNs are differentiated from mucinous cystic neoplasms of the pancreas by the lack of ovarian stroma in the former.1, 2
Similar to the well defined adenoma-to-carcinoma sequence in colorectal cancer6 and the progression of pancreatic intraepithelial neoplasia (PanIN) to pancreatic ductal adenocarcinoma,7 IPMN is thought to progress from an adenomatous stage to IPMN with dysplasia, IPMN with carcinoma in-situ and eventually invasive IPMN.8 Current estimates for time to progression from IPMN adenoma to invasive IPMN are about 5 years.3-5 The risk of harboring an invasive cancer in the setting of an IPMN is much higher with involvement of the main pancreatic duct (60% - 92%) as compared to involvement of a branch duct only.4, 5, 9-11 Consequently, the International Consensus Guidelines recommend resection for all IPMNs with main duct involvement when medically appropriate.12 Management is more controversial for branch duct IPMN, with the guidelines recommending resection for cystic lesions >3 cm. Branch duct IPMNs <3 cm should be resected only if symptomatic or associated either with a solid component or positive cytology.12
Resection of IPMN prior to progression to an invasive cancer is associated with an excellent outcome,3-5, 11, 13-15 but the natural history of invasive IPMN following resection remains unclear. This leads to ambiguous postoperative discussions with patients regarding prognosis and use of adjuvant therapy. In some studies invasive IPMN has been shown to have a favorable prognosis compared with sporadic ductal adenocarcinoma. This has led some to suggest the underlying biology is different, leading to a more indolent course for invasive cancer arising in the setting of IPMN.4, 5, 15 Other studies suggest a similar poor outcome for both invasive IPMN and sporadic ductal adenocarcinoma.11, 13, 14 Variability in the literature is most likely attributed to the small sample size reported in single institutional series. In fact, the largest published series reports on only 68 patients with invasive IPMN.16 Furthermore, studies with such small numbers do not allow for analyses to be controlled for stage or patient age which further weakens the comparison with sporadic pancreatic adenocarcinoma.
The aims of this study were: 1) To compare survival outcomes between AJCC stage-matched invasive IPMN and sporadic pancreatic adenocarcinoma following surgical resection 2) To determine adverse predictors of survival following resection of invasive IPMN. We used the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database to overcome sample size limitations of previous studies and a stage-matching strategy for a more rigorous comparison of survival outcomes.
Methods
SEER Database
SEER Program registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. Although information on radiation therapy is recorded, no information on chemotherapy is reported. SEER contains over 3 million cases from 17 geographic sites, covering approximately 26 percent of the United States population. The database was designed to reflect the overall characteristics of this country's population and is regarded as a model population-based tumor registry. The November 2007 update was used for this study, providing information from 1971-2005.17 Because our SEER-selected cohort was not linked to patient identifiers, our study was exempt from Institutional Research Board review.
Case Selection
The study period was from 1991-2005. Invasive IPMN was identified using the International Classification of Disease for Oncology (3rd ed.) codes 8050, 8260, 8450, 8453, 8471, 8480, 8481, 8503, as described elsewhere.18 Sporadic pancreatic adenocarcinoma was identified using the codes 8000, 8010, 8020, 8021, 8022, 8140, 8141, 8211, 8230, 8500 and 8521. Patients with mucinous cystic neoplasms are separately coded as 8440 and 8470 and were excluded. Our initial population was 74,894 patients with either invasive IPMN or sporadic pancreatic adenocarcinoma. We excluded patients who did not undergo surgery or underwent a ‘non-cancer directed surgery’ (e.g., bypass). Cases diagnosed on autopsy only and cases with no histological confirmation were also excluded. Although no specific field for AJCC stage in pancreatic cancer is provided in the SEER database, the information to accurately stage patients is present in other data fields. Utilizing extent of disease (EOD) data fields, we were able to determine stage according to the 6th edition of the AJCC staging manual, as our group and others have done so previously.19, 20
Statistical Analysis
All statistical analysis was performed using SPSS 16.0 statistical software (SPSS Inc.). For analysis purposes the study population was divided into invasive IPMN and sporadic adenocarcinoma. Categorical variables were compared using chi-square analysis. Differences between continuous variables were determined by using Student's t-tests. Survival analysis was performed using Kaplan-Meier method and differences in survival were compared using the log-rank test. Multivariate analysis using Cox regression modeling and assuming proportional hazards was used to identify predictors of survival. Factors included in the model were age, site of tumor, type of surgery, histology, tumor grade, tumor size and lymph node status. Predictors of lymph node involvement were identified using logistic regression including the following variables in our model: age, sex, race, site of primary, tumor grade and tumor size.
Results
Demographic and tumor characteristics of the 729 patients with invasive IPMN and the 8082 patients with sporadic adenocarcinoma groups are outlined in Table 1. The median age of patients at the time of surgical resection was 66 years for both invasive IPMN and sporadic adenocarcinoma. There was no significant intergroup difference with regard to sex and race. The most common location for both invasive IPMN and sporadic adenocarcinoma was in the head of the pancreas and correspondingly the Whipple procedure (pancreaticoduodenectomy) the most common surgical procedure performed for both. Post-operative mortality, defined as death within 30 days of resection, was similar between invasive IPMN (6.6%) and sporadic adenocarcinoma (6.8%). Overall, the annual number of resections performed for invasive IPMN increased five-fold during the span of the study from 1991 to 2005. Significant differences between the two groups were observed in mean tumor size, tumor grade and nodal status (Table 1). Mean and median tumor size was significantly larger by 1cm for invasive IPMN compared to sporadic adenocarcinoma. Invasive IPMN tended to be of lower grade. Additionally, patients with sporadic pancreatic adenocarcinoma were more likely to have positive nodes than invasive IPMN at the time of resection, even though there were no significant differences in the median number of nodes examined or in the median number of positive nodes identified in patients with nodal involvement. Adjuvant radiation therapy was used more often for sporadic adenocarcinoma as compared to invasive IPMN.
Table 1.
Demographic and tumor characteristics of resected invasive IPMN and sporadic PAC cohorts.
| Demographic and tumor characteristics | Invasive IPMN (n = 729) |
Sporadic Adenocarcinoma (n = 8082) |
p value |
|---|---|---|---|
| Age (yrs) | 0.009 | ||
| Mean ± SD | 64.1 ± 13.2 | 65.2 ± 11.0 | |
| Median | 66 | 66 | |
| Sex (%) | NS | ||
| Male | 52.1 | 50.4 | |
| Race (%) | NS | ||
| White | 84.3 | 83.3 | |
| Black | 7.6 | 9.9 | |
| Other | 8.1 | 6.8 | |
| Location of tumor (%) | <0.001 | ||
| Head | 77.2 | 85.2 | |
| Body | 6.8 | 5.2 | |
| Tail | 16 | 9.6 | |
| Surgery (%) | <0.001 | ||
| Whipple | 74.2 | 82.5 | |
| Distal pancreatectomy | 14.9 | 9.4 | |
| Total pancreatectomy | 10.9 | 8.1 | |
| Post-operative mortality (%) | 6.6 | 6.8 | <0.001 |
| Adjuvant radiation (%) | 34.5 | 41.6 | <0.001 |
| Stage (%) | <0.001 | ||
| IA | 6.5 | 4.2 | |
| IB | 14.6 | 8.2 | |
| IIA | 25.1 | 24.6 | |
| IIB | 37.1 | 47.6 | |
| III | 5.5 | 7.2 | |
| IV | 11.1 | 8.2 | |
| Tumor size (cm) | <0.001 | ||
| Mean ± SD | 4.5 ± 3 | 3.5 ± 2.0 | |
| Median | 4.0 | 3.0 | |
| Number nodes examined | NS | ||
| Mean ± SD | 8.7 ± 8.4 | 9.3 ± 8.1 | |
| Median | 7 | 8 | |
| Nodal status (%) | <0.001 | ||
| Positive | 47.2 | 59.9 | |
| Number nodes positive (%) | NS | ||
| Mean ± SD | 3.1 ± 3.4 | 2.9 ± 2.6 | |
| Median | 2 | 2 | |
| Grade (%) | <0.001 | ||
| High | 24.1 | 36.6 |
IPMN: Intraductal Papillary Mucinous Neoplasm
PAC: Sporadic pancreatic adenocarcinoma
Survival analysis
Overall survival 5 years after resection was 22% for invasive IPMN (median survival 21 months) vs. 11% for sporadic pancreatic adenocarcinoma (median survival 14 months), a statistically significant difference (p <0.001; Figure 1A). In patients with node-negative disease (Figure 1B), 5-year survival was 35% for invasive IPMN (median survival 34 months) vs. 17% for sporadic pancreatic adenocarcinoma (median survival 18 months), a significant difference (p <0.001). This survival advantage for invasive IPMN was not seen in patients with node-positive disease (Figure 1C); 5-year survival was 9% for invasive IPMN (median survival 15 months) vs. 7% for sporadic pancreatic adenocarcinoma (median survival 13 months), not significantly different (p = 0.54). When patients were matched by AJCC stage, there was a survival advantage for invasive IPMN among patients with stage IA, IB or IIA disease (node-negative) but no survival difference among patients with stage IIB disease (node-positive) (Figure 2).
Figure 1.
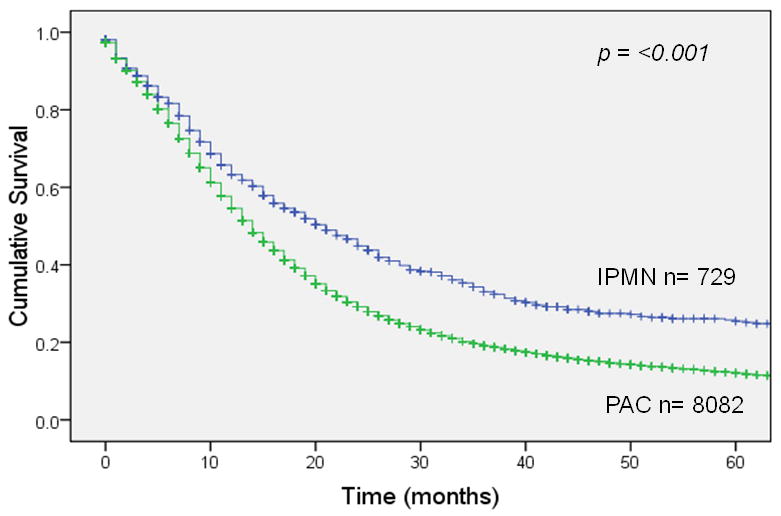
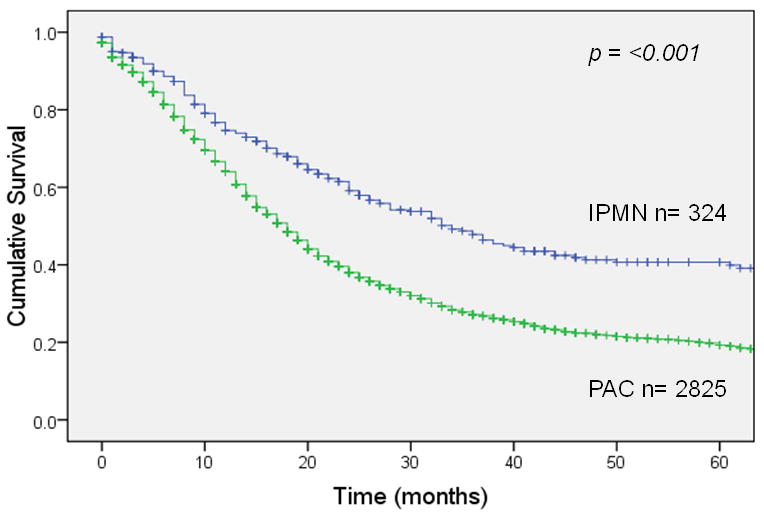

Survival after resection of invasive IPMN vs. sporadic pancreatic adenocarcinoma (PAC). Panel A: All patients. Panel B: Patients with node negative resected tumors. Panel C: Patients with node positive resected tumors.
Figure 2.
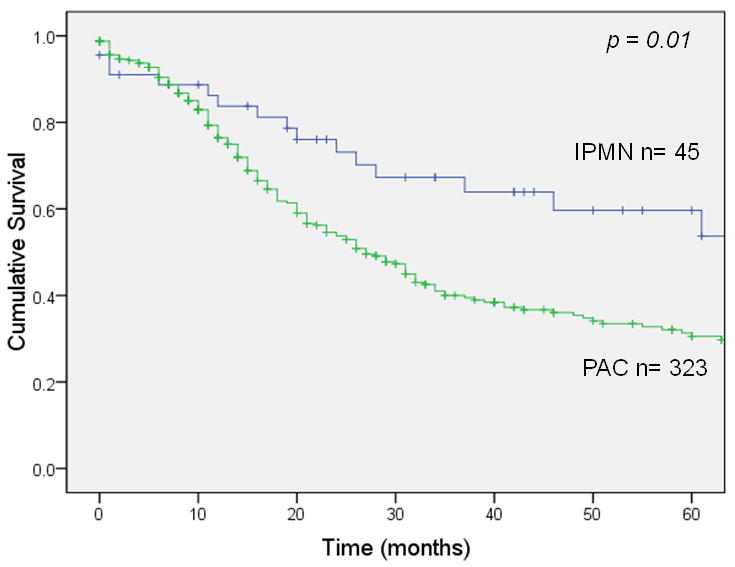
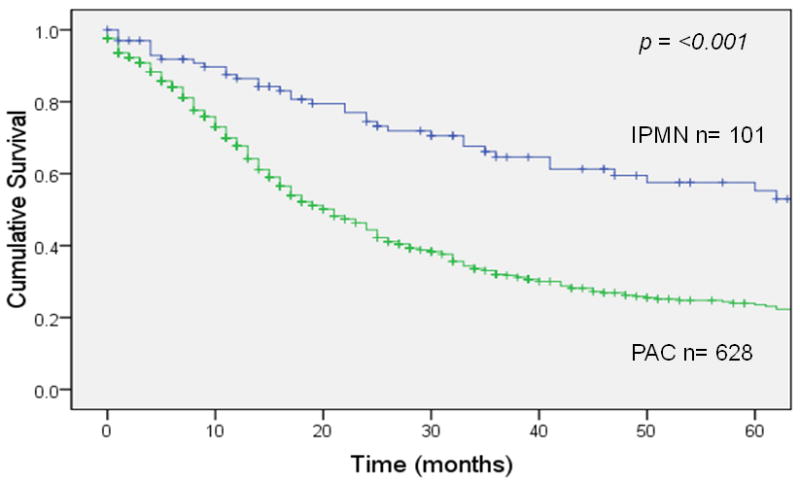


Stage-matched survival of patients with invasive IPMN or sporadic adenocarcinoma (PAC). Panel A: stage IA (p = 0.01). Panel B: stage IB (p <0.001). Panel C: stage IIA (p = 0.018). Panel D: stage IIB (p = 0.073).
Regression analysis (Table 2)
Table 2.
Cox regression analysis for adverse predictors of survival
| Population | Hazard Ratio (95% CI)∞ | p value |
|---|---|---|
| Entire cohort (n = 5695)† | ||
| Negative lymph nodes* | 1.00 | |
| Positive lymph nodes | 1.43 (1.34 - 1.52) | < 0.001 |
| Low tumor grade* | 1.00 | |
| High tumor grade | 1.41 (1.32 – 1.50) | < 0.001 |
| Tumor size ≤2cm* | 1.00 | |
| Tumor size >2 cm | 1.34 (1.24 – 1.46) | < 0.001 |
| Invasive IPMN histology* | 1.00 | |
| Sporadic PAC histology | 1.31 (1.15- 1.50) | < 0.001 |
| Age <66 years* | 1.00 | |
| Age >66 years | 1.27 (1.20 – 1.36) | < 0.001 |
| Lymph node negative only (n = 2240)† | ||
| Sporadic PAC histology | 1.63 (1.32 – 2.02) | < 0.001 |
| High tumor grade | 1.42 (1.27 – 1.57) | < 0.001 |
| Tumor size >2 cm | 1.33 (1.18 – 1.50) | < 0.001 |
| Age >66 years | 1.21 (1.09 – 1.34) | < 0.001 |
| Lymph node positive only (n = 3455)† | ||
| High tumor grade | 1.40 (1.29 – 1.51) | < 0.001 |
| Tumor size >2 cm | 1.35 (1.21 – 1.51) | < 0.001 |
| Age >66 years | 1.32 (1.22 – 1.432) | < 0.001 |
| Invasive IPMN only (n = 359)† | ||
| Positive lymph nodes | 1.98 (1.50 – 2.60) | < 0.001 |
| High tumor grade | 1.74 (1.31 – 2.31) | < 0.001 |
| Tumor size >2 cm | 1.50 (1.04 – 2.19) | 0.03 |
| Age >66 years | 1.33 (1.03 – 1.73) | 0.03 |
Significant Hazard Ratios shown only
Referent (only shown for entire cohort group)
Number entering regression analysis
IPMN: Intraductal papillary mucinous neoplasm
PAC: Sporadic ductal adenocarcinoma
Multivariate analysis of the entire cohort, including both resected invasive IPMN and sporadic pancreatic adenocarcinoma identified sporadic pancreatic adenocarcinoma histology, positive lymph nodes, high tumor grade, tumor size >2cm and age >66 years as adverse prognostic factors of survival following surgical resection. Importantly, assigning invasive IPMN as the referent histology, sporadic pancreatic adenocarcinoma histology was a significant adverse predictor of mortality (HR 1.31, 95% CI 1.15-1.50), independent of other known adverse factors.
To further investigate the relative adverse effect on survival associated with sporadic pancreatic adenocarcinoma histology, we performed separate subset analyses of node negative and node positive patients. High tumor grade, tumor size >2cm and age >66 years continued to be adverse predictors of survival for both subsets. However, sporadic pancreatic adenocarcinoma histology was an independent predictor of mortality only in the node negative patients (HR 1.63, 95% CI 1.32-2.02), but not in the node positive group.
Among patients with invasive IPMN, multivariate analysis identified positive lymph nodes, high tumor grade, tumor size >2 cm, and age >66 years as predictive of adverse survival. Because of the strong association between positive lymph nodes and adverse survival, we performed further analyses to identify predictors of lymph node positivity in the invasive IPMN cohort. Tumor grade and increasing tumor size were both predictive of lymph node positivity for invasive IPMN (Table 3). With increasing tumor size the percentage of patients with positive lymph nodes increased for both invasive IPMN as well as sporadic adenocarcinoma, although for the same tumor size, invasive IPMN was less likely to be associated with dissemination of disease to local lymph nodes (Figure 3).
Table 3.
Predictors of lymph node positivity in invasive IPMN
| Population | Odds Ratio (95% CI) | p value |
|---|---|---|
| Invasive IPMN (n = 362)† | ||
| Low tumor grade* | 1.00 | |
| High tumor grade | 2.15 (1.30 – 3.56) | 0.004 |
| Tumor size ≤1cm* | 1.00 | |
| Tumor size 1-2cm | 10.90 (1.30- 91.48) | 0.028 |
| Tumor size >2 cm | 13.12 (1.67- 104.45) | 0.015 |
Referent
Number entering analysis
IPMN: Invasive papillary mucinous neoplasm
Figure 3.

Increase in nodal positivity with increasing size of primary tumor.
Impact of grade in invasive IPMN
As shown in Table 2, high tumor grade was second only to lymph node positivity in terms of predicting adverse survival in patients with invasive IPMN. To further explore the impact of grade on invasive IPMN we performed Kaplan-Meier analysis stratified for tumor grade. For the entire invasive IPMN cohort, patients with high grade tumors had significantly lower median and 5 year survivals (11 months vs. 23 months and 6% vs. 25%, p <0.001) when compared to low grade tumors; Figure 4.
Figure 4.

Impact of tumor grade on survival in invasive IPMN
Discussion
Intraductal Papillary Mucinous Neoplasm (IPMN) of the pancreas is a relatively new disease that accounts for about 20% of pancreatic resections for malignancy performed today.5 Current knowledge of the natural history and prognosis of invasive IPMN comes from single institution series, and is severely limited by small sample sizes and short follow-up. Even though prevailing thought is that the underlying biology of invasive IPMN is different from sporadic pancreatic adenocarcinoma, it is unclear whether this translates clinically into a difference in survival for patients undergoing surgical resection. This study aimed to better define the natural history of invasive IPMN when compared to stage-matched sporadic pancreatic adenocarcinoma following surgical resection to provide a frame of reference for discussions regarding prognosis and the use of adjuvant therapy.
Our data demonstrates overall 5-year survival for invasive IPMN following resection was 22% with a 47.2% rate of lymph node positivity. Patients with invasive IPMN and metastasis to lymph nodes suffered a much worse overall survival (9% 5-year survival) compared to patients with node negative invasive IPMN who had a 35% survival at 5 years. Results from previously published single institutional series on outcomes after resection for invasive IPMN have 5-year survivals ranging from 40%-85% for node-negative invasive IPMN and 0-45% for node-positive invasive IPMN.4, 5, 11, 13, 16, 21-23 In these same studies, lymph node positivity rates are 33%-54%. Variance within published series may be due to small sample size, inadequate time of follow up, and a lack of distinction between in situ and true invasive carcinomas. Our study reports a lower 5-year survival for node-negative invasive IPMN as compared to published series which may be attributable to the population-based nature of our data, as opposed to results reported from single high-volume centers. Nevertheless, we confirm that lymph node positivity in invasive IPMN has a significant impact on survival with few long term survivors.
On direct comparison of outcomes with sporadic pancreatic adenocarcinoma, we show that survival following surgical resection is significantly better for patients with invasive IPMN vs. sporadic adenocarcinoma (22% vs. 11%). This survival advantage is magnified for node-negative disease (35% vs. 17%) but disappears for node-positive disease (9% vs. 7%). Other studies have compared postoperative outcomes for patients with invasive IPMN vs. sporadic adenocarcinoma with conflicting results. Both Sohn et al24 and Marie et al13 found a significantly better survival for all patients with invasive IPMN and for patients with node-negative invasive IPMN as compared with survival of patients with PAC. As in our study, a survival advantage was demonstrated for resected node negative invasive IPMN and node positive disease demonstrated similar poor survival for both tumor types. In contrast, both Wada et al14 and Schnelldorfer et al11 found no significant difference in survival between invasive IPMN and sporadic adenocarcinoma overall and when matched for stage. Finally Woo et al25 and Shimada et al26 reported better survival for invasive IPMN vs. sporadic adenocarcinoma, even among patients with node-positive disease.
It has been suggested that adenocarcinoma arising in the setting of an invasive IPMN is relatively more indolent than sporadic pancreatic adenocarcinoma, and more amenable to cure following resection.7, 23-25 Others contend that the putative survival advantage seen with invasive IPMN is not due to indolent biology but rather due to lead time bias secondary to earlier presentation.11, 14 To tease out the survival differences, we stage-matched patients with invasive IPMN and sporadic adenocarcinoma: survival was significantly better for patients with stage IA, IB and IIA (node-negative) invasive IPMN. This suggests that invasive IPMN may indeed have a more indolent biology than sporadic adenocarcinoma, and this translates into a clinical survival advantage following surgical resection. Further support for a different biology comes from the observation that peri-neural and vascular invasion is more common in sporadic adenocarcinoma.23 Finally, several studies have identified differences in the expression of K-ras, p 53, DPC-4 and MUC 1-7 glycoproteins between invasive IPMN and sporadic adenocarcinoma, suggesting differences in genetic make-up.27 These differences may be reflected pathologically by lower grade tumors (75.9% vs. 63.4%, p<0.001) and clinically by a lower propensity for lymph node metastases (47.2% vs. 59.9%, p<0.001) in invasive IPMN, as demonstrated in our study and shown by others as well.7, 8, 19-22 As we demonstrate in Figure 3, invasive IPMN is less likely to involve regional lymph nodes when compared with sporadic adenocarcinoma of the same size. The absence of a survival difference for patients with stage IIB (node-positive) disease suggests that any intrinsic differences in biology are moot once tumor metastasizes to local lymph nodes.
Even though others have also shown that lymph node positivity 7, 8, 19-22 and tumor size 21, 28 are adverse prognostic indicators for survival following resection of invasive IPMN, our report is the first to link high tumor grade to poor outcome. We show that high tumor grade has an adverse impact on survival second only to lymph node positivity overall and is the most important prognostic indicator in patients with localized disease and negative nodes. This poor survival associated with high grade invasive IPMN may influence future adjuvant therapy decisions in these patients.
Although this study is the largest report in the literature on the natural history of resected invasive IPMN and represents the ‘real-world’ picture, the use of population-based data has several inherent limitations. Even though we used specific ICD-10 codes for invasive IPMN to identify cases, it is possible that some cases were misclassified due to variability among pathologists in the different SEER regions. We are limited by an inability to confirm the initial diagnosis histologically and must rely on the ability of pathologists from different regions of the country over the time frame of the study period to accurately distinguish between invasive IPMN and sporadic adenocarcinoma. Misclassification of invasive IPMN may also have occurred in some cases in which an advanced presentation would have led to a loss of the classic cystic features of IPMN and categorization of the lesion as sporadic adenocarcinoma. In other cases invasive IPMN may have been ‘over-called’ by pathologists in up to 8% of cases, as has been shown by others.11 Furthermore, there may have been some variability and difficulty in measuring the size of the invasive component of the IPMN due to the cystic nature of the disease. The size of the tumor as reported to the SEER database is the size of the invasive component only and not the size of the entire cystic lesions. Because SEER records only cases with invasive cancer, no statements could be made about patients with IPMN adenomas or IPMN with in situ carcinoma. Also not recorded is information on classification of IPMN into main duct, branch duct or mixed type or information on margin status following surgery. Finally, SEER does not provide information on the receipt of adjuvant chemotherapy. In this patient population it is likely that adjuvant radiation therapy was accompanied by systemic chemotherapy and can be used as a proxy for treatment with both. The fact that a higher percentage of patients with sporadic adenocarcinoma received adjuvant radiation as compared to invasive IPMN (42% vs. 35%) may indicate that decisions on adjuvant therapy following resection are being influenced by the perceived ‘indolence’ of IPMN as compared to the well known malevolence of sporadic pancreatic adenocarcinoma.
As indicated in Table 1, 16.6% of invasive IPMN and 15.4% of sporadic pancreatic adenocarcinoma patients had either Stage III or IV disease on final pathology, and retrospectively would not be considered surgical candidates. We feel that in many of these cases, patients were assessed to be resectable based on the pre-operative work up and vessel invasion/metastatic disease only recognized intra-operatively by the surgeon- who may have already committed to performing the resection or changed the surgical intent to palliation. Although these patients were included in the overall study cohort, they would not be expected to influence the results because our analysis controls for stage and does not depend on preoperative assessment of respectability. For example, in our stage matched comparison, we are only comparing Stage IIA invasive IPMN with Stage IIA sporadic adenocarcinoma.
In this study we use a population-based database to study the largest cohort of invasive IPMN to date and show that invasive IPMN has a lower propensity for lymph node involvement and presents with lower grade tumors than sporadic pancreatic adenocarcinoma. These differences translate into a relative survival advantage for patients with invasive IPMN following surgical resection. With lymph node involvement, however, the survival advantage for invasive IPMN disappears and the prognosis is equally grim for both cancers. Along with lymph node involvement, we identify tumor grade, tumor size and patient age as predictors of survival following resection for invasive IPMN. In comparison to sporadic pancreatic adenocarcinoma, the potential for a curative resection in invasive IPMN is higher with negative lymph nodes and every effort should be made to intervene at this early stage of the disease. The results of this study help define the natural history after resection of invasive IPMN, a diagnosis with an increasing incidence. These results provide important prognostic information for both patients and clinicians and will help guide postoperative discussions regarding utilization of adjuvant therapies. Additionally, the finding that high tumor grade adversely affects overall survival of invasive IPMN with negative nodes merits consideration of this variable in decisions regarding adjuvant therapy following resection of invasive IPMN.
Acknowledgments
Supported by grant CA090848 from the National Cancer Institute and by funding from the Rod Fasone Memorial Cancer Fund (Indianapolis, IN), the Henry L. Guenther Foundation (Los Angeles, CA), the William Randolph Hearst Foundation (San Francisco, CA), the Davidow Charitable Fund (Los Angeles, CA), and Mrs. Ruth Weil (Los Angeles, CA).
Footnotes
No financial disclosures or conflict of interest for any of the authors.
Presented in part at the American Society for Clinical Oncology Gastrointestinal Symposium, January 15-17, 2009, San Francisco, CA and the annual meeting of the Society of Surgical Oncology, March 5-8, 2009, Phoenix, AZ.
Synopsis: The SEER database is used to compare the natural history of invasive IPMN to stage matched sporadic pancreatic adenocarcinoma. This study will help guide discussions regarding prognosis and decisions regarding adjuvant therapy.
References
- 1.Kloppel G, S E, Longnecker DS. World Health Organization International Classification of Tumours. Berlin: Springer; 1996. Histological typing of tumours of the exocrine pancreas; pp. 11–20. [Google Scholar]
- 2.Longnecker DS, A G, Hruban RH, Kloppel G. Intraductal papillary-mucinous neoplasms of the pancreas. In: Hamilton SR, A L, editors. World Health Organization Classification of Tumours: Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 237–240. [Google Scholar]
- 3.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123(5):1500–7. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 4.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239(5):678–85. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239(6):788–97. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;70(6 Suppl):1727–31. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Wilentz RE, H R. Pathology of cancer of the pancreas. Surg Oncol Clin N Am. 1998;7:43–65. [PubMed] [Google Scholar]
- 8.Fujii H, Inagaki M, Kasai S, et al. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 1997;151(5):1447–54. [PMC free article] [PubMed] [Google Scholar]
- 9.Couvelard A, Sauvanet A, Kianmanesh R, et al. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: a prospective evaluation. Ann Surg. 2005;242(6):774–8. doi: 10.1097/01.sla.0000188459.99624.a2. discussion 778-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246(4):644–51. doi: 10.1097/SLA.0b013e318155a9e5. discussion 651-4. [DOI] [PubMed] [Google Scholar]
- 11.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143(7):639–46. doi: 10.1001/archsurg.143.7.639. discussion 646. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6(1-2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 13.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51(5):717–22. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189(5):632–6. doi: 10.1016/j.amjsurg.2005.01.020. discussion 637. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa Y, Unger TA, Taylor S, et al. Mucus is a predictor of better prognosis and survival in patients with intraductal papillary mucinous tumor of the pancreas. J Gastrointest Surg. 2003;7(1):12–8. doi: 10.1016/S1091-255X(02)00152-X. discussion 18-9. [DOI] [PubMed] [Google Scholar]
- 16.Niedergethmann M, Grutzmann R, Hildenbrand R, et al. Outcome of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas (IPMN): a 10-year experience. World J Surg. 2008;32(10):2253–60. doi: 10.1007/s00268-008-9692-8. [DOI] [PubMed] [Google Scholar]
- 17.Insert City of Publication Here see notes. National Cancer Institute; Surveillance, Epidemiology, and End Results [database online] [Google Scholar]
- 18.Riall TS, Stager VM, Nealon WH, et al. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204(5):803–13. doi: 10.1016/j.jamcollsurg.2007.01.015. discussion 813-4. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13(9):1189–200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 20.Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Reber H, Tomlinson JS. Impact of tumor grade on prognosis in pancreatic cancer: Should we include grade in AJCC Staging? Ann Surg Oncol. doi: 10.1245/s10434-010-1071-7. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239(3):400–8. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nara S, Onaya H, Hiraoka N, et al. Preoperative evaluation of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas: clinical, radiological, and pathological analysis of 123 cases. Pancreas. 2009;38(1):8–16. doi: 10.1097/MPA.0b013e318181b90d. [DOI] [PubMed] [Google Scholar]
- 23.Nakagohri T, Kinoshita T, Konishi M, et al. Surgical outcome of intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2007;14(11):3174–80. doi: 10.1245/s10434-007-9546-x. [DOI] [PubMed] [Google Scholar]
- 24.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234(3):313–21. doi: 10.1097/00000658-200109000-00005. discussion 321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo SM, Ryu JK, Lee SH, et al. Survival and prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas: comparison with pancreatic ductal adenocarcinoma. Pancreas. 2008;36(1):50–5. doi: 10.1097/MPA.0b013e31812575df. [DOI] [PubMed] [Google Scholar]
- 26.Shimada K, Sakamoto Y, Sano T, et al. Invasive carcinoma originating in an intraductal papillary mucinous neoplasm of the pancreas: a clinicopathologic comparison with a common type of invasive ductal carcinoma. Pancreas. 2006;32(3):281–7. doi: 10.1097/01.mpa.0000202955.33483.e2. [DOI] [PubMed] [Google Scholar]
- 27.Al-Refaie WB, Choi EA, Tseng JF, et al. Intraductal papillary mucinous neoplasms of the pancreas. Med Princ Pract. 2006;15(4):245–52. doi: 10.1159/000092985. [DOI] [PubMed] [Google Scholar]
- 28.Yang AD, Melstrom LG, Bentrem DJ, et al. Outcomes after pancreatectomy for intraductal papillary mucinous neoplasms of the pancreas: an institutional experience. Surgery. 2007;142(4):529–34. doi: 10.1016/j.surg.2007.07.007. discussion 534-7. [DOI] [PubMed] [Google Scholar]


