Abstract
The Dam1 complex, also known as DASH complex, is the outer kinetochore protein complex of yeast that plays a crucial role in attachment of kinetochore to microtubule. The Dam1 complex is formed by at least nine proteins including Dam1p, Duo1p, Dad1p, Spc19p and Spc34p. In this study, domains of Spc34p that physically interact with other subunits of the complex were mapped using a high-throughput methodology. The method is a combination of two-hybrid screening of a random truncation library of the Spc34 gene and a unique PCR-based amplification that converge the selected DNA fragments to a few short fragments. Duo1p, Dam1p, Dad1p and Spc19p binding domains of Spc34p were mapped on M1-E59, M1-D47, M1-D47 or T207-E295 and S154-Q294, respectively. Most of the boundaries were located at less conserved regions among fungal Spc34p homologs, which is consistent with the boundaries of the putative secondary structures. The accuracy of the mapped domain boundaries was verified using truncated Spc34p polypeptides. The results and methodology we demonstrated herein not only shed light on the molecular architecture of the protein complex but also pave the road to the high-throughput identification of specific interaction domains of proteins whose possible interaction partners have been identified in genome-scale analyses.
INTRODUCTION
Interactions between proteins play crucial roles in the structural architecture of cells and organelles as well as functional organization of biological reactions executed in, on and between cells. An entire corpus of all the possible protein–protein interactions in Saccharomyces cerevisiae has been emerging due to comprehensive and exhaustive protein–protein interaction analyses using the yeasts two-hybrid system (1–3) or large-scale analysis of protein complexes using affinity-column chromatography coupled with mass spectrometry (4). The function of a novel protein found in an interaction cluster with well-characterized proteins can be annotated to be the same biological context as the characterized proteins. In addition to functional annotation, the molecular organization of the protein complex can be indicated by the new members of the interaction cluster. In the comprehensive two-hybrid analysis, such interaction clusters have been discovered in, for example, autophagy-related proteins (2,3), vesicular transport system (1,3), calmodulin-dependent protein kinase Cdc28 complex (2) and kinetochore-related proteins including Dam1 complex (1,3). Thus, the information from the huge protein–protein interaction network gives clues for subsequent analyses on the respective interactions or protein complexes.
One of the next important milestones in yeast’s functional genomics is the systematic analyses of ‘interaction targeting’ where the specific interactions between endogenous proteins are disrupted by constitutive/conditional knockout methods such as over-expression of the binding domain as an antagonist (reviewed by Ito et al. 5). Such methodology allows for the roles of individual interactions in a biological context to be validated. However, one of the bottlenecks to be overcome is the low throughput of interaction domain identification. Conventional methods for domain mapping usually require either preliminary information on protein structure, or time-consuming, trial-and-error analysis of domain boundaries. A novel high-throughput method with well-formatted procedure would be advantageous for the interaction domain identification. Such a high-throughput method would be useful for elucidation of the molecular orientation and structural organization of newly discovered protein complexes in a particular interaction cluster as well as the interaction targeting. The information on the structural/functional domains responsible for the inter-subunits’ binding can give important clues to allow an understanding of how the protein complex is constructed and coordinated, and how the entire function of the complex is controlled.
Dam1 complex (6,7), also known as DASH complex (8), is the yeast outer kinetochore complex consisting of Dam1p, Duo1p, Dad1p, Ask1p, Spc19p, Spc34p and some other recently found subunits such as Dad2p [also called Hsk1p (8)], Dad3p and Dad4p (9). Recent physiological and genetic analyses show that the complex is physically attached to the nuclear microtubule and central kinetochore, and plays crucial roles in metaphase and anaphase spindle integrity maintenance as well as organized segregation of sister chromosomes (6–8, reviewed in 10,11), possibly under the control of phosphorylation by aurora B kinase (Ipl1p complex; 9). The physical interactions between the subunits were originally discovered in the comprehensive interaction analysis (3). All members except for Ask1p and recently found Dad2-4 subunits show physical interaction with Spc34p (3), suggesting Spc34p plays a pivotal role as a scaffold within the Dam1 complex.
In this work, we performed an exhaustive identification of the interaction domains of Spc34p for the other subunits using a unique high-throughput methodology that can be efficiently applied to the systematic identification of interaction domains and interaction targeting. The domain boundaries mapped with this method were verified, indicating a high degree of accuracy of the mapped domain boundaries. Subunit orientations of the Dam1 complex revealed in this work and an overview of the potential molecular architecture of the complex is also discussed.
MATERIALS AND METHODS
Materials
DNA polymerases such as Taq and Ex-Taq DNA polymerases, and some other DNA modification enzymes including DNA ligase and restriction enzymes were purchased from TaKaRa (Japan) unless otherwise stated. Pfu-turbo DNA polymerase was from Stratagene. Conventional yeast two-hybrid system (MATCHMAKER Gal4 Two-Hybrid System ver. 1) was purchased from BD Biosciences (CA). Oligo nucleotides for conventional PCR, PASA-PCR and DNA sequence analysis were from FASMAC (Japan).
Construction of the prey vector pGAD424-TA
Plasmid pGAD424-TA was the derivative of a conventional vector pGAD424 (BD Biosciences). After the elimination of pGAD424 endogenous EcoRV site by site-directed mutagenesis, another EcoRV site was introduced to the multiple cloning sites by PCR using oligonucleotides (ERV-gad424f, ATCGGATCCGTCGACCTGCAGAGAT, and ERV-gad424r, ATCTGGATCCCCGGGAATTCGATCT) as primers. The resulting plasmid, termed pGAD424-TA, was verified by DNA sequencing of the multiple cloning sites and leucine complementation analysis. The resulting plasmid was digested with EcoRV, then treated with Taq DNA polymerase under a typical buffer condition (recommended in manufacturer’s protocol) in the presence of 2 mM dTTP for 2 h at 70°C to attach extra-dTs at both 3′-ends (12). Modified pGAD424-TA was purified with phenol/chloroform extraction followed by ethanol precipitation, then used as a T-vector for the following random truncation library construction.
Plasmids for baits
Yeast cDNAs for Dam1 complex subunits were prepared from total RNA of strain TM101 by reverse transcriptase–PCR with ORF-specific primers. The resulting gene fragments were cloned into the unique SmaI site of the conventional two-hybrid bait vector pGBT9. The DNA sequences of the resulting plasmids were verified, then designated as pGBT9-X (X = Duo1, Dam1, Dad1 or Spc19).
Construction of truncation library of Spc34 by DNase I random digestion and TA-cloning
Five micrograms of PCR-amplified DNA fragment containing the entire Spc34p coding region was added to a reaction mixture consisting of 20 mM Tris–HCl (pH 8.0), 150 mM NaCl, 1 mM MnCl2 and an appropriate amount of DNase I (Ambion, TX) and allowed to be randomly digested for 5 min at 37°C to give an average of one or two breaks per gene. The reaction was stopped by the addition of phenol/chloroform solution. After ethanol precipitation, the digested fragments were treated with Ex-Taq DNA polymerase in the presence of 0.2 mM dNTPs to form dA-protruding 3′ ends. The DNA fragments were then fractionated by 0.7% agarose gel electrophoresis and fragments ranging from 400 bp to full-length in size were gel-purified. The purified fragments were ligated to 100 ng of the T-vector at 12°C for 12 h, followed by transformation of Escherichia coli DH5α-FT to construct an Spc34 random truncation library. The resulting bacterial colonies grown on the LB plates containing ampicillin were pooled, and plasmid DNA was isolated using the alkaline–SDS method. The pool of plasmids, designated pGAD-Spc34frag library, was used as the prey library in the following yeast two-hybrid screening.
Yeast two-hybrid screening and convergence of the selected fragments using PASA-PCR
Two-hybrid screening of interaction-positive clones was carried out according to the manufacturer’s protocol (BD Biosciences). Briefly, S.cerevisiae strain HF7c cells carrying pGAD-Spc34frag library were transformed using the pGBT9-X and then spread on agar plates of synthetic medium that lacks leucine, tryptophan and histidine supplemented with appropriate amount of 3-AT. It should be noted that the diversity of the insert fragment was acceptably retained during the competent cell preparation, since the analysis of naïve fragment library using colony-directed PCR (n = 60) showed no significant changes in the size variation between before and after the competent cell preparation. The resulting colonies (more than 1000 clones) on the selection plates were scraped with a spreader and pooled in a tube. Plasmids were harvested from the pool using a conventional method based on cell-lytic enzyme Zymolyase (Seikagaku-Kogyo, Japan), and used as templates for PASA-PCR. PASA-PCR was carried out as described previously (13) using pGAD424-specific primers (pasa-adf, ATACCCCACCAAACCCAAAA and pasa-adr, ACGATTCATAGATCTCTGCAGG) with Pfu-Turbo DNA polymerase. The reaction condition was as follows; 30 cycles of 94°C for 30 s, slow cooling at the constant rate of 0.2°C s–1 to 58°C, and 58°C 5 s, followed by 10 cycles of 94°C 30 s, 53°C 30 s and 72°C 60 s. After the amplification, the products were mixed with EcoRV-digested pGAD424-TA, and used for the transformation of HF7c competent cells with pGBT9-X (X = Duo1, Dam1, Dad1 or Spc19). Resulting transformants on the selection plates were then subjected to DNA sequence analysis to identify the boundary sequences at the cloning site.
Sequence alignment and secondary structure prediction
Polypeptide sequence alignment of Spc34p with other closely related yeast homologs was carried out using ‘Fungal Alignment’ of the Saccharomyces Genome Database (http://www.yeastgenome.org/). The secondary structures of Spc34p were analyzed using Chou-Fasman secondary structure prediction program compiled in a conventional gene/protein analysis software (GENETYX-MAC, ver. 11.2.0., Software Development Co. Ltd, Japan). Coiled-coil structure was predicted using COILS (14; http://www.ch.embnet.org/software/COILS_form.html) or MultiCoil (15; http://multicoil.lcs.mit.edu/cgi-bin/multicoil).
RESULTS AND DISCUSSION
Design of the methodology for high-throughput identification of interaction domains
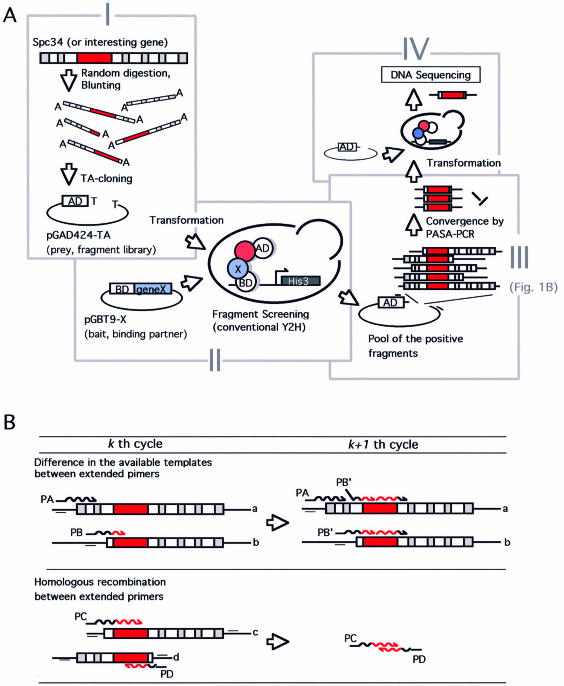
The methodology for high-throughput identification of interaction domains is schematically illustrated in Figure 1. First, the fragment library of the interesting gene is constructed to give combinatorial endpoints to the target gene sequence (Fig. 1A, step I): a fragment encompassing the entire protein coding region is prepared by PCR using gene-specific primers, then subjected to DNase I random digestion. Subsequently, the digested fragments are treated with Taq DNA polymerase with 3′–5′ exonuclease activity (e.g. TaKaRa Ex-Taq DNA polymerase) to remove single stranded ends and add additional dA at their 3′ ends. The trimmed double stranded fragments are then ligated with pGAD424-TA vector of which ends are modified in advance by Taq DNA polymerase with dTTP. The ligation reaction based on the complementary T-A pair allows stoichiometric insertion of the random digested fragments to the vectors, and prevents self-ligation of the vector as well as concatemarization of insert fragments (data not shown).
Figure 1.
(A) Schematic representation of the framework for the high-throughput identification of protein interaction domains. (I) Construction of combinatorial ‘endpoints’ library using conventional PCR amplification of the target gene (depicted as Spc34) and DNase I random digestion. The randomly truncated gene fragment was ligated with the two-hybrid vector pGAD424-TA using TA-cloning. The red box in the gene fragment represents the region responsible for the specific interaction. (II) Selection of interaction-positive fragments using conventional yeast two-hybrid screening with a specific binding partner expressing plasmid (depicted as pGBT9-X). (III) Convergence of the selected fragments using PASA-PCR with pGAD424-TA specific primers (represented as small bars on the plasmid). (IV) Polishing of converged fragments. Fragments converged in step III are mixed with EcoRV-digested pGAD424-TA then used for a second yeast transformation. Several clones on the selection plate are subjected to DNA sequence analysis to identify the position of critical endpoint. The region drawn in red represents the protein interaction domain. See text for more details. (B) Convergence of the selected (binding-positive) fragments by PASA-PCR. The mechanism of the preferential amplification of the shortest amplicon is illustrated. (Upper) Preferential amplification based on the difference in effective concentration of templates. PA, primer extended on template with long flanking sequence (template a). PB, primer extended on short template with less flanking sequence (template b). This diagram represents that only template a is available for further extension of PA in the next (k + 1th) cycle, whereas the PB can utilize the both to give further extended primer PB’, resulting in more efficient amplification. (Lower) A homologous recombination between the two primers prematurely extended in kth cycle (PC, primer extended on template c, and PD, the counterpart primer extended on template d) occurs to form a shorter fragment with the novel combination of the endpoints in (k + 1th) cycle. See text for details.
Secondly, S.cerevisiae HF7c were transformed by the fragment library plasmid and then by bait-expression plasmid pGBT9-X to perform two-hybrid screening (Fig. 1A, step II). In the two-hybrid screening, every possible clone bearing the endpoints acceptable for the specific interaction is selected. Although we employed the classical vectors and host strain (HF7c) for the two-hybrid screening to show the feasibility and generality of our system, other useful host strains and screening systems such as the diploid cell-based two-hybrid (1,16,17) or commercially available LexA-based two-hybrid system (e.g. BD Biosciences) with inducible promoters are potentially compatible.
In step III, the interaction-positive fragments are converged to a single or few clones. Although every clone selected in step II should encompass the region responsible for the specific interaction (shown in red boxes in Fig. 1), most of the selected clones have flanking sequence on either or both sides of that region. PASA-PCR (preferential amplification of the shortest amplicon using modified PCR) (13) is used to enrich the shortest fragment that carries the least flanking sequence. The amplification manner of PASA-PCR is the PCR adaptation of in vitro evolution using Qβ RNA replicase (18), where the shorter duplicating RNA molecules were propagated more efficiently by iterative cycles of in vitro selection and amplification. PASA-PCR consists of iterative cycles (typically, 30–50 cycles) of denaturation of template DNAs and an extremely abbreviated annealing/extension period (<5 s), followed by an additional 10 cycles of conventional PCR to raise the yield of the converged products. Because of the short annealing/extension period, the numbers of cycles necessary for the amplicon to duplicate are dependent on their sizes, as discussed in detail (13). The strength of PASA-PCR is its ability to facilitate homologous recombination. In PASA-PCR, primers cannot complete their extension within a single cycle, so that prematurely extended primers anneal on another template in the following cycles (template switching based on in vitro homologous recombination, Fig. 1B). As a result, the effective concentration of templates is reduced for the primer that started its extension on the templates with long flanking sequences (the primer PA in Fig. 1B) in the previous cycle, yet it remains unchanged for the primer that started on the short template (the primer PB in Fig. 1B). This difference in the effective concentration allows the shortest amplicon to duplicate more efficiently than the longer ones. In addition, the homologous recombination between the prematurely extended primers occurs, resulting in the generation of novel combinations of the endpoints (Fig. 1B). Fragments thus generated are shorter than the other fragments, so that they are amplified more efficiently in the following cycles. Moreover, such recombination means that we do not have to construct a huge initial random fragment library to cover every possible combination of endpoints. Thus, owing to this property, a relatively small (as few as 104–5 clones for the gene of <1000 bp) library is sufficient for the exhaustive screening.
In the final step, the converged fragments are polished by a second round of two-hybrid analysis with the specific binding partner (Fig. 1A, step IV). To avoid laborious gel-purification or ligation reaction, the converged fragments are mixed with pGAD424-TA vector linearized by EcoRV digestion and the mix is used directly for transformation via homologous recombination in vivo (19). Following the screening, the fragments are then amplified using colony-directed PCR from the yeast colonies and the products are used as templates for DNA sequencing.
Selection of interaction-positive Spc34 fragments and their convergence by PASA-PCR
We performed the interaction domain mapping of Spc34p for the interaction partners as shown in Figure 1. Yeast HF7c cells harboring pGAD-Spc34frag (consisting of 104 independent clones with various insert sizes) were transformed by plasmid pGBT9-X (X represents respective genes for interaction partners including Dam1p, Duo1p, Dad1p and Spc19p) then allowed to grow on selection plates. The cells showing Spc34frag-dependent growth (∼1000–1500 colonies/interaction partner) were pooled, and their plasmids were subjected to PASA-PCR-convergence (Fig. 2). It should be noted that we used a pair of pGAD424-specific primers, pasa-adf and pasa-adr (see Materials and Methods for their sequences), for PASA-PCR. These primers were designed to anneal to positions remote from the cloning site in order to facilitate in vivo homologous recombination in the following subcloning step (Fig. 1A, step IV). The fragments amplified by these primers, therefore, contain an extra 88 bp sequence derived from the prey vector besides the insert sequence.
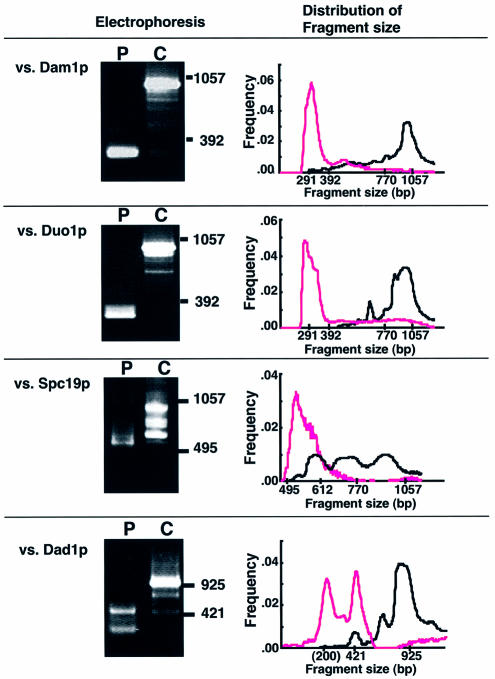
Figure 2.
Convergence of the selected fragments by PASA-PCR. The plasmid pool obtained from interaction-positive clones with the indicated binding partner (yeast colonies grown on the selection plates) was amplified by PASA-PCR (lanes P) or conventional PCR (lanes C). The amplified fragments were subjected to agarose gel electrophoresis. Bars with numbers on the right indicate the migration points of size markers (base pair). The size distribution of the amplified fragments was analyzed using gel-image analyzer (NIH image ver. 1.59), and represented as scatter diagrams (right panels). x and y axes of the graphs represent amplified fragment size (base pair, in logarithmic scale) and frequency (total = 1.0), respectively. The positions where the size markers were migrated are indicated on the x axis. Dots in magenta and black represent the frequency of the fragment in PASA-PCR and conventional PCR, respectively.
The convergence of fragments occurred successfully for each interaction partner (Fig. 2). Spc34 fragments responsible for Dam1p and Duo1p interactions appeared ∼290 bp. Amplification of the fragments by conventional PCR (lanes C) resulted in a major fragment at 1000 bp with some minor bands at the lower positions. The major band in lane C was a product from the clone bearing undigested Spc34 (data not shown). A comparison of the size-distribution of the fragments amplified by PASA-PCR with those by conventional PCR clearly shows how efficiently PASA-PCR enriches rarely existing short fragments from the pool of binding-positive fragments.
In addition to these interaction domains, converged PCR products were also obtained in Dad1p interacting fragments as templates, although the convergence was not strict (Fig. 2). The fragments converged to three fragments with distinctive sizes (bands of migration points of 380 and 250 bp with the smallest band ∼200 bp). In this case, the smallest fragments of <200 bp might be false-positive fragments likely generated by misannealing of the primers, since any insert fragments responsible for the smallest band were not found in the pool selected in the following step (data not shown). As shown below, the remaining two fragments encoded two distinct regions of Spc34p, suggesting that the two fragments were converged independently. As described above, one of the principal driving forces for the convergence is in vitro homologous recombination between prematurely extended primers, therefore, competition does not occur between two independent sequences. This is the reason why the two fragments with different (but similar) sizes were converged independently.
DNA sequence analysis of the converged fragments
Figure 3 shows the endpoint positions of the interaction-positive inserts converged by PASA-PCR. For each interaction partners, five or more clones were sequenced, and the endpoints found at the cloning site were annotated on the Spc34p amino acid sequence. In each case, the identified endpoints were focused on a single, or a few specific positions. For example, three out of five of the fragments for the Dam1p interaction had an identical C-terminal endpoint at D47. Likewise, C-terminal endpoints for Duo1p interaction were mainly mapped at E59. Further analysis of more clones (n = 18 clones) showed that nearly 90% had an identical endpoint at E59, suggesting a significant convergence. The sizes of these regions were consistent with those amplified by PASA-PCR (Fig. 2). These data also suggest that the shortest amplicon among the selected fragments was successfully enriched, so that the major endpoint in the converged fragments likely appears as a ‘critical endpoint’. Moreover, the high intensity of the convergence suggests that DNA sequence analysis of several clones is sufficient to find the critical endpoint. This would be a very convenient property for high-throughput identification of interaction domains, where many domains need to be identified simultaneously in the limited resources for DNA sequencing analysis (e.g. colony picking, template preparation, sequence data analysis, etc.). In contrast, the region for Spc19p interaction was mapped in the latter part of Spc34 sequence (around S154-Q294, depicted in black arrows). These suggest that Spc34p likely consists of two or more independent folding units.
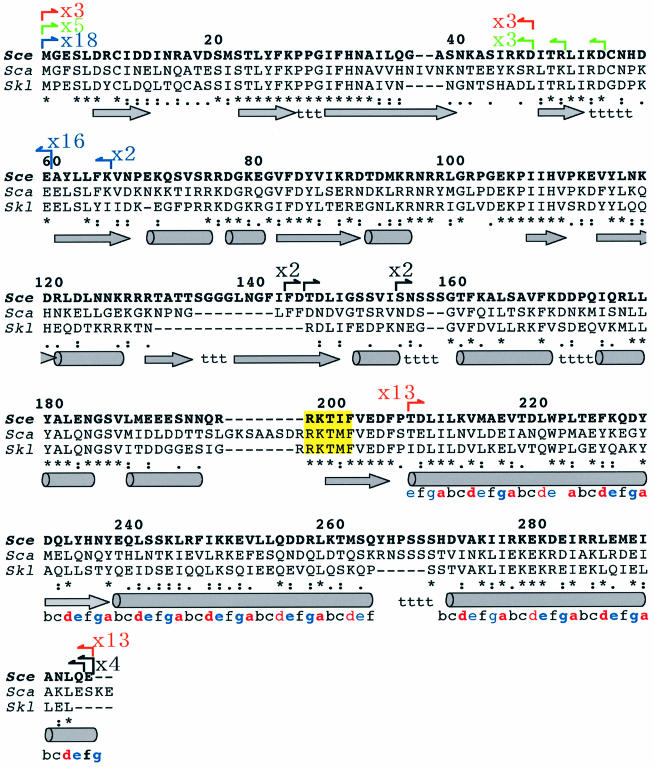
Figure 3.
Endpoint positions of the fragments amplified by PASA-PCR. Endpoint positions of the converged fragments were analyzed by DNA sequencing. The positions of the identified endpoints were indicated by arrows with vertical lines on the polypeptide sequence alignment. Orange, green, blue and black arrows represent the endpoints found in the fragments for Dad1p, Dam1p, Duo1p and Spc19p, respectively. Arrows with numerals represent the numbers of the endpoints found in the identical positions. Polypetide sequences of Spc34p homologs from S.cerevisiae (described in bold letters), S.castellii and S.kluyveri were juxtaposed. The numerals on the alignment represent the position of the amino acid residues of S.cerevisiae Spc34p, where the first methionine of the protein is defined as +1. The letters under the alignment such as ‘*’, ‘:’ and ‘.’ indicate identical, strongly similar and similar amino acid residues, respectively. Cylinders, arrows in gray, and t under the sequences indicate putative α helix, β strand and turns, respectively, predicted by Chou-Fasman method (see Materials and Methods) using the S.cerevisiae Spc34p polypeptide sequence. The repeating serial alphabets from T207 to E295 (C-terminal) with colors represent the predicted coiled-coil sequence. The letters a and d in red indicate the positions for the hydrophobic amino acid residues in the canonical coiled-coil structure. Blue e and g are the positions for the hydrophilic residues. The conserved residues consistent with the periodic rule are typed in bold letters. The conserved amino acid sequence highlighted in yellow (five residues centered on T199) indicates the recognition sequence of aurora B kinase (9).
While the convergence in their endpoints was obviously detected, the region responsible for Dad1p interaction domain was mapped to two distinct regions (Fig. 3, depicted in orange arrows). Three out of 16 of the selected fragments (19%) contained the region for N-terminal 47 residues of Spc34p (M1-D47), and the rest (81%) had the C-terminal 89 residues (T207-E295). The respective sizes of the allocated regions were consistent with the sizes of the fragments amplified by PASA-PCR (Fig. 2). Their appearance frequencies in the selected clones were likely consistent with the intensities of their amplified products (Fig. 2). These results suggest that Dad1p subunit can attach to either or both of the regions. In addition, the positions of these critical endpoints were mapped on the region where the local amino acid sequence was less conserved among closely related homologous proteins (Fig. 3), suggesting correlation of the endpoints with the structure of Spc34p.
A relationship between putative secondary structures of Spc34p and the converged endpoints are also shown in Figure 3. Except for those at the N- or C-termini of Spc34p, the most critical endpoints were found at the boundaries of predicted secondary structures. For example, the C-terminal endpoints of the fragments for Dam1p or Dad1p interaction appeared at the same position (D47), where is the boundary of a putative loop and β-strand. Similarly, the critical endpoint for Duo1p occurred between a putative β-turn and β-strand, and the endpoints for Spc19p were spread within the putative domain linker where serine residues appear in high frequency. The coincidences between critical endpoints and the boundaries of putative secondary structures were also found when we used alternative secondary structure-prediction programs such as GOR 3 (19), or GOR 4 (20), whereas somewhat less significant coincidences were found when GOR 1 (21) or PSIPRED (22) was used instead (data not shown).
Interestingly, another critical endpoint for Dad1p interaction was found at T207 from where the putative α-helix starts. The α-helix and the following two α-helices contain periodical appearance of hydrophobic and hydrophilic amino acid residues (Fig. 3, represented as serial alphabets colored in red and blue, respectively), suggesting that these three putative helices can be involved in coiled-coil structures to form interaction surface(s) (14). The predicted structures for these putative coiled coils (15) indicate their propensity to form trimeric coils rather than dimeric coils. Although the prediction program does not find any typical coiled structures in the opposite Dad1p sequence, the periodical hydrophobic residues interrupted by glycines and an intervening sequence is located in the center of the Dad1p sequence as follows: V27-LQEINETMNSILNGLNGLNISLESSIAVGREFQSVSDLWKTLYDGLESLSDEA-P81, where letters in bold represent the periodical hydrophobic residues. The periodicity of hydrophobic residues with the intervening sequence is completely conserved among Dad1p homologs of the closely related yeasts including S.bayanus and S.paradoxus (Fungal Alignment, SGD webpage). The conserved sequence is also found in distantly related homologous proteins such as a putative gene product of Ashbya gossypii (accession no. E66080) and Candida albicans (accession no. AX488771), suggesting that the importance of irregular repeat with intervening sequence in Dad1p function (data not shown), and the possible interaction manner between Spc34p C-terminal region and Dad1p is coiled-coil. Although it is no more than a speculation, the first coil-like structure of Spc34p as well as its homologs also has an atypical repeat disrupted by P222, which may correspond to the irregular repeat in Dad1p.
On the other hand, another region for Dad1p interaction was mapped on the N-terminal of Spc34p, which is identical to the one identified for Dam1p interaction. The coincidence of the critical endpoints in the prey molecule (in this case, it is Spc34p fragment) may suggest that either of the bait subunits (Dam1p or Dad1p) requires the other as a bridge to form bait–prey interaction complex. It should be noted that, as mentioned by Vidal and Legrain (23), it is possible that a two-hybrid analysis of two indirectly associated proteins can give a positive signal occasionally by the bridge of yeast’s endogenous protein. In that case, the identified binding domain against the given bait is, in reality, the domain for the bridging protein. The same domain is, therefore, mapped when the bridge protein is used as the bait instead. In our case, however, it is not obvious which subunit is the bridge for the other. Since the ambiguity in the composition of bait–prey complex as well as less accuracy in interaction-intensity quantification are intrinsic to two-hybrid assay, another extensive in vitro analysis using purified subunits is required to clarify the precise organization of subunits.
The region responsible for Spc19p binding was mapped on S154-Q294. This region encompasses the entire coiled-coil structures on which the Dad1p binding site was mapped. According to the coiled-coil prediction program, Spc19p also has three obvious coiled-coil structures for trimeric coils. Therefore, it is possible that Spc19p and Dad1p likely form trimeric coils together with the C-terminal region of Spc34p. However, in addition to the coiled-coil interaction, our data indicate that an additional sequence (S154-P206) besides the coiled-coil region is required to interact with Spc19p. In fact, N-terminal truncation of Spc34p to D170 showed a significant loss of binding affinity to Spc19p (13), clearly indicating that the coiled-coil interaction is not sufficient to stabilize the putative trimer. Together with the irregular periodicity in Dad1p subunits, the evidence suggests that the architecture on the C-terminal region of Spc34p would be more complicated than a typical coiled-coil interaction.
Accuracy of the domain boundaries mapped as critical endpoints
Accuracy of the domain boundaries is another important criterion as well as the throughput of the process. Pin-point identification of the domain boundary guarantees reliable structural information that can give insight to the function of the polypeptide, and is essential for elucidating structure–function relationships of proteins.
While the ‘critical’ endpoints of Spc34p fragments for Duo1p and Dam1p interactions were different (Fig. 3), they were located close to each other. The endpoints for Dam1p mainly converged at D49 with variations in R52 and D56, whereas the ones for Duo1p show higher convergence at E59, which is just outside of the region mapped for Dam1p binding. We constructed a series of truncation mutants of Spc34p (Fig. 4) on the basis of the predicted secondary structures and sequence similarity between the homologous proteins (Fig. 3), and tested their interactions with the binding partners.
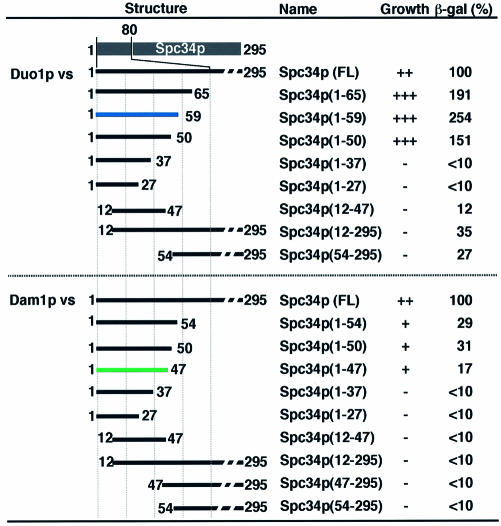
Figure 4.
Verification of the critical endpoints converged by PASA-PCR. A series of truncated Spc34p mutants (listed in the figure) were prepared, and their interaction with Duo1p (upper panels) and Dam1p (lower panels) were analyzed using two-hybrid analysis. The growth rate on the selection plate and β-galactosidase activity of each truncation mutant is summarized and shown as a list. The constructs having the critical endpoints converged from Duo1p and Dam1p interacting clones are highlighted in blue and green, respectively. The value in β-galactosidase activity is the percentage of the β-galactosidase activity of each clone toward that obtained using full-length of Spc34p [also included in the list as Spc34p (FL)].
Figure 4 shows the result of the binding assay between truncated mutants of Spc34p and Duo1p or Dam1p. These data clearly indicate that the critical endpoints converged by PASA-PCR are on the boundaries of the respective interaction domains. In both cases, there were no binding regions outside of the converged region. Further deletions within the critical endpoints did not allow the host cells to grow on the selection plate except for the truncation mutant termed Spc34p(1-50). Nevertheless, the expression level of the reporter β-galactosidase in the clone was significantly reduced compared to that of the clone expressing Spc34p(1-59). The result suggests the importance of the putative β-turn (K53-H57, Fig. 3) proximal to the critical endpoints (E59) in the efficient interaction between Spc34p and Duo1p. In addition, the C-terminal endpoint giving the highest β-galactosidase activity (E59, Fig. 4) was coincident with the position that converged by PASA-PCR. This suggests the endpoint that gave maximum binding activity was preferentially enriched by PASA-PCR and the following two-hybrid assay.
The binding assay with pGBT9-Dam1 showed the weak β-galactosidase activity compared with full-length of Spc34p (Fig. 4), suggesting that the rest of the region (D47-E295) is required to facilitate/stabilize the interaction between the N-terminal region of Spc34p and Dam1p. Since the D47-E295 region did not show obvious signs of direct interaction with Dam1p (Fig. 4), another factor that interacts within the D47-E295 region may stabilize the binding between N-terminal region and Dam1p. Dad1p is the primary candidate for the stabilizing molecule, because of the following reasons: (i) a physical interaction between Dad1p and Dam1p has been reported (3). (ii) Dad1p had two independent binding regions on Spc34p (Fig. 3). Moreover, (iii) the boundary of Spc34p for Dad1p interaction was coincident with that for Dam1p interaction (Fig. 3). Although the coincidence of the boundary may suggest the bridging effect as discussed above, it also likely suggests co-operative interaction of Dad1p and Dam1p with the N-terminal region of Spc34p to form a stable trimeric cluster.
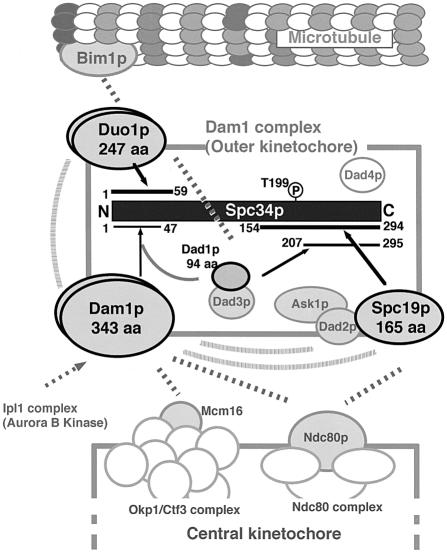
Subunit orientations and molecular architecture of the Dam1 complex
A summary of the structural information of the Dam1 complex formed around Spc34p revealed in this work is illustrated in Figure 5. Other Dam1 complex members not involved in direct interaction with Spc34p as well as those whose specific interaction partners are not yet clarified are included in this figure. Recent findings in inter-subunit network (depicted in gray) reported by another research group (24) are also involved.
Figure 5.
Model of the subunits orientation and molecular architecture of Dam1 complex. The subunits orientation of Dam1 complex with surrounding molecular machineries (microtubules and central kinetochore compexes) involved in the Dam1 complex function is illustrated based on the results obtained in Figure 3 and other information from the literature (2,3,9,24). The black rectangle in the center indicates amino acid sequence of Spc34p. The respective subunits interacting with Spc34p are drawn as ellipses with bold letters. The respective interaction sites for the interacting subunits are represented as lines on the Spc34p sequence. The numbers on the ends of the lines indicate the positions of critical endpoints. The interactions identified in this work are drawn as black arrows. Dotted gray lines indicate the inter-subunits and inter-complex interactions identified in the comprehensive analysis of yeast interactome (2,3), aurora kinase target analysis by Cheeseman et al. (9), or recent findings obtained by Shang et al. using immunoprecipitation of in vitro synthesized subunits (24). Known interactions between Spc19p–Dad2p–Ask1p (3), and between Dad1p and Dad3p (24) are represented as overlapping ellipses. Dad4p (9) is represented as isolated gray ellipses, since its specific interaction partners have not been found so far. Possible dimerization of Dam1p and Duo1p, which is also reported by Shang et al. (24) are represented as bi-layered ellipses. It should be noted that dimerization of Spc34p is also suggested by this group (24).
The foundation is built upon the N-terminal 59 residues and C-terminal 140 residues of Spc34p. The binding ‘scaffolds’ in the termini can be spatially located in proximity by the simultaneous interaction of the small Dad1p (94 aa). An hypothesis of the co-operative assembly of Dad1p–Dam1p on the N-terminal scaffold is also represented in this figure.
Duo1p is another member that interacts with Spc34p in the N-terminal scaffold. The interaction manner between Duo1p–Spc34p and Dam1p/Dad1p–Spc34p is neither competitive nor exclusive. First, physical interaction of Duo1p–Dam1p has been found experimentally (3,24, also depicted in Fig. 5). Secondly, the proteins are simultaneously immunoprecipitated as constituents of the Dam1 complex with stoichiometric (1:1) molecular ratio (7,9). Therefore, Duo1p and Dam1p/Dad1p possibly interact with the scaffold simultaneously, while it is not apparent whether they assemble on the scaffold independently or synergistically.
The function of the outer kinetochore Dam1 complex is to tether microtubule with central kinetochore complexes (review 10,11, also shown in Fig. 5). The Dam1p subunit plays important role in this function. The subunit likely acts as an interface between the Dam1 complex and central kinetochore protein complexes (3). It is known that the subunit executes the association/dissociation of the complex with/from the center kinetochore under the control of aurora B kinase (9). Spc19p could also be another interface involved in the complex–complex interaction, since the physical interaction with Ndc80p has been found in the comprehensive interaction analysis (3). However, the mechanism of regulation of the interaction between Spc19p and Ndc80p is unclear, since Spc19p is not aurora B kinase substrate (9). In addition, an Spc19 mutant strain lacking potential sites for phosphorylation in Spc19p does not show significant changes in phenotype (9), suggesting that interaction between Spc19p and Ndc80p is not regulated by Spc19p phosphorylation. Interestingly, Spc34p has an aurora B kinase-target site at T199 (9), where the Spc19p-binding domain was mapped (Fig. 3). A detailed analysis of the relationship between Spc34p phosphorylation at T199 and the function of Spc19p would elucidate a novel regulation mechanism of the complex–complex interaction.
Thus, the accurate and high-throughput mapping of interaction domains provides structural insight into the molecular orientations and architectural design of protein complexes. Further information of the complexes will be obtained by the systematic interaction targeting, where the conditional/ constitutive antagonists for the specific interactions are designed on the basis of the domain information obtained in the high-throughput identification. Our methodology is sufficiently applicable and amenable to the high-throughput identification. The process is a combination of the construction and functional screening of combinatorial endpoints libraries (i.e. combinatorial bioengineering) and convergence of selected clones to enrich the critical endpoints (i.e. in vitro evolution). We believe this is a powerful tool in functional genome analysis, where innovations in high-throughput processing techniques have made significant breakthroughs.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Dr Andrew Hayhurst and Karl Griswold and Lluis Masip for their comments on the manuscript. We are grateful to Dr H. Nakano and Y. Iwasaki for fruitful discussion. This work was supported by a grant from Ministry of Agriculture, Forestry, and Fishery of Japan to Y.K.
REFERENCES
- 1.Ito T., Tashiro,K., Muta,S., Ozawa,R., Chiba,T., Nishizawa,M., Yamamoto,K., Kuhara,S. and Sakaki,Y. (2000) Toward a protein-protein interacton map of the budding yeast. Proc. Natl Acad. Sci. USA, 97, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uetz P., Giot,L., Cagney,G., Mansfield,T.A., Judson,R.S., Knight,J.R., Lockshon,D., Narayan,V., Srinivasan,M., Pochart,P., Qureshi-Emili,A., Li,Y., Godwin,B., Conover,D., Kalbfleisch,T., Vijayadamodar,G., Yang,M., Johnston,M., Fields,S. and Rothberg,J.M. (2000) A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature, 403, 623–627. [DOI] [PubMed] [Google Scholar]
- 3.Ito T., Chiba,T., Ozawa,R., Yoshida,M., Hattori,M. and Sakaki Y. (2001) A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl Acad. Sci. USA, 98, 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavin A.C., Bosche,M., Krause,R., Grandi,P., Marzioch,M., Bauer,A., Schultz,J., Rick,J.M., Michon,A.M., Cruciat,C.M., Remor,M., Hofert,C., Schelder,M., Brajenovic,M., Ruffner,H., Merino,A., Klein,K., Hudak,M., Dickson,D., Rudi,T., Gnau,V., Bauch,A., Bastuck,S., Huhse,B., Leutwein,C., Heurtier,M.A., Copley,R.R., Edelmann,A., Querfurth,E., Rybin,V., Drewes,G., Raida,M., Bouwmeester,T., Bork,P., Seraphin,B., Kuster,B., Neubauer,G. and Superti-Furga,G. (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature, 415, 141–147. [DOI] [PubMed] [Google Scholar]
- 5.Ito T., Ota,K., Kubota,H., Yamaguchi,Y., Chiba,T., Sakuraba,K. and Yoshida,M. (2002) Roles for the two-hybrid system in exploration of the yeast protein interactome. Mol. Cell. Proteomics, 1, 561–566. [DOI] [PubMed] [Google Scholar]
- 6.Cheeseman I.M., Enquist-Newman,M., Muller-Reichert,T., Drubin,D.G. and Barnes,G. (2001) Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol., 152, 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janke C., Ortiz,J., Tanaka,T.U., Lechner,J. and Schiebel,E. (2002) Four new subunits of the Dam1-Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J., 21, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Bachant,J., Alcasabas,A.A., Wang,Y., Qin,J. and Elledge,S.J. (2002) The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev., 16, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheeseman I.M., Anderson,S., Jwa,M., Green,E.M., Kang,J.-S., Yates,J.R.,III, Chan,C.S.M., Drubin,D.G. and Barnes,G. (2002) Phospho-regulation of kinetochore-microtubule attachments by aurora kinase Ipl1p. Cell, 111, 163–172. [DOI] [PubMed] [Google Scholar]
- 10.Cheeseman I.M., Drubin,D.G. and Barnes,G. (2002) Simple centromere, complex kinetochore: linking spindle microtubules and centromeric DNA in budding yeast. J. Cell Biol., 157, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleveland D.W., Mao,Y. and Sullivan,K.F. (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell, 112, 407–421. [DOI] [PubMed] [Google Scholar]
- 12.Marchuk D., Drumm,M., Saulino,A. and Collins,F.S. (1990) Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res., 19, 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawarasaki Y., Sasaki,Y., Ikeuchi,A., Yamamoto,S. and Yamane,T. (2002) A method for functional mapping of protein-protein binding domain by preferential amplification of the shortest amplicon using PCR. Anal. Biochem., 303, 34–41. [DOI] [PubMed] [Google Scholar]
- 14.Lupas A., Van Dyke,M. and Stock,J. (1991) Predicting coiled coils from protein sequences. Science, 252, 1162–1164. [DOI] [PubMed] [Google Scholar]
- 15.Wolf E., Kim,P.S. and Berger,B. (1997) Multicoil: a program for predicting two- and three-stranded coiled coils. Protein Sci., 6, 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walhout A.J.M. and Vidal,M. (2001) High-throughput yeast two-hybrid assays for large scale protein interaction mapping. Methods, 24, 297–306. [DOI] [PubMed] [Google Scholar]
- 17.Soellick T.-M. and Uhrig,J.F. (2001) Development of an optimized interaction-mating protocol for large-scale yeast two-hybrid analyses. Genome Biol., 2, 0052.1–0052.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills D.R., Peterson,R.L. and Spiegelman,S. (1967) An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc. Natl Acad. Sci. USA, 58, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibrat J.F., Garnier,J. and Robson,B. (1987) Further developments of protein secondary structure prediction using information theory. New parameters and consideration of residue pairs. J. Mol. Biol., 198, 425–443. [DOI] [PubMed] [Google Scholar]
- 20.Garnier J., Gibrat,J.F. and Robson,B. (1996) GOR secondary structure prediction method version IV. Methods Enzymol., 266, 540–553. [DOI] [PubMed] [Google Scholar]
- 21.Garnier J., Osguthorpe,D.J. and Robson,B. (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J. Mol. Biol., 120, 97–120. [DOI] [PubMed] [Google Scholar]
- 22.McGuffin L.J., Bryson,K. and Jones,D.T. (2000) The PSIPRED protein structure prediction server. Bioinformatics, 16, 404–405. [DOI] [PubMed] [Google Scholar]
- 23.Vidal M. and Legrain,P. (1999) Yeast forward and reverse ‘n’-hybrid systems. Nucleic Acids Res., 27, 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang C., Hazbun,T.R., Cheeseman,I.M., Aranda,J., Fields,S., Drubin,D.G. and Barnes,G. (2003) Kinetochore protein interactions and their regulation by the aurora kinase Ipl1p. Mol. Biol. Cell, 14, 3342–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]