Abstract
Objective
TLR2/TLR4-mediated innate immunity serves in frontline antimicrobial host defense, but also modulates tissue remodeling and repair responses to endogenous ligands released in low-grade inflammatory diseases. Here, we assessed if the endogenous TLR2/TLR4 ligands low molecular weight hyaluronan (LMW-HA) and high mobility group box protein 1 (HMGB1), which are increased in osteoarthritic (OA) joints, drive pro-catabolic chondrocyte responses dependent on TLR2 and TLR4 signaling through the cytosolic adaptor MyD88.
Methods
We studied mature femoral head cap cartilage explants and immature primary knee articular chondrocytes from TLR2/TLR4 double knockout (TLR2/TLR4−/−), MyD88 knockout (MyD88−/−) and congenic wild type mice.
Results
IL-1β and TLR2 and TLR4 ligands induced both HMGB1 release from chondrocytes and extracellular LMW-HA generation in normal chondrocytes. TLR2/TLR4−/− and MyD88−/− mouse cartilage explants and chondrocytes lost the capacity to mount pro-catabolic responses to both LMW-HA and HMGB1, demonstrated by >95% suppression of nitric oxide (NO) production (p<0.01), and attenuated induction of MMP-3 and MMP-13. Combined deficiency of TLR2/4, or of MyD88 alone, also attenuated release of NO and blunted induction of MMP-3 and MMP-13 release. Last, MyD88 was required for HMGB1 and hyaluronidase 2 (hyal2) (which generates LMW-HA) to induce chondrocyte hypertrophy, which is implicated in OA progression.
Conclusions
MyD88-dependent TLR2/TLR4 signaling is essential for pro-catabolic responses to LMW-HA and HMGB1, and MyD88 drives chondrocyte hypertrophy. Therefore, LMW-HA and HMGB1 act as innate immune cytokine-like signals with the potential to modulate chondrocyte differentiation and function in OA progression.
Introduction
Manifestations of low-grade inflammation, including varying degrees of synovitis, as well as inflammatory cytokine expression within articular cartilage, contribute to pathogenesis and disease progression in OA (1–6). Chondrocytes, the unique cellular component of articular cartilage within the "organ" structure of the synovial joint, are responsible for normal maintenance and remodeling of articular cartilage and extracellular matrix. Primitive Toll-like receptor 2 (TLR2) and TLR4-mediated innate immune responses are not only in the first line of host antimicrobial defense but also trigger and shape a multitude of chronic inflammatory and tissue repair responses to endogenous ligands, as exemplified in lung injury and atherosclerosis (7–10). Human articular chondrocytes express multiple TLRs, including TLR2 and TLR4 (11,12). Moreover, expression of TLR2 and TLR4 are increased in human knee OA cartilage (12) and induced by inflammatory mediators such as IL-1, TNFα, and TLR2 and TLR4 agonists in cultured chondrocytes (12).
MyD88 is an essential intracellular signaling adaptor for all TLRs, with the exception of TLR3 and the MyD88-independent pathway of TLR4 signaling (7). MyD88 signaling promotes expression of NF-κB-dependent genes including iNOS, and certain cytokines and MMPs held to modulate pathogenesis of OA (7, 13–15). CPPD and monosodium urate crystals are endogenous TLR2 ligands that induce NO production in chondrocytes via MyD88 signaling, which may contribute to cartilage pathology in chondrocalcinosis and gout, respectively (13).
At least two endogenous shared TLR2/TLR4 ligands (LMW-HA and HMGB1)(16,17) can be increased in the joint in OA (18, 19). HA, a major glycosaminoglycans of cartilage extracellular matrix (ECM), is a high molecular weight polymer subject to rapid degradation at sites of tissue injury and inflammation (20). The primary receptor for HA is CD44, which is required to clear HA following tissue or cellular injury (21) and also mediates physiologic cell-cell interactions (21). LMW-HA induces nitric oxide (NO) production and MMP-13 expression in cultured articular chondrocytes by alternative CD44-dependent and undefined CD44-independent mechanisms (22,23).
HMGB1 was originally identified as a DNA-binding protein critical for proper transcriptional regulation (24,25). HMGB1 can be either passively released into the extracellular milieu by necrotic and damaged cells, or actively secreted with delay by activated cells (24,25). Extracellular HMGB1 represents an optimal “necrotic marker” selected by the innate immune system to recognize tissue damage and initiate reparative responses, and HMGB1 also acts as a pro-inflammatory cytokine (24,25). Purified HMGB1 induces MMP-13 in chondrocytes, mediated only partly by receptor for advanced glycation endproducts (RAGE) signaling (26). In addition, treatment of cartilage explants with HMGB1 up-regulates the production of NO, prostaglandin E2, IL-6 and IL-8 (19). Moreover, HMGB1 induces type × collagen, the core marker of chondrocyte hypertrophy, in cultured chondrocytes (3). Dysregulated chondrocyte differentiation to hypertrophy, a state associated with altered ECM biosynthesis and increased MMP-13 expression and promoted by several inflammatory mediators (1–4), appears to contribute to OA progression (27–29).
Here, we observe LMW-HA and HMGB1 to provide cytokine-like signals that modulate matrix catabolism and chondrocyte hypertrophic differentiation critically through innate immune signaling via MyD88, TLR2, and TLR4. Our results reveal a new model of how innate immunity in cartilage has the capacity to affect the phenotype of tissue remodeling and repair in OA.
Materials and Methods
Reagents
All chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated. Low molecular weight HA (Select HA™ 50K Low Endotoxin) was from Hyalose L.L.C. (Oklahoma City, OK). Purified human recombinant HMGB1 (rHMGB1) was from R&D Systems, Inc. (Minneapolis, MN). LMW-HA and rHMGB1 were confirmed to contain < 0.1 EU/ml and 0.05 EU/ml Endotoxin, respectively, by limulus lysate assay. Human hyal2 and HMGB1 cDNAs were from OriGene Technologies, Inc. (Rockville, MD). Antibodies to MMP-3, MMP-13 and type X collagen were obtained from Millipore (Billerica, MA), BioVision Inc. (Mountain View, CA) and CalBiochem (San Diego, CA), respectively.
Studies of mouse femoral head cartilage explants and immature knee chondrocytes
All animal studies were humanely performed in compliance with an institutionally reviewed and approved protocol. TLR2 knockout (TLR2−/−), TLR4−/− and MyD88−/− mice on a C57BL/6 background were kindly provided by Dr. Shizuo Akira (University of Osaka, Japan) and TLR2 and TLR4 knockout mice were crossbred to produce TLR2/TLR4 double knockout (TLR2/TLR4−/−) mice. All mice were carried under specific pathogen-free conditions. Femoral head cartilage caps from two month old mice were isolated as described (30) and placed in 0.1 ml DMEM/high glucose supplemented with 10% FCS, 1% L-glutamine, 100U/ml penicillin, and 50 µg/ml streptomycin at 37°C in 5% CO2 a 96-well plate containing for 24 hours prior to the described treatments. Nitric oxide (NO) production was measured in conditioned media as the concentration of nitrites in conditioned media by the Griess reaction (13) using NaNO2 as standard. Immature mouse primary knee articular chondrocytes were isolated, validated for chondrocytic differentiation, and cultured as previously described in detail (4).
Transfection of immature mouse knee articular chondrocytes
Primary immature mouse knee articular chondrocytes were maintained in Dulbecco’s modified Eagle’s (DMEM) high glucose medium with 10% fetal calf serum (FCS), 100 µg/ml of streptomycin, and 100 IU/ml of penicillin at 37°C for at least 3 days prior to transfection experiments. Primary immature mouse knee articular chondrocytes were transfected using the Amaxa Nucleofection™ system (Amaxa Inc., Gaithersburg, MD) according to the manufacturer protocol, with transfection efficiency of > 70% in chondrocytes, as assessed by counting GFP positive cells after transfection with the vector control DNA that has a GFP tag.
Analyses of MMP-3, MMP-13, and type × collagen and of HA degradation
SDS-PAGE and Western blot analyses of MMP-3 and MMP-13 in conditioned media, and type × collagen in cell lysates, were as previously described (3), using enhanced chemiluminescence (ECL) (Pierce, Rockford, IL). Conditioned media levels of degraded HA were measured by ELISA (R&D Systems, Inc. Minneapolis, MN).
Real-time quantitative RT-PCR
For real-time quantitative RT-PCR, total RNA isolation was followed by reverse transcription and real-time PCR with SYBR Green I master mix (Roche) and the Roche LightCycler system. GAPDH was used as a reference gene. Primer sequences used: type II collagen: Forward 5'- CAAGGAGAAGCCGGACA-3', Reverse 5'-AGCAGCTCCAGGGAATC-3'; type × collagen: Forward 5'-CCAGGTCTCAATGGTCCCTAAG-3', Reverse 5'- GGTATTCCAGGTTCACCTCT-3'; MMP-13: Forward 5'-TGTTCACTTTGAGGATACAGGC-3', Reverse 5'-CATAGACAGCATCTACTTTATCACC-3'; Runx2: Forward 5'GATGCTCTGTTTCTTTCTTTCAGG-3', Reverse 5'-CTCCAGCATTTCATGCTAGT-3'; GAPDH: Forward 5'-CATCCCAGAGCTGAACG-3', Reverse 5'-CTGGTCCTCAGTGTAGCC7-3'.
Statistical analyses
Triplicate replicates or greater were assayed in all cell culture experiments where numerical data were obtained, and such data were uniformly expressed as mean ± SD. Statistical analyses were performed by one way ANOVA.
Results
Chondrocytes degrade HA and release HMGB1 in response to certain inflammatory mediators
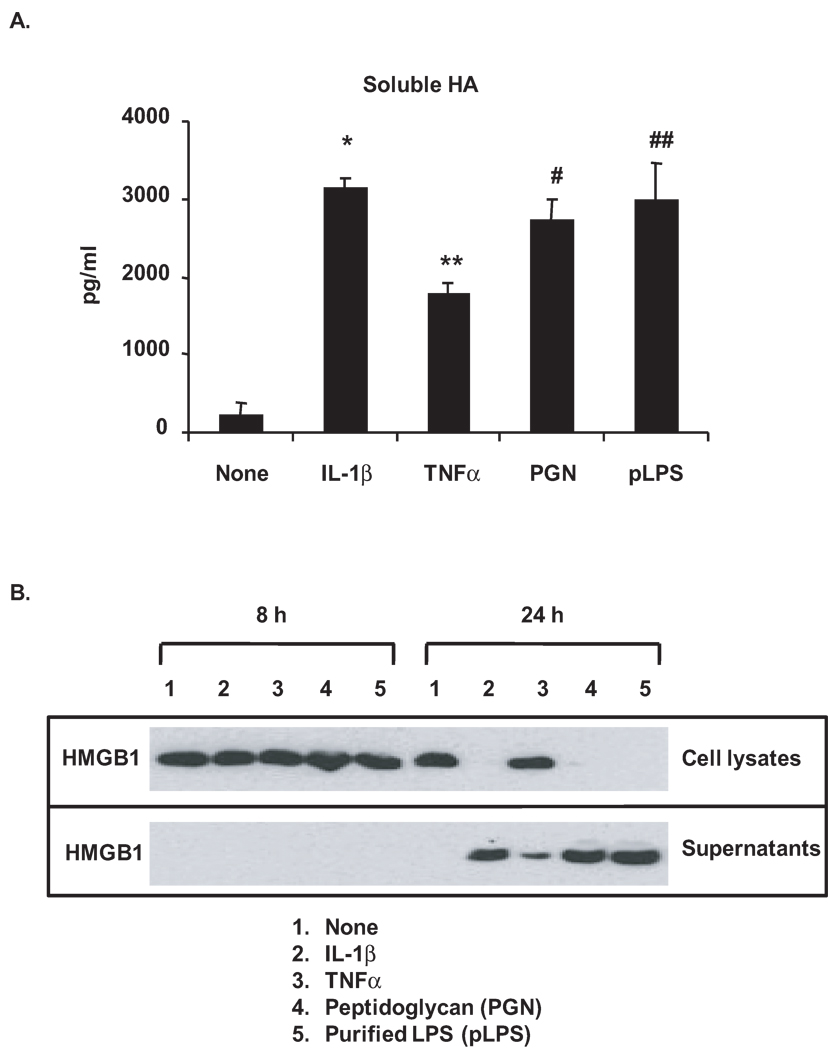
Inflammatory mediator-stimulated degradation of HA to generate LMW-HA was indicated by the appearance of LMW-HA in conditioned media of immature mouse knee articular chondrocytes in response to IL-1β (10 ng/ml) or TNFα (10 ng/ml), as well as the TLR2 ligand peptidoglycan (PGN, 2 µg/ml) and the TLR4 ligand purified LPS (pLPS, 1 µg/ml) (Figure 1A). Findings were comparable in mature bovine knee articular chondrocytes (data not shown). In parallel studies, HMGB1 was detected in the conditioned media but became reduced in cell lysates in chondrocytes after 24 hours stimulation with IL-1β, PGN and pLPS, consistent with robust release of cellular stores of HMGB1 (Figure 1B). TNFα induced partial release of HMGB1 after 24 hours, as evidenced by detection of HMGB1 expression in both cell lysates and conditioned media.
Figure 1.
Degradation of HA and release of HMGB1 in chondrocytes in response to inflammatory mediators. Immature mouse (C57B/L6) knee articular chondrocytes were stimulated with IL-1β (10 ng/ml), TNFα(10 ng/ml), peptidoglycan (PGN, 2 µg/ml) and purified LPS (pLPS, 1 µg/ml) for either 3 days (A) or 8 and 24 hours (B). Degradation of HA was assessed by ELISA analysis of soluble HA released in the conditioned media after 24 hours treatment (A). *, **, #, ## p<0.05 relative to none (non-treated control). Expression of HMGB1 was examined by Western blotting of cell lysates and conditioned media (B). Data for A and B were representative of 3 individual experiments.
TLR2/TLR4- and MyD88-dependent pro-catabolic responses to LMW-HA and HMGB1
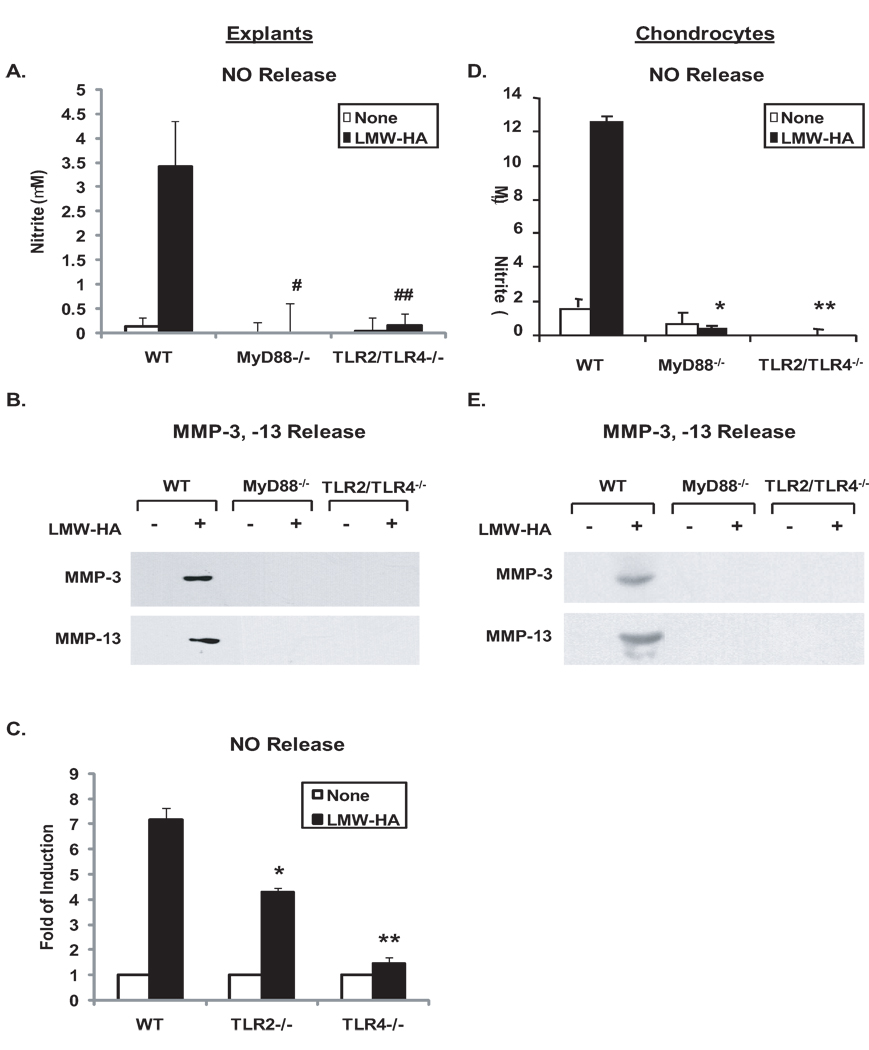
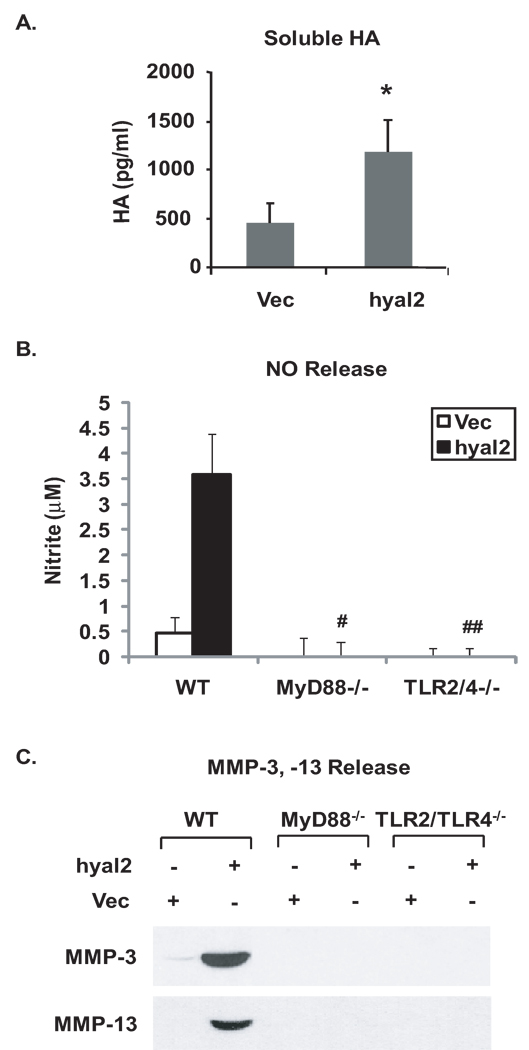
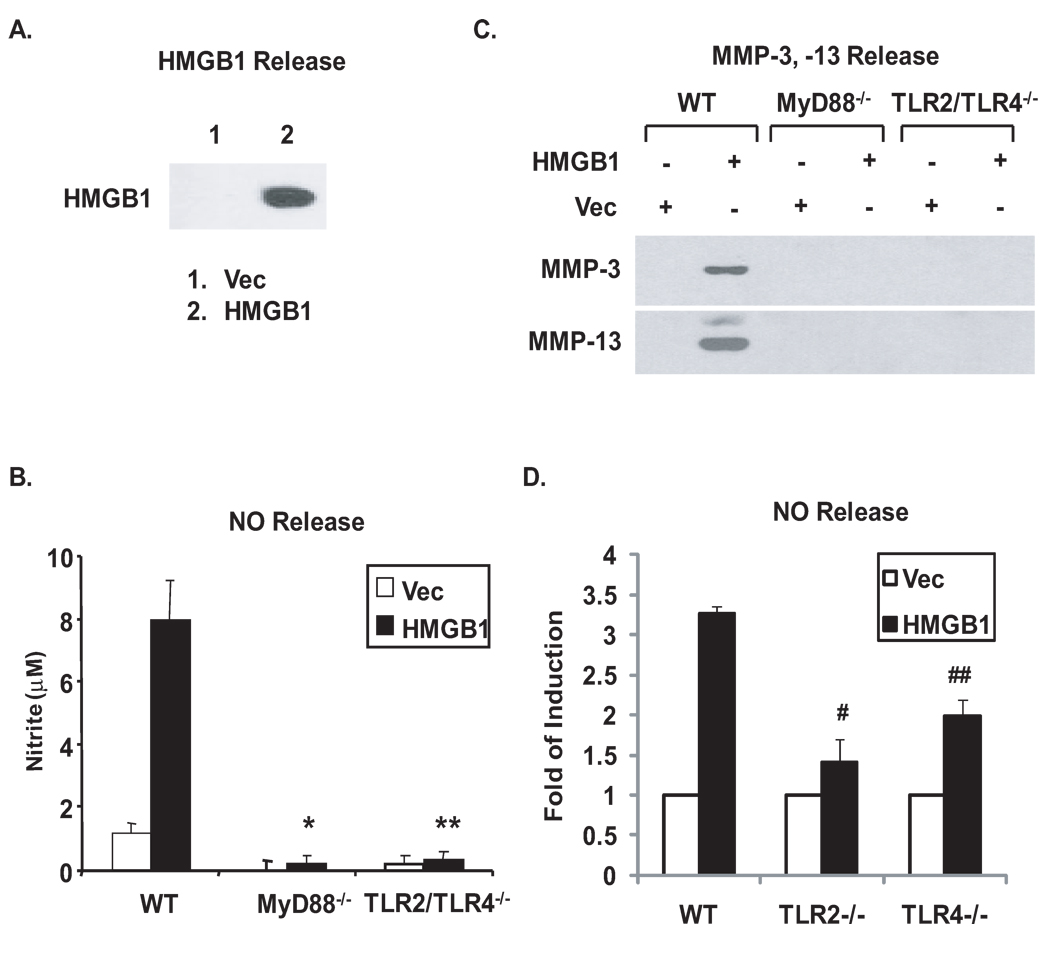
First, the effects of MyD88 knockout and TLR2/TLR4 double knockout on catabolic responses to exogenous LMW-HA were examined in vitro. LMW-HA, at concentrations of 100 µg/ml previously used in chondrocytes (22,23), markedly induced NO, MMP-3, MMP-13 release responses in WT cartilage explants (Figure 2A, B) and chondrocytes (Figure 2C, D) that were attenuated both by knockout of MyD88 or double knockout of TLR2 and TLR4. Next, the effects of single TLR2 and TLR4 knockout were assessed on responses to LMW-HA. NO release in response to LMW-HA decreased by ~47% and ~92% from the conditioned media of TLR2−/− and TLR4−/− cartilage explants, respectively, relative to WT controls (Figure 2E), and there was a significant difference in LMW-HA-induced NO release between TLR2−/− and TLR4−/− cartilage explants (p=0.03), suggesting predominant role of TLR4 in mediating LMW-HA to induce NO release. Similarly, MMP-3 and MMP-13 release was partially inhibited in TLR2−/− and significantly attenuated in TLR4−/− cartilage explants under the same condition (data not shown). Since hyal2 is the predominant hyaluronidase isoenzyme in chondrocytes (31), mouse chondrocytes were next transfected with hyal2 cDNA, which induced degradation of HA, as detected by ELISA of conditioned media (Figure 3A). Transfection of hyal2 cDNA but not the plasmid vector control led to significant induction of NO generation and release of MMP-3, MMP-13 in WT but not MyD88−/− and TLR2/TLR4−/− chondrocytes (Figure 3B, C).
Figure 2.
TLR2/TLR4- and MyD88-dependent pro-catabolic responses to exogenous LMW-HA. Mouse femoral head cartilages isolated from TLR2/TLR4−/−, TLR2−/−, TLR4−/− and congenic WT mice (A, B, E), or immature mouse chondrocytes isolated from MyD88−/−, TLR2/TLR4−/− and respective congenic WT mice (C, D) were stimulated with 100 µg/ml LMW-HA for 5 and 3 days, respectively. Conditioned media were analyzed for release of NO (n=8 for explants, n=3 for chondrocytes), MMP-3 and MMP-13 (all data shown were representative of 3 individual experiments) by Griess reaction and Western blot analyses. Statistics for NO release in A, #, ## p<0.008 for LMW-HA treated MyD88−/− and TLR2/TLR4−/− relative to WT mice explants, respectively. Statistics for NO release in B, * and ** p<0.01 for LMW-HA treated MyD88−/− and TLR2/TLR4−/− relative to WT mouse chondrocytes, respectively. Statistics for NO release in E, *p<0.05 and **p<0.004 for LMW-HA treated TLR2−/− and TLR4−/− relative to WT mice explants, respectively.
Figure 3.
TLR2/TLR4- and MyD88-dependent pro-catabolic responses to endogenous degraded HA. Immature mouse chondrocytes isolated from MyD88−/−, TLR2/TLR4−/− and congenic WT mice were transfected with hyal2 cDNA, with an empty vector plasmid DNA used as a control. Forty-eight hours after transfection, the cells were placed onto poly-HEME coated plates. After three more days, degradation of HA (A) was confirmed from the conditioned media of chondrocytes by ELISA analysis. The conditioned media were analyzed for release of NO (B), MMP-3 and MMP-13 (C). Data for degradation of HA (n=3), for NO release from chondrocytes (n=3), and for MMP-3 and MMP-13 release from chondrocytes were representative of 3 individual experiments. Statistics for concentration of degraded HA in A, * p<0.04 relative to the vector control. Statistics for NO release in B, #, ## p<0.02 for hyal2 transfected MyD88−/− and TLR2/TLR4−/− relative to WT chondrocytes, respectively.
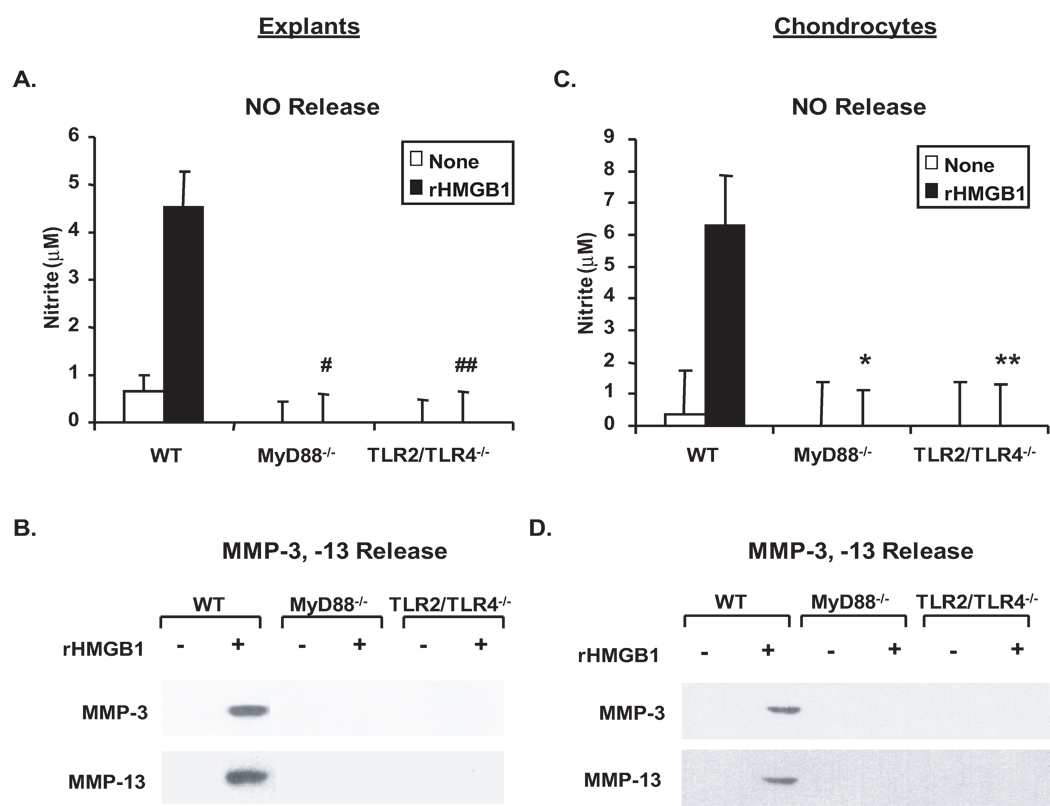
In parallel, the effects of MyD88 knockout and TLR2/TLR4 double knockout on catabolic responses to HMGB1 were also determined. Similarly, release of NO, MMP-3 and MMP-13 was dramatically induced in WT but not in MyD88−/− or TLR2/TLR4−/− cartilage explants (Figure 4A, B) and chondrocytes (Figure 4C, D) in response to recombinant HMGB1 (rHMGB1) at the concentration of 10 µg/ml previously used in chondrocytes (26) in vitro. Transfection of HMGB1 cDNA but not the plasmid vector control enforced release of HMGB1, as demonstrated by Western blot analysis of conditioned media from the transfected chondrocytes (Figure 5A), which resulted in induction of release of NO, MMP-3 and MMP-13 in WT but not MyD88−/− or TLR2/TLR4−/− chondrocytes (Figure 5B, C). Noticeably, NO release induced by HMGB1 transfection was decreased by ~82% and ~57% in TLR2−/− and TLR4−/− chondrocytes, respectively, relative to WT controls (Figure 5D). Unlike the predominant role of TLR4 in mediating catabolic responses to LMW-HA, there was no significant difference in release of NO between TLR2−/− and TLR4−/− chondrocytes in response to transfection of HMGB1 (p=0.09). In addition, partial inhibition of release of MMP-3 and MMP-13 was observed in both TLR2−/− and TLR4−/− chondrocytes (data not shown). These results indicated differential responses through TLR2 and TLR4 for NO release and other pro-catabolic responses to each of these ligands.
Figure 4.
TLR2/TLR4- and MyD88-dependent pro-catabolic responses to recombinant HMGB1. Mouse femoral head cartilages (A,B) or immature mouse chondrocytes (C,D) isolated from MyD88−/−, TLR2/TLR4−/− and congenic WT mice were stimulated with purified recombinant HMGB1 (10 µg/ml) for 3 days. Conditioned media were analyzed for release of NO, MMP-3, and MMP-13 as above. Data for NO release from cartilage explants and chondrocytes (n=5 and 3, respectively), and for MMP-3 and MMP-13 release from both explants and chondrocytes were representative of 3 individual experiments. Statistics for NO release in A, #, ##p<0.01 for rHMGB1-treated MyD88−/− and TLR2/TLR4−/− relative to WT explants. Statistics for NO release in B, *,** p<0.01 for rHMGB1-treated MyD88−/− and TLR2/TLR4−/− relative to WT chondrocytes.
Figure 5.
TLR2/TLR4- and MyD88-dependent pro-catabolic responses to endogenously produced HMGB1. Immature mouse chondrocytes isolated from MyD88−/−, TLR2/TLR4−/−, TLR2−/−, TLR4−/− and congenic WT mice were transfected with HMGB1 cDNA, with an empty vector plasmid DNA used as a control. Forty-eight hours after transfection, the cells were placed onto poly-HEME coated plates. After three more days, release of HMGB1 was confirmed form the conditioned media by Western blot analysis (A). The conditioned media were analyzed for release of NO (B), MMP-3 and MMP-13 (C). Data for NO release from chondrocytes (n=3), and for MMP-3 and MMP-13 release from chondrocytes were representative of 3 individual experiments. Statistics for NO release in B, *, **p<0.005 for HMGB1 transfected MyD88−/− and TLR2/TLR4−/− relative to WT chondrocytes, respectively. Statistics for NO release in D, #p< 0.01 and ## p<0.04 for HMGB1 transfected TLR2−/− and TLR4−/− relative to WT chondrocytes, respectively.
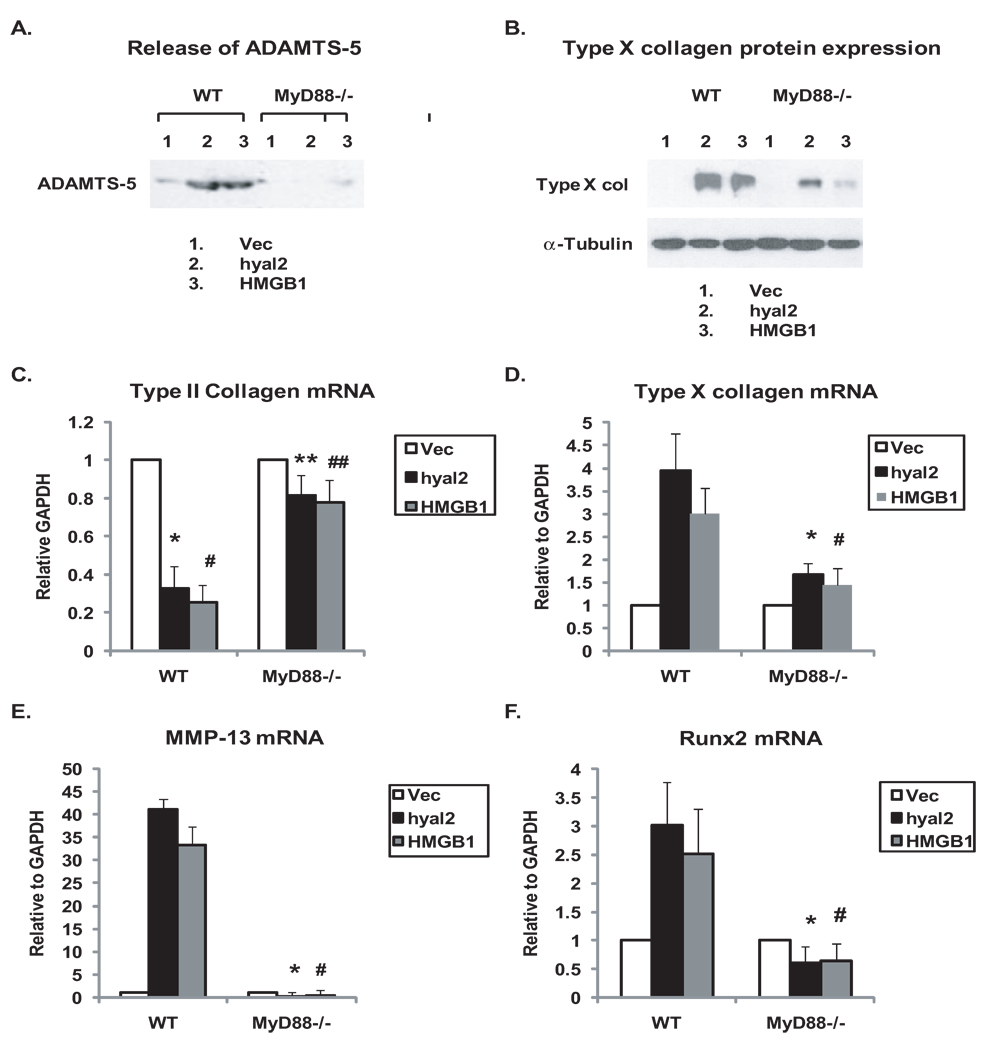
Transfection of hyal2 and HMGB1 cDNA but not the plasmid vector control also induced release in WT chondrocytes of the aggrecanase ADAMTS5, which is central to the progression of experimental mouse OA (30), and suppression of type II collagen mRNA expression, but these responses were attenuated in MyD88−/− chondrocytes (Figure 6A,C). Under the same conditions, type × collagen protein expression was induced in WT chondrocytes, but less so in MyD88−/− chondrocytes (Figure 6B), with quantitative RT-PCR analysis determining ~80% suppression of type × collagen mRNA in MyD88−/− chondrocytes (Figure 6D). In addition, MyD88 deficiency attenuated induction of mRNA expression of the chondrocyte hypertrophy marker MMP-13 (Figure 6E), and of Runx2 (Figure 6F) which is essential for mouse growth plate chondrocyte hypertrophy in vivo and is a critical promoter of both articular chondrocyte hypertrophy and progression of experimental OA in vivo (28).
Figure 6.
MyD88 mediates ADAMTS5 release and maturation to hypertrophy in chondrocytes in response to transfection of hyal2 and HMGB1. Immature mouse chondrocytes isolated from MyD88−/− and congenic WT mice were transfected with either hyal2 or HMGB1, with an empty vector plasmid DNA used as a control. Forty-eight hours after transfection, the cells were placed onto poly-HEME coated plates. Release of ADAMTS5 (A) and type × collagen protein expression (B) were determined from conditioned media after two more days and cell lysates after three more days, respectively, by Western blot analyses. The mRNA expression of type II and type × collagen, MMP-13 and Runx2 (C–F) were analyzed by quantitative RT-PCR after two more days. Data for release of ADAMTS5 and type × collagen protein expression in A and B were representative of 3 individual experiments. Data for mRNA expression of type II and type × collagen, MMP-13 and Runx2 (C–F) were from chondrocytes (n=3). Statistics for type II mRNA expression in C, *p<0.02 and #p<0.01 for WT chondrocytes transfected with hyal2 and HMGB1 relative to WT chondrocytes transfected with the vector control, respectively; **, ##P<0.01 comparing MyD88−/− with WT chondrocytes in response to transfection with hyal2 and HMGB1, respectively. For mRNA expression of type × collagen, MMP-13, and Runx2, *,# p<0.05 (Figure D), *,#p<0.001 (Figure E), and *,#p<0.02 (Figure F) comparing MyD88−/− with WT chondrocytes in response to transfection with hyal2 and HMGB1, respectively.
Discussion
In this study, we discovered that the endogenous TLR2/TLR4 ligands LMW-HA and HMGB1 are able to stimulate cartilage matrix catabolism in a manner dependent on TLR2 and TLR4 and their signaling adaptor MyD88. Previously, CD44 has been demonstrated to form a signaling complex with TLR4 and the TLR4 extracellular adaptor protein MD2 (32). The complex of CD44, TLR4, and MD2 recognizes released HA fragments in sterile injury, by acting to enhance or stabilize the interaction between HA and TLR4 (32). CD44 was previously implicated in induction of NO production and MMP-13 expression by LMW-HA in articular chondrocytes in vitro (22,23). Therefore, our results suggest the possibility, not yet directly tested, that CD44 may function cooperatively with TLR2 and TLR4 to induce cartilage catabolism in response to LMW-HA. Such a signaling mechanism could shift cartilage homeostatic chondrocyte responses to LMW-HA to pro-catabolic responses. Alternatively, effects of cell surface hyaluronan receptors other than CD44 such as receptor for hyaluronan-mediated motility (RHAMM) (33) or extracellular matrix hyaluronan binding proteins (34) could, in conjunction with TLR2 and TLR4, modulate chondrocyte pro-catabolic responses to LMW-HA.
Our results, which demonstrated the capacity of HMGB1 to induce NO, MMP-3, MMP-13 and ADAMTS5 release, suggest that chondrocyte secretion of HMGB1 may allow it to exert pro-catabolic effects similar to those of "conventional" inflammatory cytokines such as IL-1β and TNFα. HMGB1 mRNA expression is increased in response to several forms of inflammatory stress, for example, exposure to IL-1β and TNFα(35). This study further confirmed that several inflammatory mediators including IL-1β and TNFα induce release of HMGB1 (36,37) in chondrocytes, although TNFα only partially did that. The limited capacity of TNFα to induce HMGB1 release was also reported previously in Raw264.7 cells (38). We speculate that TNFα may not induce HMGB1 directly, but rather via its induction of other inflammatory mediators. Since HMGB1 expression in both synovium and cartilage is increased in the collagen induced arthritis (CIA) model of inflammatory arthritis (39), HMGB1 could promote cartilage matrix catabolism in primary inflammatory arthropathies such as RA.
Chondrocytes in OA cartilage recapitulate some aspects of growth plate chondrocyte differentiation (27,29). Stage-specific secretion of HMGB1 by growth plate cartilage chondrocytes has been shown to regulate endochondral ossification (40). Our results suggest that both HMGB1 and LMW-HA have potential capacity to regulate cartilage maturation to hypertrophy in OA cartilage, implicated by their role in induction of type × collagen, MMP-13 and Runx2.
HMGB1 is a ligand not only for TLR2 and TLR4, but also for RAGE, and RAGE signaling mediates HMGB1-induced MMP-13 expression (26) and hypertrophic differentiation (4) in chondrocytes in vitro. However, HMGB1-induced MMP-13 expression is only partially inhibited by soluble RAGE (26), indicating that other receptors are involved, likely, in view of the results of this study, to include TLR2 and TLR4. Signaling transduced by RAGE, TLR2 and TLR4 promote NF-κB activation (41). Moreover, TLR2 and TLR4 are each necessary for chondrocyte pro-catabolic responses to HMGB1. Hence, we speculate that TLR2 and TLR4 and RAGE cooperatively mediate chondrocyte pro-catabolic responses to HMGB1. Notably, the domain of HMGB1 responsible for interaction with RAGE has been localized to amino acids 150–183, immediately preceding the acidic C-terminus of HMGB1 (42). It is possible that the HMGB1 domain recognized by RAGE is not the same as that recognized by TLR2 and TLR4.
Recent studies indicated that highly purified recombinant HMGB1 has little pro-inflammatory activity, but HMGB1 (100 to 500 ng/ml) forms complexes with pro-inflammatory molecules such as ssDNA, LPS, IL-1β and nucleosomes that interact with TLR9, TLR4, IL-1R and TLR2 respectively, greatly potentiating their biological activity (43–47). This study determines the role of TLR2, TLR4 and MyD88 in HMGB1-induced catabolic responses, and further study will be needed to sort out actions of HMGB1 mediated by forming a complex with other molecules to affect signaling via MyD88-dependent IL-1R or TLR pathways.
MyD88, TLR2 and TLR4 mediated chondrocyte catabolic responses to LMW-HA and HMGB1 were observed in chondrocytes in response to not only exogenous LMW-HA and rHMGB1 (Figure 2 and 4), but also endogenously produced LMW-HA and HMGB1 via hyal2 and HMGB1 transfection (Figure 3 and 5). The concentrations of exogenous LMW-HA (100 µg/ml) and the endogenously produced degraded HA from the chondrocytes with enforced expression of hyal2 (about 1.25 ng/ml) were in the physiological range, since the in vivo degraded HA concentration from synovial fluid of both rheumatoid arthritis (RA) and OA patients was between 0.17 to 1.32 mg/ml (48). The concentration of endogenously produced HMGB1from the chondrocytes with forced expression of HMGB1 was not quantitatively determined here. The level of rHMGB1 (10 µg/ml) applied in our in vitro study was based on the concentration previously used in chondrocytes, which was higher than the in vivo concentration of HMGB1 in OA joints (nanogram/ml range) (49). However, up to 10 µg/ml of HMGB1 in synovial fluid of RA patients has been observed (50).
This study examined cartilages and chondrocytes of knockout mice to discover consequences of loss of function. One limitation of the work done is that we did not isolate the knee articular cartilages from adult mice, due to small yield in numbers of mature chondrocytes in knees (i.e., <1000 primary cells/mouse per pair of isolated knees) (50). Instead, we assessed immature knee chondrocytes, providing >25-fold gain in efficiency of chondrocyte isolation from the mouse knee, but presenting limitations due to lack of mouse epiphyseal closure. Since partial inclusion of epiphyseal with articular chondrocytes cannot be ruled out, this limitation was addressed by complementing studies of immature mouse chondrocytes with analyses of mature mouse articular femoral head cartilage explants.
We conclude that TLR2, TLR4 and their adaptor molecule MyD88 are central to chondrocyte matrix catabolism and chondrocyte hypertrophy in response to the endogenous TLR2/TL4 ligands LMW-HA and HMGB1. Our results support a novel model of innate immunity as significant regulators of chondrocyte differentiation and cartilage catabolic remodeling and repair responses, activated by multiple endogenous TLR ligands that arise in the joint during OA progression. Examples of such endogenous TLR2 andTLR4 ligands, other than those tested, include fibronectin type III repeat extra domain A (Fn-EDA), group V secretory phospholipase A2 (sPLA2) and free fatty acids (51–53). Our results indicate that need for teasing out the in vivo roles of TLR2/TLR4, and of MyD88, in the progression of OA.
Acknowledgments
Studies supported by the Research Service of the Department of Veterans Affairs and NIH grants AR1067966 (RLB), AR54135, PAG07996 (RT)
References
- 1.Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 2.Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005;52:144–154. doi: 10.1002/art.20748. Erratum in: Arthritis Rheum. 2006;54:2320. [DOI] [PubMed] [Google Scholar]
- 3.Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J Immunol. 2006;175:8296–8302. doi: 10.4049/jimmunol.175.12.8296. [DOI] [PubMed] [Google Scholar]
- 4.Cecil DL, Terkeltaub R. Transamidation by transglutaminase 2 transforms S100A11 Calgranulin into a catabolic cytokine for chondrocytes. J. Immunol. 2008;180:8378–8385. doi: 10.4049/jimmunol.180.12.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 6.Borzì RM, Mazzetti I, Marcu KB, Facchini A. Chemokines in cartilage degradation. Clin Orthop Relat Res. 2004;427 Suppl:S53–S61. doi: 10.1097/01.blo.0000143805.64755.4f. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Paul-Clark MJ, McMaster SK, Sorrentino R, Sriskandan S, Bailey LK, Moreno L, Ryffel B, Quesniaux VF, Mitchell JA. Toll-like receptor 2 is essential for the sensing of oxidants during inflammation. Am J Respir Crit Care Med. 2009;179:299–306. doi: 10.1164/rccm.200707-1019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wikén M, Grunewald J, Eklund A, Wahlström J. Higher Monocyte Expression of TLR2 and TLR4, and Enhanced Pro-inflammatory Synergy of TLR2 with NOD2 Stimulation in Sarcoidosis. J Clin Immunol. 2009;29:78–89. doi: 10.1007/s10875-008-9225-0. [DOI] [PubMed] [Google Scholar]
- 10.Curtiss LK, Tobias PS. Emerging role of toll-like receptors in atherosclerosis. J Lipid Res. 2008 Nov 1; doi: 10.1194/jlr.R800056-JLR200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobacz K, Sunk IG, Hofstaetter JG, Amoyo L, Toma CD, Akira S, Weichhart T, Saemann M, Smolen JS. Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of toll-like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum. 2007;56:1880–1893. doi: 10.1002/art.22637. [DOI] [PubMed] [Google Scholar]
- 12.Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY, Oh HJ, Kim HY. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 13.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. 2005;174:5016–5023. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad R, Sylvester J, Zafarullah M. MyD88, IRAK1 and TRAF6 knockdown in human chondrocytes inhibits interleukin-1-induced matrix metalloproteinase-13 gene expression and promoter activity by impairing MAP kinase activation. Cell Signal. 2007;19:2549–2457. doi: 10.1016/j.cellsig.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Sacre SM, Andreakos E, Kiriakidis S, Amjadi P, Lundberg A, Giddins G, Feldmann M, Brennan F, Foxwell BM. The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am J Pathol. 2007;170:518–525. doi: 10.2353/ajpath.2007.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7:1271–1285. doi: 10.1016/j.intimp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Venable RO, Stoker AM, Cook CR, Cockrell MK, Cook JL. Examination of synovial fluid hyaluronan quantity and quality in stifle joints of dogs with osteoarthritis. Am J Vet Res. 2008;69:1569–1573. doi: 10.2460/ajvr.69.12.1569. [DOI] [PubMed] [Google Scholar]
- 19.Attur M, Dave MN, Akamatsu M, Nakagawa N, Miki J, Yang H. Differential expression of high mobility group protein in human normal and arthritic cartilage; functional genomic analysis. Trans Orthop Rse Soc. 2003;28:18. (abstract) [Google Scholar]
- 20.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 21.Knudson CB, Knudson W. Hyaluronan and CD44: modulators of chondrocyte metabolism. Clin Orthop Relat Res. 2004;427 Suppl:S152–S162. [PubMed] [Google Scholar]
- 22.Iacob S, Knudson CB. Hyaluronan fragments activate nitric oxide synthase and the production of nitric oxide by articular chondrocytes. Int J Biochem Cell Biol. 2006;38:123–133. doi: 10.1016/j.biocel.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFkappaB and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2006;281:17952–17960. doi: 10.1074/jbc.M602750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Yamada S, Maruyama I. HMGB1, a novel inflammatory cytokine. Clin Chim Acta. 2007;375:36–42. doi: 10.1016/j.cca.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13:632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O'Keefe RJ, Zuscik M, Chen D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2007;434:644–648. doi: 10.1038/nature03369. Erratum in: Nature. 2007;446:102. [DOI] [PubMed] [Google Scholar]
- 31.Chow G, Knudson CB, Knudson W. Expression and cellular localization of human hyaluronidase-2 in articular chondrocytes and cultured cell lines. Osteoarthritis Cartilage. 2006;14:849. doi: 10.1016/j.joca.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 33.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 34.Dowthwaite GP, Edwards JC, Pitsillides AA. An essential role for the interaction between hyaluronan and hyaluronan binding proteins during joint development. J Histochem Cytochem. 1998;46:641–651. doi: 10.1177/002215549804600509. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 36.Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006;3:e17. doi: 10.1371/journal.pmed.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–392. [PubMed] [Google Scholar]
- 38.Wähämaa H, Vallerskog T, Qin S, Lunderius C, LaRosa G, Andersson U, Harris HE. HMGB1-secreting capacity of multiple cell lineages revealed by a novel HMGB1 ELISPOT assay. J Leukoc Biol. 2007;81:129–136. doi: 10.1189/jlb.0506349. [DOI] [PubMed] [Google Scholar]
- 39.Palmblad K, Sundberg E, Diez M, Söderling R, Aveberger AC, Andersson U, Harris HE. Morphological characterization of intra-articular HMGB1 expression during the course of collagen-induced arthritis. Arthritis Res Ther. 2007;9:R35. doi: 10.1186/ar2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi N, Yoshida K, Ito T, Tsuda M, Mishima Y, Furumatsu T, Ronfani L, Abeyama K, Kawahara K, Komiya S, Maruyama I, Lotz M, Bianchi ME, Asahara H. Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol Cell Biol. 2007;27:5650–5663. doi: 10.1128/MCB.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility groupB1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 42.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–4811. [PubMed] [Google Scholar]
- 43.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol. 2008;180:5067–5074. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 45.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 46.Urbonaviciute V, Fürnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 48.Hamada T, Torikai M, Kuwazuru A, Tanaka M, Horai N, Fukuda T, Yamada S, Nagayama S, Hashiguchi K, Sunahara N, Fukuzaki K, Nagata R, Komiya S, Maruyama I, Fukuda T, Abeyama K. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 2008;58:2675–2685. doi: 10.1002/art.23729. [DOI] [PubMed] [Google Scholar]
- 49.Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furie R, Chiorazzi N, Tracey KJ, Andersson U, Harris HE. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002;46:2598–2603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- 50.Johnson KA, van Etten D, Nanda N, Graham RM, Terkeltaub RA. Distinct transglutaminase 2-independent and transglutaminase 2-dependent pathways mediate articular chondrocyte hypertrophy. J Biol Chem. 2003;278:18824–18832. doi: 10.1074/jbc.M301055200. [DOI] [PubMed] [Google Scholar]
- 51.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 52.Kikawada E, Bonventre JV, Arm JP. Group V secretory PLA2 regulates TLR2-dependent eicosanoid generation in mouse mast cells through amplification of ERK and cPLA2alpha activation. Blood. 2007;110:561–567. doi: 10.1182/blood-2006-10-052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]