Summary
The establishment of Drosophila embryonic dorsal-ventral (DV) polarity relies on serine proteolytic activity in the perivitelline space between the embryonic membrane and the eggshell [1]. Gastrulation Defective cleaves and activates Snake, which processes and activates Easter, which cleaves Spätzle to form the activating ligand for the Toll receptor. Ventral restriction of ligand formation depends on the Pipe sulfotransferase, which is expressed in ventral cells of the follicular epithelium surrounding the developing oocyte [2]. Pipe modifies components of the developing eggshell to produce a ventral cue embedded in the vitelline membrane [3]. This ventral cue is believed to promote one or more of the proteolysis steps in the perivitelline space. By examining the processing of transgenic, tagged versions of the perivitelline proteins during DV patterning we find that the proteolysis of Easter by Snake is the first Pipe-dependent step and therefore the key ventrally-restricted event in the protease cascade. We also find that Snake and Easter associate together in a complex in both wild-type and pipe mutant-derived embryos. This observation suggests a mechanism in which the sulfated target of Pipe promotes a productive interaction between Snake and Easter, perhaps by facilitating conformational changes in a complex containing the two proteins.
Results and Discussion
Pipe function is required for the processing of Easter and Spätzle but not for the processing of GD or Snake
To determine the first step in the protease cascade at which Pipe exerts its effects on the dorsal group serine protease cascade, we generated functional versions of Gastrulation defective (GD), Snake, Easter and Spätzle, tagged at their carboxy termini with either the enhanced M6 version of GFP [4] or with a peptide tag consisting of three tandem copies of the HA epitope from Influenza Hemagglutinin [5]. When expressed in the germline of females homozygous for their corresponding dorsal group mutations, each of the tagged proteins was capable of restoring lateral and/or ventral pattern elements to progeny embryos that would otherwise have been completely dorsalized (data not shown).
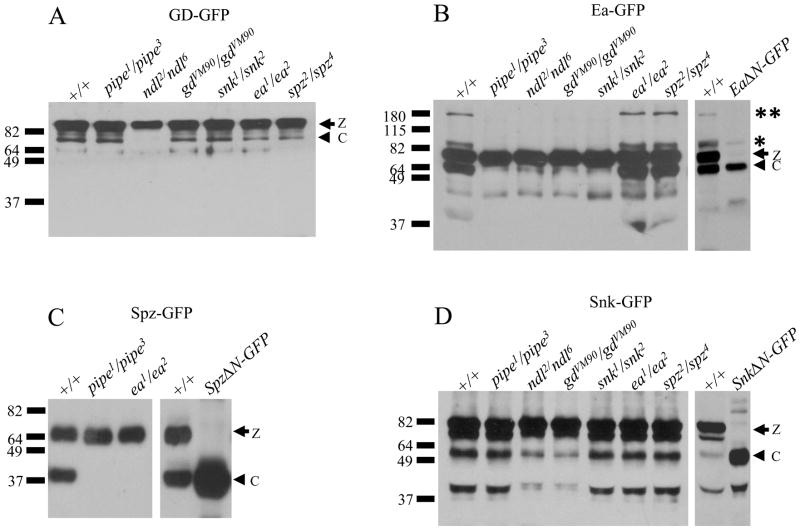
The germline-specific Gal4 driver, nos-Gal4:VP16 [6], was used to express each of the tagged dorsal group proteins in females carrying loss-of-function alleles of various dorsal group genes. Extracts prepared from blastoderm stage progeny embryos were subsequently subjected to SDS-PAGE followed by Western blot analysis using antibodies specific for either GFP or for the HA epitope. Western blot analysis of embryonic extracts derived from wild-type females expressing GD-GFP demonstrated the presence of an unprocessed zymogen form of the transgenic protein of 90 kD, as well as a 73 kD species corresponding to a processed form of the protein (Figure 1A). The processed form of GD-GFP was present in embryos from females carrying loss-of-function alleles of pipe, gastrulation defective (gd), snake (snk), easter (ea) and spätzle (spz), but not in the progeny of females lacking nudel (ndl) function. These results are consistent with the observations of Lemosy et al., 2001 [7], who detected a 46 kD putative processed form of HA-tagged GD in embryos from wild-type and pipe mutant females, but not in embryos from GD-HA-expressing ndl mutant females. Thus, the Nudel protease domain appears to be necessary for the processing of GD, but neither pipe nor any of the other dorsal group proteins are required. It is presently unclear whether the Nudel protease acts directly to mediate the processing of GD [7], or alternatively, if the function of Nudel in the modification and maturation of the eggshell [8] provides a permissive environment for GD proteolysis. Although GD has also been observed to undergo a Snake dependent cleavage event in cultured cells [7, 9], the snk alleles that we used in this analysis carry stop codons near the N-terminus of the Snake open reading frame [10] showing that Snake is not required for GD processing in vivo.
Figure 1. Processing of Easter and Spätzle, but not of Gastrulation Defective and Snake, is dependent upon Pipe activity.
(A) Processing of GD-GFP in embryos from females expressing GD-GFP in various dorsal group mutant backgrounds. Maternal dorsal group phenotypes are shown at the top of each lane. The position of molecular weight markers (in kD) is shown at left. In this and all subsequent panels showing the results of Western blot analysis, the arrow followed by “z” indicates the position of the unprocessed, zymogen form of the fusion protein while the arrowhead followed by “c” indicates the position of the cleaved form of the fusion protein.
(B) Processing of Ea-GFP in embryos from females expressing GD-GFP in various dorsal group mutant backgrounds. Note the presence of bands representing the Ea-GFP-X and Ea-GFP-XX forms of the protein, corresponding to processed forms of the protein complexed with Spn27A (marked with a single asterix and two asterixes, respectively). The identification of the 63 kD species as the cleaved form of Ea-GFP, not bound to Spn27A, is confirmed by its co-migration with the EaΔN-GFP construct (far right lane), which corresponds to the putative processed catalytic region of Easter fused to GFP.
(C) Processing of Spz-GFP in embryos from females expressing Spz-GFP in wildtype, pipe, and ea mutant backgrounds. The identity of the cleaved form of Spz-GFP is confirmed by its co-migration with SpzΔN-GFP, which corresponds to the putative Toll-binding domain of Spätzle, fused to GFP (far right lane).
(D) Processing of Snk-GFP in embryos from females expressing Snk-GFP in various genetic backgrounds. The identity of the processed form of Snake-GFP is confirmed by its co-migration with SnkΔN-GFP, which corresponds to the catalytic domain of Snake, fused to GFP (far right lane).
Embryos produced by wild-type females expressing Easter-GFP (Ea-GFP) contained an unprocessed zymogen form of the transgenic protein of 78 kD, as well as a 63 kD species corresponding to the cleaved, activated form of the protein (Figure 1B). The ability to visualize the lower molecular weight, processed form of Ea-GFP contrasts with the situation for wild-type endogenous Easter protein in which all processed Easter is observed in a high molecular weight species of 80–85 kD, Easter-X [11], which represents a covalently-linked complex between activated Easter and the serine protease inhibitor Serpin27A [12, 13]. In wild-type embryos, processed Easter rapidly forms a covalent linkage with Spn27A, and the proportion of the 35 kD processed form of Easter that is not bound to Spn27A is undetectable on Western blots. The ability to detect cleaved Ea-GFP that is not complexed with Spn27A in our experiments is likely due to the high levels of Ea-GFP expressed under the control of nos-Gal4:VP16, and may also be enhanced by increased stability of GFP-tagged protein. Embryos from wild-type females expressing Ea-GFP also contained two higher molecular weight species detected with anti-GFP. One of these species has a mass consistent with Ea-GFP-X (see position of asterix), while the other species, referred to here as Ea-GFP-XX, has a much larger molecular weight of approximately 180 kD (paired asterixes). Both of these higher molecular weight species also contain Spn27A (data not shown).
The processed form of Ea-GFP was not detected in embryos from females mutant for pipe, ndl, gd, or snk, nor were either of the Spn27A-associated versions of processed Ea-GFP. In contrast, all three forms of processed Ea-GFP were present in the extracts of embryos from females mutant for ea and spz. These results agree with those of LeMosy [14], who showed that Easter processing is dependent upon the presence of pipe activity. Further, since active processed Easter is required for the cleavage of Spätzle, we expected that a tagged version of Spätzle (Spz-GFP) would fail to exhibit processing in embryos from females mutant for either pipe or easter. This result, shown in Figure 1C, is consistent with results of Morisato and Anderson [15] who showed that endogenous Spätzle protein is not processed in embryos produced by females homozygous for mutations in any of the genes that act upstream of Toll in the pathway, including pipe and easter.
In contrast to Ea-GFP and Spz-GFP, both the 80 kD Snk-GFP zymogen and the 58 kD processed form of Snk-GFP were detected in all genetic backgrounds tested (Figure 1B). Strikingly, the levels of processed protein in embryos from pipe mutant mothers were indistinguishable from those produced by wild-type-derived embryos. In contrast, the levels of processed Snk-GFP were detectably lower in embryos from females mutant for either gd and ndl. This is consistent with studies carried out in cell culture in which co-expression of Snake and Gastrulation Defective resulted in processing of Snake [7, 9]. We also observed a similar low level of processing in embryos from wild-type females expressing a version of Snk-GFP in which the amino acid sequence of the putative zymogen cleavage site, serine-valine-proline-leucine, was converted to serine-valine-lysine-lysine (data not shown). Snake carrying this alteration fails to undergo cleavage by Gastrulation Defective when the two proteins are expressed together in Drosophila S2 tissue culture cells [7]. This suggests that the low level of Snake cleavage that occurs in the absence of Gastrulation Defective and Nudel is due to another protease in embryos that is capable of processing Snake at a nearby site.
Pipe activity determines the level of Easter and Spätzle processing
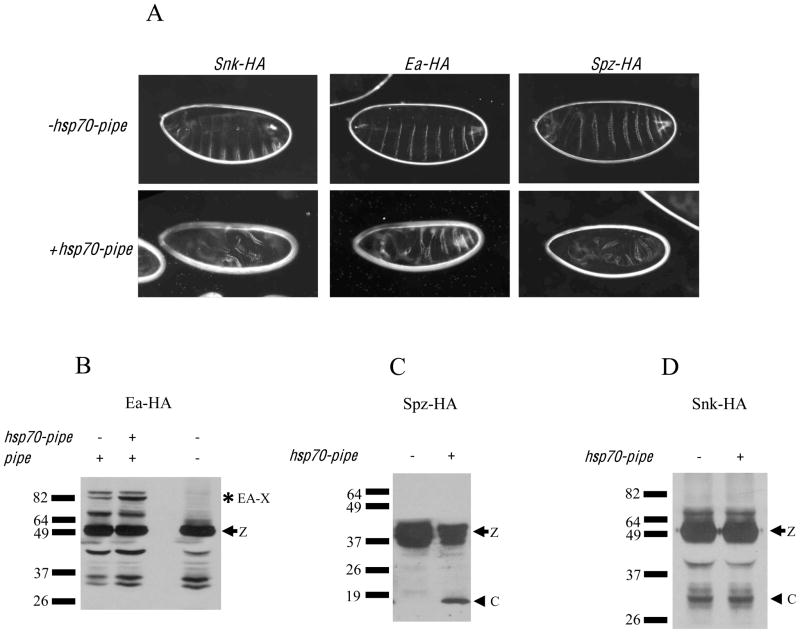
Our results indicate that Pipe activity is required for the processing of Easter and consequently for the processing of Spätzle, but not for the processing of Gastrulation Defective and Snake. If the processing of Easter by Snake is the initial Pipe-dependent, ventrally-restricted processing event then the processing of Easter and Spätzle, but not that of Gastrulation Defective or Snake, should be enhanced by an increase in the level of Pipe activity. To test this, we used the female germline-specific Gal4 driver, pCOG-Gal4:VP16 [6], to express HA-tagged versions of Snake, Easter and Spätzle together with hsp70-pipe, a transgene that is expressed constitutively throughout the follicle cell layer [16]. Embryos from females that expressed Snake-HA (Snk-HA), Easter-HA (Ea-HA), or Spätzle-HA (Spz-HA) together with hsp70-pipe exhibited an increase in their relative level of ventralization in comparison to the phenotypes of embryos from females expressing these transgenes in the absence of hsp70-pipe (Figure 2A). As shown in Figure 2B and Figure 2C, higher levels of processing of Ea-HA and Spz-HA were observed in embryonic extracts derived from females expressing the transgenes together with hsp70-pipe. The enhancement in Ea-HA processing was detected in the form of increased levels of the 82 kD Ea-GFP-X. In these experiments, Ea-HA expressed under the control of pCOG-Gal4:VP16 was present at much lower levels than Ea-GFP expressed with nos-Gal4:VP16. Moreover, Ea-HA appeared to be unstable, in comparison to Ea-GFP, as indicated by the presence of multiple Ea-HA degradation products (Figure 2B). Presumably, sufficient Spn27A was present to complex with all of the EaΔN-HA that was produced. Similarly, the ability to detect processed Spz-GFP, when expressed under the control of nos-Gal4:VP16 (Figure 1C), but not Spz-HA expressed under the control of pCOG-Gal4:VP16 (Figure 2A), presumably resulted from the lower levels of Spz-HA expression directed by pCOG-Gal4:VP16 and from decreased stability of the HA-tagged protein.
Figure 2. Increased Pipe expression leads to an increase in the processing of Easter and Spätzle, but not to increased processing of Snake.
(A) Cuticle preparations of embryos from females expressing Snk-HA (left column), Ea-HA (middle column), and Spz-HA (right column), in the absence (top row) or presence (bottom row) of hsp70-pipe. Note that for each transgene, expression in females together with the hsp7-pipe transgene leads to the production of embryos with a more strongly ventralized phenotype.
(B) Processing of Ea-HA in embryos from females expressing the fusion protein in an otherwise wild-type background (lane 1 at left), in a wild-type background together with the hsp70-pipe transgene (lane 2) and in a pipe mutant background (pipe genotype = pipe1/pipe3). The arrow followed by “z” indicates the position of the zymogen form of the protein. Note that the increase in Ea-HA processing observed in the presence of hsp70-pipe is detected as increased amounts of Ea-HA-X (asterix). No Ea-HA-X is detected in extracts of embryos from pipe mutant mothers.
(C) Processing of Spz-HA in embryos from females expressing the fusion protein in the absence (left lane) or presence (right lane) of the constitutively expressed hsp70-pipe transgene. The arrow followed by “z” indicates the position of the zymogen form of the protein, while the arrowhead followed by “c” indicates the position of the cleaved form of the protein.
(D) Processing of Snk-HA in embryos from females expressing the fusion protein in the absence (left lane) or presence (right lane) of the constitutively expressed hsp70-pipe transgene. The position of molecular weight markers, in kD, are shown at left. The arrow followed by “z” indicates the position of the zymogen form of the protein, while the arrowhead followed by “c” indicates the position of the cleaved form of the protein.
In contrast to the situation for Ea-HA and Spz-HA, the levels of processed Snk-HA were not influenced by the presence or absence of hsp70-pipe in mother flies (Figure 2D). Together, these results support our conclusion that the first step in the dorsal group serine protease cascade that is regulated by pipe, and therefore restricted to the ventral side of the perivitelline space, is the processing of Easter by Snake.
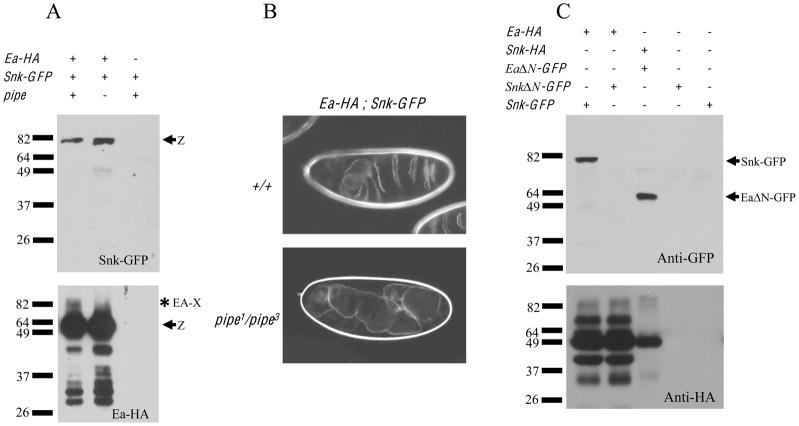
Easter-HA and Snake-GFP are present in a complex
To test whether the role of Pipe in DV patterning is to facilitate the formation of a complex between Snake and Easter, Ea-HA and Snk-GFP were expressed together in wild-type females using the nos-Gal4:VP16 driver. When an anti-HA antibody was used to precipitate Ea-HA from wild-type-derived embryos (Figure 3A, lower panel), Snk-GFP was observed to co-precipitate (Figure 3A, upper panel). In addition to the Ea-HA zymogen, we also detected the precipitation of a larger molecular weight species, likely to correspond to Ea-HA-X. Ea-HA and Snk-GFP also co-precipitated from extracts of embryos produced by pipe mutant females. In this instance, no Ea-HA-X appeared to be precipitated, consistent with the absence of Ea-HA activation. We note that higher levels of Ea-HA and Snk-GFP precipitated from pipe-derived embryos than from wild-type-derived embryos. Additionally, a lower molecular weight protein, likely to correspond to the cleaved form of Snk-GFP, was detected in precipitates from pipe-derived embryos. The activated proteases have been reported to be de-stabilized following zymogen cleavage [7]. In the pipe mutant background, where Easter-HA is not processed, higher levels of unprocessed and processed Snake-GFP would be expected to be present. Moreover, in wild type embryos the precipitated Ea-HA-X, covalently bound to Spn27A, would be unable to bind Snake-GFP, thus decreasing the amount of co-precipitated Snk-GFP in the wild-type versus the pipe mutant-derived background. Importantly, while wild-type females expressing Ea-HA and Snk-GFP produced ventralized embryos, pipe mutant females expressing the two proteins produced dorsalized progeny (Figure 3B, bottom panel) despite the two proteins being present in a complex.
Figure 3. Co-precipitation of Snake and Easter is not dependent upon Pipe activity.
(A) Extracts from embryos produced by females expressing Snk-GFP either with (left and middle lanes) or in the absence of Ea-HA (right lane) were subjected to immune precipitation with anti-HA. Extracts were divided into two portions and subjected to western blot analysis with anti-HA (to detect the precipitated Ea-HA)(bottom panel) and with anti-GFP (to detect the co-precipitating Snk-GFP)(top panel). Note that Snk-GFP co-precipitated with Ea-HA from extracts of embryos from otherwise wild-type (left panel) or pipe1/pipe3 females (middle panel).
(B) Cuticle preparations of embryos from females expressing Ea-HA together with Snk-GFP in otherwise wild-type (top panel) or pipe1/pipe3 females (bottom panel). Note that despite the fact that Ea-HA and Snk-GFP co-precipitate in embryos from pipe mutant mothers (see Figure 3A), the embryos are dorsalized.
(C) Extracts of embryos from otherwise wild-type female expressing various fusion proteins (indicated by +’s at top) were subjected to immune precipitation using anti-HA. Immune precipitates were subjected to Western blot analysis using anti-GFP to detect co-precipitating proteins. The positions of Snk-GFP and EaΔN-GFP are shown at right. Note that co-precipitation of Snake by Easter required the presence of the Snake prodomain (compare lanes 1 and 2) but not the prodomain of Easter (lane 3). Bottom panel: lanes 1 and 2 show Ea-HA, while lane 3 shows Snk-HA.
Finally, we carried out additional co-precipitation experiments to determine which segments of Easter and Snake were necessary for the two proteins to be present in a complex. Easter lacking its prodomain (EaΔN-GFP) was present in a complex with Snk-HA (Figure 3C, lane 3), while Snake lacking its prodomain (SnkΔN-GFP) was not in a complex with Ea-HA (Figure 3C, lane 2). These results indicate that the Easter catalytic domain and the Snake prodomain are required for this interaction, while the Easter prodomain region is dispensible.
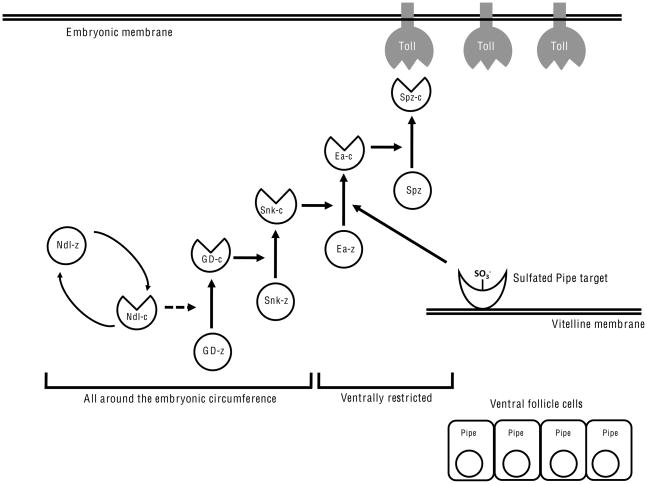
A single protein isoform derived from the pipe locus is essential for embryonic DV polarity [17]. The expression of this isoform in ventral follicle cells of the egg chamber leads to the sulfation of eggshell components that become incorporated into the eggshell nearby their site of synthesis [3]. These sulfated species facilitate the formation of the Toll ligand adjacent to the ventral side of the embryo. Our results indicate that the processing of Easter by Snake is the event that is regulated by the sulfated target(s) of Pipe. Importantly, this result indicates that the proteolytic pathway leading to Spätzle activation is not linear. Rather, it is branched, with both the Nudel/GD dependent processing of Snake and the Pipe-dependent facilitation of Easter cleavage by processed Snake being distinct and essential activating inputs (Figure 4).
Figure 4.
Functional interactions between the perivitelline space-localized components of the dorsal group signal transduction pathway. z and c refer to the zymogen and cleaved, activated forms of Nudel, GD, Snake, Easter and Spätzle. Vertical arrows indicate processing events in which precursor proteins are converted to their active products. Horizontal and diagonal arrows indicate the effectors required for each of the processing events. The curved arrows that connect Ndl-z and Ndl-c indicate that Ndl undergoes activation by autoprocessing. The dotted line indicates that while processed Ndl is required for the formation of processed GD, it is unknown whether Ndl directly processes GD. Other structures and molecules are as labeled in the figure. Note that the pathway leading to processed Spz is branched. Snake processing and Pipe-target mediated facilitation of Easter processing by Snake are independently required for the formation of the Toll ligand.
Three likely regulatory mechanisms could control the ventral processing of Easter by Snake. First, the sulfated Pipe target could act to localize Snake and/or Easter to the ventral side of the embryo. This seems unlikely, as none of the GFP-tagged proteins examined here were enriched in the ventral perivitelline space of embryos from wild-type females (data not shown).
A second possible control mechanism would involve a serine protease inhibitor of Snake protein whose effects were counteracted on the ventral side of the embryo by the sulfated target of Pipe. A series of dominant ventralizing alleles of ea have been identified which carry mutations near the active site of the catalytic region of the protein [18]. These are considered to perturb the mutant proteins’ abilities to interact with Spn27A [19]. However, no dominant ventralizing mutant alleles of snake have been isolated, which suggests that a similar dedicated protease inhibitor does not exist for Snake.
Based on the results reported here, we favor a third scenario in which Snake and Easter, and perhaps other components of the dorsal group protease cascade, form a diffusible complex ine the perivitelline space. We hypothesize that Easter cannot be processed until the complex encounters the ventrally localized sulfated target of Pipe, at which time the complex undergoes a conformational change that facilitates the cleavage of Easter by Snake, perhaps by exposing the zymogen cleavage target site of Easter or by bringing Snake and Easter into juxtaposition. The observation that transgenically-expressed SnkΔN-GFP does not form a stable complex with Easter-HA may seem inconsistent with a model in which the sulfated Pipe target modulates the interaction between activated Snake and Easter zymogen. However, a disulfide bond is believed to link the prodomain and catalytic regions of full-length Snake [20]. After Snake zymogen processing, the two Snake protein fragments are predicted to remain linked to one another. As we have shown, co-precipitation between Easter and Snake requires the Snake prodomain (Figure 3C). Additionally, we have detected co-precipitation of processed Snake by Easter (Figure 3A). Presumably there exists a population of Easter zymogen that is complexed to processed Snake via the cleaved Snake prodomain, which remains disulfide bonded to the catalytic domain. This is likely the complex that is acted upon by the sulfated Pipe target.
Although the dependence of Spätzle cleavage upon Pipe may be indirect and arise solely through the Pipe-dependent processing of its activating protease, we cannot rule out the possibility that Spätzle is also present in a complex together with Snake and Easter, with a Pipe dependent conformational change in the complex also acting directly to enhance Spätzle cleavage by Easter, perhaps by facilitating an interaction between processed Easter and the precursor form of Spätzle. Ongoing experiments are directed towards determining the extent to which conformational changes affecting components of the perivitelline dorsal group proteins, brought about by Pipe action, lead to the processing of Easter and Spätzle and, in turn, to the formation of the Drosophila embryonic DV axis.
Experimental Procedures
Drosophila strains and maintenance
All stocks were maintained employing standard conditions and procedures. Details on fly stocks used in this study are provided in the Supplemental Experimental Procedures.
Plasmid constructs
Details on the generation of expression constructs used in this study are provided in the Supplemental Experimental Procedures. Transgenic fly lines carrying insertions of the various expression constructs were generated by conventional P-element mediated transformation [21]. In some cases, microinjections were performed at GenetiVision, Inc. (Houston, Texas).
Western blotting and co-immunoprecipitation studies
For Western blot analysis of fusion proteins, eggs were collected on yeasted apple juice/agar plates. To achieve uniformity in studies of fusion proteins, precisely staged embryos collected by hand were used in the preparation of embryonic extracts. For each embryo extract, a volume corresponding to exactly 100 μg of protein was subjected to SDS polyacrylamide gel electrophoresis, followed by electroblotting to nitrocellulose membranes, which were subjected to Western blot analysis with detection by either monoclonal anti-GFP or anti-HA epitope.
For co-immunoprecipitation studies embryos were hand staged, collected, and homogenized as for Western blot analysis of extracts. Following determination of protein concentrations, a volume of extract containing 100 μg protein was incubated at 4°C overnight together with rabbit polyclonal anti-HA antibody. Antibody/protein complexes were incubated together with protein G-agarose beads for 2 hours and collected by centrifugation. Precipitates were then divided into two aliquots and subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting with monoclonal anti-GFP and monoclonal anti-HA, respectively, as described above. More detailed descriptions of the Western blotting and co-Immunoprecipitation studies are provided in the Supplemental Experimental Procedures.
Examination of embryonic phenotypes
For the examination of embryonic phenotypes, larval cuticles were prepared according to Van der Meer (1977)[22]. Classification of embryonic Dorsal/Ventral phenotypes was carried out as described in Zhang et al., 2009 [17].
Supplementary Material
Acknowledgments
We are grateful to Kathryn Anderson, Andrea Brand, Robert DeLotto, Carl Hashimoto, Ellen LeMosy, Donald Morisato, Jean-Marc Reichhart and Pernille Rorth for providing Drosophila stocks and/or DNA clones. This work was supported by a grant from the National Institutes of Health (GM077337).
Footnotes
Supplemental information includes Supplemental Experimental Procedures and can be found with this article online at xxxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo: Shaping and transducing a morphogen gradient. Curr Biol. 2005;15:R887–R899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–481. doi: 10.1016/s0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Stevens LM, Stein D. Sulfation of eggshell components by Pipe defines dorsal-ventral polarity in the Drosophila embryo. Curr Biol. 2009;19:1200–1205. doi: 10.1016/j.cub.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuldt AJ, Adams JH, Davidson CM, Micklem DR, Haseloff J, St Johnston D, Brand AH. Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev. 1998;12:1847–1857. doi: 10.1101/gad.12.12.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly ML, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–768. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 6.Rorth P. Gal4 in the Drosophila female germ line. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 7.LeMosy EK, Tan YQ, Hashimoto C. Activation of a protease cascade involved in patterning the Drosophila embryo. Proc Nat Acad Sci USA. 2001;98:5055–5060. doi: 10.1073/pnas.081026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeMosy EK, Hashimoto C. The Nudel protease of Drosophila is required for eggshell biogenesis in addition to embryonic patterning. Dev Biol. 2000;217:352–361. doi: 10.1006/dbio.1999.9562. [DOI] [PubMed] [Google Scholar]
- 9.Dissing M, Giordano H, DeLotto R. Autoproteolysis and feedback in a preotease cascade directing Drosophila dorsal-ventral cell fate. EMBO J. 2001;20:2387–2393. doi: 10.1093/emboj/20.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith C, Giordano H, DeLotto R. Mutational analysis of the Drosophila snake protease: an essential role for domains within the proenzyme polypeptide chain. Genetics. 1994;136:1355–1365. doi: 10.1093/genetics/136.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misra S, Hecht P, Maeda R, Anderson KV. Positive and negative regulation of Easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development. 1998;125:1261–1267. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto C, Kim DR, Weiss LA, Miller JW, Morisato D. Spatial regulation of developmental signaling by a serpin. Dev Cell. 2003;5:945–950. doi: 10.1016/s1534-5807(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 13.Ligoxygakis P, Roth S, Reichhart JM. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr Biol. 2003;13:2097–2102. doi: 10.1016/j.cub.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 14.LeMosy EK. Spatially dependent activation fo the patterning protease, Easter. FEBS Letters. 2006;580:2269–2272. doi: 10.1016/j.febslet.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisato D, Anderson KV. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76:677–688. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- 16.Sen J, Goltz JS, Konsolaki M, Schüpbach T, Stein D. Windbeutel is required for function and correct subcellular localization of the Drosophila patterning protein Pipe. Development. 2000;127:5541–5550. doi: 10.1242/dev.127.24.5541. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Zhu X, Stevens LM, Stein D. Distinct functional specificities are associated with protein isoforms encoded by the Drosophila dorsal-ventral patterning gene pipe. Development. 2009;136:2779–2789. doi: 10.1242/dev.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin YS, Anderson KV. Dominant and recessive alleles of the Drosophila easter gene are point mutations at conserved sites in the serine protease catalytic domain. Cell. 1990;60:873–881. doi: 10.1016/0092-8674(90)90100-s. [DOI] [PubMed] [Google Scholar]
- 19.Chang AJ, Morisato D. Regulation of Easter activity is required for shaping the Dorsal gradient in the Drosophila embryo. Development. 2002;129:5635–5645. doi: 10.1242/dev.00161. [DOI] [PubMed] [Google Scholar]
- 20.Smith CL, Giordano H, Schwartz M, DeLotto R. Spatial regulation of Drosophila Snake protease activity in the generation of dorsal-ventral polarity. Development. 1995;121:4127–4135. doi: 10.1242/dev.121.12.4127. [DOI] [PubMed] [Google Scholar]
- 21.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable elements. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer JM. Optical clean and permanent whole mount preparations for phase-contrast microscopy of cuticular structures of insect larvae. Dros Info Serv. 1977;52:160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.