Abstract
The purpose of the study is to screen the interactions of fourth generation fluoroquinolone-gatifloxacin with efflux pumps i.e. P-gp, MRP2 and BCRP. Mechanism of gatifloxacin interaction with efflux transporters may explain its acquired resistance. Such clarification may lead to the development of strategies to overcome efflux and enhance its bioavailability at target site. This process will aid in the reduction of dose volume, further eliminating the chances of systemic toxicity from topical gatifloxacin eye drops. MDCK cell lines transfected with the targeted efflux transporters were used for this study. [14C] Erythromycin was selected as a model substrate for P-gp and MRP2 whereas Hoechst 33342 was employed as a substrate for BCRP. Uptake and transport studies of these substrates were performed in the presence of gatifloxacin to delineate its interaction with efflux transporters. Further the efflux ratio in the presence of gatifloxacin was calculated from bidirectional transport studies. The concentration of [14C] erythromycin and Hoechst 33342 were measured using scintillation counter and fluorescence plate reader respectively. A concentration dependent inhibition effect in the presence of gatifloxacin was revealed on [14C] erythromycin uptake. The efflux ratio (BL-AP/AP-BL) of substrates was found to approach unity at higher gatifloxacin concentrations. Increased concentration of gatifloxacin did not elevate uptake of Hoechst 33342. All these studies were validated with known inhibitors as positive control. Uptake and transport studies support the hypothesis that gatifloxacin is a substrate for P-gp, MRP2 but not for BCRP. Possible interactions of gatifloxacin with P-gp and MRP2 may be a possible mechanism for acquired resistance of gatifloxacin. This information can be further extended to design prodrugs or formulations in order to prevent development of acquired resistance and improve therapeutic efficacy with its reduction in side effects.
Keywords: Gatifloxacin, Efflux transporters, P-glycoprotein, Multidrug resistance protein, Breast cancer resistance protein
1. Introduction
Fluoroquinolones are widely prescribed antimicrobials for prophylaxis and treatment of various ocular infections. These compounds possess broad spectrum bactericidal activity and concentration dependent effect (Hooper 1999a; 2000; Hooper and Wolfson 1993; Turnidge 1999). Depending on their range of activity, the agents are categorized into four different generations. Nalidixic acid was the first quinolone to be introduced. Since then, structural modifications have resulted in the development of later generations. The second and third generation drugs like ofloxacin and levofloxacin are mainly indicated in the treatment of bacterial keratitis (Baker et al. 1996; Leibowitz 1991). But the development of bacterial resistance against these drugs necessitated the introduction of fourth generation fluoroquinolones in combating ocular infections. These antibiotics were originally introduced for treating systemic infections (Goldstein et al. 1999; Saravolatz and Leggett 2003).
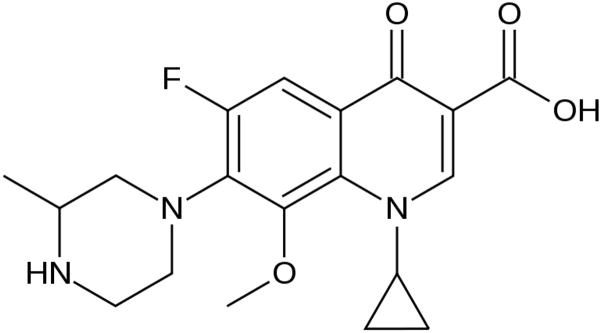
The fourth generation drug, gatifloxacin is approved for the treatment of acute sinusitis, chronic bronchitis, community acquired pneumonia and other respiratory infections. Gatifloxacin is a new 6-fluoro-8-methoxyquinolone that is two- to fourfold more active than the other quinolones (Ba et al. 2006). It shows increased activity against gram positive and gram negative bacteria. Furthermore, the drug also showed activity against atypical pathogens and anaerobic organisms (Bauernfeind 1997; Ednie et al. 1998). This expanded microbicidal activity makes it a potent drug in the treatment of ocular infections caused primarily by gram positive bacteria. Gatifloxacin inhibits both topoisomerase II (DNA Gyrase) and topoisomerase IV. Due to its dual site of action, two genetic mutations are required for the development of bacterial resistance unlike other fluoroquinolones which require only single mutation (Hooper 1999; Mather et al. 2002). This mechanism offers gatifloxacin an additional path to resist mutation based development of bacterial resistance. Its structure is outlined in Fig. 1. Methyl substituted piperazinyl group at C-7 position leads to increased stability, half life of the drug and improved pharmacokinetic and safety parameters over other fluoroquinolones. Moreover, absence of halide substitution at C-8 position reduces photosensitivity compared to other fluoroquinolones (Blondeau 1999; Domagala 1994; Lipsky and Baker 1999).
Fig 1.
Structure of gatifloxacin.
Gatifloxacin has a high intrinsic activity with minimum inhibitory concentration (MIC) of 0.1μg/ml against many bacterial species. Despite this expanded activity, the use of gatifloxacin in ocular infections is limited by systemic toxicity upon administration. Subtherapeutic concentrations in the corneal aqueous humor and iris-ciliary body can result from multi drug resistance (MDR) associated efflux pumps. These pumps expel antimicrobial agents out of cell, leading to suboptimal eradication of microbes. An escalating dose may result in systemic toxicity. Several efflux pumps have been identified in the eye. The ATP binding cassette (ABC) transporters derive their energy from the hydrolysis of ATP and utilize this energy to transport the drug molecules out of the cell, thus imparting drug resistance. Though these transporters are considered as drug-resistant pumps, they are expressed in many normal tissues suggesting their role in cellular transport of many endogenous substrates and therapeutic agents. (Gottesman et al. 2002). Several members of this family i.e. P-glycoprotein (P-gp/MDR1/ABCB1), multidrug resistance associated protein 2 (MRP2/ABCC2/cMOAT) and breast cancer resistance protein (BCRP/ABCG2/MXR) have been identified on the rabbit and human cornea (Dey et al. 2003; Karla et al. 2009; Karla et al. 2007). These efflux pumps can act either alone or synergistically contributing to multi drug resistance.
Understanding interaction of gatifloxacin with these efflux transporters is important to better delineate the mechanism of its acquired resistance which can lead to the development of novel strategies to enhance drug bioavailability at target site. This study can also aid in reduction of dose volume, further eliminating the chances of systemic toxicity from topical gatifloxacin eye drops.
2. Materials and Methods
2.1 Materials
Gatifloxacin was obtained from Bosche Scientific (New Brunswick, NJ, USA). MDCK transfected cell lines expressing P-gp, MRP2 and BCRP were generous gifts from Drs. A. Schinkel and P. Borst (Netherlands Cancer Institute, Amsterdam). [14C]Erythromycin (Specific activity: 51.3 mCi/mMol) was obtained from Moravek Biochemicals (Brea, CA, USA). Quinidine and MK-571 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Hoechst 33342, Ko-143, TrypLE™ Express, Dulbecco’s modified Eagle’s medium (DMEM) and ATP determination kit were obtained from Invitrogen (Carlsbad, CA, USA). Fetal Bovine Serum (FBS) was purchased from Atlanta biologicals (Lawrenceville, GA, USA). Culture flasks (75cm2 growth area), 12-well plates (3.8cm2 growth area per well), polyester transwells (pore size of 0.4μm and 12mm diameter), and 96-well plates (0.32cm2 growth area per well) were purchased from Corning Costar Corp. (Cambridge, MA, USA). All other chemicals were products of commercial grade and were obtained from Sigma Chemicals (St. Louis, MO, USA) and Fisher Scientific.
2.2 Cell culture
Madin-Darby canine kidney (MDCK) cell line is derived from dog renal epithelial cells. It is extensively used as a model to study membrane permeation (Irvine et al. 1999). The cell line has been transfected with human MDR1, MRP2 and BCRP genes to generate MDCK cells over expressing P-gp, MRP2 and BCRP respectively (Evers et al. 1997; Evers et al. 1998; Pavek et al. 2005). These transfected cell lines have been extensively employed to study drug efflux mechanisms (Guo et al. 2002; Tang et al. 2002). MDCKII-MDR1, MDCK-MRP2 and MDCK-BCRP cell lines of passage numbers 5–15, 5–15 and 5–10 respectively were used for our studies. Cells were cultured in T-75 flasks at 37°C in a humidified atmosphere using 5% CO2 and were maintained with DMEM medium supplemented with 10% fetal bovine serum (heat inactivated), 1% nonessential amino acids, 20 mM HEPES, 29 mM sodium bicarbonate and 100μg/ml of penicillin and streptomycin. The medium was changed every alternate day. Cells were allowed to reach 80–90% confluence which was confirmed with a microscope and then passaged using TrypLE™ Express solution. The cells were seeded at a density of 25,000/cm2 in 12-well tissue culture-treated plastic plates and collagen coated Transwell® inserts and at a density of 10,000/cm2 in 96-well plates. These cells were then allowed to grow for 5–7 days and used for further studies.
2.3 Preparation of drug solutions
[14C] Erythromycin was selected as a model substrate to study MDR1 and MRP2 mediated efflux (Dey et al. 2004; Hariharan et al. 2009) and Hoechst 33342 was employed as a model substrate for BCRP mediated efflux (Robey et al. 2009). Uptake of these substrates was quantified in the presence of various concentrations of Gatifloxacin (1μM-10mM). Specific inhibitors such as quinidine (75μM), MK-571 (100μM) and Ko-143 (10μM) were selected for MDR1, MRP2 and BCRP respectively to delineate interaction of efflux pumps with gatifloxacin.
2.4 Uptake studies
Uptake studies were performed in 12-well tissue culture-treated plates with confluent cells, usually 5–7 days post seeding. The medium was aspirated from wells and cells were washed (3 ×10 min) with 2ml of Dulbecco’s phosphate-buffered saline (DPBS) containing 130 mM NaCl, 2.5 mM KCl, 7.5 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgSO4, 5 mM glucose, and 20 mM HEPES at pH 7.4. Then 1ml of drug solutions (each containing 0.25 μCi/mL of [14C] Erythromycin) were added to the cells and incubated at 37°C for 30 min. Then, solutions were removed and uptake was terminated with ice-cold stop solution (200 mM KCl and 2 mM HEPES). Later, cells were lysed overnight at room temperature with 1 mL of 0.1% (v/v) Triton-X in 0.3 N sodium hydroxide solution. Aliquots (500μL) of the cell lysate were then added to scintillation vials containing 3 mL of scintillation cocktail (Fisher Scientific, Fair Lawn, NJ) and radioactivity was measured with a scintillation counter (Model LS-6500; BeckmanCounter, Fullerton, CA), which can measure [14C] compounds with an efficiency of 95%. Uptake in each well was normalized to protein content, estimated by the Bio-Rad protein estimation kit using bovine serum albumin as the standard.
2.5 Accumulation of Hoechst 33342
Uptake study with Hoechst 33342 was performed in 96 well plates 5–7 days post seeding. The culture medium was removed and cells were washed 3 times with DPBS buffer maintained at pH 7.4. Then cells were incubated for 15 min at 37°C with 100 μL of Hoechst 33342 (5μM) alone, in the presence of Ko-143 (10μM) and various concentrations of gatifloxacin. Solutions were then removed and uptake process was terminated with ice cold stop solution. Later, cells were lysed with 100 μL of 0.1% (v/v) Triton-X in 0.3 N sodium hydroxide solution. Intracellular accumulation of the dye was measured with a fluorescence spectrophotometer with excitation and emission filters set at 370 and 450nm respectively. Uptake in each well was normalized to the protein content.
2.6 Transport studies
Transport of [14C] Erythromycin was quantified in the presence of varying concentrations of gatifloxacin and in the presence of specific inhibitors at pH 7.4 and 37°C. Transport studies were carried out with cells seeded on 12 well transwell inserts precoated with collagen. Five to seven days post seeding, the medium was removed and the cell monolayers were washed with DPBS (3 ×10 min). For apical (AP)-basolateral (BL) transport, 0.5 ml of test solution was added to the AP side and 1.5 ml of DPBS was added to the BL side. For BL-AP transport, 1.5 ml of test solution was placed in BL side and 0.5 ml of DPBS was added to the AP side. These transport experiments were conducted for a total time period of 3 hours. Samples (100μL) were withdrawn from the receiver chamber at time intervals of 15, 30, 45, 60, 90, 120, 150 and 180 min and replaced with the same volume of DPBS to maintain sink conditions. To the samples, 3 mL of scintillation cocktail was added and radioactivity was measured with a scintillation counter.
2.7 Data analysis
[14C] Erythromycin accumulated inside the cells or transported across the cell monolayers in the presence of various concentrations of gatifloxacin and specific inhibitors were calculated according to Eq. 1
| Eq.1 |
CPM sample and CPM donor denote average values of CPM counts of sample and donor (in quadruplicate) respectively; C donor represents the concentration of donor used and C sample represents the concentration of sample.
Calculation of Ki and Vmax
[14C] Erythromycin concentration obtained from uptake studies is fitted to Michaelis-Menten equation as shown in Eq.2
| Eq.2 |
V represents the observed rate of uptake at a concentration [C] and Vmax is the maximum rate of uptake and Ki is the apparent inhibition coefficient of the respective efflux pump.
Calculation of Efflux ratios
Cumulative amounts of radioactive substance transported across the cell monolayers for the total time period was calculated using Eq.3
| Eq.3 |
Cs is the amount of radioactive substance transported at a time t, Vp is the volume of the sample withdrawn and Vt is the total volume in the receiver chamber.
Flux value (J) is calculated according to Eq.4
| Eq.4 |
ΔQ/Δt represents the amount of radioactive substance transported per unit time across the cell monolayers with a cross sectional area A.
Permeability (P) value was then calculated according to Eq.5
| Eq.5 |
Thus the efflux ratio was calculated from AP-BL and BL-AP permeability studies as shown in Eq. 6
| Eq.6 |
2.8 ATP Assay
The cells were seeded in 96-well plates at a density of 10,000/cm2 and allowed to reach confluence. 5–7 post seeding, the medium was removed and the cells were washed with DPBS (3 × 10 min). The cells were exposed to various concentrations of drug solutions (100 μL) at 37° C for 30 minutes. Later, drug solutions were removed and lysis solution (100 μL) was added. The lysate from the cells was then used for quantitative detection of ATP using ATP Determination Kit (Molecular Probes, Invitrogen). ATP solution (5mM) available in the kit served as a control and ATP inhibitors such as ouabain (1mM), sodium azide (1mM) and 2,4-DNP (1mM) served as positive inhibitors. Luminescence was then measured using a microtiter plate reader (SpectraFluor Plus; Maennedorf, Switzerland) and it was normalized to protein count, estimated by the Bio-Rad protein estimation kit using bovine serum albumin as the standard.
2.9 Statistical analysis
All the experiments were conducted at least in quadruplicate (n = 4), and results are expressed as mean ± the standard deviation (SD). Statistical comparison of mean values was performed with one-way analysis of variance (ANOVA) or a Student’s t test (Graph Pad INSTAT, version 3.1). A P-value of <0.05 was considered to be statistically significant.
3. Results
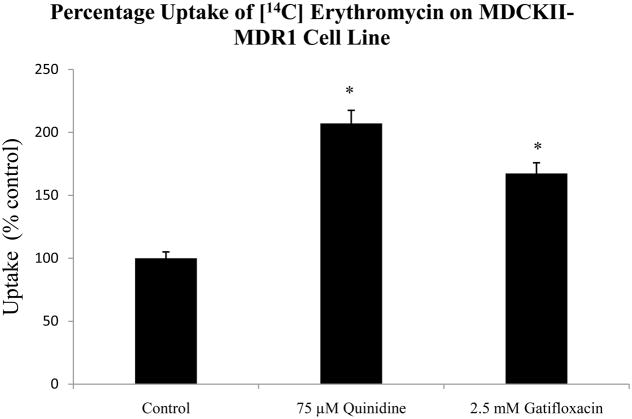
3.1 Uptake of [14C] erythromycin in the presence of 75 μM quinidine and 2.5mM gatifloxacin in MDCKII-MDR1 cells
[14C] Erythromycin uptake was performed in the presence of 75 μM quinidine and 2.5mM gatifloxacin. Uptake of [14C] erythromycin was higher by 107% and 67% in the presence of 75 μM quinidine and 2.5mM gatifloxacin respectively (Fig. 2).
Fig 2.
Uptake of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) in MDCKII-MDR1 cell monolayers in the presence of 75 μM quinidine and 2.5mM gatifloxacin. *Indicates P < 0.05. Values represent mean ± standard deviation (n =4).
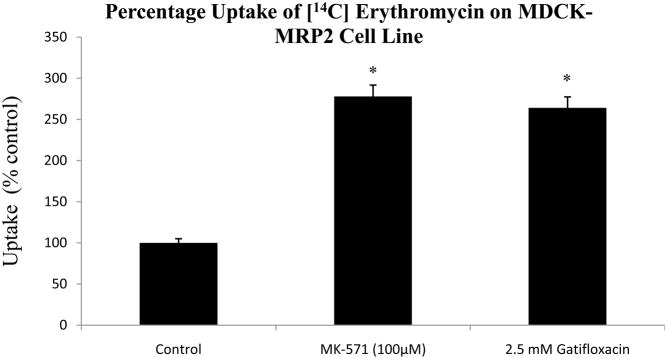
3.2 Uptake of [14C] erythromycin in the presence of 100 μM MK571 and 2.5mM gatifloxacin in MDCK-MRP2 cells
[14C] Erythromycin uptake was performed in the presence of 100 μM MK571 and 2.5mM gatifloxacin. Uptake of [14C] erythromycin was significantly higher by 177% and 164% in the presence of 100 μM MK571 and 2.5mM gatifloxacin respectively (Fig. 3).
Fig 3.
Uptake of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) in MDCK-MRP2 cell monolayers in the presence of 100 μM MK-571 and 2.5mM gatifloxacin. *Indicates P < 0.05. Values represent mean ± standard deviation (n =4).
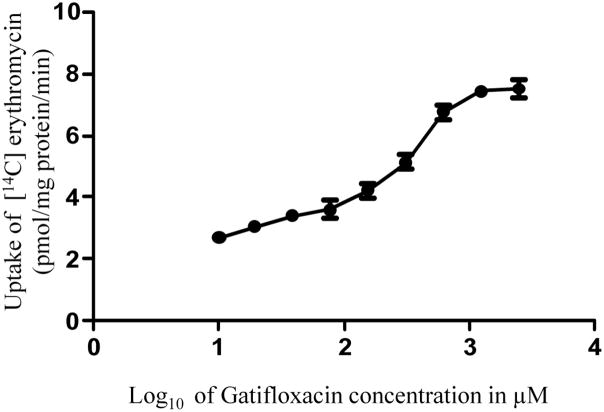
3.3 Dose-dependent inhibition of [14C] erythromycin uptake in MDCKII-MDR1 cells in the presence of quinidine and gatifloxacin
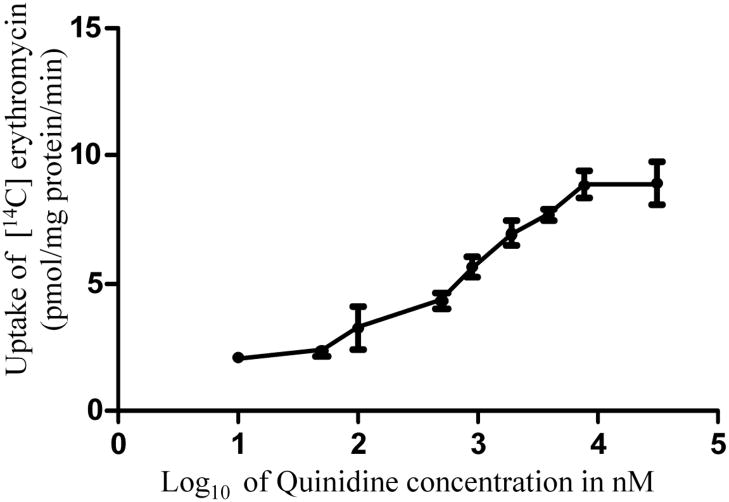
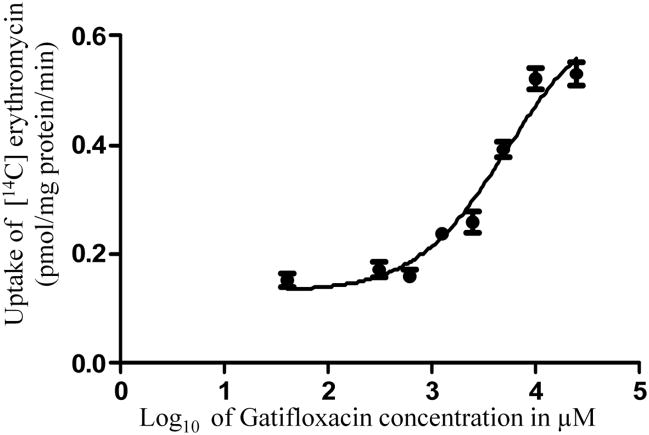
Concentration dependent uptake of [14C] erythromycin in MDCKII-MDR1 cells was studied with varying concentrations of quinidine and gatifloxacin. IC50 values of gatifloxacin and quinidine from the dose-response curves (Figs. 4 and 5) were calculated to be 359.0 ± 90.1 μM and 981.2 ± 115.67 nM respectively. Ki values of gatifloxacin and quinidine were calculated by the method of Cheng and Prusoff (Cheng and Prusoff 1973) and were estimated to be 385.5 ± 88.6 μM and 1.23 ± 0.161 μM respectively.
Fig 4.
Dose dependent inhibition of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) uptake in MDCKII-MDR1 cell monolayers in presence of gatifloxacin. Values represent mean ± standard deviation (n= 4).
Fig 5.
Dose dependent inhibition of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) uptake in MDCKII-MDR1 cell monolayers in presence of quinidine. Values represent mean ± standard deviation (n= 4).
3.4 Dose-dependent inhibition of [14C] erythromycin uptake in MDCK-MRP2 cells in the presence of MK571 and gatifloxacin
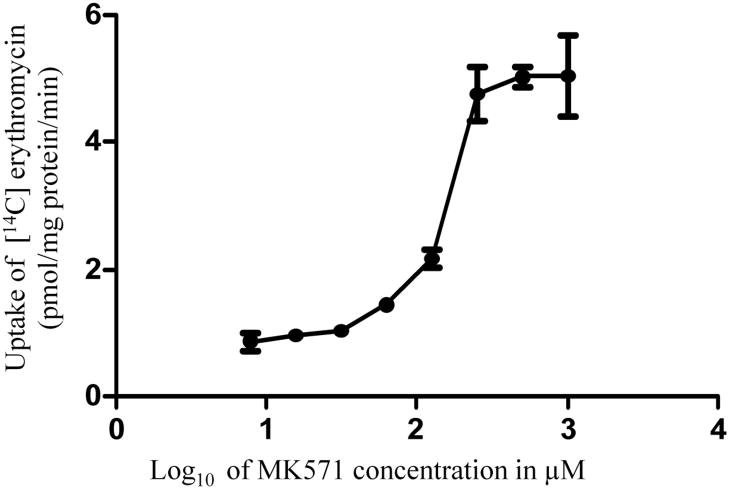
Concentration dependent uptake of [14C] erythromycin in MDCK-MRP2 cells was studied in the presence of varying concentrations of MK571 and gatifloxacin. IC50 values of gatifloxacin and MK571 from the dose-response curves (Figs. 6 and 7) were calculated to be 5232 ± 390.5 μM and 188.4 ± 28.23 μM respectively. Ki values of gatifloxacin and MK571 were calculated by the method of Cheng and Prusoff (Cheng and Prusoff 1973) and were estimated to be 5365 ± 218.4 μM and 174.71 ± 18.17 μM respectively.
Fig 6.
Dose dependent inhibition of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) uptake in MDCK-MRP2 cell monolayers in presence of gatifloxacin. Values represent mean ± standard deviation (n= 4).
Fig 7.
Dose dependent inhibition of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) uptake in MDCK-MRP2 cell monolayers in presence of MK-571. Values represent mean ± standard deviation (n= 4).
3.5 Polarized [14C] erythromycin transport across MDCKII-MDR1 cell monolayers in presence of quinidine and varying concentrations of gatifloxacin
Bidirectional transcellular transport of [14C] erythromycin across MDCKII-MDR1 in presence of gatifloxacin (0.25, 0.5, 1 and 2.5 mM) was performed. [14C] Erythromycin transport alone was considered as the negative control and [14C] erythromycin in the presence of 75 μM quinidine served as the positive inhibitor for MDR1. A significant polarization in the transport of [14C] erythromycin across MDCKII-MDR1 cell monolayer was evident. Apparent permeability (Papp) value of [14C] erythromycin transport in the B–A direction was more than fivefold higher than the AP–BL direction (Fig. 8). Transport of [14C] erythromycin from apical to basolateral side across MDCKII-MDR1 monolayers was significantly elevated in the presence of gatifloxacin (0.25, 0.5, 1 and 2.5 mM) (Fig. 8). No significant difference in [14C] erythromycin permeability was observed in the presence of 0.5 mM to 2.5 mM in AP-BL direction. The same concentrations of gatifloxacin also showed marked inhibition of the BL–AP transport of [14C] erythromycin across the cell monolayers. Efflux ratios calculated as the ratio of Papp in AP-BL direction to BL-AP direction of the substrate were found to progressively decrease and approach unity with increasing concentrations of gatifloxacin.
Fig 8.
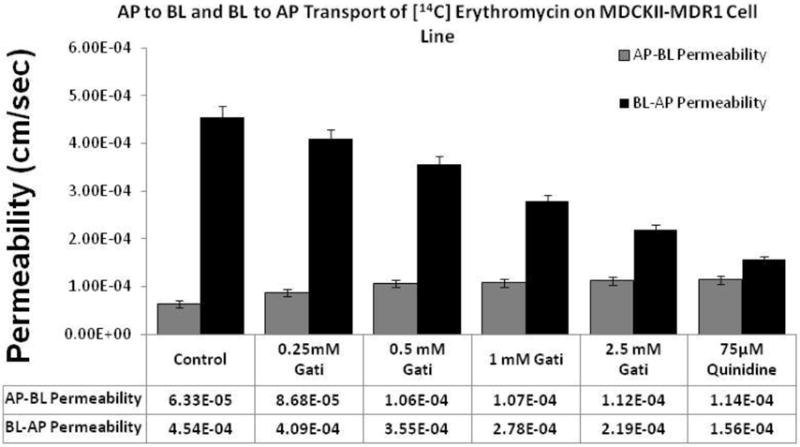
Dose dependent effect of gatifloxacin (0.25, 0.5, 1 and 2.5 mM) on the apparent permeability of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) across MDCKII-MDR1 cell monolayers. Control represents transport of [14C] erythromycin alone, transport of [14C] erythromycin in presence of quinidine (75 μM) is the positive control. Transcellular transport of [14C] erythromycin was measured in the AP–BL and BL–AP direction. Values represent mean ± standard deviation (n= 4).
3.6 Polarized [14C] erythromycin transport across MDCK-MRP2 cell monolayers in the presence of MK-571 and varying concentrations of gatifloxacin
Bidirectional transcellular transport of [14C] erythromycin across MDCK-MRP2 cells in presence of gatifloxacin (0.25, 0.5, 1 and 2.5 mM) was monitored. [14C] Erythromycin transport alone served as the negative control and [14C] erythromycin in the presence of 100 μM MK-571 was considered as the positive inhibitor for MRP2. A significant difference in polarized transport of [14C] erythromycin across MDCK-MRP2 cell monolayer was noted. Papp value of [14C] erythromycin transport in the BL-AP direction was more than 7 fold larger than in the AP-BL direction (Fig. 9). Transport of [14C] erythromycin from apical to basolateral side across MDCK-MRP2 monolayers was significantly elevated in the presence of gatifloxacin (0.25, 0.5, 1 and 2.5 mM) (Fig. 9). Same concentrations of gatifloxacin also showed marked inhibition in BL-AP directional transport of [14C] erythromycin. Efflux ratios continuously became lower and approached unity with increasing concentrations of gatifloxacin.
Fig 9.
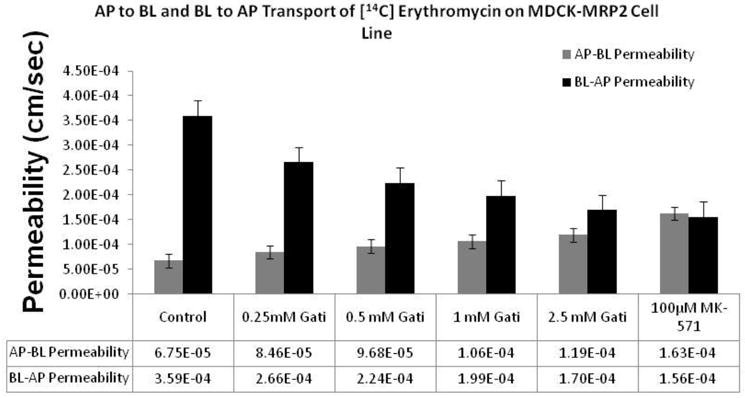
Dose dependent effect of gatifloxacin (0.25, 0.5, 1 and 2.5 mM) on the apparent permeability of [14C] erythromycin (0.25 μCi/mL, 51.3 mCi/mmol) across MDCK-MRP2 cell monolayers. Control represents transport of [14C] erythromycin alone, transport of [14C] erythromycin in presence of MK-571 (100μM) is the positive control. Transcellular transport of [14C] erythromycin was measured in the AP–BL and BL–AP direction. Values represent mean ± standard deviation (n= 4).
3.7 Accumulation of Hoechst 33342
Accumulation of Hoechst 33342 (a specific BCRP substrate) was determined in confluent MDCK-BCRP cells. ABCG2 specific inhibitor Ko-143 (10μM) was added as a positive control for the inhibition studies. Hoechst 33342 uptake was not found to be significantly different in a concentration dependent manner in presence of gatifloxacin (0.5, 1, 2.5, 5 and 10 mM) (Fig. 10). In presence of Ko-143 (10μM), the dye uptake was found to rise by 2.5 fold (Fig. 10).
Fig 10.
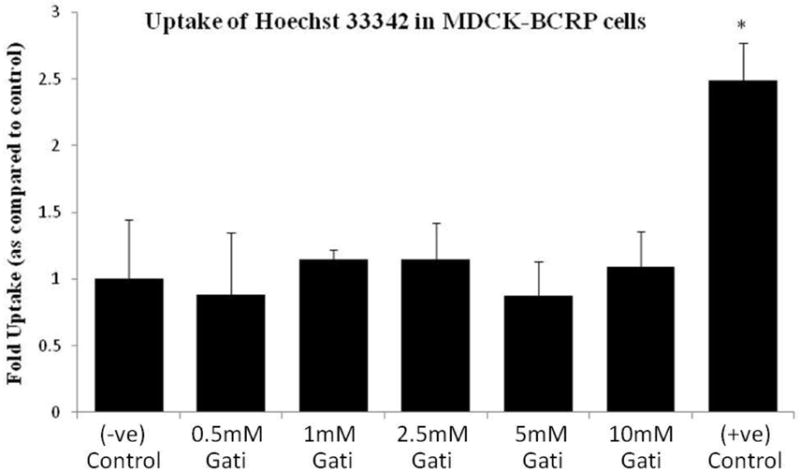
Accumulation of 5μM Hoechst 33342 in MDCK-BCRP cells alone (−ve) and in presence of gatifloxacin (0.5, 1, 2.5, 5 and 10 mM). Ko143 (10μM) was used as a (+ve) inhibitor of BCRP mediated efflux. * Indicates P < 0.05. Values represent mean ± standard deviation (n =6).
3.8 ATP activity assay
An ATP activity assay was performed in all the MDCK cell lines post thirty minute treatment with gatifloxcacin (0.75, 1.25, 2.5 and 5mM). Ouabain, sodium azide and 2,4 dinitro phenol at a concentration of 1mM were used as positive inhibitors of ATP activity. No significant difference was observed in the ATP activity of the treated cells when compared to the vehicle control. Significant difference was observed in the activity in the presence of all the three inhibitors compared to the control (Fig. 11).
Fig 11.
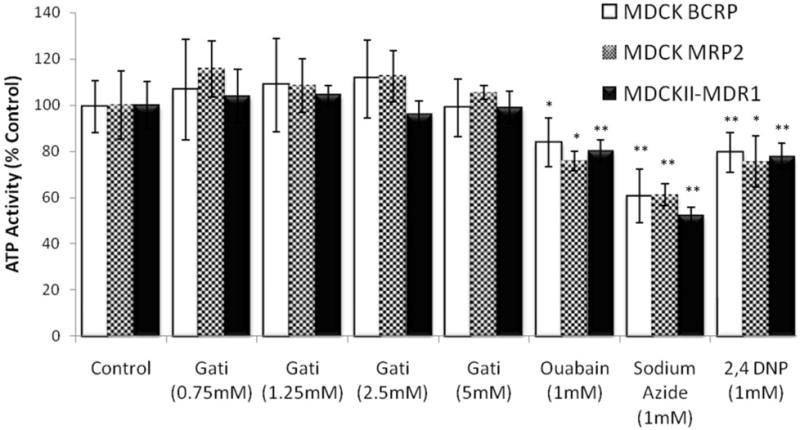
ATP activity assay in MDCKII-MDR1, MDCK-MRP2 and MDCK-BCRP cells lines post 30 min treatment with different concentrations of gatifloxacin. Ouabain, sodium azaide, and 2,4 dinitro phenol were used as positive inhibitors of ATP. *Indicates P < 0.05. **Indicates P < 0.01. Values represent mean ± standard deviation (n =8).
4. Discussion
Gatifloxacin is a highly effective broad spectrum antibiotic indicated in treatment or prophylaxis of ophthalmic infections. The drug suffers from severe systemic toxicity issues (Mohr et al. 2008; Onyenwenyi et al. 2008) even though it shows negligible ocular toxicity (Choi and Chung 2009; Donnenfeld et al. 2004). Development of drug resistance in ocular therapy has required an increase in dose which in turn may lead to systemic toxicity. The aim of the present study was to delineate the role of efflux transporters (P-gp, MRP2 and BCRP) in the development of gatifloxacin resistance. MDCK cells transfected with individual efflux transporters were selected as in vitro models to study the drug resistance.
Cellular uptake of [14C] erythromycin across MDCKII-MDR1 cells was enhanced by almost 2-fold in the presence of quinidine (75μM). Gatifloxacin (2.5 mM) also raised the uptake of [14C] erythromycin by 1.6-fold even though at a much higher concentration (Fig. 2). This concentration of gatifloxacin is still less than the concentration used in marketed ophthalmic solution ZYMAR® (0.3% w/v or approx. 8.0 mM). This 2-fold increase in the rate of erythromycin uptake by the MDCKII-MDR1 cell line in the presence of quinidine, a known substrate and/or inhibitor (Jain et al. 2004), confirmed that MDCKII-MDR1 cells express P-gp. A significant rise in erythromycin uptake by higher concentrations of gatifloxacin also suggests that this fluoroquinolone is a substrate of P-gp.
In a similar study, cellular uptake of [14C] erythromycin across MDCK-MRP2 cells was also higher by 1.8-fold in the presence of MK571 (100μM), a known inhibitor of MRPs (Luders et al. 2009). Gatifloxacin (2.5 mM) also elevated the uptake of [14C] erythromycin by 1.7-fold (Fig. 3). These results confirm that MDCK-MRP2 cells express MRP2 and gatifloxacin is a substrate of MRP2.
Affinity pattern of [14C] erythromycin in the presence of both quinidine and gatifloxacin was determined by dose-dependent studies in MDCKII-MDR1 cells. Lineweaver-Burk transformation of quinidine suggests noncompetitive inhibition of erythromycin efflux by P-gp where as gatifloxacin appears to cause competitive inhibition, suggesting that quinidine and gatifloxacin may or may not share a common site of binding on the transporter (data not shown). A comparison of Ki values indicates that quinidine probably exhibits much higher affinity toward P-gp relative to gatifloxacin (Figs. 4 and 5). Similarly affinity pattern of [14C] erythromycin in the presence of both MK-571 and gatifloxacin was determined by dose-dependent studies in MDCK-MRP2 cells. The Lineweaver-Burk transformation in this case suggested noncompetitive inhibition for MK-571 and revealed competitive inhibition for gatifloxacin. These results suggest that MK-571 and gatifloxacin may or may not share a common binding site on MRP2 (data not shown). A comparison of Ki values indicates that MK-571 probably has a much higher affinity toward MRP relative to gatifloxacin (Figs. 6 and 7).
A classical indication of the involvement of efflux in transport kinetics is the difference in permeation rates of the substrates in the apical to basolateral and basolateral to apical directions (Polli et al. 2001). If a compound is a substrate for P-gp, the apical to basolateral transport (AP-BL) is considerably lower than the basolateral to apical transport (BL-AP). The ratio of this transepithelial transport (AP-BL) vs. (BL-AP) is represented as efflux ratio and this ratio approaches one when the transport of a substrate at both directions remains same. Transepithelial bidirectional transport of [14C] erythromycin across MDCKII-MDR1 cells reveals that the transport in the absorptive direction (AP to BL) was significantly lower than in the secretory direction (BL to AP) (Fig. 8). This asymmetric permeation is due to the apically polarized P-gp efflux transporter, for which erythromycin is a very good substrate. In the presence of quinidine (75 μM), the apparent permeability (Papp) for secretory transport of erythromycin decreased significantly where as the absorptive transport increased significantly bringing the efflux ratio down to 1.3 as compared to 7.2 in the control (Fig. 8). A similar concentration dependent decrease in the rate of secretory transport as well as increase in the absorptive transport rate was observed in the presence of gatifloxacin in MDCKII-MDR1 cells (Fig. 8).
In a similar study performed in MDCK-MRP2 cell monolayers, the transepithelial permeability of [14C] erythromycin in the basolateral to apical and apical to basolateral direction again generated an efflux ratio of 5.3. Further, the efflux ratio across MDCK-MRP2 cells in the presence of MK-571 (100 μM) decreased significantly (efflux ratio=1). A similar trend in decrease of (BL-AP) transport as well as increase in the (AP-BL) transport rate was evident in the presence of increasing concentrations of gatifloxacin (Fig. 9). These results further confirm our earlier observations that gatifloxacin is recognized by both P-gp and MRP2 as a substrate.
Uptake of the fluorescent substrate was found to rise significantly in presence of Ko-143 confirming the presence of BCRP in MDCK-BCRP cells. However, such uptake was ineffective in the presence of different concentrations of gatifloxacin suggesting that this fluoroquinolone is not a substrate of BCRP (Fig. 10).
One possible reason for the inhibition of efflux observed in the presence of gatifloxacin can be its action on ATP. Since these efflux transporters are ATP dependent proteins, any decrease in amount or activity of ATP can result in the potential decrease in the sensitivity of the substrate for the transporter. To test this hypothesis an ATP assay was performed in the presence of various gatifloxacin concentrations in all the MDCK cell lines used during this study. The ATP activity did not diminish in any of the cells treated with gatifloxacin where as a significant decrease in the ATP activity was observed in the presence of all the three ATP inhibitors. Hence this negates the possibility of an indirect inhibition of P-gp and MRP2 mediated efflux by gatifloxacin via ATP inhibition (Fig. 11).
In this study we attempted to delineate the possible role of major efflux proteins in the acquired drug resistance of gatifloxacin. Initial uptake studies suggested a possible interaction between gatifloxacin and efflux transporters (P-gp, MRP2). Concentration dependent affinity analysis further suggested that gatifloxacin may act as a substrate for both P-gp and MRP2. This hypothesis was further supported by the concentration dependent reduction in efflux ratios in both MDCKII-MDR1 and MDCK-MRP2 cells.
Earlier reports by Li et al. studied the interaction of gatifloxacin with P-gp but did not find any significant involvement of the transporter in the translocation of gatifloxacin (Li et al. 2009). The cell line used in that study was rat endothelial cell line (RBMEC) which expresses relatively lesser amounts of P-gp. For this reason transfected cell lines were used in this study so that the expression of the efflux transporters can be assured.
In conclusion, this report indicates that efflux transporters may play an important role in the development of gatifloxacin resistance which in turn may limit drug concentration at the target site or may inhibit drug uptake by the microbes. Knowledge of drug interaction with these efflux transporters can aid in the development of strategies to better circumvent these efflux transporters.
Acknowledgments
This work has been supported by Missouri Life Sciences research fund and NIH grant RO1EY009171-16
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ba BB, Arpin C, Vidaillac C, Chausse A, Saux MC, Quentin C. Activity of gatifloxacin in an in vitro pharmacokinetic-pharmacodynamic model against Staphylococcus aureus strains either susceptible to ciprofloxacin or exhibiting various levels and mechanisms of ciprofloxacin resistance. Antimicrob Agents Chemother. 2006;50(6):1931–1936. doi: 10.1128/AAC.01586-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RS, Flowers CW, Jr, Casey R, Fong DS, Wilson MR. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Arch Ophthalmol. 1996;114(5):632–633. doi: 10.1001/archopht.1996.01100130624031. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12–8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40(5):639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- Blondeau JM. Expanded activity and utility of the new fluoroquinolones: a review. Clin Ther. 1999;21(1):3–40. doi: 10.1016/s0149-2918(00)88266-1. discussion 41–42. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Choi JA, Chung SK. Safety of intracameral injection of gatifloxacin, levofloxacin on corneal endothelial structure and viability. J Ocul Pharmacol Ther. 2009;25(5):425–431. doi: 10.1089/jop.2009.0010. [DOI] [PubMed] [Google Scholar]
- Dey S, Gunda S, Mitra AK. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J Pharmacol Exp Ther. 2004;311(1):246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44(7):2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother. 1994;33(4):685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- Donnenfeld E, Perry HD, Chruscicki DA, Bitterman A, Cohn S, Solomon R. A comparison of the fourth-generation fluoroquinolones gatifloxacin 0.3% and moxifloxacin 0.5% in terms of ocular tolerability. Curr Med Res Opin. 2004;20(11):1753–1758. doi: 10.1185/030079904X5959. [DOI] [PubMed] [Google Scholar]
- Ednie LM, Jacobs MR, Appelbaum PC. Activities of gatifloxacin compared to those of seven other agents against anaerobic organisms. Antimicrob Agents Chemother. 1998;42(9):2459–2462. doi: 10.1128/aac.42.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers R, Cnubben NH, Wijnholds J, van Deemter L, van Bladeren PJ, Borst P. Transport of glutathione prostaglandin A conjugates by the multidrug resistance protein 1. FEBS Lett. 1997;419(1):112–116. doi: 10.1016/s0014-5793(97)01442-7. [DOI] [PubMed] [Google Scholar]
- Evers R, Kool M, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, Borst P. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest. 1998;101(7):1310–1319. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology. 1999;106(7):1313–1318. [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Guo A, Marinaro W, Hu P, Sinko PJ. Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT) Drug Metab Dispos. 2002;30(4):457–463. doi: 10.1124/dmd.30.4.457. [DOI] [PubMed] [Google Scholar]
- Hariharan S, Gunda S, Mishra GP, Pal D, Mitra AK. Enhanced corneal absorption of erythromycin by modulating P-glycoprotein and MRP mediated efflux with corticosteroids. Pharm Res. 2009;26(5):1270–1282. doi: 10.1007/s11095-008-9741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC. Mode of action of fluoroquinolones. Drugs. 1999;58(Suppl 2):6–10. doi: 10.2165/00003495-199958002-00002. [DOI] [PubMed] [Google Scholar]
- Hooper DC. Quinolones. In: Mandell GL, Bennet JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia: Elsevier Churchill Livingstone; 2000. [Google Scholar]
- Hooper DC, Wolfson JS. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1993. Mechanisms of quinolone action and bacterial killing. [Google Scholar]
- Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J Pharm Sci. 1999;88(1):28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- Jain R, Majumdar S, Nashed Y, Pal D, Mitra AK. Circumventing P-glycoprotein-mediated cellular efflux of quinidine by prodrug derivatization. Mol Pharm. 2004;1(4):290–299. doi: 10.1021/mp049952s. [DOI] [PubMed] [Google Scholar]
- Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr Eye Res. 2009;34(1):1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karla PK, Pal D, Mitra AK. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp Eye Res. 2007;84(1):53–60. doi: 10.1016/j.exer.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Leibowitz HM. Clinical evaluation of ciprofloxacin 0.3% ophthalmic solution for treatment of bacterial keratitis. Am J Ophthalmol. 1991;112(4 Suppl):34S–47S. [PubMed] [Google Scholar]
- Li Y, Liu L, Li J, Xie L, Wang GJ, Liu XD. Transport of gatifloxacin involves Na+/Ca2+ exchange and excludes P-glycoprotein and multidrug resistance associated-proteins in primary cultured rat brain endothelial cells. Eur J Pharmacol. 2009;616(1–3):68–72. doi: 10.1016/j.ejphar.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28(2):352–364. doi: 10.1086/515104. [DOI] [PubMed] [Google Scholar]
- Luders AK, Saborowski R, Bickmeyer U. Inhibition of multidrug/xenobiotic resistance transporter by MK571 improves dye (Fura 2) accumulation in crustacean tissues from lobster, shrimp, and isopod. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150(3):368–371. doi: 10.1016/j.cbpc.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol. 2002;133(4):463–466. doi: 10.1016/s0002-9394(02)01334-x. [DOI] [PubMed] [Google Scholar]
- Mohr JF, 3rd, Peymann PJ, Troxell E, Lodise TP, Ostrosky-Zeichner L. Risk factors for hyperglycemia in hospitalized adults receiving gatifloxacin: a retrospective, nested case-controlled analysis. Clin Ther. 2008;30(1):152–157. doi: 10.1016/j.clinthera.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Onyenwenyi AJ, Winterstein AG, Hatton RC. An evaluation of the effects of gatifloxacin on glucose homeostasis. Pharm World Sci. 2008;30(5):544–549. doi: 10.1007/s11096-008-9205-8. [DOI] [PubMed] [Google Scholar]
- Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312(1):144–152. doi: 10.1124/jpet.104.073916. [DOI] [PubMed] [Google Scholar]
- Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299(2):620–628. [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61(1):3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravolatz LD, Leggett J. Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones. Clin Infect Dis. 2003;37(9):1210–1215. doi: 10.1086/378809. [DOI] [PubMed] [Google Scholar]
- Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MRP2 gene a good model of the human intestinal mucosa? Pharm Res. 2002;19(6):773–779. doi: 10.1023/a:1016192413308. [DOI] [PubMed] [Google Scholar]
- Turnidge J. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs. 1999;58(Suppl 2):29–36. doi: 10.2165/00003495-199958002-00006. [DOI] [PubMed] [Google Scholar]