Abstract
Objective
Spleen tyrosine kinase (Syk) is involved in membrane-mediated signaling in various cells, including immune cells. It is overexpressed in T cells from patients with systemic lupus erythematosus (SLE), and its inhibition has been shown to improve T cell function as well as to improve disease manifestations in (NZB × NZW)F1 lupus-prone mice and in patients with rheumatoid arthritis. While clinical trials examining Syk inhibition in patients with SLE are being considered, the aim of our experiments was to determine whether the therapeutic effects of Syk inhibition extend to other strains of lupus-prone mice and whether they result in improvement in skin disease and modification of established disease.
Methods
Female MRL/lpr or BAK/BAX mice were studied. Starting either at age 4 weeks (before disease) or at age 16 weeks (after established disease) and continuing for up to 16 weeks, mice were fed chow containing the Syk inhibitor R788 or control chow.
Results
We found that inhibition of Syk in MRL/lpr and BAK/BAX mice prevented the development of skin disease and significantly reduced established skin disease. Similarly, Syk inhibition reduced the size of the spleen and lymph nodes, suppressed the development of renal disease, and suppressed established renal disease. Discontinuation of treatment resulted in extended suppression of skin disease for at least 8 weeks and suppression of renal disease for 4 weeks.
Conclusion
Syk inhibition suppresses the development of lupus skin and kidney disease in lupus-prone mice, suppresses established disease in lupus-prone mice, and may represent a valuable treatment for patients with SLE.
Spleen tyrosine kinase (Syk) is a member of the Src family of nonreceptor tyrosine kinases (1). Syk is widely expressed in the hematopoietic system and is involved in a variety of signal transduction pathways (2,3), including receptor signaling in mast cells (4), monocytes (5), osteoclasts (6,7), and T and B cells (8–10).
Recently, a highly specific Syk inhibitor, known as R406, was established (11,12), and it has been shown to block Fc receptor (11,12) and T cell receptor (13,14) signaling. Syk inhibition has also been shown to correct aberrant T cell receptor/CD3–initiated signaling in patients with systemic lupus erythematosus (SLE) (13). In animal models, R406 has been shown to be of potential clinical value in the treatment of allergic diseases (15), rheumatoid arthritis (16), and lymphoma (17,18). R788 is a small-molecule, water-soluble prodrug of the biologically active R406 and a potent inhibitor of Syk (19,20). R788 has been shown to inhibit the progression of kidney disease in lupus-prone (NZB × NZW)F1 mice (21).
We present here evidence that Syk inhibition suppresses the development of skin disease in lupusprone MRL/lpr and BAK/BAX mice and suppresses established skin disease. Similarly, Syk inhibition suppressed the development of kidney disease and improved established renal disease. The clinical benefit lasted 4 weeks for renal disease and at least 8 weeks for skin disease after treatment was discontinued.
MATERIALS AND METHODS
Mice and materials
Female MRL/lpr mice, BAK/BAX double-knockout mice, and C57BL/6 mice were purchased from The Jackson Laboratory and were housed in the animal facility of Beth Israel Deaconess Medical Center. Double-stranded DNA (dsDNA) antibody and mouse IgG antibody were purchased from Sigma. Syk inhibitors R788 and R406 were provided by Rigel Pharmaceuticals.
Treatment of MRL/lpr mice with the Syk inhibitor R788
Starting at the age of 4 weeks and continuing for 16 weeks, female MRL/lpr mice (n = 8 per group) were fed chow containing R788 (3 gm/kg of chow or 10 gm/kg of chow) or were fed control chow. The chow was prepared by Research Diets. In experiments to determine the effect of R788 on established skin injury and nephritis, female MRL/lpr mice were treated for 8 weeks beginning at the age of 16 weeks (10 gm/kg of chow). Mice were killed at end of the experiment, and serum, skin, and kidney samples were collected for examination. During the experimental period, mice were monitored for urinary protein content and mortality.
Histologic assessment
Histopathologic examination of skin and kidney samples was done after routine fixation and paraffin embedding of the tissues. Tissue sections from the skin were cut and stained with hematoxylin and eosin. All slides were coded and evaluated in a blinded manner with regard to identity of the sample. Severity of skin inflammation was scored on a scale of 0–4, where 0 = normal, 1 = hyperplasia of the epidermis, and 2–4 = increasing numbers of infiltrating inflammatory cells in the skin. Pathologic changes in the kidney were graded according to the presence of glomerular, interstitial, and perivascular inflammation. Scores ranging from 0 (normal) to 4 (most severely inflamed) were assigned for each of the 3 features. A minimum of 100 glomeruli were assessed to determine the glomerular index in each mouse.
Assessment of urine
The mice in each group were placed overnight in a Nalgene metabolic cage to collect urine. Urine was measured with Multistix 10 SG and analyzed by Clinitek Status analyzer (both from Bayer Healthcare). Proteinuria was graded on a scale of 0–4, where 0 = none, 1 = 30–100 mg/dl, 2 = 100–300 mg/dl, 3 = 300–2,000 mg/dl, and 4 = >2,000 mg/dl.
Measurement of serum IgG and anti-DNA antibody
Serum IgG and anti-dsDNA and anti–single-stranded DNA (anti-ssDNA) antibodies were detected by enzyme-linked immunosorbent assay (ELISA). For these assessments, 96-well plates coated with either calf thymus dsDNA (1.5 mg/ml; Sigma) or mouse IgG antibody (0.1 mg/ml; Sigma) in phosphate buffered saline (PBS) were incubated overnight at 4°C. After blocking with 1% bovine serum albumin for 2 hours at room temperature, serum samples were added to the plates and incubated for 2 hours at room temperature. After washing 4 times with PBS, horseradish peroxidase–conjugated goat anti-mouse IgG antibody was added to the plates and incubated for 1 hour at room temperature. Antibody binding was visualized using 3,3′,5,5′-tetramethylbenzidine (Sigma), and plates were read at an optical density of 450 nm.
Immunohistochemistry staining
For immunohistochemical examination, deparaffinized, ethanol-dehydrated tissue sections were boiled for 2 minutes in 10 mM sodium citrate buffer using a microwave oven. Sections were then allowed to cool to room temperature and rinsed in a detergent solution (0.5% Tween 20 in PBS) for 10 minutes. After deparaffinization and antigen retrieval, samples were stained with antibody to Syk, followed by incubation with biotinylated secondary antibodies and avidin–biotin–peroxidase complexes and 3-amino-9-ethyl-carbazole containing H2O2. All sections were counterstained with Mayer’s hematoxylin. Dendritic cells were fixed and permeabilized and then stained with monoclonal anti-CD11c conjugated to fluorescein isothiocyanate (Novus Biologicals).
Statistical analysis
Statistical evaluations of the severity of skin and kidney inflammation were performed using the Mann-Whitney test. P values less than or equal to 0.05 were considered significant.
RESULTS
Syk expression in the skin lesions of MRL/lpr mice
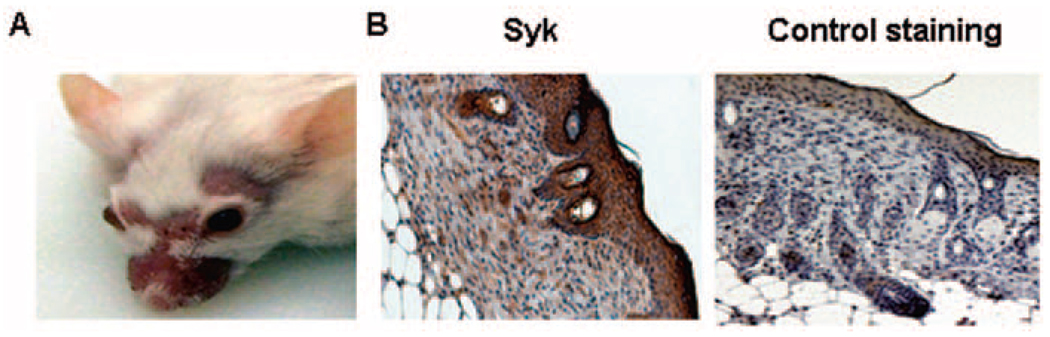
MRL/lpr mice spontaneously develop skin lesions (Figure 1A). At the beginning of the experiments, we used immunohistochemical analysis to determine whether Syk was expressed in these skin lesions. As shown in Figure 1, staining with anti-Syk antibody and isotype control revealed the presence of Syk in the epidermis as well as in the dermis-infiltrating inflammatory cells. In previous studies, we had shown that Syk was present in the glomeruli of MRL/lpr mice (22).
Figure 1.
Syk expression in the skin lesions of female MRL/lpr mice. A, Photograph showing representative skin lesions in MRL/lpr mice. B, Syk expression in skin lesions from MRL/lpr mice stained with anti-Syk antibody and isotype control. Syk is present in the epidermis as well as in the dermis-infiltrating inflammatory cells. Representative photomicrographs are shown (original magnification × •••).
Prevention of the development of skin injury in MRL/lpr mice by treatment with Syk inhibitor R788
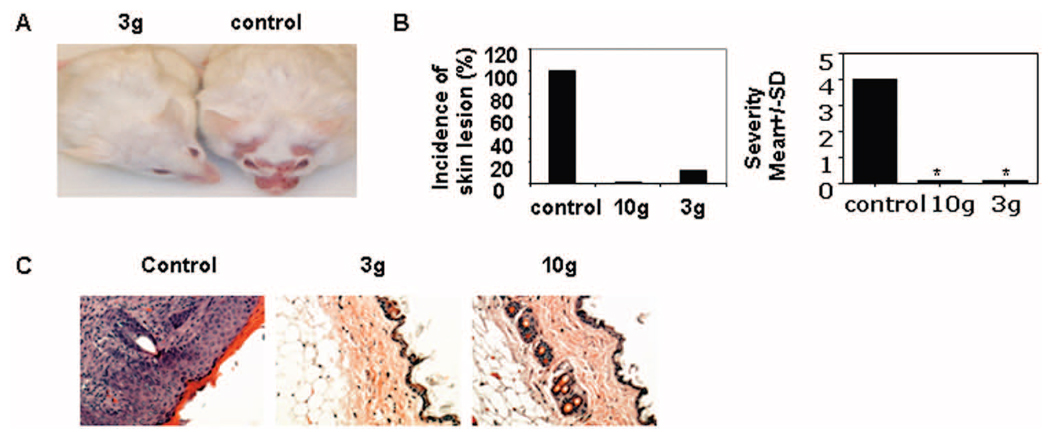
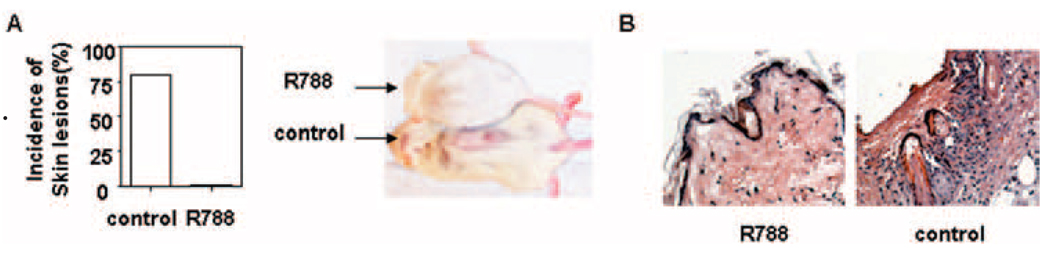
To determine whether treatment with the Syk inhibitor R788 improves skin lesions in lupus-prone mice, we fed chow containing the Syk inhibitor (3 gm or 10 gm of inhibitor per kg of chow) or control chow to MRL/lpr mice. Treatment started at the age of 4 weeks and continued for 16 weeks. We found that skin injury was abrogated in MRL/lpr mice treated with R788 (3 gm/kg or 10 gm/kg of chow), but not in mice fed control chow (Figures 2A–C). Histopathologic examination demonstrated a lack of skin damage in MRL/lpr mice treated with R788 (Figure 2C). These data indicate that Syk inhibition can efficiently prevent the development of skin injury in MRL/lpr mice.
Figure 2.
Prevention of the development of skin lesions in female lupus-prone MRL/lpr mice by treatment with Syk inhibitor R788. A, Photograph showing representative MRL/lpr mice treated with R788 (3 gm/kg of chow) or control chow. B, Incidence and severity of skin lesions in MRL/lpr mice treated with R788 (3 gm/kg or 10 gm/kg of chow) or control chow. Values are the mean and SD of 8 mice per group. * = P < 0.01 versus control. C, Histopathologic features of skin lesions from MRL/lpr mice treated with R788 (3 gm/kg or 10 gm/kg of chow) or control chow. Representative photomicrographs are shown (original magnification × •••).
Suppression of established skin injury in MRL/lpr mice by treatment with Syk inhibitor R788
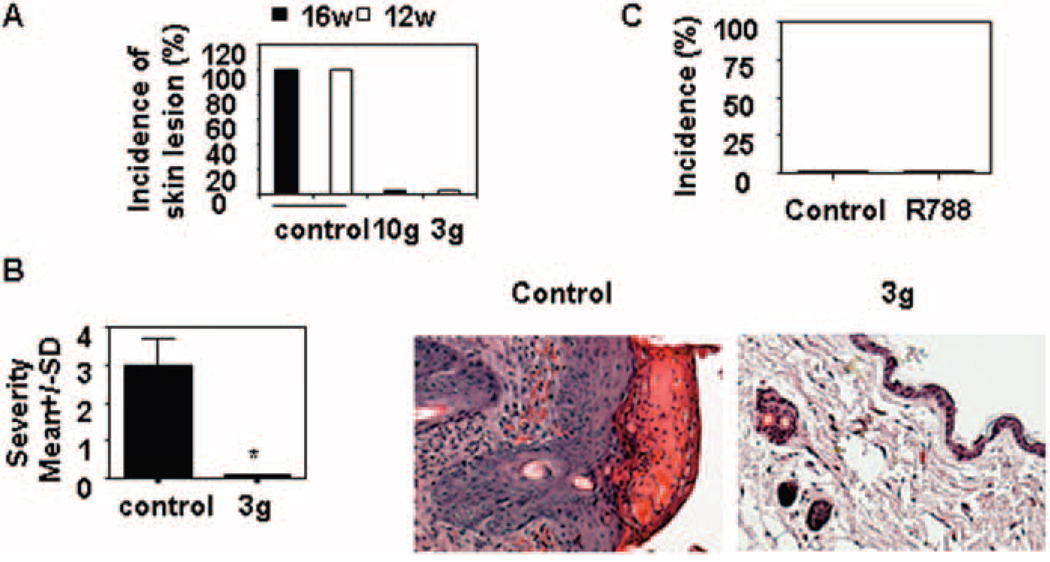
Next, we investigated whether treatment with a Syk inhibitor can improve established skin disease. To this end, the mice were fed chow containing R788 starting at the age of 12 weeks (3 gm) or at the age of 16 weeks (10 gm) and continuing for 8 weeks. We observed that treatment resulted in significant improvement in skin injury (Figures 3A and B), suggesting that Syk inhibition can reverse established disease.
Figure 3.
Marked improvement in established skin lesions in female lupus-prone MRL/lpr mice following treatment with Syk inhibitor R788. A, Incidence of skin lesions in MRL/lpr mice treated for 6 weeks with 3 gm of R788 per kg of chow starting at age 12 weeks, with 10 gm of R788 per kg of chow starting at age 16 weeks, or with control chow starting at age 12 weeks or 16 weeks. Values are the mean and SD of ••• mice per group. B, Severity and histopathologic features of skin lesions from MRL/lpr mice treated for 6 weeks with R788 (3 gm/kg of chow) or with control chow starting at age 12 weeks. Values are the mean and SD of ••• mice per group. * = P < 0.01 versus control. Representative photomicrographs are shown (original magnification × •••). C, Incidence of skin lesions at 14 weeks. Mice were treated for 6 weeks with Syk inhibitor and were then divided into 2 subgroups: one continued Syk inhibitor and the other received control chow for 8 weeks. The incidence of skin injury was then recorded. Values are the mean and SD of ••• mice per group.
Extended clinical benefit after discontinuation of treatment with Syk inhibitor R788
To determine whether there was lasting clinical benefit once treatment was discontinued, MRL/lpr mice that had been treated with R788 (3 gm/kg of chow) for 8 weeks were divided into 2 groups. In one group, treatment was discontinued, and the mice were switched to control chow. In the other group, treatment was continued at the same dose. We observed that 8 weeks later, none of the mice developed skin injury (Figure 3C), which suggests that Syk inhibition has extended clinical benefit.
Suppression of kidney disease in MRL/ lpr mice by treatment with Syk inhibitor R788
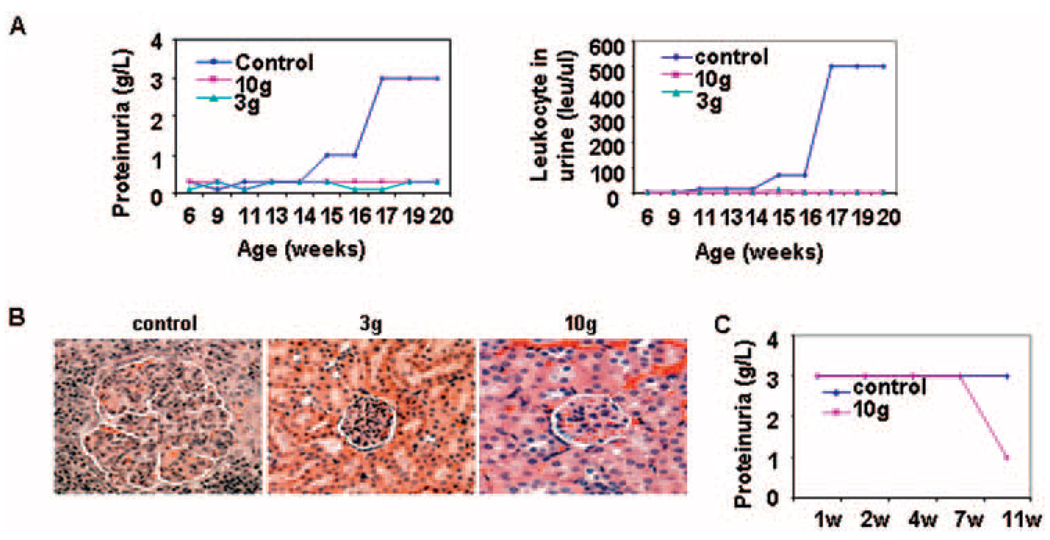
Female MRL/lpr mice started receiving chow containing the Syk inhibitor (3 gm/kg or 10 gm/kg of chow) at the age of 4 weeks. Both treated groups failed to develop proteinuria or pyuria by the time they reached the age of 20 weeks (Figure 4A). Histopathologic examination demonstrated that the presence of R788 in the chow suppressed pathologic changes in the kidney (Figure 4B). In mice in which R788 was added to the chow at the age of 16 weeks, when kidney disease was already established, proteinuria improved after 7 weeks of treatment (Figure 4C), suggesting that Syk inhibition may have clinical value in the treatment of patients with SLE.
Figure 4.
Effects of Syk inhibitor R788 on kidney disease in female lupus-prone MRL/lpr mice. A, Levels of proteinuria and urinary leukocytes in MRL/lpr mice treated with R788 (3 gm/kg or 10 gm/kg of chow) or control chow. Values are the mean of ••• mice per group. B, Histopathologic features of kidney sections from MRL/lpr mice treated with R788 (3 gm/kg or 10 gm/kg of chow) or control chow. Representative photomicrographs are shown (original magnification × •••). C, Proteinuria in MRL/lpr mice treated for 6 weeks with R788 (10 gm/kg of chow) or control chow starting at the age of 16 weeks. Values are the mean of 4 mice per group.
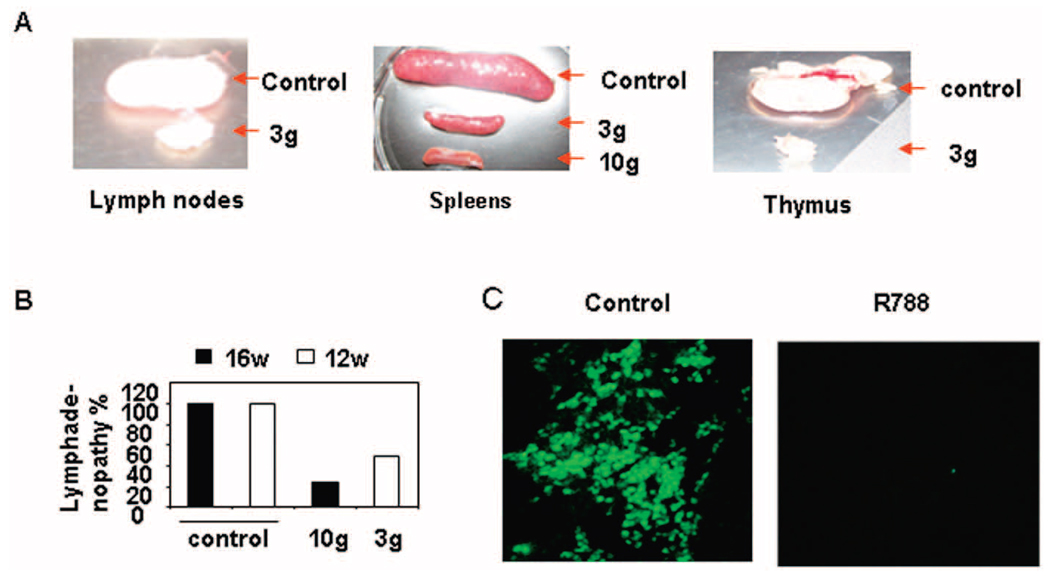
Effect of Syk inhibitor R788 on lymphadenopathy, splenomegaly, thymus size, and autoantibody production in MRL/lpr mice
The sizes of the lymph nodes, spleen, and thymus were significantly smaller in MRL/lpr mice that had been treated with R788 as compared with MRL/lpr mice in the control group (Figure 5A). Furthermore, we observed that the Syk inhibitor decreased established lymphadenopathy in MRL/lpr mice. As shown in Figure 5B, addition of R788 to the chow beginning at age 12 weeks or at age 16 week decreased the size of the lymph nodes. We also measured serum titers of IgG and anti-dsDNA/anti-ssDNA antibody in MRL/lpr mice treated with the Syk inhibitor. Surprisingly, we did not observe any change in the serum levels of IgG or anti-dsDNA/anti-ssDNA in mice in the active treatment group (data not shown).
Figure 5.
Reduction of lymphadenopathy, splenomegaly, and thymus size in female lupus-prone MRL/lpr mice following treatment with Syk inhibitor R788. A, Representative photographs of lymph nodes, spleens, and thymuses from MRL/lpr mice treated with Syk inhibitor R788 (3 gm/kg or 10 gm/kg of chow) or control chow. B, Incidence of lymphadenopathy in MRL/lpr mice treated with 3 gm of R788 per kg of chow starting at age 12 weeks, with 10 gm of R788 per kg of chow starting at age 16 weeks, or with control chow starting at age 12 weeks or 16 weeks. Values are the mean and SD of 4 mice per group. C, Reduced numbers of dendritic cells in skin lesions of MRL/lpr mice following treatment with Syk inhibitor R788. Immunofluorescence staining of anti-CD11c expression in skin lesions from MRL/lpr mice treated for 16 weeks with 3 gm of R788 per kg of chow or with control chow starting at age 6 weeks. Representative photomicrographs are shown (original magnification × •••).
Since dendritic cells are the first to respond to pathogens in the presence of skin inflammation (23), we examined whether R788 treatment affected the presence of dendritic cells in the skin lesions. Using immunofluorescence and an anti-CD11c antibody, we found that treatment with R788 eliminated CD11c+ cells from the skin of MRL/lpr mice (Figure 5C).
Amelioration of skin injury and induction of the development of gray fur color in BAK/BAX double-knockout mice by treatment with Syk inhibitor R788
The BAK/BAX double-knockout mouse has been reported to develop lupus-like disease, including skin rash, spontaneously (24,25). Treatment of BAK/BAX mice with R788 (3 gm/kg or 10 gm/kg of chow) significantly prevented the development of skin injury as compared with those fed control chow (Figure 6). Similar to the observations in the MRL/lpr mice, treatment of BAK/BAX double-knockout mice with R788 reduced splenomegaly and lymphadenopathy. These observations extend the beneficial effects of Syk inhibition to yet another strain of lupus-prone mice.
Figure 6.
Improvement of skin lesions in female BAK/BAX double-deficient mice following treatment with Syk inhibitor R788. A, Incidence of skin lesions (left) and representative photograph showing the appearance of skin lesions (right) in BAK/BAX double-deficient mice treated for 12 weeks with 3 gm of R788 per kg of chow or with control chow starting at age 6 weeks. Values are the mean and SD of 4 mice per group. B, Histopathologic features of skin lesions from BAK/BAX double-deficient mice treated with 3 gm of R788 per kg of chow or with control chow. Representative photomicrographs are shown (original magnification × •••)
DISCUSSION
Building on previous studies showing that Syk inhibition reverses aberrant T cell signaling in SLE patients (13) and improves autoimmune manifestations and pathologic changes in the kidney of (NZB × NZW)F1 mice (21), we present herein evidence that oral administration of the Syk inhibitor R788 improves kidney disease in another lupus-prone mouse model, the MRL/lpr mouse. In addition, we found that R788 has a significant effect on the expression of skin disease not only in the MRL/lpr mouse, but also in the BAK/BAX mouse. Importantly, we found that R788 treatment suppresses established skin and renal disease and that its clinical effects continue for at least 1 month following discontinuation of treatment.
Inhibition of Syk signaling affects the function of diverse cells. Syk inhibition has been shown to induce apoptosis of B cells (17) and tumor cells (26), and it is possible that the reduction of splenomegaly and lymphadenopathy in the MRL/lpr and BAK/BAX mice is the result of increased cell death in the presence of the Syk inhibitor. Both strains have a profound defect in apoptosis (22,25). In this study, we found that CD11c+ cells did not enter the skin of R788-treated mice, which suggests that there are broader effects of Syk inhibition in the immune system. Since Syk is widely expressed in diverse cell types, including T cells, B cells, macrophages, and dendritic cells, we think that the Syk inhibitor R788 exerts its effects on some combination of T cells, B cells, and dendritic cells.
Interestingly, Syk inhibition did not lead to significant inhibition of the production of anti-dsDNA/anti-ssDNA in the MRL/lpr mouse. This observation is consistent with those in previous reports of another lupus-prone mouse and a collagen-induced model of arthritis. Specifically, while Syk inhibition suppressed lupus nephritis in the (NZB × NZW)F1 mouse, it did not affect autoantibody titers (21). In the collagen-induced arthritis model, Syk inhibition had a profound effect on the development of clinical arthritis, bone erosions, pannus formation, and synovitis, but no effect on the anticollagen antibody levels (16). These observations indicate that the clinical effect of Syk inhibitors does not rely on the production of antibody, but rather, on immune and nonimmune cells (14).
Syk inhibition in patients with rheumatoid arthritis resulted in prompt clinical improvement (20), whereas the main side effects were limited to the gastrointestinal tract as well as neutropenia. Clinical benefit has also been reported in patients with idiopathic thrombocytopenia (27). The results of studies examining T cells from SLE patients (13), of preclinical studies reported previously (21) and those reported herein, as well as phase II studies in rheumatoid arthritis and thrombocytopenia make a compelling case for exploration of the use of Syk inhibitors in the treatment of patients with SLE.
Acknowledgments
Supported by the PHS/NIH (grant R01-AI-42269), the Mary Kirkland Center for Lupus Research, and Rigel Pharmaceuticals.
Footnotes
Dr. Pine owns stock or stock options in Rigel Pharmaceuticals and is coinventor with Rigel Pharmaceuticals on a patent for R788, a small-molecule inhibitor of Syk (US 2700 4626A1). Dr. Tsokos has received consulting fees, speaking fees, and/or honoraria from Genentech, Rigel, GeneWay, and Human Genome Sciences (less than $10,000 each).
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Tsokos had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Deng, Bahjat, Pine, Tsokos.
Acquisition of data. Deng, Liu.
Analysis and interpretation of data. Deng, Bahjat, Tsokos.
REFERENCES
- 1.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, et al. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk-and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 4.Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 5.Darby C, Geahlen RL, Schreiber AD. Stimulation of macrophage FcγRIIIA activates the receptor-associated protein tyrosine kinase Syk and induces phosphorylation of multiple proteins including p95Vav and p62/GAP-associated protein. J Immunol. 1994;152:5429–5437. [PubMed] [Google Scholar]
- 6.Reeve JL, Zou W, Liu Y, Maltzman JS, Ross FP, Teitelbaum SL. SLP-76 couples Syk to the osteoclast cytoskeleton. J Immunol. 2009;183:1804–1812. doi: 10.4049/jimmunol.0804206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurosaki T, Hikida v. Tyrosine kinases and their substrates in B lymphocytes. Immunol Rev. 2009;228:132–148. doi: 10.1111/j.1600-065X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 11.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks Fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 12.Cha HS, Boyle DL, Inoue T, Schoot R, Tak PP, Pine P, et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther. 2006;317:571–578. doi: 10.1124/jpet.105.097436. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan S, Juang YT, Chowdhury B, Magilavy A, Fisher CU, Nguyen H, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. 2008;181:8145–8152. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyttaris VC, Tsokos GC. Syk kinase as a treatment target for therapy in autoimmune diseases. Clin Immunol. 2007;124:235–237. doi: 10.1016/j.clim.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsubara S, Li G, Takeda K, Loader JE, Pine P, Masuda ES, et al. Inhibition of spleen tyrosine kinase prevents mast cell activation and airway hyperresponsiveness. Am J Respir Crit Care Med. 2006;173:56–63. doi: 10.1164/rccm.200503-361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pine PR, Chang B, Schoettler N, Banquerigo ML, Wang S, Lau A, et al. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin Immunol. 2007;124:244–257. doi: 10.1016/j.clim.2007.03.543. [DOI] [PubMed] [Google Scholar]
- 17.Young RM, Hardy IR, Clarke RL, Lundy N, Pine P, Turner BC, et al. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood. 2009;113:2508–2516. doi: 10.1182/blood-2008-05-158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig TE, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheridan C. Small molecule challenges dominance of TNF-α inhibitors. Nat Biotechnol. 2008;26:143–144. doi: 10.1038/nbt0208-143. [DOI] [PubMed] [Google Scholar]
- 20.Weinblatt ME, Kavanaugh A, Burgos-Vargas R, Dikranian AH, Medrano-Ramirez G, Morales-Torres JL, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:3309–3318. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 21.Bahjat FR, Pine PR, Reitsma A, Cassafer G, Baluom M, Grillo S, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum. 2008;58:1433–1444. doi: 10.1002/art.23428. [DOI] [PubMed] [Google Scholar]
- 22.Deng GM, Tsokos GC. Cholera toxin B accelerates disease progression in lupus-prone mice by promoting lipid raft aggregation. J Immunol. 2008;181:4019–4026. doi: 10.4049/jimmunol.181.6.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Bravo M, Ardavin C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29:343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Nazareth M, Fanti P, Schwach C, Poppenberg K, Janis K, Aronica SM. Altered Bax expression and decreased apoptosis in bone marrow cells of lupus-susceptible NZB/W mice. Lupus. 2001;10:785–793. doi: 10.1177/096120330101001105. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ, et al. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M, et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 27.Gao C, Boylan B, Bougie D, Gill JC, Birenbaum J, Newman DK, et al. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcγRIIa and the integrin β3 cytoplasmic domain. J Clin Invest. 2009;119:504–511. doi: 10.1172/JCI36745. [DOI] [PMC free article] [PubMed] [Google Scholar]