Abstract
To determine whether data quality is meaningfully reduced by high electrode impedance, EEG was recorded simultaneously from low- and high-impedance electrode sites during an oddball task. Low-frequency noise was found to be increased at high-impedance sites relative to low-impedance sites, especially when the recording environment was warm and humid. The increased noise at the high-impedance sites caused an increase in the number of trials needed to obtain statistical significance in analyses of P3 amplitude, but this could be partially mitigated by high-pass filtering and artifact rejection. High electrode impedance did not reduce statistical power for the N1 wave unless the recording environment was warm and humid. Thus, high electrode impedance may increase noise and decrease statistical power under some conditions, but these effects can be reduced by using a cool and dry recording environment and appropriate signal processing methods.
In event-related potential (ERP) studies, researchers have traditionally minimized noise in the recordings by reducing the impedance between the recording electrodes and the living skin tissue (Luck, 2005; Picton et al., 2000). When large numbers of electrodes are used, however, the process of reducing the impedances becomes very time consuming, and this has led manufacturers of electroencephalogram (EEG) recording systems to develop systems that can tolerate high electrode impedances. These systems have become quite popular, but many researchers are concerned that the quality of the EEG will be poorer with high electrode impedances than with low electrode impedances. High electrode impedances do not meaningfully reduce the size of the EEG signal (Johnson et al., 2001), but they might increase the noise level, resulting in a lower signal-to-noise (S/N) ratio. The goal of the present study was to determine whether the S/N ratio is meaningfully reduced when the EEG is recorded with high compared to low electrode impedances.
If the S/N ratio of the EEG is lower, more trials will need to be averaged together to obtain a given S/N ratio in the averaged ERPs. That is, a decline in the S/N ratio of the EEG recordings will necessitate an increase in the number of trials tested in each subject or an increase in the number of subjects tested in an experiment to achieve a given S/N ratio in the averaged ERPs. To put this in terms that reflect the “bottom line” for most ERP researchers, recording the EEG under conditions that yield a lower S/N ratio will either decrease the probability of obtaining a statistically significant experimental effect (if the number of trials is held constant) or increase the amount of recording time necessary to obtain a significant effect (if the statistical power is held constant). In most cases, researchers would like to maintain the probability of obtaining a significant effect. However, the increased number of trials required for this is often higher than one might expect, because the S/N ratio of an average does not increase linearly with the number of trials in the average but instead increases with the square root of the number of trials (see Luck, 2005). If, for example, the S/N ratio of the raw EEG recording is half as great with high electrode impedances as with low electrode impedances, then it would be necessary to record four times as many trials with high electrode impedances as with low electrode impedances to achieve the same S/N ratio and hence the same statistical power in the averaged ERPs (all else being equal).
However, it is not clear whether the S/N ratio will actually be meaningfully reduced when high electrode impedances are used in conjunction with an EEG recording system that was designed to tolerate these high impedances (which we will call high-impedance systems). There are several published papers describing the properties and performance of these systems (Ferree, Luu, Russell, & Tucker, 2001; Metting van Rijn, Peper, & Grimbergen, 1990; Tucker, 1993), but these papers do not provide direct, empirical, and quantitative comparisons of low and high electrode impedances under the conditions of a typical ERP experiment. For example, the study of Ferree et al. (2001) simply recorded the resting EEG and assessed the power in different frequency bands as a function of impedance.
The goal of the present study was to provide a quantitative evaluation of the effects of electrode impedance on the S/N ratio of ERP recordings by an independent laboratory with no significant ties to manufacturers of EEG recording equipment1. In particular, the study was designed to determine whether more trials must be averaged together to obtain a significant experimental effect with high electrode impedances than with low electrode impedances. To provide a direct test of the effects of electrode impedance, unconfounded by other factors, we compared high and low electrode impedances within a single recording system rather than comparing a high-impedance system with a low-impedance system (as in the study of Johnson et al., 2001). A comparison across different systems would confound factors other than electrode impedance that vary across systems (e.g., amplifier noise levels, electrode composition, shielding effectiveness, analog-to-digital converter precision, etc.). Thus, we addressed the general issue of the effects of electrode impedance on S/N ratio rather than comparing the performance of specific commercial EEG recording systems. This is an important distinction, because the finding of a decreased S/N ratio when the electrode impedance is higher does not mean that the S/N ratio of a given high-impedance system will be lower than the S/N ratio of a completely different low-impedance system. There are simply too many other factors that differ across systems to draw such a conclusion. However, the finding of a large impact of electrode impedance on data quality would have important implications for the procedures used to collect data with a given EEG recording system.
Before describing the study, we will define the term impedance and consider the reasons why high electrode impedance might or might not be expected to yield a lower S/N ratio.
What is Impedance?
Impedance is opposition to alternating current (AC) flow, and it has two components, resistance and reactance. Resistance by itself is opposition to direct current (DC) flow, and in the context of impedance is a frequency-independent opposition to AC current flow. A volume control on a radio, for example, is typically a device that creates a variable resistance. Reactance is a combination of capacitance and inductance, which oppose AC current flow in a manner that depends on the frequency content of the AC current. Because the EEG contains a strong AC signal, ERP researchers measure impedance rather than merely resistance.
In the context of EEG recordings, impedance is typically measured by passing a small 10-Hz current between two or more electrodes and measuring the opposition to the flow of this current (for more details, see Chapter 3 in Luck, 2005). The goal is to measure the impedance between the electrode and the highly conductive living skin tissue that immediately overlies the skull (i.e., the electrode impedance). The living skin tissue is covered by a layer of dead skin cells, and these dead skin cells provide a relatively high-impedance interface between the electrode2 and the living skin tissue. When impedance is measured between two electrodes, the measured value reflects the impedance of everything between the two electrodes, which includes the impedance between each electrode and the living skin tissue lying right beneath it (with a small contribution from the impedance of the tissues between the living skin under the electrodes). However, it is possible to estimate the impedance between each individual electrode and the underlying living skin (see Method section below and Chapter 3 in Luck, 2005).
High Impedance and Common Mode Rejection
We will now consider why the electrode impedance might be expected to impact the S/N ratio of EEG recordings. Two key issues are commonly raised in this context, namely common mode rejection and cephalic skin potentials. Common mode rejection refers to the ability of a recording system to reject noise that is in common to the active and reference electrodes. That is, any noise sources that are identical in the active and reference recording electrodes are attenuated in a differential amplifier, because the output of the amplifier subtracts the voltage measured at the reference electrode from the voltage measured at the active electrode3. This primarily eliminates noise induced by electrical devices in the recording environment (e.g., lights, video displays, wiring, etc.) rather than biological noise generated by the subject (e.g., muscle activity, eye blinks, etc.). As the common mode rejection of an amplifier increases, the contribution of noise signals decreases and the S/N ratio increases.
To eliminate noise that is in common to the active and reference sites, the signals arising from these sites must be treated equivalently. The ability of an EEG amplifier to accomplish this depends, in part, on the ratio of the electrode impedance to the amplifier’s input impedance. The amplifier’s input impedance is its tendency to oppose the flow of current from the electrodes through the amplifier, and it is determined by the electronics used in the amplifier. The amplifier’s input impedance is a fixed value, typically in the Megohm range or higher. The electrode impedance, in contrast, is determined by the properties of the skin, which can vary considerably across individuals, across electrode sites, and across time. As the electrode impedance increases, the common mode rejection of the system decreases, and the S/N ratio of the recording decreases. This is primarily due to the fact that random differences between the impedances at the different electrode sites are typically magnified when the impedances are increased (Ferree et al., 2001). Thus, all else being equal, more trials will be needed to achieve a given level of statistical significance with high electrode impedances than with low electrode impedances.
With traditional low-impedance EEG recording systems, this problem is typically solved by cleansing and abrading the skin. Abrasion of the skin reduces impedance by disrupting the external layer of dead skin cells, providing a more direct contact with the underlying living skin tissue. Oils on the surface of the skin may also play a role in impedance, and cleansing the skin may reduce the contribution of these oils.
When large numbers of electrodes are used, a significant amount of time is required to reduce the impedance at each electrode site. In addition, abrasion of the skin makes it possible for blood-borne pathogens to be transferred from the subject to the electrodes and vice versa, and this could potentially lead to the transfer of illnesses such as hepatitis and AIDS from one subject to the next. Disinfection of the electrodes between subjects can reduce the possibility of disease transmission, but it is not practical to completely sterilize the electrodes and thereby completely eliminate the possibility of disease transmission. In addition, abrasion of the skin can leave a red mark or even a scab, which is particularly problematic for studies of infants and young children. For these reasons, many investigators would like an alternative to low-impedance recordings.
To deal with the problem of decreased common mode rejection with high electrode impedances, it is possible to use amplifiers with a higher input impedance, thus yielding the same ratio of electrode impedance to input impedance that would be obtained in a traditional low-impedance system. In addition, high-impedance recording systems often include features that reduce sensitivity to induced noise from electrical devices near the subject, such as preamplifiers built into the electrodes (active electrodes) and shielding of the electrode cables (see review by Metting van Rijn et al., 1990).
It should be noted that most of the induced electrical noise in most ERP experiments arises from AC devices near the subject or electrode cables (called line noise). This noise has a frequency of 50 or 60 Hz, depending on the nature of a country’s electrical system (e.g., 50 Hz in Europe, 60 Hz in North America). Consequently, when high electrode impedances lead to reduced common mode rejection, any increase in the noise level is typically largest at the 50- or 60-Hz line frequency.
High Impedance and Cephalic Skin Potentials
High electrode impedances may lead to a second problem that cannot be solved by means of changes to the amplifier’s input impedance, namely an increase in skin potential artifacts. Skin potentials arise because of the standing electrical potential that is normally present between the inside and the outside of the skin (Edelberg, 1972). The magnitude of this potential depends on the conductance of the skin, which in turn depends on factors such as the thickness of the skin, the number of sweat glands and hair follicles, and the degree of skin hydration (Fowles, 1971; Tregear, 1966). When the voltage is recorded between two electrodes on the surface of the skin, any differences in the conductance of the skin under these two electrodes will lead to a different voltage offset for each electrode, which creates an electrical potential between the two electrodes. This potential will vary over time if the conductance of the skin under one electrode varies over time in a different manner than the conductance of the skin under the other electrode.
Sweat glands form a variable-resistance bridge between the inside and the outside of the skin and play an important role in these changes in skin potential over time (see review by Fowles, 1986). When the air temperature is in the low-to-normal range and stress levels are low, the individual’s sweat glands will contain relatively little sweat, and they will not serve as good conductors. Under these conditions, the impedance between the outside and the inside of the skin will ordinarily be high (because conductance is inversely related to impedance). If the individual’s body temperature or stress level increases, sweat will begin to fill the sweat glands, and this will increase the conductance and thereby decrease the impedance, even if no sweat is actually excreted from the sweat gland onto the surface of the skin. As the impedance between the inside and the outside of the skin changes, the electrical potential also changes, creating a very large artifact (often several hundred microvolts). Thus, changes in temperature or psychological state may cause changes in the potential recorded between the scalp electrodes.
Skin potentials usually consist of slow shifts in voltage over a period of many seconds. They are especially pronounced in certain body parts, such as the palms of the hands, and they also occur across the surface of the head (where they are termed cephalic skin potentials). Skin potentials become much more prominent under warm and humid recording conditions, where they provide a significant source of noise, distorting the amplitudes of relatively slow ERP components such as the P3 wave and the contingent negative variation (Corby, Roth, & Kopell, 1974; Picton & Hillyard, 1972). They also distort the baseline and therefore add noise to the measurement of faster ERP components as well.
There are two main ways to decrease skin potential artifacts in EEG recordings. First, one can reduce the occurrence of changes in the sweat level within the sweat glands. For example, keeping the recording environment consistently cool and dry will reduce the occurrence of skin potentials. Second, one can reduce the size of the voltage measured at the recording electrodes when the sweat level changes. Abrasion of the skin under the electrode will have this effect, because it creates a low-impedance voltage shunt between the living skin tissue and the electrode. That is, because electricity tends to follow the path of least resistance, a low-impedance connection between the outside and the inside of the skin in one location will minimize the voltage change produced by a change in the impedance at a nearby location. Picton and Hillyard (1972) note that puncturing the skin with a needle at the recording site is the most effective means of eliminating skin potentials; gentle abrasion of the skin also appears to be quite effective at minimizing skin potentials and can be accomplished with little or no discomfort (see Chapter 3 in Luck, 2005).
The Present Study
Unlike the problem of common mode rejection, the problem of increases in low-frequency noise due to skin potentials is not as easy to solve by means of changes to the circuitry of an EEG recording system4. Thus, it is possible that the use of high electrode impedances will result in an increase in low-frequency noise even in recording systems that are designed to tolerate these high impedances, and that this will decrease the S/N ratio and increase the number of trials needed to obtain a statistically significant experimental effect. This problem would be expected to be more severe under warm recording conditions, especially when the humidity is high, because these conditions increase the incidence and magnitude of skin potentials. Thus, the present study was designed to quantify the S/N ratio and the number of trials required to obtain a statistically significant experimental effect in low- versus high-impedance recordings, using both cool and dry recording conditions and warm and humid recording conditions. It should be noted that the comparison of low and high electrode impedances by Ferree et al. (2001) did not examine frequencies below 1 Hz and did not report the temperature and humidity of the recording environment, so it does not provide any information about the potential problem of skin potential artifacts.
High electrode impedances may also lead to an increase in 50- or 60-Hz line noise. Our laboratory is well shielded to reduce sources of line noise, and so we were unable to accurately assess the effects of electrode impedance on line noise. This limitation will be addressed further in the Discussion.
To make the present experiment relevant for a large number of ERP researchers, we used the most common ERP paradigm, the oddball task. The stimulus sequences consisted of 20% target stimuli and 80% standard stimuli. Our main analyses were focused on the P3 wave, which was expected to be larger for the targets than for the standards. We used a high-impedance recording system, but we abraded the skin under the electrodes in one hemisphere so that we could obtain low-impedance recordings from that hemisphere simultaneously with high-impedance recordings from the other hemisphere. Thus, one hemisphere served as the control for the other hemisphere, minimizing the contribution of global state factors and individual differences to the results. In addition, we kept the recording environment cool and dry for half of each recording session and warm and humid for the other half5, allowing us to assess interactions between impedance and recording environment.
Our main question was whether more trials would be necessary to obtain a statistically significant difference in P3 amplitude between targets and standards in the high-impedance hemisphere than in the low-impedance hemisphere. We predicted that the S/N ratio would be somewhat worse for the high-impedance recordings than for the low-impedance recordings under cool and dry conditions, with a further decline under warm and humid conditions. In addition, we predicted that these changes in S/N ratio would lead to increases in the number of trials required to obtain a statistically significant effect of probability on P3 amplitude. Because the P3 probability effect is much larger than most ERP effects, we also examined the effects of impedance on statistical significance for the somewhat subtler interaction between probability and electrode site. In addition, we also examined the N1 component to determine whether changes in impedance would also influence a smaller, earlier, shorter-duration ERP component.
Method
Participants
Seventeen subjects between the ages of 18 and 30 were tested. Our laboratory always excludes any subject who has artifacts on greater than 25% of trials under our typical cool and dry testing conditions, and five subjects were eliminated for this reason in the present study. All reported analyses are from the remaining 12 subjects.
Stimuli and Task
The stimuli were black letters and digits, each measuring 2.5 × 2.5° of visual angle, presented at the center of a cathode ray tube video monitor. The monitor was viewed at a distance of 70 cm and had a light gray background and a continuously visible fixation point. Each stimulus was presented for 200 ms, followed by a blank intertrial interval of 1100–1500 ms (rectangular distribution). Subjects alternated between blocks in which letters were 80% probable and digits were 20% probable, and blocks in which this was reversed. The starting point was randomized across subjects. Subjects pressed a button with the index finger of the dominant hand for one stimulus category and with the middle finger of the dominant hand for the other stimulus category; the assignment of stimuli to buttons was counterbalanced. Each block contained 160 trials, and a rest break was provided every 40 trials. For both the cool and dry condition and the warm and humid condition, each subject received 4 blocks, yielding a total of 128 target stimuli and 512 standard stimuli in each recording environment.
Recording and Analysis
The EEG was recorded inside an Eckel C-15A sound attenuating chamber with an RF shielding package and no windows. The video monitor was enclosed within a Faraday cage (see pp. 114–115 in Luck, 2005) and powered via a shielded AC cable. Lighting was provided by strips of DC-powered light emitting diodes (LEDs; Nemalux LED Lighting, Model GSLED24-12-W).
The temperature and humidity inside the recording chamber were either decreased (using fans and the building ventilation system) or increased (using a space heater and humidifiers) to achieve a cool and dry environment or a warm and humid environment. The fans, space heater, and humidifiers were turned off during the actual recordings. We measured the temperature and humidity at the beginning and end of each temperature/humidity condition. At the start of the cool and dry testing condition, the temperature in the recording chamber was lowered to between 19.5 and 23° C (67–73° F), with a mean of 21.3° C (70.3° F) and a standard error of 0.29° C. At the end of the cool and dry testing condition, the temperature in the recording chamber was between 19.8 and 22.2° C (67.6–71.9° F) with a mean of 21.2° C (70.1° F) and a standard error of 0.20° C. At the start of the warm and humid testing condition, the temperature in the recording chamber was between 26.0 and 29.5° C (79–85 °F), with a mean of 27.7° C (81.8° F) and a standard error of 0.29° C. At the end of the warm and humid testing condition, the temperature in the recording chamber was between 25.0 and 27.6° C (77–81.7° F) with a mean of 26.1° C (79.0 ° F) and a standard error of 0.21° C. The absolute humidity in the cool and dry testing session was a mean of 7.67 g/m3 and a standard error of 0.376 at the beginning of the session and a mean of 7.83 g/m3 and a standard error of 0.373 at the end of the session. For the warm and humid testing session, the absolute humidity was an average of 11.5 g/m3 with a standard error of 0.564 at the beginning of the session and an average of 9.11 g/m3 with a standard error of 0.339 at the end of the session.
Given that 25° C (78° F) is a typical recommended summertime room temperature in the U.S., the average warm temperature of 27.7° C (81.8° F) in the warm and humid condition is well within the range of temperatures one might expect inside a small EEG recording chamber, especially in a poorly ventilated building. Moreover, the humidity level in this condition (which corresponds to 44% relative humidity) is also within a normal range for much of the world. It should be noted that warmer temperatures may make subjects drowsier and less attentive. However, because we were recording from low and high impedance electrode sites simultaneously, any differences in subject state between the cool and warm conditions would have equivalent effects on the low and high impedance sites.
The order of the testing conditions was counterbalanced across subjects. Subjects completed both conditions in the same testing session, with a one-hour break between conditions to allow for the temperature and humidity of the recording chamber to be adjusted.
To determine the effects of electrode impedance on data quality, we lowered electrode impedances in one hemisphere of each subject to less than 5 KΩ, and electrode impedances in the other hemisphere were allowed to remain at their naturally high levels (ranging from 10–190 KΩ). The impedances were lowered in the left hemisphere for half of the subjects and in the right hemisphere for the other half. Impedances were lowered using traditional scalp abrasion techniques and were measured using a Grass F-EZM4A impedance meter. Electrode impedances were measured before and after each half of the session, for a total of four times per subject. Table 1 provides the means and standard errors for the low- and high-impedance measurements across time points.
Table 1.
Mean electrode impedance (Z) as a Function of Recording Environment and Electrode Site (SEM in parentheses)
| Electrode Site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F3/4 | C3/4 | P3/4 | P9/10 (reference) | CMS | DRL | |||||
| Session | Low Z | High Z | Low Z | High Z | Low Z | High Z | Low Z | High Z | Low Z | Low Z |
| Before Cool/Dry Block | 3.11 (0.52) | 56.44 (13.25) | 2.82 (0.55) | 68.48 (13.41) | 3.85 (0.45) | 88.84 (18.86) | 2.99 (0.47) | 81.02 (17.58) | 3.45 (0.65) | 2.49 (0.29) |
| After Cool/Dry Block | 2.51 (0.40) | 29.43 (6.89) | 2.43 (0.38) | 34.2 (8.43) | 3.03 (0.38) | 43.83 (7.96) | 2.65 (0.44) | 24.72 (6.35) | 2.90 (0.40) | 2.03 (0.21) |
| Before Warm/Humid Block | 2.52 (0.38) | 32.95 (8.27) | 2.38 (0.46) | 33.27 (9.50) | 2.83 (0.36) | 43.70 (11.60) | 4.20 (1.61) | 34.26 (5.84) | 2.97 (0.33) | 2.44 (0.36) |
| After Warm/Humid Block | 2.63 (0.39) | 42.68 (12.82) | 2.41 (0.44) | 34.5 (10.35) | 2.90 (0.42) | 38.12 (7.56) | 2.78 (0.45) | 33.53 (6.82) | 5.76 (1.80) | 4.25 (1.16) |
The EEG was recorded using a Biosemi ActiveTwo EEG recording system (Biosemi B.V., Amsterdam, Netherlands). This system has a number of features that are designed to optimize data quality, including a preamplifier within each electrode, a driven right leg circuit, very high electrical isolation, and a low bias current. In contrast to most EEG amplifiers, which amplify the difference between the active-ground voltage and the reference-ground voltage, the Biosemi Active Two system amplifies and measures the single-ended voltage between each electrode site and a common mode sense (CMS) electrode. All referencing is accomplished offline.
The electrodes were mounted in an elastic cap using a subset of the International 10/20 System sites (F3, C3, P3, P9, F4, C4, P4, P10), as depicted in Figure 1. Signa Gel (Parker Labs, Fairfield, NJ) was used to create a stable electrical connection between each electrode and the scalp. The electrode offset was kept below 40 mV. The CMS electrode was located at site FC1, with a driven right leg (DRL) electrode located at site FC2. The impedance of the CMS and DRL electrodes were both lowered to less than 5 K; any effect of the impedance at the CMS and DRL sites will equivalently affect both the low- and high-impedance sites.
Figure 1.
Electrode recording montage.
The single-ended signals were converted to differential signals offline, with right hemisphere electrodes referenced to electrode P10 (near the right mastoid) and left hemisphere electrodes referenced to electrode P9 (near the left mastoid). The impedance of the reference electrode for a given hemisphere was lowered if the impedances of the other electrodes in that hemisphere were lowered. The horizontal electrooculogram (EOG) was recorded from electrodes placed lateral to the external canthi and was used to measure horizontal eye movements. The vertical EOG was recorded from electrodes above and below the right eye to detect blinks. The monopolar EOG signals were converted to bipolar signals offline (left minus right for horizontal EOG and lower minus upper for vertical EOG). The EEG and EOG were low-pass filtered using a fifth order sinc filter with a half-power cutoff at 204.8 Hz and then digitized at 1024 Hz with 24 bits of resolution.
Because the Biosemi ActiveTwo system uses active electrodes, in which each electrode contains a preamplifier, electrode impedances cannot be measured with the standard electrodes. Consequently, we purchased a set of passive electrodes from Biosemi and used them when we were measuring the impedances6.
Impedance is typically measured by passing a small alternating current between two or more electrodes connected to the skin. Thus, the measured impedance reflects contributions from more than a single electrode. To determine the impedance at one specific electrode, we tested impedances in sets of two electrodes and measured each electrode separately against two additional electrodes. We then computed the impedance of each individual electrode using a simple algebraic expression. Specifically, we defined A+B as the impedance measured between electrodes A and B, A+C as the impedance measured between electrodes A and C, and B+C as the impedance measured between electrodes B and C. With these three measurements, the impedance at electrode A can be computed as [(A+B)+(A+C)−(B+C)]÷2.
Monte Carlo Analyses
One of our primary goals was to assess the number of trials necessary to achieve statistical significance with low and high electrode impedances under cool and dry conditions and under warm and humid conditions. To achieve this goal, we performed Monte Carlo analyses in which we simulated experiments with varying numbers of trials by sampling random subsets of the large number of trials that were recorded from each subject. We then determined whether a given simulated experiment yielded a significant difference in P3 amplitude between the target and standard stimulus categories using a conventional paired t-test. To obtain a robust estimate of the likelihood of achieving statistical significance with a given number of trials, we simulated 1000 experiments for a given number of trials by sampling different random subsets of trials for each simulated experiment. This allowed us to estimate the statistical power (e.g., the probability of attaining p < .05) for a given number of trials. This was done separately for the four combinations of electrode impedance (low versus high) and recording condition (cool and dry versus warm and humid). In our P3 analyses, we simulated experiments with 10, 20, 30, 40, 50, 60, and 70 artifact-free trials (target:standard ratios of 2:8, 4:16, 6:24, 8:32, 10:40, 12:48, and 14:56 trials, preserving the 20% target and 80% standard stimulus probabilities in the experimental design).
Because the data were recorded in a highly shielded environment with an EEG recording system that is designed to minimize induced electrical noise, we expected that electrode impedance would primarily impact low-frequency noise. That is, although increased electrode impedance would be expected to yield increased 60-Hz line noise, other aspects of the recording system and environment were expected to yield such a low level of line noise that any increase would be difficult to assess. To determine whether filtering would mitigate the expected low-frequency noise and to assess the optimal filter cutoff value, we repeated the simulation after applying a high-pass filter with a half-amplitude cutoff value of 0.01, 0.05, 0.10, 0.50, or 1.0 Hz (noncausal Butterworth impulse response function, −24 dB/octave). The DC offset was removed before filtering by subtracting the mean voltage across the entire trial block. No low-pass filtering was applied. In total, we simulated 140,000 experiments, 1000 for each combination of electrode impedance, recording temperature, number of trials, and filter setting.
The EEG data from each trial were sorted according to whether the stimulus was in the target category or in the standard category, and epochs were extracted from −1000 to 2000 ms. Each epoch was then baseline-corrected by subtracting the mean voltage from the 400 ms prior to stimulus onset. This is necessary because the EEG voltage fluctuations ride on top of a relatively large, slowly changing voltage offset, which would add enormous variance to the ERP measurements if not removed. Trials containing artifacts, defined as voltages exceeding ±100 μV, were marked for rejection and were excluded from all analyses described below7. Trials with incorrect behavioral responses were also excluded. After these trials were excluded, we randomly selected the appropriate number of target and standard trials from the set of trials obtained from a given subject (e.g., 10 target trials and 40 standard trials when simulating an experiment with 50 trials). We then computed the averaged ERP waveforms from these trials. From these averaged waveforms, we measured P3 amplitude as the mean voltage between 350 and 650 ms at the P3 or P4 electrode site (depending on which hemisphere had the impedance that was currently being tested). This set of procedures was repeated for each subject, and we then performed a paired-samples t-test to compare the amplitudes of the target and standard trials. This same procedure was repeated 1000 times with different random selections of trials for each combination of electrode impedance, recording temperature, number of trials, and filter setting. All simulations were conducted in Matlab (The Mathworks, Inc., Natick, MA) using the EEGLAB Toolbox (Delorme & Makeig, 2004) and custom routines.
To determine whether our results would generalize to other ERP components and other types of experimental effects, we conducted a similar set of analyses examining the scalp distribution of the N1 wave. Rather than assessing the differences between target and standard trials at a single electrode site, we assessed the differences in voltage between two electrode sites (P3 versus C3 or P4 versus C4, depending on which hemisphere had the appropriate impedance level) using only the data from the standard trials. N1 amplitude was measured as the mean voltage between 80 and 100 ms. We simulated experiments with 16, 32, 48, 64, 80, 96, and 112 artifact-free trials from each electrode site. The simulations were otherwise identical to those described for the P3 wave.
Although we conducted simulations of 140 combinations of conditions for each of two ERP components, there are many other useful ways in which the data could be analyzed (e.g., different filter settings, measurement approaches, statistical analyses, etc.). We have therefore made the full data set available at http://erpinfo.org/impedance so that interested researchers can test other signal processing and data analysis approaches.
Results
Basic ERP Results
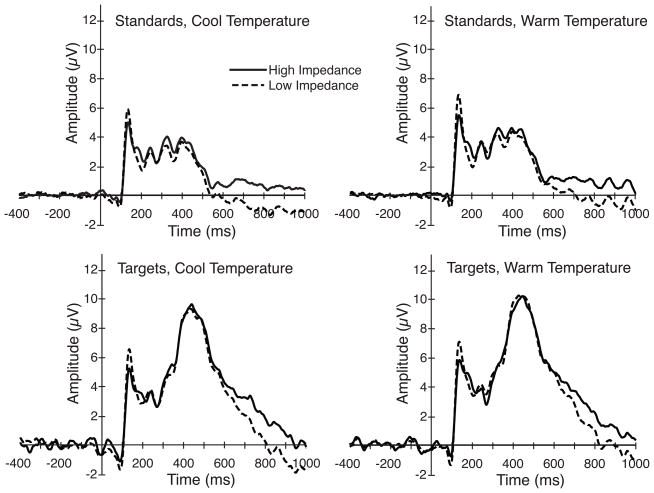
Figure 2 shows the grand average ERP waveforms for the target and standard stimuli, based on all of the artifact-free trials for each subject. As would be expected on the basis of thousands of prior studies, the target stimuli elicited a greater positivity than the standard stimuli in the P3 latency range (see below for statistical analyses). The same basic pattern was observed for all four combinations of impedance and recording environment. Due to the large number of trials per condition, the noise level evident in the prestimulus baseline period was quite low, although it was clearly higher for the target stimuli than for the standard stimuli (reflecting the smaller number of target trials).
Figure 2.
Grand average ERP waveforms for low- and high-impedance parietal electrode sites for the standard and target stimuli in the cool and warm recording environments. The waveforms were low-pass filtered with a half-amplitude cutoff of 30 Hz (noncausal Butterworth impulse response function, −24 dB/octave) in this and all subsequent figures.
As has been described previously (Johnson et al., 2001), higher impedance did not produce a substantially smaller overall signal size. However, the P2 wave (ca. 150 ms) was somewhat smaller for the high-impedance electrode sites than for the low-impedance electrode sites for both the target and standard stimuli in both the cool and warm recording environments. This effect was primarily caused by a single subject who exhibited an extremely large hemispheric asymmetry in the P2 wave, and this effect was not statistically significant. Specifically, an ANOVA on the mean amplitude from 125–175 ms with factors of probability, impedance level, and recording environment yielded a significant main effect of probability (F(1,11) = 7.09, p = .022), with no significant main effects of impedance (p = .650) or recording environment (p = .254) and no significant interactions between probability and impedance (p = .203), probability and recording environment (p = .155), impedance and recording environment (p = .946), or the three-way interaction between probability, recording environment and impedance (p = .495). Similarly, an ANOVA on the mean amplitude from 350–650 ms yielded a significant main effect of probability (F(1,11) = 50.69, p < .001). The main effects of impedance (p = .627) and recording environment (p = .385), and the interactions between probability and impedance (p = .189), probability and recording environment (p = .979), impedance and recording environment (p = .759), and the three-way interaction between probability, recording environment and impedance (p = .920) all failed to reach significance. An analysis of the mean amplitude from 650–1000 also yielded a significant main effect of probability (F(1,11) = 15.75, p = .002), with no significant main effects of impedance (p = .161) or recording environment (p = .639) and no significant interactions between probability and impedance (p = .788), probability and recording environment (p = .944), impedance and recording environment (p = .720), and no significant three-way interaction between probability, impedance and recording environment (p = .688).
These results indicate that the use of high electrode impedances does not produce a global attenuation of the ERP signal.
EEG Quality
To determine whether there was an increase in low-frequency voltage fluctuations in the high-impedance conditions, as would be expected from an increase in skin potentials, we visually inspected the data on a time scale that displayed the EEG over an entire trial block (approximately 5 minutes). Figure 3 shows the EEG from the low- and high-impedance electrode sites in a representative subject, recorded during a warm temperature, high humidity block and a cool temperature, low humidity block. Very slow voltage drifts can be seen across the 5-minute recording period for all four combinations of impedance and recording environment. These very slow drifts did not appear to vary systematically across conditions when the data from all subjects were inspected, and they were much slower and larger than the skin potentials typically caused by changes in the level of sweat in the sweat glands. Instead, they likely reflect slower processes, such as changes in skin hydration (Fowles & Venables, 1970).
Figure 3.
Raw EEG data from a representative subject for the first block of testing for both low- and high-impedance parietal electrode sites in each of the recording environments. Data were downsampled to 10 Hz to illustrate the low-frequency voltage fluctuations over time. The sharp downward spikes are eyeblink artifacts. Note that the data from the high- and low-impedance sites were collected simultaneously, whereas the data from the warm and cool recording environments were collected in separate trial blocks.
Faster changes in voltage can be seen in the EEG from the high-impedance electrode in the warm and humid condition. These voltage fluctuations occurred over periods of tens of seconds, which is typical for skin potentials (Edelberg, 1972). They often consisted of quite large and relatively sudden changes in voltage (see arrows in Figure 3). As in the individual subject shown in Figure 3, almost all of the subjects exhibited more pronounced voltage fluctuations of this nature in the high-impedance recordings than in the low-impedance recordings, especially in the warm and humid recording condition. Quantitative support for these observations will be provided in the following sections.
The waveforms in Figure 3 also show a number of small, sharp, spike-like potentials. Closer inspection of the data revealed that these were eyeblink artifacts (as indicated by the scalp distribution and opposite polarity above versus below the eye).
Frequency Analysis
To quantify these increases in low-frequency voltage fluctuations in the high-impedance conditions, we performed a Fast Fourier Transform (FFT) on the data from the standard stimuli to assess the amplitude density in each frequency band. The raw EEG was epoched with a window of −500 ms to 1500 ms relative to stimulus onset and baseline corrected by subtracting the mean prestimulus voltage from the entire epoch to remove the DC offset. Epochs containing eyeblink artifacts were excluded. FFTs were computed on the remaining epochs, averaged across trials for each subject, and then averaged across subjects. The resulting grand average FFTs for the standards8 are shown in Figure 4A, using a log scale for the x-axis to enhance the visibility of the low frequencies. The amplitude density was similar across conditions for the higher frequencies (10–100 Hz) but differed in the lower frequencies. For the low frequencies, the amplitude density was lowest in the low-impedance conditions (with no effect of temperature), intermediate in the high impedance, cool temperature condition, and largest in the high impedance, warm temperature condition. A 3-way ANOVA with factors of impedance, recording environment, and frequency range (binned into fifty 2-Hz wide bins from 0–100 Hz) yielded significant main effects of impedance (F(1, 11) = 9.02, p = .012) and frequency (F(49, 539) = 34.21, p < .001). In addition, there was a significant two-way interaction between recording environment and frequency (F(49, 539) = 6.70, p = .025).
Figure 4.
Amplitude density as a function of frequency, derived from Fast Fourier transforms (FFTs) from the parietal electrode sites for the standard trials as a function of recording environment and impedance. The X axis plots frequency on a log scale. Note that the low-impedance cool and warm recording environment values overlap almost completely. Panel A shows the FFT based on all epochs except those with eyeblink artifacts. Panel B shows the FFT after also excluding epochs with large voltage excursions in the parietal channels.
It should be noted that the amplitude density in the 60-Hz line frequency band was quite low in all conditions (between 0.96 and 1.10 μV, which was only slightly higher than the amplitude density in surrounding frequency bands). This suggests that high electrode impedances did not lead to a meaningful increase in induced activity from environmental electrical sources, such as lights and video monitors (i.e., there was no clear reduction in common mode rejection). However, the low level of 60-Hz noise is probably also the result of the specific EEG recording system and the use of extensive shielding. High electrode impedances may yield meaningful increases in line noise in other recording systems and in poorly shielded recording environments.
ERP researchers often reject epochs with large voltage excursions, such as those marked by the arrows in Figure 3. We therefore asked whether the differences in low-frequency noise would remain if we excluded epochs with voltages that exceeded ±100 μV in the EEG from the P3 and P4 sites (after referencing and baseline correction). The resulting FFTs are shown in Figure 4B. Although rejecting epochs with large voltage excursions did indeed reduce the low-frequency activity in the warm temperature, high impedance condition, the low-frequency activity remained substantially higher in the high-impedance electrode sites compared to the low-impedance sites. A 3-way ANOVA with factors of impedance, recording environment, and frequency range (binned into fifty 2-Hz wide bins from 0–100 Hz) yielded significant main effects of impedance (F(1, 11) = 19.95, p = .001) and frequency (F(49, 539) = 104.37, p < .001). In addition, there was a significant two-way interaction between impedance and frequency (F(49, 539) = 37.10, p < .001). Thus, removal of epochs with large voltage excursions can greatly reduce low-frequency noise in high-impedance recordings in a warm environment, but it does not reduce this noise to the level obtained in low-impedance recordings. Because this type of artifact rejection is common practice in ERP research, all subsequent analyses were performed only on the artifact-free epochs.
RMS Noise Level in the EEG and ERP Data
To provide an additional measure of the overall noise level in the EEG recordings, we measured the root mean square (RMS) voltage on the same baseline-corrected, artifact-free epochs used for the FFT analyses. RMS voltage provides an overall measure of the magnitude of the signal being recorded, irrespective of frequency. Because the ERP signal was approximately the same across impedance levels and recording environments (see Figure 2), any differences in the single-trial EEG magnitude must reflect differences in the noise level. Thus, a larger RMS voltage is an indication of larger noise. Baseline correction was necessary prior to computation of RMS voltage to eliminate the contribution of the DC offset, which does not influence the S/N ratio of the averaged ERPs (because the averaged ERPs are computed from baseline-corrected EEG epochs).
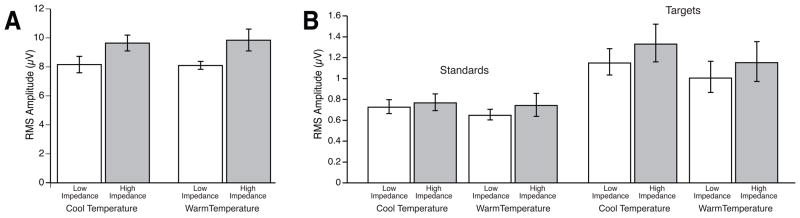
Figure 5A shows that the RMS amplitude was higher for the high-impedance recordings than for the low-impedance recordings, and this was confirmed by a 2-way ANOVA with factors of impedance level and recording environment, which yielded a significant main effect of impedance level (F(1,11) = 19.55, p = .001). Although the RMS voltage at the high-impedance electrode was slightly larger for the warm and humid condition than for the cool and dry condition, the main effect of recording environment and the interaction between impedance level and recording environment did not reach significance (p = .832 and .552, respectively). These results indicate that the voltage had greater variability over time at the high-impedance sites than at the low-impedance sites, which presumably reflects a higher noise level.
Figure 5.
(A) Root mean square (RMS) amplitude measured from the EEG epochs, excluding epochs with eyeblink artifacts or large voltage excursions, for low- and high-impedance parietal electrode sites as a function of recording environment. (B) RMS amplitude measured from the prestimulus baseline period (−400 ms to 0 ms) in the averaged ERPs for the standards and targets as a function of impedance and recording environment.
To assess whether these differences in noise could be detected after averaging, we also measured the RMS voltage during the 400-ms prestimulus period in the averaged ERP waveforms from each subject (all artifact-free trials were included in these averages). This analysis was conducted because the S/N ratio of the averaged ERPs is more relevant than the S/N ratio of the EEG for most ERP studies. This analysis was limited to the prestimulus interval, in which any deviation from zero should entirely reflect noise, so that the RMS amplitude values would not be influenced by the actual ERP signals. In general, the noise level of the prestimulus baseline period provides a useful metric of the overall noise level of the data.
Figure 5B shows that the RMS voltage was larger for the targets than for the standards, presumably reflecting the smaller number of trials in the averages for the target stimuli. This effect was confirmed in a 3-way ANOVA with factors of probability, impedance, and recording environment, which yielded a significant main effect of probability (F(1,11) = 31.36, p < .001). The RMS voltage was also larger for the high-impedance recordings than for the low-impedance recordings, leading to a significant main effect of impedance (F(1,11) = 5.75, p = .035). However, none of the main effects or interactions involving recording environment reached significance. This demonstrates that the higher RMS noise level observed in the EEG for the high-impedance recordings has a significant effect on the averaged ERP waveforms.
Both the RMS and FFT analyses indicated that the noise level was higher at the high-impedance electrodes than at the low-impedance electrodes, but this effect was more pronounced in the warm and humid recording environment in the FFT analyses but not in the RMS analyses. This probably reflects the fact that RMS amplitude collapses activity across all frequency bands, and the interaction with recording environment was present only in the lower frequencies. This may have diluted the effects of recording environment on the RMS amplitude measures.
Monte Carlo Simulations of Statistical Significance for the Effect of Probability on P3 Amplitude
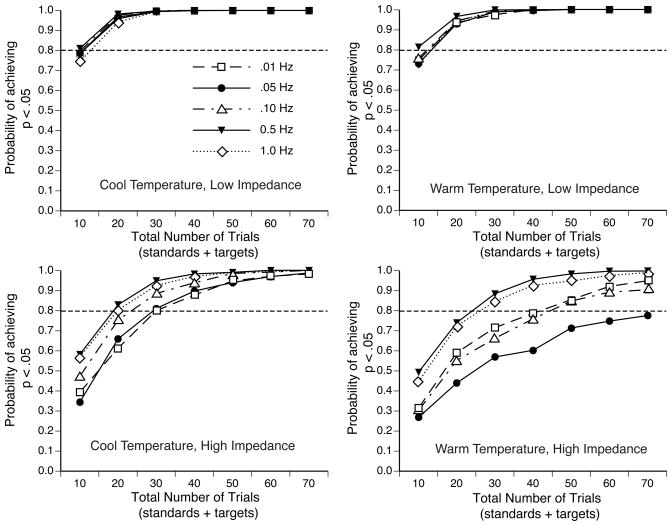
To ascertain whether the increased low-frequency noise and greater voltage variability observed at the high-impedance electrode sites affects the ability to detect a significant experimental effect, we conducted Monte Carlo simulations to determine how many trials were necessary to find a significant target-versus-standard mean amplitude difference, as described in the Method section. Figure 6A shows the probability of obtaining a significant effect as a function of the number of trials with the unfiltered EEG data. The low-impedance conditions required the smallest number of trials to detect a significant experimental effect and exhibited no effect of recording environment; the high impedance conditions required substantially more trials to achieve a given likelihood of statistical significance, with somewhat more trials required under warm and humid conditions than under cool and dry conditions.
Figure 6.
(A) Probability of obtaining a significant P3 amplitude difference between the standards and targets in the Monte Carlo simulations as a function of the number of trials in the simulated experiment. (B) Probability of obtaining a significant P3 probability (target versus standard) X electrode site (F3/4, C3/4, P3/4) interaction effect in the Monte Carlo simulations as a function of the number of trials in the simulated experiment.
Figure 6A also indicates the number of trials required to obtain an 80% likelihood of having a significant effect (i.e., a statistical power of .80). The 80% point was reached for the two low-impedance conditions in approximately 12 trials; however, almost three times as many trials (approximately 35) were required for the high impedance conditions. Thus, all else being equal, substantially more trials may be necessary to achieve statistical significance with high electrode impedances than with low electrode impedances, at least for some kinds of experiments.
Note that these Monte Carlo simulations were performed after epochs with ocular artifacts or large voltage excursions were excluded. We also conducted this set of simulations without excluding trials with large voltage excursions in the P3 and P4 channels. The probability of obtaining a significant p-value was much lower in these simulations for the high impedance, warm temperature condition (but not for the other conditions). A close examination of the averaged ERP waveforms from these simulations indicated that, although these large voltage excursions were relatively rare, they occurred often enough to produce a very large distortion in the waveforms from 1–2 of the subjects in many of the simulated experiments. These distortions led to outlier values that prevented the target-versus-standard difference from reaching significance in a large proportion of the simulated experiments. Fortunately, it is trivial to exclude epochs containing these artifacts, and they are rare enough that this does not produce a substantial decline in the number of trials available for averaging9. Thus, these artifacts are unlikely to be a serious problem in most ERP experiments.
The FFTs shown in Figure 4 demonstrated that the main difference between the low- and high-impedance conditions lies in the low frequencies. Therefore, we examined whether high-pass filters that remove the low frequency noise could improve the quality of the high-impedance data. It is important to note, however, that high-pass filters with cutoffs above 0.1 Hz will tend to attenuate the P3 wave (as described many years ago by Duncan-Johnson & Donchin, 1979) and can produce substantial distortions of the entire ERP waveform (see Chapter 5 in Luck, 2005). To illustrate this, Figure 7 shows how the averaged ERPs for the target trials varied as a function of the filter cutoff in the high impedance, warm temperature condition. There was very little effect as the filter cutoff increased from DC to 0.1 Hz. When the filter cutoff was increased beyond 0.1 Hz, however, the amplitude of the P3 wave became attenuated, ranging from approximately 9 μV for the unfiltered waveforms to approximately 4 μV with a cutoff of 1.0 Hz. In addition, the target-minus-standard amplitude difference also decreased as the filter cutoff was increased. Therefore, although high-pass filtering attenuated the low-frequency noise in the high-impedance conditions, it also attenuated the signal being measured, limiting the ability to detect a significant target-versus-standard P3 amplitude effect.
Figure 7.
Effect of different high-pass filter cut-offs on the grand average ERP waveform for the high-impedance parietal electrode site in the warm and humid recording environment. The pattern shown here was also found in the other conditions.
Figure 8 shows how the different high-pass filter settings impacted the Monte Carlo simulation results. In the two low impedance conditions, filtering had a negligible effect on the ability to detect a significant effect. This is exactly what would be expected given the low levels of low-frequency noise in these conditions. In the high impedance, cool temperature condition, the probability of obtaining a significant effect increased as the filter cutoff was increased from 0.01 Hz through 0.5 Hz. This is as would be expected given the high level of low-frequency noise in this condition. It is important to note that, as illustrated in Figure 7, the reduction in noise achieved by high-pass filtering is also accompanied by an attenuation of P3 amplitude. As a result, the probability of obtaining a significant effect in the cool temperature, high impedance condition decreased at the 1.0-Hz filter setting relative to the 0.5-Hz filter setting. In the high impedance, warm temperature condition, the probability of obtaining a statistically significant effect was again highest for the 0.5-Hz filter setting and slightly lower for the 1.0-Hz setting, but the effects of the lower filter settings were less systematic (perhaps reflecting the longer time constants of these filters, which could cause the occasional large voltage excursions to spread to adjacent epochs).
Figure 8.
Effect of high-pass filter cutoff on the probability of obtaining a significant P3 amplitude difference between the standards and targets in the Monte Carlo simulations.
It is important to note that the tradeoff between reduction in noise and attenuation of signal depends on many factors that will vary across experiments. Although the 0.5 Hz filter cutoff yielded the best tradeoff for the high-impedance conditions in the present experiment, this cutoff will not necessarily be optimal in other experiments. Furthermore, as shown in Figure 7, a cutoff greater than 0.1 Hz can severely distort the waveform (note, e.g., the artifactual negative potential produced from −50 to +100 ms by the 0.5-Hz and 1.0-Hz filters). Thus, although a high-pass filter with a cutoff of 0.5 Hz or higher may maximize statistical significance, researchers must be very careful when using such filters.
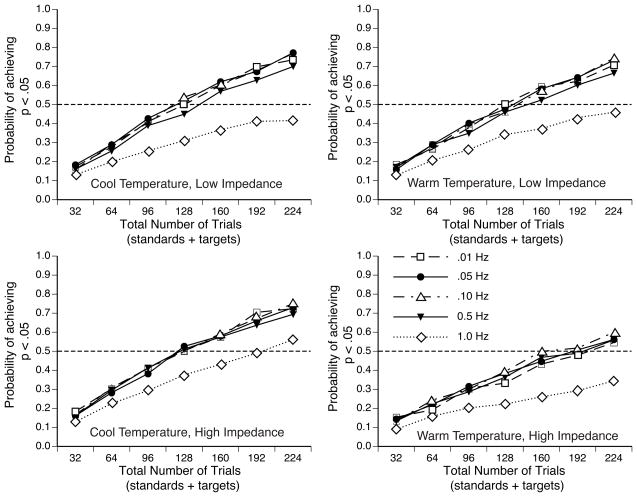
Monte Carlo Simulations of Statistical Significance for P3 Scalp Distribution
Given the large nature of the P3 probability main effect, the low-impedance conditions reached a high likelihood of obtaining significance even with the smallest number of trials tested. To assess the effect of electrode impedance under more typical conditions, we conducted an additional set of simulations examining the more modest electrode site X probability interaction effect. Specifically, we conducted Monte Carlo simulations for electrode sites F3/4, C3/4 and P3/4 to determine how many trials were necessary to obtain a significant electrode site X probability interaction effect in a repeated measures ANOVA. These simulations were conducted with the unfiltered data, excluding epochs with artifacts.
Figure 6B shows the probability of obtaining a significant interaction effect as a function of the number of trials. In general, statistical power was highest for the low-impedance recordings, independent of the recording environment, was substantially reduced for high-impedance recordings in the cool and dry recording environment, and was further reduced for high-impedance recordings in the warm and humid recording environment. For the two low-impedance conditions, the probability of an interaction effect increased steadily as the number of trials increased, with an 80% likelihood of a significant interaction effect achieved with between 60 and 70 trials. For the high-impedance, cool temperature condition, the probability of an interaction effect reached 50% likelihood only with the largest number of trials tested. Furthermore, for the high-impedance, warm temperature condition, only a 27% likelihood of a significant interaction effect was reached with the largest number of trials tested. Thus, high electrode impedances can have a substantial effect on statistical power for relatively subtle effects, especially when the recording environment is warm and humid. Additional analyses indicated that this general pattern of results was not influenced by high-pass filtering.
The effect of high electrode impedance was magnified in the warm and humid recording environment in this analysis and in the FFTs, but not in the analyses of the main effect of probability on P3 amplitude. The reason for this is not clear. One possibility is that the main effect of probability is so large that it is less sensitive to some types of noise. It is also possible that skin potentials are more problematic at the frontal and central electrode sites that were included in the electrode site X probability interaction analyses (perhaps because the P3 is smaller at those sites). Further research is needed to identify the specific conditions under which high electrode impedances will be particularly problematic.
Monte Carlo Simulations of Statistical Significance for the N1 Wave
Because the FFT analyses indicated that the noise in the high-impedance recordings was primarily present in relatively low frequencies (< 5 Hz), one might suppose that relatively short-duration components would be unaffected by the noise or that high-pass filtering might do a better job of improving the S/N ratio. However, transient ERP responses contain a broad range of frequencies; indeed, an infinitesimally brief response contains equal amounts of all frequencies. Moreover, low-frequency noise may have a large impact on the baseline voltage used in measuring a given component, and noise in the baseline will be propagated to the measurement of the component. Moreover, filtering out the lower frequency components of an ERP waveform may create artifactual voltage deflections that distort the timing and amplitude of the higher frequency components (see Chapter 5 in Luck, 2005). Thus, it is not obvious whether relatively short-duration components would be significantly degraded by low-frequency noise in the data and, if so, whether they would be significantly aided by high-pass filtering.
We therefore repeated the Monte Carlo simulations on the N1 wave, determining the probability of obtaining a significant difference in amplitude between the central and parietal electrode sites for the standard stimuli as a function of the number of trials (16–112 per electrode site). Figure 9 overlays grand average waveforms from the central (C3/4) and parietal (P3/4) electrode sites, based on averages that included all of the artifact-free trials for each subject. The mean amplitude of the N1 was larger at the C3/4 electrode sites compared with the P3/4 sites. A 3-way ANOVA with factors of electrode site, impedance, and recording environment yielded a significant main effect of electrode site (F(1,11) = 34.79, p < .001). The main effects of impedance (p = .489) and recording environment (p = .560), and the interactions between electrode site and impedance (p = .182), electrode site and recording environment (p = .170), impedance and recording environment (p = .570), and the three-way interaction between electrode site, impedance and recording environment (p = .409) all failed to reach significance.
Figure 9.
Grand average ERP waveforms for the central and parietal electrode sites as a function of impedance and recording environment.
Figure 10 shows the simulation results for the different high-pass filter settings in the four conditions. The probability of obtaining a significant difference in amplitude between the central and parietal electrode sites for a given number of trials was approximately the same in all conditions, except that more trials were needed to obtain a given probability of significance in the high impedance, warm temperature condition. The broken line in Figure 10 shows the point at which a 50% probability of a significant effect was reached (50% was used rather than 80% because the N1 effect was much smaller than the P3 effect). Approximately 50% more trials were necessary to reach this probability in the high impedance, warm temperature condition than in the other three conditions. The cutoff of the high-pass filter had little influence on the probability of obtaining a significant effect, except that the probability was reduced when the filter cutoff was increased to 1.0 Hz10. These results demonstrate that short-duration components like the N1 may be relatively unaffected by low-frequency noise in high impedance recordings, especially when the recording environment is cool and dry. Note, however, that a much larger reduction in the probability of statistical significance was obtained when we did not first exclude epochs containing large voltage deflections in the C3/4 and P3/4 sites. Thus, it is essential that these artifacts are removed in high-impedance recordings.
Figure 10.
Effect of high-pass filter cutoff on the probability of obtaining a significant N1 amplitude difference between the central and parietal electrode sites in the Monte Carlo simulations.
Discussion
By recording ERPs with low- and high-impedance electrodes simultaneously in each subject, this study was able to quantify the effects of impedance on data quality while holding all other factors constant. The main finding was that high electrode impedances led to a poorer S/N ratio and reduced statistical power when P3 amplitude was measured, especially under warm and humid recording conditions and especially when the electrode site X probability interaction was examined. When N1 amplitude was measured, however, high electrode impedances produced no substantial decline in statistical power under cool and dry recording conditions and only a modest decline under warm and humid conditions. In addition, high electrode impedances produced an increase in the noise level of the EEG, which was confined primarily to relatively low frequencies (< 5 Hz). Given that skin potentials consist primarily of low-frequency voltage changes and are known to be influenced by electrode impedance (Picton & Hillyard, 1972), this pattern of results suggests that the reduced data quality observed in the high-impedance recordings was caused in large part by an increased size or incidence of skin potentials. However, the effects of electrode impedance on low-frequency noise observed here are equally important whether or not they are a result of skin potentials.
The present study found no detectable impact of electrode impedance on 60-Hz line noise, but the recording environment was so well shielded that the line noise was too small to be accurately assessed. Thus, the present results do not provide conclusive evidence about the effects of electrode impedance on line noise. However, a previous study similarly found that increased electrode impedance yielded only a small and statistically insignificant increase in 60-Hz noise (Ferree et al., 2001). Moreover, moderate amounts of line noise are only a minor problem for most ERP experiments, because frequencies above 30 Hz can usually be filtered with little impact on the ERP waveform (see Chapter 5 in Luck, 2005). However, very high levels of line noise would be problematic in many ERP experiments, and even small amounts of line noise can be problematic for analyses of short-latency components such as the auditory brainstem responses and for frequency-domain analyses that focus on the gamma band. Thus, investigators who wish to record with high electrode impedances should consider whether shielding and other noise-reduction measures will be necessary to ensure that line noise does not degrade the data quality.
It is important to note that the design of the present experiment rules out many alternative explanations of the results. That is, because we recorded simultaneously from low- and high-impedance electrode sites, differences in subject state or related factors cannot explain the results. For example, any additional sweat secreted in the warm and humid condition would have impacted the electrolyte equally at the low- and high-impedance sites. Similarly, any increase in drowsiness in the warm and humid condition would have had equivalent effects on the recordings from the low- and high-impedance electrodes.
The two most important metrics of data quality for most ERP researchers are the probability of reaching statistical significance for a given number of trials and the number of trials required to achieve a particular probability of achieving significance. The influence of impedance on these metrics will depend on the nature of the effect being assessed in a given experiment, and it will also depend on the nature of the signal processing operations that are applied to the data (e.g., the filter settings). For example, increases in low-frequency noise would be expected to have a larger impact on components that are dominated by relatively low frequencies; consistent with this, we found much larger effects of electrode impedance on a large, late, long-duration effect (the P3 probability effect and the P3 electrode site X probability interaction effect) than on a small, early, short-duration effect (the N1 scalp distribution effect). In addition, we found that attenuating the low-frequency noise by increasing the half-amplitude cutoff frequency of the high-pass filter to 0.1 or 0.5 Hz greatly improved the statistical power in the high-impedance recordings when P3 mean amplitude was measured. However, further increases in the filter cutoff (up to 1.0 Hz) caused a decrease rather than an increase in statistical power. Moreover, substantial distortion of the ERP waveforms was observed with cutoffs of 0.5 Hz and above, impacting both the N1 and P3 waves, and a 0.1-Hz filter may therefore be optimal in most cases. If very large amounts of low-frequency noise are present—as may be the case for high-impedance recordings obtained in a warm recording environment—the probability of obtaining a statistically significant experimental effect may be increased by using a cutoff as high as 0.5 Hz. However, such severe filters distort the time course of the ERP waveforms and must be used with caution, even when relatively short-latency components such as the N1 wave are the focus of the analyses.
It would be tempting to conclude from the present results that low-impedance recording systems are superior to high-impedance recording systems (when used with low and high electrode impedances, respectively). Such a conclusion would be unwarranted. The present study shows that, all else being equal, the low-frequency noise level is lower for low electrode impedances than for high electrode impedances. However, all else is not usually equal when comparing a given low-impedance system with a given high-impedance system (or when comparing two high-impedance systems). Factors such as the use of preamplifiers in the electrodes, a stable conductive medium between the electrode and the skin, and a driven right leg circuit may have a substantial effect on data quality. Thus, recordings obtained with high electrode impedances from an optimized system may be as good as, or even better than, recordings obtained with low electrode impedances from an inferior system. Impedance is only one of several factors that influence data quality, and the impact of impedance will depend on the nature of the recording environment and the ERP measures being analyzed. However, the present data clearly indicate that researchers who are considering recording with high electrode impedances should think carefully about the impact this might have on the number of trials needed to reach statistical significance.
This brings up an important issue, namely the degree to which the present results can be generalized. For example, would the same pattern of results be obtained using a different EEG recording system, a different experimental paradigm, a different set of signal processing procedures, etc.? There is no obvious reason to believe that the decline in S/N ratio observed here for high electrode impedances—and the interaction between impedance and the temperature of the recording environment—would be more severe than in other recording systems, paradigms, etc. Indeed, by using an optimized recording system and a highly shielded recording environment, the present results may represent a best-case scenario for high-impedance recordings. It would be worthwhile, however, for other researchers to replicate this study under different conditions to establish the generality of the findings. In addition, it would be worthwhile for other investigators to apply different signal processing techniques to the data from the present experiment (available at http://erpinfo.org/impedance) to see if the reduction in statistical power resulting from high electrode impedances can be mitigated by means of offline data processing.
It is important to note that the difference in statistical power for low- versus high-impedance recordings was relatively modest under the cool and dry recording conditions for the N1 wave. For researchers who can maintain a cool and dry recording environment and who focus on relatively fast components, the benefits of high electrode impedances (reduced electrode application time, reduced subject discomfort, and reduced probability of disease transmission) may outweigh this reduction in statistical power. However, the decrease in statistical power was quite substantial for the P3 wave, even under cool and dry recording conditions. For the main effect of probability, achieving 80% power required 2–3 times as many trials for high-impedance recordings compared to low-impedance recordings (see Figure 6A). Similarly, the probability of obtaining a statistically significant probability X electrode site interaction for a given number of trials was approximately half as great for high-impedance recordings as for low-impedance recordings under cool and dry recording conditions (see Figure 6B). Thus, the advantages of high electrode impedances may be more than offset by the reduction in statistical power for researchers who are focusing on relatively slow components such as P3 and N400. Moreover, the reduction in statistical power with high electrode impedances is even larger in a warm and humid recording environment. Therefore, researchers who focus on relatively slow components and who wish to use high electrode impedances would be well advised to spend the time and effort needed to ensure that the recording environment is cool and dry. This could halve the number of trials needed to obtain statistical significance in some experiments.
Unfortunately, ensuring a cool and dry recording environment is often quite difficult, because most laboratories are located in large buildings with a single, centralized air cooling system. These systems are not designed to provide substantially different levels of cooling to different rooms. When a subject and electronic devices such as a video display are placed inside a small, closed recording chamber, substantial inflow of cool air from the ventilation system may be necessary to maintain a cool chamber temperature, and it may not be possible for a given air cooling system to provide this inflow without making other parts of the building uncomfortably cool. Indeed, many ERP recording chambers are not connected to the central cooling system and rely on a small fan to bring cool air into the chamber from the surrounding room. Thus, even in an air-conditioned building that is generally kept at a comfortable temperature (e.g., 25° C or 77° F), it may be difficult to maintain a cool temperature (e.g., 21° C or 70° F) in the recording chamber. Although we have not systematically investigated the effects of intermediate temperatures between the 21° C and 28° C values tested in the present study, our informal observations suggest that data quality is highest toward the low end of this range. Substantial effort may be necessary to ensure temperatures in this range, especially in locations with a hot and humid climate during significant portions of the year. At a minimum, researchers who record with high electrode impedances should carefully track the temperature within the recording environment.
Researchers who are unable to ensure consistently cool and dry recording conditions will need to choose between (a) high electrode impedances and a concomitant increase in the number of trials needed to achieve statistical significance, and (b) low electrode impedances and a concomitant increase in electrode application time and in the risk of disease transmission. For some researchers, it may simply be impractical to obtain low electrode impedances because of the need to record from many channels or because of concerns about disease transmission or subject discomfort. For other researchers, however, large numbers of electrodes may be unnecessary, which minimizes the problem of increased electrode application time when the skin must be abraded. Indeed, the use of large numbers of electrodes may make it more difficult to ensure that high-quality data are being recorded at each site, because it may be difficult to carefully monitor the incoming EEG signals from many dozens of sites. For many research questions, it may be more important to have high-quality data from a small number of electrodes rather than low-quality data from a large number of electrodes. In addition, the risk of disease transmission is quite low in most research settings, and it can be further reduced by adequate disinfection procedures. Thus, the choice of high versus low electrode impedances will depend on the specific needs of a researcher.
Researchers who are concerned about the temperature in the recording environment may consider whether they actually need to use an electrically shielded recording chamber11. It may be much easier to provide adequate ventilation in a moderately large room than in a much smaller recording chamber, and the present findings indicate that lowering the temperature can dramatically reduce the difference in data quality between low- and high-impedance recordings. If a chamber is not used, induced electrical noise from the environment will almost certainly increase. However, this noise can be substantially reduced by means of active electrodes, shielding of cables and video monitors, DC-powered lighting systems, etc. Indeed, many researchers record inside shielded chambers but bring sources of line noise (e.g., video monitors) inside the chamber without shielding them. Induced electrical noise may be eliminated much less expensively by shielding the AC devices than by purchasing a shielded chamber, especially if AC devices are present in the chamber (see Chapter 3 in Luck, 2005). Moreover, in most ERP studies, a modest amount of 50- or 60-Hz noise arising from the ambient electrical environment may be much easier to filter than a large amount of low-frequency noise arising from skin potentials. Exceptions to this would include studies of gamma band activity and short- and mid-latency auditory evoked responses, in which line-frequency noise would overlap extensively with the frequency content of the signal being recorded.
It is also possible that the increase in low-frequency noise we obtained with high-impedance recordings under warm and humid conditions could be reduced, at least to some degree, by the use of an electrode gel with a higher salt concentration. We have not tested this possibility, and we will probably not have the opportunity to provide a systematic test. However, we would encourage researchers who are using high-impedance recording systems in warm recording environments to provide a systematic test of different electrolytes and to publish the results so that the entire ERP community can benefit from the knowledge gained.
It is also worth asking whether psychological factors (e.g., emotional responses) could trigger skin potentials following specific types of stimuli in an experiment, leading to slow voltage changes that are interpreted as being ERPs even though they arise from the skin instead of the brain. This is unlikely in most cases, because electrodermal responses typically begin more than 1000 ms after the onset of the eliciting stimulus (see e.g., Lim et al., 1997). Thus, stimulus-elicited skin potentials will not have an impact on most ERP components. However, they may be problematic in studies examining very slow ERPs (e.g., the contingent negative variation). Skin potentials triggered by one stimulus may also overlap with the ERPs elicited by subsequent stimuli, influencing the pre-stimulus baseline period and even the post-stimulus waveform for the subsequent stimuli. These overlapping skin potentials will distort the data in the same general manner as overlapping ERP components (see Woldorff, 1993, for an extensive discussion), except that the skin potentials will be slower and last longer. Consequently, overlapping skin potentials will typically be a problem only if they differ systematically across conditions, and the overlap can be minimized by the use of an appropriate high-pass filter.
We would like to stress that it should be possible for manufacturers of EEG recording equipment to produce systems that are designed for use with high electrode impedances but that also make it convenient to abrade the skin and obtain low electrode impedances. This would involve providing a reasonably large access hole for each electrode so that an implement for abrading the skin could be inserted. It would also involve providing a means of conveniently measuring the impedance at each electrode. Such a system would allow researchers to use high electrode impedances for experiments in which statistical power is high or in which large numbers of electrodes are necessary, but to switch to low electrode impedances for experiments in which statistical power would otherwise be too low.
Acknowledgments
This study was made possible by a grant to S.J.L. from the National Institute of Mental Health (R01 MH076226) and by a graduate research fellowship to E.S.K. from the National Science Foundation.
We would like to thank Coen Metting van Rijn, Lloyd Smith, Ingmar Gutberlet, Phil Holcomb, and an anonymous reviewer for comments on this manuscript.
Footnotes
When evaluating studies like this, it is important to consider whether the researchers have any potential conflicts of interest that might lead to intentional or unintentional bias in the design, analysis, or presentation of the study. We would therefore like to make the following disclosures. Our laboratory uses high-impedance EEG recording systems manufactured by BioSemi but has not received any free or discounted equipment or any other financial considerations from BioSemi or from any other manufacturers. We lead a yearly summer workshop on ERP methods (the ERP Boot Camp), which has received a donation of a few electrode caps and a small amount of money from Cortech Solutions, the U.S. distributor for Biosemi. The ERP Boot Camp has also received modest financial support from several other vendors of ERP recording and analysis systems, including Brain Products GmbH, EasyCap GmbH, and Advanced Neuro Technologies, and software has been provided for the ERP Boot Camp by Compumedics Neuroscan, Megis GmbH, and Brain Products GmbH. These donations have been modest, have been used to support the ERP Boot Camp rather than our laboratory, and have come from vendors of both low- and high-impedance recording systems. Consequently, we believe that the research reported here was not significantly biased by financial, scientific, or personal ties to manufacturers or distributors of EEG systems.
The electrode does not typically directly contact the skin. Instead, a conductive gel or liquid typically provides the electrical connection between the electrode and the skin. We will use the term electrode to refer to the combination of the electrode and the conductive gel or liquid.
Some EEG recording systems now use single-ended amplifiers rather than differential amplifiers, meaning that they digitize the active and reference signals separately. The difference between active and reference can then be computed offline. However, the same issues apply to these systems as to differential amplification systems.
Some EEG recording systems have a very large input range, which indirectly helps to solve one consequence of skin potentials, namely saturation of the amplifier that can occur when a skin potential brings the voltage out of the amplifier’s operating range. However, the use of a large input range does not address the primary consequence of skin potentials, namely large voltage shifts.
It did not seem worthwhile at this time to separately assess the effects of temperature and humidity. Cooling systems typically reduce humidity levels, so humidity and temperature will tend to covary under real-world conditions.
Additional testing demonstrated that the removal and reinsertion of electrodes has a negligible effect (< 1% change) on impedances measured for both low- and high-impedance sites.
The same trials were rejected for both the low- and high-impedance recording sites and across the different filter settings. This makes it simpler to compare across sites and across filter settings, because the comparisons were based on identical trials. The raw unfiltered EEG was used to determine which trials contained artifacts.
The FFTs for the targets showed the same pattern of results.
We could not objectively quantify the number of trials that contained these large voltage excursions because eyeblinks also produce large voltage excursions.
The reduction in the probability of obtaining statistical significance produced by the 1.0-Hz high-pass cutoff occurred because this filter took the low-frequency energy of the P3 wave, inverted it, and spread it both forward and backward in time, so that it added variance in the N1 latency range.
We thank Lloyd Smith for this suggestion.
References
- Corby JC, Roth WT, Kopell BS. Prevalence and methods of control of the cephalic skin potential EEG artifact. Psychophysiology. 1974;11:350–360. doi: 10.1111/j.1469-8986.1974.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson C, Donchin E. The time constant in P300 recording. Psychophysiology. 1979;16:53–55. doi: 10.1111/j.1469-8986.1979.tb01440.x. [DOI] [PubMed] [Google Scholar]
- Edelberg R. Electrical activity of the skin: Its measurement and uses in psychophysiology. In: Greenfield NS, Sternbach RA, editors. Handbook of Psychophysiology. New York: Rinehart and Winston; 1972. pp. 367–418. [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fowles DC. The reduction of palmar skin potential by epidermal hydration. Psychophysiology. 1971;7:254–261. doi: 10.1111/j.1469-8986.1970.tb02231.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. The eccrine system and electrodermal activity. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology. New York: Guilford Press; 1986. pp. 51–96. [Google Scholar]
- Fowles DC, Venables PH. The reduction of palmar skin potential by epidermal hydration. Psychophysiology. 1970;7:254–261. doi: 10.1111/j.1469-8986.1970.tb02231.x. [DOI] [PubMed] [Google Scholar]
- Johnson MH, de Haan M, Oliver A, Smith W, Hatzakis H, Tucker LA, et al. Recording and analyzing high-density event-related potentials with infants using the Geodesic Sensor Net. Developmental Neuropsychology. 2001;19:295–323. doi: 10.1207/S15326942DN1903_4. [DOI] [PubMed] [Google Scholar]
- Lim CL, Rennie C, Barry RJ, Bahramali H, Lazzaro I, Manor B, et al. Decomposing skin conductance into tonic and phasic components. International Journal of Psychophysiology. 1997;25:97–109. doi: 10.1016/s0167-8760(96)00713-1. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Metting van Rijn AC, Peper A, Grimbergen CA. High quality recording of bioelectric events. I: interference reduction, theory and practice. Med & Biol Eng & Comput. 1990;28:389–397. doi: 10.1007/BF02441961. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Picton TW, Hillyard SA. Cephalic skin potentials in electroencephalography. Electroencephalogray and Clinical Neurophysiology. 1972;33:419–424. doi: 10.1016/0013-4694(72)90122-8. [DOI] [PubMed] [Google Scholar]
- Tregear RT. Physical Properties of the Skin. London: Academic Press; 1966. [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalography & Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Woldorff M. Distortion of ERP averages due to overlap from temporally adjacent ERPs: Analysis and correction. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]