Abstract
Background:
Hepatic injury after ischemia/reperfusion is attributed to the development of oxygen free radical (OFR)-mediated lipid peroxidation - a process that can be measured through its byproducts, specifically malondialdehyde. The use of free radical scavengers can offer significant protection against OFR-induced liver injury. We hypothesize that a new potent OFR scavenger, polyethylene glycol-superoxide dismutase (PEG-SOD), can inhibit OFR-mediated lipid peroxidation in hepatic ischemia/reperfusion injury.
Methods:
Twelve male Sprague-Dawley rats (300-350 g) were subjected to occlusion of the left and middle hepatic arteries and portal veins for 90 min, followed by 120 min reperfusion. PEG-SOD (5000 units/kg) was given intravenously before vascular occlusion and again immediately upon reperfusion to six rats. Normal saline was given to the remaining six rats to be used as a control group. The right hepatic lobe (used as internal control) and left hepatic lobe were harvested separately and tissue malondialdehyde was measured.
Results:
A marked increase in lipid peroxide was found in the normal saline group after 2 h reperfusion. Treatment with PEG-SOD prevented the rise in tissue malondialdehyde. The mean difference in the malondialdehyde between the left and right hepatic lobes were 13.20 ± 6.35 and 1.70 ± 3.65 nmol/g in the normal saline (control) and PEG-SOD groups, respectively. This difference was found to be statistically significant (P < 0.005) using Student's t-test.
Conclusions:
PEG-SOD can effectively attenuate hepatic ischemia/reperfusion injury by inhibiting OFR-mediated lipid peroxidation.
Keywords: ischemia, lipid peroxidation, liver, oxygen free radicals, reperfusion injury
Introduction
Total interruption of hepatic blood flow is often necessary during hepatic surgery. Upon revascularization and reoxygenation, the organ undergoes a process termed reperfusion injury that further impairs organ function. One mechanism of this injury is explained by oxygen free radical (OFR) damage to cellular membranes, which leads to the generation of byproducts of lipid peroxidation, such as malondialdehyde [1,2,3]. An initial OFR formed from molecular oxygen, with the aid of the enzyme xanthine oxidase, is superoxide anion. This can lead to the generation of other reactive oxygen species, such as hydroxyl radical and hydrogen peroxide [1,4]. Protection from these OFRs comes from endogenous scavengers such as super-oxide dismutase (SOD), glutathione peroxidase, and catalase. These scavengers are rapidly depleted by the high amount of reactive oxygen agents produced by the reentry of oxygenated blood into the ischemic tissue, however [5]. Restitution of these OFR scavengers can attenuate the ischemia/reperfusion injury.
The administration of exogenous SOD has been tested in several experimental models, but the results are still controversial, primarily because of the short half-life of native SOD (6 min) [5,6,7,8,9]. Recently, a longer-acting form of SOD with a half-life of 14 h was developed through its conjugation to polyethylene glycol (PEG). We hypothesize that PEG-SOD, a potent superoxide anion scavenger, can inhibit OFR-mediated lipid peroxidation in hepatic ischemia/reperfusion injury.
Materials and methods
Preparation of ischemic rats
Twelve male Sprague-Dawley rats (Harlan Sprague Dawley Inc, Indianapolis, IN, USA) weighing 300-350 g were used. The animals were housed in wire-bottomed cages and allowed free access to standard laboratory chow and water. The animals were not fasted overnight and were allowed to drink water freely.
An injection of 50 mg/kg pentobarbital sodium (Nembutal 50mg/ml; Abbott Laboratories, North Chicago, Illinois, USA) was given intraperitoneally for anesthesia. The hair over the abdomen and neck was clipped for exposure. Next, the left carotid artery was cannulated with a 20 cm segment of polyethylene tube (Clay Adams, Parsippany, NJ, USA) and used to administer heparin (200 IU/kg body weight). Attention was then turned to the abdomen where the hepatic and portal vessels supplying the median and left lateral hepatic lobes were occluded for 90 min with a loop of 0 silk suture, which was secured with a clamp and a flanged polyethylene tube.
Before vascular occlusion, six of the animals received a 5000 units/kg bolus of PEG-SOD and the other six received an equivalent volume of normal saline (control group). Reflow was achieved by removal of the clamp, polyethylene tube, and suture. Immediately upon reperfusion, another 5000 units/kg bolus of PEG-SOD was given to the group that received PEG-SOD earlier and an equivalent volume of normal saline was given to the control group. Continuous blood pressure measurements were obtained using the arterial catheter. The systolic blood pressure was noted to be greater than 90 mmHg at all times.
After 120 min of reperfusion, the left and right lobes of the liver were harvested and stored in a refrigerated container and the animals were decapitated. The liver specimens were kept in a -80°C freezer overnight for 16 h to be analyzed the following day.
Polyethylene glycol-superoxide dismutase
The preparation was provided by the Sigma-Aldrich Corporation (St Louis, MO, USA). This SOD enzyme is derived from bovine erythrocytes and coupled to methoxy-polyethylene glycol through secondary amine linkage. PEG, when conjugated to protein, shields the protein from interaction with other macromolecules. This generally decreases immunogenicity and proteolysis.
The enzyme has an activity of approximately 4000 units/mg protein. This was diluted in normal saline to a concentration of 4000 units of activity per ml.
Assay of lipid peroxide
The assay of lipid peroxide was performed according to a modified version of the method of Masugi and Nakamura [10]. The measurement of liver lipid peroxide by a colorimetric reaction with thiobarbituric acid is described in detail below, and the determined lipid peroxide is referred to as malondialdehyde.
After thawing the frozen liver, 2 ml of 0.05 mol/l phosphate buffer (pH 7.4) was added to 1 g liver. The resulting mixture was homogenized using a teflon-pestled homogenizer. The homogenate was used for experiments. To 0.5 ml liver homogenate in a teflon-stoppered test tube, 2.5 ml of 20% trichloroacetic acid solution and 1 ml of 0.67% thiobarbituric acid solution were added. The color of thiobarbituric acid pigment was developed in a water bath at 100°C for 30 min.
After cooling with tap water to room temperature, 4 ml n-butanol was added and shaken vigorously. After centrifugation, the color of butanol layer was measured at 535 nm. 1,1,3,3-tetraethoxypropane was used as a standard of malondialdehyde.
Statistical analysis
Statistical analysis was performed using Student's t-test. P < 0.05 was considered statistically significant. Results are presented as mean ± standard deviation.
Results
Evaluation of the degree of lipid peroxidation was determined by measuring tissue malondialdehyde in nmol/g wet liver weight. In the control group, which was given normal saline, the mean malondialdehyde in the left hepatic lobe increased significantly compared with the right lobe, with values of 59.95 ± 6.67 and 46.75 ± 2.79 nmol/g, respectively. The group that was given PEG-SOD did not show a statistically significant difference in malondialdehyde values between the left and right hepatic lobes, with levels of 47.80 ± 1.68 and 46.10 ± 3.31 nmol/g, respectively (Fig. 1).
Figure 1.
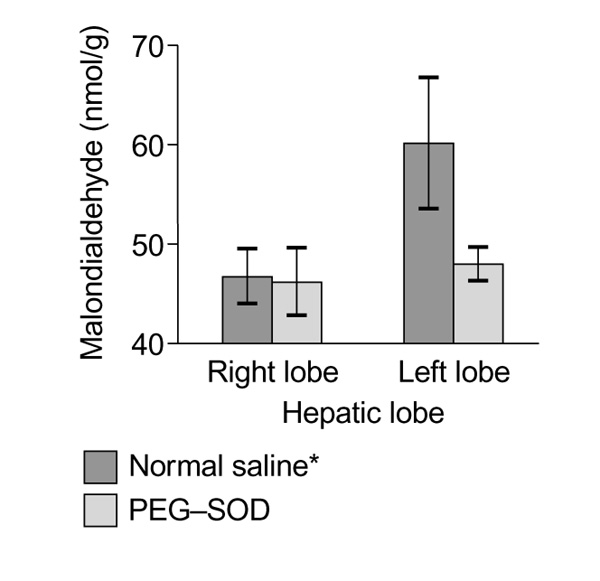
Effect of polyethylene glycol-superoxide dismutase (PEG-SOD) and normal saline on lipid peroxidation. Values are presented as means ± standard deviation. *P < 0.005 for left lobe versus right lobe in normal saline group.
A more appropriate analysis that incorporates both the internal and external controls and compares the mean difference in malondialdehyde between the normal saline group and the PEG-SOD group may be seen in Table 1. This showed that the mean difference in malondialdehyde between the left and right hepatic lobes in the control group was significantly higher than that in the PEG-SOD group, with levels of 13.20 ± 6.35 and 1.70 ± 3.65 nmol/g, respectively.
Table 1.
Mean difference in malondialdehyde between the left hepatic lobe and the right hepatic lobe
| Normal saline | PEG-SOD | |
| Mean difference in | 13.20 ± 6.35 | 1.70 ± 3.65* |
| MDA (nmol/g liver tissue) |
Comparison of the mean difference in malondialdehyde (MDA) between the left hepatic lobe and the right hepatic lobe in the normal saline group and polyethylene glycol-superoxide dismutase (PEG-SOD) group. *P < 0.005, versus normal saline.
Discussion
Hepatic ischemia is a common event in modern day surgery. It is evident in transplantation, trauma, and states of low blood flow or shock. The re-establishment of blood flow is necessary in rescuing ischemic tissues, because this permits both the restoration of cell charge and the washout of toxic metabolites. However, reperfusion of ischemic tissues also leads to a sequence of events that, paradoxically, injures tissues.
One proposed mechanism of ischemia/reperfusion injury is OFR-mediated lipid peroxidation of cellular membranes, a process that can be measured through the generation of malondialdehyde, a relatively stable intermediate of lipid peroxidation that has been widely accepted as a marker of OFR-mediated lipid peroxidation [1,11,12,13].
Other nonspecific markers of ischemia/reperfusion injury have also been used because of their organ-specific utility and have been shown to correlate well with malondialdehyde levels. A few examples of these markers are transaminases for the liver, creatinine for the kidney, and amylase for the pancreas. In the past, conclusions have been drawn regarding the effectiveness of antioxidants in attenuating OFR-mediated ischemia/reperfusion injury solely based on the theoretical mechanism of injury, mechanism of drug action, and the diminished levels of organ-specific markers of injury. Because evidence of oxidant stress is only implied and not documented, these conclusions regarding the appropriate and effective use of these drugs remain in question. The use of malondialdehyde as our marker of injury allowed us to demonstrate the appropriate and effective role of PEG-SOD in inhibiting OFR-mediated lipid peroxidation.
Active research continues to clarify the source of OFRs and its relative contribution to ischemia/reperfusion injury. Possible sources include hepatocytes, endothelial cells, Kupffer cells, and activated neutrophils called upon to repair the injured organ. The use of various drugs, cytokines, and monoclonal antibodies to suppress the activity of inflammatory cells has been shown to be effective in attenuating ischemia/reperfusion injury, especially if given before the injury [14,15]. Regardless of the precise source of OFRs, tissue reoxygenation after ischemia leads to the generation of superoxide anion as an important initial OFR. Other reactive oxygen intermediates that may play a role in ischemia/reperfusion injury are hydrogen peroxide and hydroxyl radical; their production usually originates from the generation of superoxide anion, however. In essence, the administration of PEG-SOD can attenuate ischemia/reperfusion injury by scavenging superoxide anion and halting the deleterious chain reaction that would lead to the generation of other reactive oxygen intermediates.
PEG-SOD has other characteristics that would make it an ideal OFR scavenger. First, SOD is a potent endogenous superoxide anion scavenger. Second, its binding to PEG allows it to resist degradation by proteolysis. Third, it is specific for superoxide anion; therefore, it will not produce adverse effects from generalized inhibition of inflammation as seen with corticosteroids or other immunosuppressants. Lastly, there are no currently known specific adverse effects from the administration of SOD or PEG-SOD.
PEG-SOD has already been tested in experimental models of ischemia/reperfusion in muscle, brain, and isolated perfused heart, as well as cold ischemia/reperfusion damage in isolated pig livers. Results showed a reduced drop in adenosine triphosphate and lipid peroxide production, a higher percentage of tissue survival in muscle, improved immediate kidney and liver function, and a smaller brain and myocardial infarction volume in PEG-SOD treated animals than in untreated ones [16,17,18].
In the present model, the protective effect of PEG-SOD was evaluated in the setting of ischemia/reperfusion injury from clamping and unclamping of the vessels to the left lateral and median hepatic lobes. This hepatic insult has been extensively studied and is appropriately suited to evaluate the process of lipid peroxidation because of the unique features of the liver. Markers of hepatic injury that have also been evaluated using this model include adenosine triphosphate depletion, transaminase elevation, increased polymorphonuclear leukocyte infiltration, and malondialdehyde elevation, all of which correlate appropriately [19]. The present, first use of PEG-SOD in this setting appears to completely inhibit OFR-mediated lipid peroxidation. Works from other researchers [1,20,21,22,23] have shown that byproducts of lipid peroxidation can function as chemoattractants for neutrophils. This may explain the 'late phase' of ischemia/reperfusion injury. By inhibiting the production of these byproducts, PEG-SOD may also attenuate this 'late phase' of ischemia/reperfusion injury. Unpublished data from our laboratory has shown that malondialdehyde levels rise modestly after an hour of reperfusion, and then briskly to peak after 2 h of reperfusion in saline-treated groups. In the group that received PEG-SOD, there was no rise in malondialdehyde levels throughout its reperfusion period. This finding supports the view that both 'early and late' ischemia/reperfusion injury can be attenuated with PEG-SOD.
In conclusion, the present study shows that PEG-SOD is an ideal drug to attenuate hepatic ischemia/reperfusion injury through its action on superoxide anion, which appears to play a central role in lipid peroxidation. Further studies are necessary to determine optimal dosage, administration time, and combination with other anti-inflammatory regimens to obtain the most effective protection from hepatic ischemia/reperfusion injury, especially in larger animals. These therapies may provide a better understanding in areas of hepatic surgery, preservation, and rejection.
References
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72:65–83. doi: 10.1016/s0039-6109(16)45628-8. [DOI] [PubMed] [Google Scholar]
- Toledo-Pereyra LH, Suzuki S. Neutrophils, cytokines, and adhesion molecules in hepatic ischemia and reperfusion injury. J Am Coll Surg. 1994;179:758–762. [PubMed] [Google Scholar]
- Atalla SL, Toledo-Pereyra LH, MacKenzie GH, et al. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985;40:584–590. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- Ar'Rajab A, Dawidson I, Fabia R. Reperfusion injury. New Horizons. 1996;4:224–234. [PubMed] [Google Scholar]
- Morpurgo E, Cadrobbi R, Morpurgo M, et al. Protective effect of superoxide dismutase and polyethylene glycol-linked superoxide dismutase against renal warm ischemia/reperfusion injury. . Transplantation. 1996;62:1221–1223. doi: 10.1097/00007890-199611150-00006. [DOI] [PubMed] [Google Scholar]
- Heberer M, Jorgensen J, Mihatsch MJ, et al. Protective effect of allopurinol and superoxide dismutase in renal isografts in cyclosporine A-treated rats. Renal Fail. 1991;13:233–242. doi: 10.3109/08860229109022159. [DOI] [PubMed] [Google Scholar]
- Pollak R, Andrisevic JH, Maddux MS, et al. A randomized double-blind trial of the use of human recombinant superoxide dismutase in renal transplantation. Transplantation. 1993;53:57–60. doi: 10.1097/00007890-199301000-00011. [DOI] [PubMed] [Google Scholar]
- McEnroe CS, Pearce FJ, Ricotta JJ, et al. Failure of oxygen-free radical scavengers to improve postischemic liver function. . J Trauma. 1986;26:892–896. doi: 10.1097/00005373-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Caldwell-Kenkel JC, Currin RT, Coote A, et al. Reperfusion injury to endothelial cells after cold storage of rat livers: protection by mildly acidic pH and lack of protection by antioxidants. Transplant Int. 1995;8:77–85. doi: 10.1007/BF00344415. [DOI] [PubMed] [Google Scholar]
- Masugi F, Nakamura T. Effect of vitamin E deficiency on the level of superoxide dismutase, glutathione peroxidase, catalase and lipid peroxide in rat liver. Int J Vit Nutr Res. 1976;46:187–191. [PubMed] [Google Scholar]
- Rauen U, Viebahn R, Lauchart W, et al. The potential role of reactive oxygen species in liver ischemia/reperfusion injury following liver surgery. Hepato-Gastroenterology. 1994;41:333–336. [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and hydroxymonenal. Methods in Enzymology. Edited by Parcker L, Glazer A. New York: Academic Press, 1991;186(B):407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malonaldehyde determination as index of lipid peroxidation. Methods in Enzymology. Edited by Parcker L, Glazer A. New York: Academic Press, 1991;186(B):421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Nonami T, Harada A. Mechanism and prevention of ischemia-reperfusion injury of the liver. Semin Surg Oncol. 1996;12:179–182. doi: 10.1002/(SICI)1098-2388(199605/06)12:3<179::AID-SSU6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Marubayashi S, Dohi K. Therapeutic modulation of free radical-mediated reperfusion injury of the liver and its surgical implications. Surg Today. 1996;26:573–580. doi: 10.1007/BF00311659. [DOI] [PubMed] [Google Scholar]
- Giardino R, Capelli S, Fini M, et al. Biopolymeric modification of superoxide dismutase (mPEG-SOD) to prevent muscular ischemia-reperfusion damage. Int J Artif Org. 1995;18:167–172. [PubMed] [Google Scholar]
- Galinanes M, Qiu Y, Ezrin A, et al. PEG-SOD and myocardial protection. Studies in the blood- and crystalloid-perfused rabbit and rat hearts. Circulation. 1992;86:672–682. doi: 10.1161/01.cir.86.2.672. [DOI] [PubMed] [Google Scholar]
- Cosenza C, Wu GH, Tuso PJ, et al. Protective effect of polyethylene glycol conjugated superoxide dismutase for cold ischemia-reperfusion damage in isolated pig livers. Transplant Proc. 1993;25:1881–1882. [PubMed] [Google Scholar]
- Marubayashi S, Oshiro Y, Maeda T, et al. Protective effect of mono-clonal antibodies to adhesion molecules on rat liver ischemia-reperfusion injury. Surgery. 1997;122:45–52. doi: 10.1016/s0039-6060(97)90263-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman BJ, Grisham MB, Granger DN. Role of oxidants in ischemia/reperfusion-induced granulocyte infiltration. Am J Physiol. 1990;258:G185–G190. doi: 10.1152/ajpgi.1990.258.2.G185. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Granger DN, Benoit JN, Suzuki M, et al. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol. 1989;257:G683–G688. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Inauen W, Kvietys PR, et al. Superoxide mediates reperfusion-induced leukocyte-endothelial cell interaction. . Am J Physiol. 1989;257:H1740–H1745. doi: 10.1152/ajpheart.1989.257.5.H1740. [DOI] [PubMed] [Google Scholar]


