Abstract
Purpose
Investigate a novel chemoradiation regimen designed to maximize locoregional control (LRC) and minimize toxicity for patients with advanced head and neck squamous cell carcinoma (HNSCC).
Patients and Methods
Patients received hyperfractionated intensity modulated radiation therapy (HIMRT) in 1.25 Gy fractions bid to 70 Gy to high-risk planning target volume (PTV). Intermediate and low-risk PTVs received 60 Gy and 50 Gy, at 1.07 and 0.89 Gy per fraction, respectively. Concurrent cisplatin 33 mg/m2/week was started week 1. Patients completed the Quality of Life Radiation Therapy Instrument prior to (PRE), at end of treatment (EOT), and at 1, 3, 6, 9, and 12 months. Overall survival (OS), progression-free (PFS), LRC, and toxicities were assessed.
Results
Thirty of 39 patients (77%) were alive without disease at median follow-up of 37.5 months. Actuarial 3-year OS, PFS, and LRC were 80%, 82%, and 87%, respectively. No failures occurred in the electively irradiated neck and there were no isolated neck failures. Head and neck QOL was significantly worse in 18 of 35 patients (51%): mean 7.8 PRE versus 3.9 EOT. By month 1, H&N QOL returned near baseline: mean 6.2 (sd=1.7). Most common acute grade 3+ toxicities were mucositis (38%), fatigue (28%), dysphagia (28%) and leukopenia (26%).
Conclusions
Hyperfractionated IMRT with low-dose weekly cisplatin resulted in good LRC with acceptable toxicity and QOL. Lack of elective nodal failures despite very low dose per fraction has led to an attempt to further minimize toxicity by reducing elective nodal doses in our subsequent protocol.
Keywords: Hyperfractionation, IMRT, chemoradiation, head and neck cancer
INTRODUCTION
Chemoradiation (CRT) has become the standard of care for most patients with locally advanced head and neck squamous cell cancer (HNSCC). Multiple phase III trials and two meta-analyses have shown significantly improved locoregional control (LRC) with CRT over radiotherapy alone, with at least two showing an overall survival (OS) benefit as well.1-6 Altered fractionation schemes including accelerated and hyperfractionated radiation therapy (AFRT) have also shown benefits over standard fractionation.7-9 Unfortunately, the improved efficacy that results from either CRT or AFRT comes at the price of higher rates of treatment-related toxicity. Both CRT and AFRT increase the risk of acute severe mucositis and skin toxicity compared to RT alone. Long-term toxicities of xerostomia and swallowing dysfunction are also major problems with these regimens.
While AFRT or CRT result in higher efficacy and toxicity compared to conventionally fractionated RT alone, it remains to be proven whether incorporating AFRT into CRT regimens has any additive or synergistic effect. Does CRT that incorporates AFRT improve survival over conventionally fractionated CRT? Are these regimens associated with acceptable toxicity? These topics were addressed in the Radiation Therapy Oncology Group (RTOG) 0129 phase III trial which has closed to accrual but not been reported. In the meantime, the current standard CRT arm for the ongoing RTOG 0522 trial utilizes AFRT via concomitant boost technique, one of the “winning” arms of RTOG 90-03, delivered with concurrent cisplatin at 100 mg/m2 on days 1 and 21.
Our institutional CRT standard for patients with locally advanced HNSCC at New Hanover Regional Medical Center differs from that of the RTOG. In 1998, the group at Duke University published their phase III trial testing hyperfractionated RT alone versus AFRT at 1.25 Gy twice daily to 70 Gy with concurrent cisplatin and fluorouracil.10 Chemotherapy was delivered in the hospital during weeks 1 and 5. In this study, CRT resulted in significantly improved LRC and progression-free survival (PFS) with a trend toward improved OS. Previously, we reported our results in the community setting utilizing this CRT regimen in 50 patients with stage III and IVa HNCCC.11 While 2-year actuarial OS was 80%, significant toxicities were recorded including 100% grade 3 acute mucositis and 14% chronic pharyngeal stricture at a median follow-up of 23 months. Xerostomia was also a common long-term complaint for these patients who were treated without the potential benefit of intensity modulated radiation therapy (IMRT). In sum, this intensive regimen was highly efficacious but toxic.
In designing the new CRT protocol reported herein, the authors hoped to maintain high rates of LRC and survival while minimizing toxicity and any negative impact on QOL in three ways. First, intensity modulation was incorporated into the accelerated, hyperfractionated RT (HIMRT) in an attempt to decrease to the volume of non-target tissues within the RT field. Second, a nonstandard, very low dose per fraction was selected to be delivered to both intermediate and low-risk PTVs. Third, the chemotherapy regimen was altered by eliminating 5-fluorouracil and changing concurrent cisplatin dose to 33 mg/m2 delivered weekly. The current report is the only study in the medical literature to our knowledge that has combined these components of weekly cisplatin without 5FU, IMRT, and low radiation dose per fraction given twice daily for treatment of patients with HNSCC. The purpose of this study was to evaluate QOL, efficacy, and toxicity of this novel CRT regimen.
PATIENTS AND METHODS
Patient Eligibility
Adults with newly diagnosed, biopsy-proven stage III and IVa squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx were eligible. In order to minimize the risk of underdosing target tissues with parotid-sparing IMRT planning, patients with stage N2c and N3 neck disease were ineligible. Patients with nasopharynx and unknown primary carcinomas were excluded. Patients were also ineligible if they had prior head and neck radiation therapy, prior chemotherapy, other invasive malignancies (excluding nonmelanoma skin cancer) within the last 5 years, or symptomatic heart disease within the past 6 months. Other eligibility criteria included Eastern Cooperative Oncology Group performance status of 0 or 1, absolute neutrophil count greater than 1.5 × 109/L, platelet count greater than 100 × 109/L, bilirubin less than 1.5 mg/dL, and serum creatinine less than 1.5 mg/dL. The study was opened at New Hanover Regional Medical Center (NHRMC) in Wilmington, NC in August, 2004. In January 2007, the study was opened for enrollment to patients at the University of North Carolina Hospital in Chapel Hill, NC. The study was approved by institutional review boards at both institutions. In addition, a Data and Safety Monitoring Board at NHRMC provided independent oversight for the trial. Each patient gave written informed consent prior to enrollment.
Pre-Treatment Evaluation
All patients were evaluated by otolaryngology, radiation oncology, medical oncology, oral surgery and nutrition services. Laboratory evaluation consisted of complete blood count, electrolytes, magnesium, creatinine, total protein, pre-albumin, alkaline phosphatase, total bilirubin, AST, and ALT. Staging included neck CT, barium swallow, and chest X-ray prior to treatment. Positron emission tomography (PET)/CT was optional.
Radiation Therapy
Immobilization for RT planning was via an Accufix device to ensure minimal in-field motion during simulation and treatment. CT planning was performed with 3-mm axial images obtained from the top of the head through the top of the aortic arch. PET/CT data sets were imported for image fusion planning at the discretion of the treating physician.
General definitions of gross tumor volume, clinical target volume, and planning target volume were according to ICRU report #50. Hyperfractionated radiation therapy was administered utilizing intensity modulation (HIMRT) in fractions of 1.25 Gy delivered twice daily, 5 days per week, to a total dose of 70 Gy to the high-risk planning target volume (PTV70). Intermediate and low-risk target volumes in the neck received 60 Gy (PTV60) and 50 Gy (PTV50) at 1.07 and 0.89 Gy per fraction, respectively. A single treatment plan was utilized. A separate anterior supraclavicular field with central blocking over the larynx was utilized to treat the low neck whenever possible (generally for all primary disease sites other than hypopharynx or larynx). The conformal supraclavicular field was treated to 44 Gy at 2 Gy per fraction, matched to the primary IMRT fields utilizing a common isocenter technique.
Chemotherapy
Patients received cisplatin 33 mg/m2 IV infusion, at a rate of 1mg per minute once weekly during the course of HIMRT, started during week 1. Six total weekly cycles were planned. Delivery of a seventh cycle was optional during the final half week of HIMRT, at the discretion of the treating medical oncologist. Standard hydration measures and premedications were utilized to prevent significant nausea, vomiting and/or renal insufficiency.
Quality of Life Assessment
The Quality of Life Radiation Therapy Instrument (QOL-RTI), a validated QOL questionnaire, consisted of 39 questions, including the head and neck module.12,13 Of these, 24 questions were general QOL, one overall, and 14 head and neck specific, of which two specifically addressed swallowing function. Potential responses to all questions were presented on an 11 point Likert-type scale, ranging from 0 (“not at all”) to 10 (“very much so”). Most questions were scored with 10 as positive, where a higher score equates to a better QOL. However, several negatively worded questions were scored by subtracting the response from 10. Patients completed the questionnaire prior to treatment (PRE), at end of treatment (EOT), and at one month (M01), 3 months (MO3), 6 months (M06), 9 months (MO9) and 12 months (M012) following completion of CRT.
Statistical Considerations
The primary design was a single group, single intervention study using a convenience sample of up to 40 consecutive patients over a 36-month period. The primary hypothesis was that reduction in QOL caused by treatment intervention would be minimized by utilization of HIMRT, very low elective nodal dose per fraction and low dose weekly cisplatin alone, with simultaneous achievement of acceptable measures of LRC, PFS, and OS.
The primary endpoint was QOL, both general and head and neck specific. An a priori definition of significant reduction of QOL was established based on results from the principal investigator's prior experience and review of the literature, such that an individual patient's change from PRE to EOT or subsequent follow-up of at least 50% would constitute a significant reduction in QOL.
Secondary endpoints of LRC, PFS, and OS were determined by Kaplan Meier method, with survival time calculated from date of registration to date of relapse or death. NCI common terminology criteria for adverse events (CTCAE) version 3.0 were utilized to score all chemotherapy and radiation toxicities, both acute and late, associated with the protocol.
RESULTS
Study Population/ Treatment Compliance
Between 2006 and 2008, thirty-nine patients enrolled and completed therapy according to protocol. Patient characteristics are listed in Table 1. Thirty-seven of 39 patients (95%) completed HIMRT as prescribed. One patient declined hyperfractionation in favor of daily IMRT during the first week of treatment and received 69.5 Gy total. The other patient discontinued treatment after 66 Gy, following a 3-week treatment interruption due to intractable nausea and vomiting requiring hospitalization (grade 4).
Table 1.
Distribution of Patient Demographic and Clinical Tumor Characteristics
| N |
39 |
| Age (median, range) |
57, 28-72 |
| Gender (male, female) |
32, 7 |
| Race (W, AA, NA) |
32, 6, 1 |
| T stage | |
| 1 | 7 |
| 2 | 14 |
| 3 | 11 |
| 4 |
7 |
| N stage | |
| 0 | 5 |
| 1 | 12 |
| 2a | 8 |
| 2b |
14 |
| AJCC Stage | |
| III | 15 |
| IVa |
24 |
| Primary Site | |
| Oropharynx | 23 |
| Larynx | 8 |
| Hypopharynx | 5 |
| Oral Cavity |
3 |
| Treatment planning PET/CT? | 14 |
Thirty patients (77%) completed 6-7 weekly cycles of cisplatin as prescribed per protocol. Seven patients (18%) received 5 cycles and two patients (5%) received 4 cycles, with further cisplatin held due to neutropenia.
Ten patients underwent selective lymph node neck dissection (SLND) following completion of CRT. One of these 10 patients had positive PET/CT post-CRT and was found to have synchronous thyroid carcinoma without residual HNSCC. Seven of the remaining 9 patients (78%) had residual disease at SLND; five of whom had isolated tumor cells in a single lymph node. All patients with residual disease at SLND were initial stage N2 and all had residual disease on clinical exam prior to surgery.
QOL
Thirty-five patients (90%) were deemed evaluable for QOL outcomes, having completed QOL questionnaires PRE and EOT at minimum. Compilation of patients’ mean scores for all QOL categories and time points are shown in Table 2. Compliance with questionnaire completion waned between M03 and MO12. Mean general QOL (based on 24 questions) PRE was 7.7 versus 6.2 EOT. Overall QOL (1 question) was also minimally affected. Mean H&N QOL (14 questions) PRE of 7.8 decreased to 3.9 EOT, significantly worse in 18 of 35 patients (51%). Swallowing QOL was also significantly worsened in 53% of patients: mean PRE of 7.6 versus 3.4 EOT. By month 1 post-treatment, both mean H&N and swallowing scores returned near baseline: mean 6.2 and 6.1, respectively.
Table 2.
Quality of Life Radiation Therapy Instrument (QOL-RTI) Summary Descriptive Statistics Using all Available Data
| pre | eot | m1 | m3 | m6 | m9 | m12 | |
|---|---|---|---|---|---|---|---|
| QOL | |||||||
| n | 35 | 35 | 34 | 33 | 31 | 31 | 24 |
| mean (sd) | 7.7(1.32) | 6.2(1.34) | 7.0(1.44) | 7.5(1.43) | 7.7(1.74) | 7.7(1.72) | 8.0(1.75) |
| Overall | |||||||
| n | 35 | 35 | 34 | 33 | 31 | 31 | 24 |
| mean (sd) | 7.2(2.62) | 5.6(2.48) | 7.1(2.51) | 7.9(2.05) | 7.9(2.13) | 8.1(2.13) | 7.9(2.52) |
| H&N | |||||||
| n | 35 | 35 | 35 | 33 | 31 | 30 | 24 |
| mean (sd) | 7.8(1.86) | 3.9(1.50) | 6.2(1.71) | 6.8(1.78) | 7.2(1.62) | 7.3(2.07) | 7.8(1.70) |
| Worse* (#,%) | na | 18 (51) | 3 (9) | 2 (6) | 0 | 2 (7) | 1 (4) |
| Swallow | |||||||
| n | 35 | 34 | 35 | 33 | 31 | 30 | 24 |
| mean (sd) | 7.6(2.81) | 3.4(2.62) | 6.1(2.64) | 7.3(2.59) | 7.4(2.09) | 7.5(2.14) | 7.6(2.50) |
| Worse* (#,%) | na | 18 (53) | 7 (20) | 4 (12) | 1 (3) | 2 (7) | 3 (12) |
Variables:
QOL = average rating on 24 general items, rated from 0-10, where 10 is positive.
Overall = one item rating of overall quality of life, rated from 0-10, 10 = very good.
H&N = average rating on 14 items specific to head and neck; rated from 0-10, where 10 is positive.
Swallow = average rating on 2 items specific to the patients ability to swallow; rated from 0-10, where 10 is positive.
Worsening = greater than 50% reduction from pretreatment in QOL H&N or QOL Swallow
Efficacy
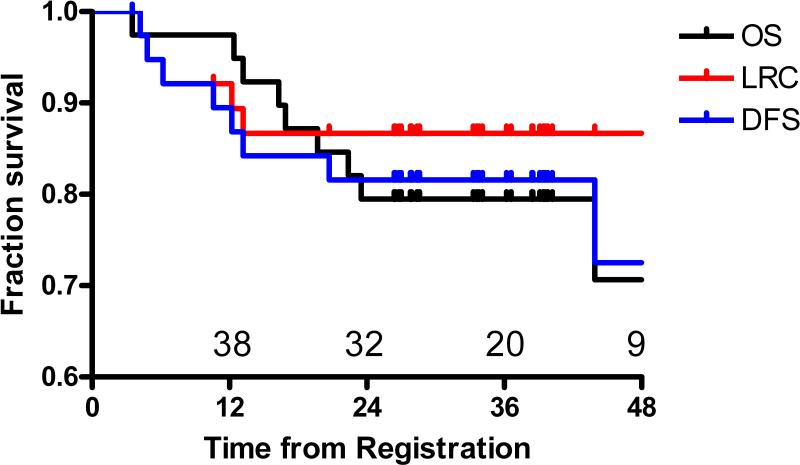
After a median follow-up of 37.5 months (range 24-59 months), we observed 9 deaths, 8 of which were due to cancer. Figure 1 details the patterns of failure. No patient failed in the electively treated neck as any component of failure and there were no isolated neck failures. No patient with a complete clinical response failed in the neck. Actuarial 3-year OS was 80%, PFS was 82%, and LRC was 87% (Figure 2).
Figure 1.
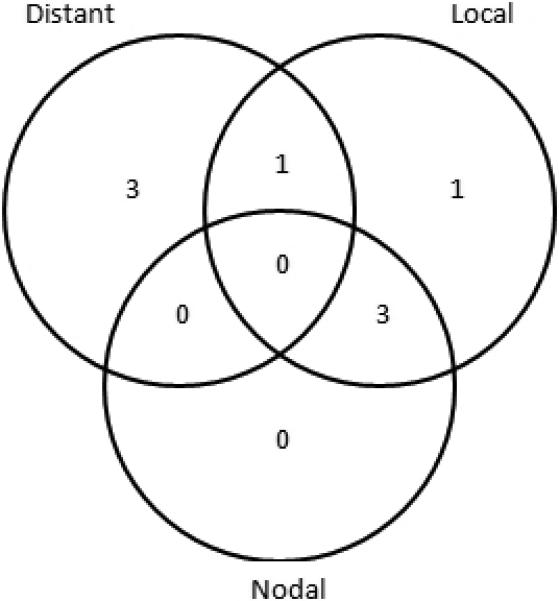
Patterns of failure for patients with advanced head and neck cancer treated with hyperfractionated IMRT and concurrent weekly cisplatin
Figure 2.
Locoregional control (LRC), disease-free survival (DFS), and Overall survival (OS) for patients with advanced head and neck cancer treated with hyperfractionated IMRT and concurrent weekly cisplatin
Toxicity
Acute grade 3 and 4 toxicities are listed in Table 3. In addition, one patient with T4N0 carcinoma of the larynx died immediately following treatment. Since examination one week prior to completion of CRT revealed no evidence of disease, this patient's death was categorized as grade 5 toxicity, a treatment-related death. A request for postmortem examination was declined by the patient's family.
Table 3.
Acute Grade 3 & 4 Adverse Effects of Hyperfractionated IMRT with Concurrent Cisplatin Observed in 39 Patients
| Adverse Effect | Grade 3 (%) | Grade 4 (%) |
|---|---|---|
| Mucositis | 15 (38) | 0 |
| Fatigue | 11 (28) | 0 |
| Dysphagia/ Dehydration | 11 (28) | 0 |
| Leukopenia | 10 (23) | 1 (3) |
| Nausea & Vomiting | 7 (18) | 0 |
| Infection | 3 (8) | 0 |
| Constipation | 3 (8) | 0 |
| Thrombosis | 1 (3) | 1 (3) |
| Dermatitis | 2 (5) | 0 |
| Xerostomia | 2 (5) | 0 |
| Mental Status Changes | 0 | 1 (3) |
| Upper GI Hemorrhage | 1 (3) | 0 |
| Elevated Creatinine | 1 (3) | 0 |
| Elevated Protime/INR | 1 (3) | 0 |
| Hypokalemia | 1 (3) | 0 |
| Voice Alteration/Dysarthria | 1 (3) | 0 |
Severe late toxicity was uncommon, consisting of one case of radionecrosis that occurred 6 months after completion of CRT, and one case of cellulitis requiring hospital admission 12 months after CRT. There were no clinical pharyngeal strictures. No patient required dilation for swallowing dysfunction. Median duration of feeding tube maintenance was 5 months (range 2-13 months).
DISCUSSION
QOL
Following survival and toxicity endpoints, health-related quality of life (QOL) is a critical outcome for patients with advanced HNSCC. Both patient-related symptoms at baseline and treatment-related factors significantly impact QOL.14-17 Multiple authors have reported significant QOL improvements with IMRT over conventional RT in terms of xerostomia and head and neck-related QOL.18-23 The impact of IMRT on swallowing dysfunction and related QOL in particular has been studied elegantly by the groups at University of Michigan and Daniel den Hoed Cancer Center.24 As expected, the regimen reported herein which incorporates both AFRT and concurrent cisplatin chemotherapy resulted in acute decrements in mean H&N QOL. Specifically, swallowing QOL was also significantly worsened (>50% decrease compared to baseline). Surprisingly though, mean scores for both H&N and swallowing QOL returned near baseline by the end of the first month after completion of CRT.
A drawback of the current study was the lack of a prospective control group in this regard. The authors’ definition of significant worsening of QOL as at least 50% decrement from baseline likely also down-played the negative impact of the CRT regimen on QOL relative to other reported series.
Efficacy & Toxicity
In the treatment of locally advanced HNSCC, both altered fractionation and combination CRT have improved efficacy over conventional radiotherapy. The standard treatment arm of the ongoing RTOG 0522 protocol is conventionally fractionated radiotherapy with concurrent cisplatin at 100 mg/m2 delivered once every 3 weeks. This chemotherapy choice dates back to the dramatic improvement in OS observed with this regimen (over conventionally fractionated RT alone) for patients with nasopharyngeal carcinoma in Intergroup 00-99.25 It remains to be seen whether incorporating AFRT with concurrent boost is superior to the current standard of care for patients with locally advanced HNSCC. We await final report of the RTOG 0129 trial in that regard.
In the meantime, however, the accumulating evidence suggests relative efficacy but significant toxicity from regimens incorporating both AFRT and concurrent chemotherapy. The authors have selected several important studies which utilized AFRT with cisplatin-based chemotherapy, shown in Table 4. The Duke University trial resulted in respectable 3 year OS of 55% and PFS of 61% in the CRT arm.10 However, only 57% of patients completed the 4 cycles of chemotherapy prescribed and late toxicity was not reported in detail. Our community experience with this regimen resulted in excellent survival but universal grade 3 acute mucosal toxicity as well as more disturbing 14% late pharyngeal stricture rate.11
Table 4.
Selected Literature Review of Hyperfractionated RT with Concurrent Cisplatin for Locally Advanced Head and Neck Squamous Cell Carcinoma
| Institution; Primary Author; (Reference #) | n | RT (Gy) | Fx | IMRT | Concurrent Cisplatin Chemotherapy | Grade 3+ Toxicity | Isolated Neck Failure (elective RT dose) | 2yr LC % | 2yr DFS % | 2yr OS % | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univ of Leuven, Belgium; Nuyts (28) | 90 | 72 | 2 Gy daily to 40 Gy, then 1.6 Gy bid | 16% | 100 mg/m2 q3 weeks | -Mucositis 75% -Dysphagia 82% -Late dysphagia 15% -Xerostomia 22% |
1% (46.4 Gy) | 70 | 60 | 74 | Stage IV = 75% N2 & N3 = 68% Hospitialization = 39% Median PEG = 5 mos. |
| Duke Univ., NC; Brizel (10) | 56 | 70 | 1.25 Gy bid | 0 | 12 mg/m2 & 5FU 600 mg × 5 days, weeks 1 & 5 | -Mucositis 77% -Grade 5 = 2% -Late dysphagia NR |
0 (40-60 Gy) | 70 (3y) | 61 (3y) | 55 (3y) | N2 & N3 = 44% Selective LND = 14 (3 +) |
| Univ. Kragujevic; Jeremic (37) | 65 | 77 | 1.1 Gy bid | 0 | 6 mg/m2/day | -Stomatitis 49% -Dysphagia 25% -Xerostomia 22% -Late dysphagia NR -Grade 5 = 4% |
NR (50.6 Gy) | 61 | NR | 68 | N2 & N3 = 57% No selective LND |
| RTOG 99-14; Garden (27) | 76 | 72 | 1.8 Gy daily, then 1.5 Gy concurrent boost | 0 | 100 mg/m2 q3 weeks | -Xerostomia 14% -Late dysphagia 18% |
NR (50-54 Gy) | 67 | 54 | 70 | Stage IV = 88% 1 yr PEG = 41% |
| Univ. Wurzburg, Germany; Beckmann (31) | 37 | 69.9 | 1.8 Gy daily, then 1.5 Gy concurrent boost | 0 | 40 mg/m2/week | -Mucositis 86% -Grade 5 = 5% |
11% (50.4 Gy) | NR | 58 | 67 | Oropharynx & hypopharynx only Conventional fx 16% |
| Univ. Zurich; Hugenin (38) | 112 | 74.4 | 1.2 Gy bid | 0 | 20 mg/m2 × 5 days, weeks 1 & 5 | -Mucositis 61% -Xerostomia 17% -Late dysphagia 23% |
NR (50-62 Gy) | 55 | 45 | 59 | N2 & N3 = 60% |
| New Hanover Regional; Maguire (11) | 50 | 70 | 1.25 Gy bid | 0 | 12/mg/m2 & 5FU 600 mg × 5 days, weeks 1 & 5 | -Mucositis 100% -Late stricture 14% |
2% (44-60 Gy) | NR | 75 | 80 | N2 & N3 = 42% Selective LND = 8 (5 +) |
| New Hanover Regional & Univ. North Carolina; Maguire | 39 | 70 | 1.25 Gy bid PTV70; 1.07 Gy bid PTV60; 0.87 Gy bid PTV50 | 100% | 33 mg/m2/week | -Mucositis 38% -Dysphagia 28% -Grade 5 = 3% -Late dysphagia 0 |
0 (44-60 Gy) | 87 (3y) | 82 (3y) | 80 (3y) | Stage IV = 62% N2 = 56% N2c & N3 excluded Median PEG = 5 mos. |
Most recent clinical trials have deleted 5-fluorouracil from their concurrent regimens due to its significant mucosal toxicity. However, even with single agent cisplatin delivered at 100 mg/m2 once every 3 weeks (the current RTOG standard), significant toxicity remains. The RTOG 99-14 trial, reported initially by Ang and updated by Garden, resulted in 14% late grade 3 salivary and 18% late grade 3 esophageal/swallowing toxicity, as well as 3 (4%) treatment-related deaths.26,27 Of particular note, 41% of evaluable patients had feeding tubes still in place at 1 year post-treatment. The group from the University of Leuven in Belgium reported a strikingly similar toxicity profile albeit without grade 5 toxicities.28 Acute grade 3 toxicities included 75% mucositis and 82% dysphagia, with late grade 3 xerostomia 22% and dysphagia 15%.
Several groups have moved to weekly cisplatin dosing in their CRT regimens for patients with locally advanced HNSCC. Theoretical benefits include improved radiosensitization and decreased toxicity compared to the RTOG standard. Precedent exists for weekly dosing of concurrent cisplatin with RT in squamous cell carcinoma of the cervix.29,30 The group from the University of Wurzburg reported a trial of 37 patients with HNSCC treated with concurrent boost AFRT and cisplatin at 40 mg/m2/week.31 While the regimen was efficacious, it still resulted in 86% acute grade 3 mucosal toxicity. Most of the patients in this trial required hospitalization during the boost phase of treatment. The authors’ experience with low-dose weekly cisplatin in the current trial has been favorable, with good QOL outcomes during the first year post-treatment as detailed above. It is unlikely that a phase 3 trial testing cisplatin low-dose weekly versus high-dose once every 3 weeks concurrent with RT will be done. In fact, the proposed successor trial to RTOG 0522 reportedly will incorporate weekly rather than once every 3 week concurrent cisplatin dosing.
The two major late toxicities of CRT for HNSCC are xerostomia and swallowing dysfunction. Although the authors did not objectively evaluate salivary flow in the current study, patients’ QOL questionnaires indicate that the rate of significant xerostomia was low. IMRT has been shown to decrease late xerostomia by minimizing dose to the contralateral parotid gland. In the current trial, we were able to maintain a contralateral mean parotid dose <26 Gy, an accepted standard based on work by Eisbruch et al.32
IMRT has not specifically been shown to significantly decrease the risk of long-term swallowing dysfunction. However, the group at University of Michigan, among others, has shown a correlation between chronic swallowing dysfunction and the RT dose to both pharyngeal constrictor muscles and larynx.33-36 In the present series, no attempt was made to avoid pharyngeal constrictor muscles within the PTV for fear of under-dosing tumor. None of these patients required pharyngeal/esophageal dilation due to chronic swallowing dysfunction, a significant improvement over our previous regimen as noted above. Median time to feeding tube removal was 5 months, a time that we consider to be quite acceptable.
Minimizing Elective Nodal IMRT Dose
One of the most interesting aspects of the treatment regimen reported herein is the low dose per fraction delivered to sites of microscopic disease. Within a single IMRT plan, intermediate and low-risk PTVs received 60 Gy at 1.07 Gy per fraction and 50 Gy at 0.89 Gy per fraction, respectively, in a concerted effort to minimize toxicity. Initially, there was some concern that these low doses per fraction might not eradicate microscopic disease. However, it appears our concerns were unfounded in this small series. No patient failed in any electively irradiated portion of the neck. There are no comparable series in the medical literature in this regard.
In conclusion, HIMRT with concurrent weekly cisplatin resulted in acute decrements in both H&N and swallowing QOL that returned near baseline by one month post-treatment. The combination of hyperfractionated IMRT at low doses per fraction (particularly to intermediate and low-risk volumes) and weekly cisplatin was quite efficacious with acceptable toxicity. In an effort to further minimize toxicity while maintaining good LRC for our future patients with HNSCC, our next clinical trial will further lower both fraction size and total dose delivered to elective nodal sites.
Acknowledgments
Supported by Cancer Disparities Research Partnership Grant, No. CA03018 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification:
The authors do not have any actual or potential conflicts of interest.
REFERENCES
- 1.Wendt TG, Grabenbauer GG, Rodel CM, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol. 1998;16:1318–1324. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 2.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced stage oropharynx carcinoma. J Natl Cancer Inst. 1999;24:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 3.Adelstein DJ, Lavertu P, Saxton JP, et al. Mature results of a phase III randomized trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer. 2000;88:876–883. doi: 10.1002/(sici)1097-0142(20000215)88:4<876::aid-cncr19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitant chemoradiotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayed S, Nelson N. Adjuvant and adjunctive chemotherapy in the management of squamous cell carcinoma of the head and neck region: a meta-analysis of prospective and randomized trials. J Clin Oncol. 1996;14:838–847. doi: 10.1200/JCO.1996.14.3.838. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, Bourhis J, Designe L, et al. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: three meta-analyses of up-dated individual data. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 7.Horiot JC, LeFur R, Nguyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25:231–241. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 8.Horiot JC, Bontemps P, Van Den Bogaert W, et al. Accelerated fractionation compared to conventional fractionation improves locoregional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol. 1997;44:111–121. doi: 10.1016/s0167-8140(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 9.Fu KK, Pajak TF, Trotti A, et al. A RTOG phase III study to compare hyperfractionation and two variants fo accelerated fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 10.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Eng J Med. 1998;338:1798–1803. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 11.Maguire PD, Meyerson MB, Neal CR, et al. Toxic cure: hyperfractionated radiotherapy with concurrent cisplatin and fluorouracil for stage III and IVa head and neck cancer in the community. Int J Radiat Oncol Biol Phys. 2004;58:698–704. doi: 10.1016/S0360-3016(03)01576-1. [DOI] [PubMed] [Google Scholar]
- 12.Johnson D, Casey L, Noriega B. A pilot study of patient quality of life during radiation therapy treatment. Quality of Life Research. 1994;3:267–272. doi: 10.1007/BF00434900. [DOI] [PubMed] [Google Scholar]
- 13.Trotti A, Johnson DJ, Gwede C, et al. Development of a head and neck companion module for the quality of life-radiation therapy instrument (QOL-RTI). Int J Radiat Oncol Biol Phys. 1998;42:257–261. doi: 10.1016/s0360-3016(98)00224-7. [DOI] [PubMed] [Google Scholar]
- 14.Murry T, Madasu R, Martin A, et al. Acute and chronic changes in swallowing and quality of life following intraarterial chemoradiation for organ preservation in patients with advanced head and neck cancer. Head Neck. 1998;20:31–37. doi: 10.1002/(sici)1097-0347(199801)20:1<31::aid-hed6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Ronis DL, Duffy SA, Foler KE, et al. Changes in quality of life over year 1 in patients with head and neck cancer. Arch Otol Head Neck Surg. 2008;134:241–248. doi: 10.1001/archoto.2007.43. [DOI] [PubMed] [Google Scholar]
- 16.Dirix P, Nuyts S, Vander Poorten V, et al. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Supp Care Control. 2008;16:171–179. doi: 10.1007/s00520-007-0300-5. [DOI] [PubMed] [Google Scholar]
- 17.Langendijk JA, Doornaert P, Verdonck-deLeeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 18.Jabbari S, Kim HM, Feng M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Raidat Oncol Biol Phys. 2005;63:725–731. doi: 10.1016/j.ijrobp.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 19.Yao M, Karnell LH, Funk GF, et al. Health-related quality of life outcomes following IMRT versus conventional radiotherapy for oropharyngeal squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2007;69:1354–1360. doi: 10.1016/j.ijrobp.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Vergeer MR, Doornaert PA, Rietveld DH, et al. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74:1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 21.van Rij CM, Oughlane-Heemsbergen WD, Ackerstaff AH, et al. Parotid-gland sparing IMRT for head and neck cancer improves xerostomia related quality of life. Radiat Oncol. 2008;9:3–4. doi: 10.1186/1748-717X-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Lin A, Kim HM, Terrell JE, et al. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Bil Phys. 2003;57:61–70. doi: 10.1016/s0360-3016(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 24.Eisbruch A, Levendag PC, Feng FY, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Inr J Radiat Oncol Biol Phys. 2007;69(Supp):S40–42. doi: 10.1016/j.ijrobp.2007.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Sarraf M, LeBlanc M, Giri PS, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: A phase III randomized intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 26.Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced had and neck carcinomas: rsadiation therapy oncology group phase II trial99-14. J Clin Oncol. 2005;23:3008–3015. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 27.Garden AS, Harris J, Trotti A, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase II trial of the radiation theapy oncology group (RTOG 99-14). Int J Radiat Oncol Biol Phys. 2008;71:1351–1355. doi: 10.1016/j.ijrobp.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuyts S, Dirix P, Clement PM, et al. Impact of adding concomitant chemotherapy to hyperfractionated accelerated radiotherapy for advanced head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1088–1095. doi: 10.1016/j.ijrobp.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Eng J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 30.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Eng J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 31.Beckmann GK, Hoppe F, Pfreunder L, et al. Hyperfractionated accelerated radiotherapy in combination with weekly cisplatin for locally advanced head and neck cancer. Head Neck. 2005;27:36–43. doi: 10.1002/hed.20111. [DOI] [PubMed] [Google Scholar]
- 32.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:77–87. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 33.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphasia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68(Supp):1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 34.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;15:1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 35.Dirix P, Abbeel S, Vanstraelen B, et al. Dysphagia after chemoradiotherapy for head-and neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009:74. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 36.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2009:74. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Jeremic B, Milicic B, Dagovic A, et al. Radiation therapy with or without concurrent low-dose daily chemotherapy in locally advanced, non-metastatic suamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22:3540–3548. doi: 10.1200/JCO.2004.10.076. [DOI] [PubMed] [Google Scholar]
- 38.Huguenin P, Beer KT, Allal A, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol. 2004;22:4665–4673. doi: 10.1200/JCO.2004.12.193. [DOI] [PubMed] [Google Scholar]