Abstract
Background & Aims
Although serological analysis is used in diagnosis of celiac disease, histopathology is considered most reliable. We performed a prospective study to determine the clinical, pathological and serological spectrum of celiac disease in a general population (Kalixanda study).
Methods
A random sample of an adult general population (n=1000) was analyzed by upper endoscopy, duodenal biopsy, and serological analysis of tissue transglutaminase (tTg) levels; endomysial antibody (EMA) levels were analyzed in samples that were tTg+. The cutoff values for diagnosis of celiac disease were villous atrophy with 40 intraepithelial lymphocytes (IELs)/100 enterocytes (ECs).
Results
Samples from 33 subjects were tTg+ and 16 were EMA+. Histological analysis identified 7/1000 subjects (0.7%) with celiac disease; all were tTg+ and 6/7 were EMA+. Another 26 subjects were tTg+ (7/26 EMA+). This was addressed by a second quantitative pathology study, (nested case-control design) using a threshold of 25 IELS/100 ECs. In this analysis, all 13 samples that were tTg+ and EMA+ had ≥25 IELs/100ECs. In total, 16 subjects (1.6%) had serological and histological evidence of gluten-sensitive enteropathy. IELs were quantified in duodenal biopsy samples from seronegative individuals (n=500); 19 (3.8%) had >25 IELs and lymphocytic duodenosis (LD).
Conclusions
Measurement of ≥25 IELs/100 ECs correlated with serological indicators of celiac disease; a higher IEL threshold could miss 50% of cases. Quantification of tTg is a sensitive test for celiac disease; diagnosis can be confirmed by observation of ≥25 IELs/100ECs in duodenal biopsies. Lymphocytic enteropathy (celiac disease and LD) is common in the population (5.4%).
Keywords: Celiac disease, Lymphocytic enteropathy, Serology, Histology, Epidemiology
Background
Celiac disease, previously thought to be rare, may occur in up to 1% of the adult Caucasian population. 1 In rigorous studies estimates of the prevalence of celiac disease are based on the rate of clinically diagnosed cases using either a geographically restricted or birth cohort denominator. 2–6,7,8 However, even these studies are still likely to underestimate the prevalence of undiagnosed celiac disease. 9
There are two main strategies for detecting celiac disease, firstly, testing members of the population with increased risk,10 and secondly, screening general population samples by serology.11 The case detection and confirmation in these studies is limited by the accuracy of serologic methods. Over the last two decades serology for celiac disease has evolved to more specific and sensitive autoantibody detection, using endomysial (EMA) or tissue transglutaminase antibodies (tTg), however, the performance of these is still variable. The sensitivity may not as good as originally reported, and may be less for individuals with partial villous atrophy. 12 13 14 15 16 Conversely, serologic tests including the most specific endomysial antibody may be positive in the absence of the architectural changes that defines celiac disease. In some individuals, biopsies reveal evidence of an inflammatory response such as increased intraepithelial lymphocytosis without architectural change or increased immunoglobulin A (IgA) deposition. 17,18
The histological features of established celiac disease are easily recognized using established criteria, 19, 20 but subtle and early changes are difficult to diagnose with assurance. In particular, the “normal” intraepithelial lymphocyte (IEL) count has raised considerable debate, with the normal range of IELs/ 100 enterocytes (ECs) quoted from 10–40 in varying studies in different centers. 21, 22
Celiac disease is defined by the enteropathy that occurs in response to ingestion of gluten and resolves when it is removed from the diet. 23 An important characteristic is that of increased IELs without which celiac disease is considered unlikely even with substantial villous changes.24 However, the converse, increased IELs without architectural changes, has been included in the spectrum of so-called gluten sensitivity. 19 Increased IELs in the absence of other histological features of celiac disease, hereafter referred to as lymphocytic duodenosis (LD) is only associated with celiac disease in a minority and may be a response to other inflammatory process in the gut. 25 The clinical implications of LD are largely unknown, apart from some disease associations, but are a frequent issue given the widespread use of upper endoscopy with duodenal biopsies for the investigation of iron deficiency anemia and chronic diarrhea. 25, 26 Possible etiologies of LD are legion including infection, drugs and autoimmune disease. 25, 27–31 The prevalence of LD, whether associated with gluten sensitivity or otherwise in the normal population is unknown.
There are no prospective studies where celiac serology and duodenal biopsies have been performed in parallel in a sample of a normal general population to determine the spectrum of lymphocytic enteropathy that includes celiac disease and lymphocytic duodenosis. The Kalixanda study is a unique study of the demographics, upper gastrointestinal pathology and symptoms in a random sample of general adult population in Northern Sweden. 32
The aims of this study are to define the prevalence of celiac disease in the general population correlating histology and serology, and to explore the frequency of lymphocytic enteropathy, to define the true nature of the celiac iceberg. Additionally, we correlated symptoms with serological and pathological findings.
Materials and Methods
Study population
The population is from two neighboring communities in Northern Sweden, Kalix and Haparanda, with 18,408 and 10,580 inhabitants, respectively (December 1998). The demographics were similar to the national average in Sweden in both communities. The study was approved by the ethics committees of Umeå University, Sweden, and the Mayo Clinic and conducted in accordance with the revised Declaration of Helsinki.
Random sampling
Using the computerized national population register, a representative sample was generated. Every seventh adult (n = 3000) from the target population (20–80 years of age, n = 21 610) was identified, equivalent to random sampling.
Study design and response rate
A total of 2122 individuals (response rate 74.2%) completed the validated postal questionnaire, the Abdominal Symptom Questionnaire (ASQ) 33 Of these subjects, 1001 underwent endoscopy. Of the 1001 subjects with complete EGD and extended ASQ, one refused biopsies and another two not evaluable due to missing data. Age and gender distribution in the 1001 subjects who responded to the questionnaire at both assessments (488 males [48.8%], mean age 54 years) reflected the local and Swedish population. 32
Esophagogastroduodenoscopy
Upper gastrointestinal endoscopy was performed by three experienced endoscopists using a predefined endoscopy protocol. 32 At endoscopy, biopsies were taken from the stomach (Sydney protocol) and 2 biopsies each from the bulb (D1) and second part (D2) of the duodenum. 34
Celiac Serology
All subjects in the study (n=1001) had serum saved at the time of endoscopy. The basic screening test used was based on the recombinant human tissue transglutaminase (tTg) ELISA IgA antibodies assay (The Binding Site, UK). In all samples that were either weak positive (20–30 U/ml), or positive (>30 U/ml), endomysial antibody (EMA) testing was performed by incubating diluted (1:5, 1:10 and 1:20) serum samples on 5 µm cryostat sections of monkey esophagus. The immunofluorescent antibody test was interpreted while unaware of the identity or dilution of the sera.
Histopathology
Sections were stained with haematoxylin and eosin. H. pylori was detected in gastric biopsies by Warthin-Starry staining. Gastric pathology was recorded as per the Sydney system. 34
Histopathology I (all subjects)
There were 2 separate pathological examinations of the duodenal biopsies from D1 and D2. In the first stage, all specimens were assessed for architectural change, (total, partial or no villous atrophy) alongside an estimation of IEL counts using the then contemporary criteria for diagnosis of celiac disease. 20
Histopathology II (nested case-control based on serology)
After serology, with changing criteria for celiac disease, a systematic re-evaluation of duodenal biopsies (blinded to serological results) a nested case control study was undertaken, each positive serological case matched to 2 seronegative controls, matched for age and gender. 21, 35 IEL counts were noted for all cases in D1 and D2 biopsies. The histological criteria used were: Non atrophic (grade A), and atrophic (grade B), Grade B subdivided into B1 –villus: crypt ratio less than 3:1 with detectable villi, and B2 with flat mucosa i.e. partial and total villous atrophy. Non atrophic (grade A) lesions were characterised by an increase in intraepithelial lymphocytes (>25) with normal villous architecture. 36. Villus height: crypt depth ratio and crypt hyperplasia were also recorded.
Validation of IEL counting method
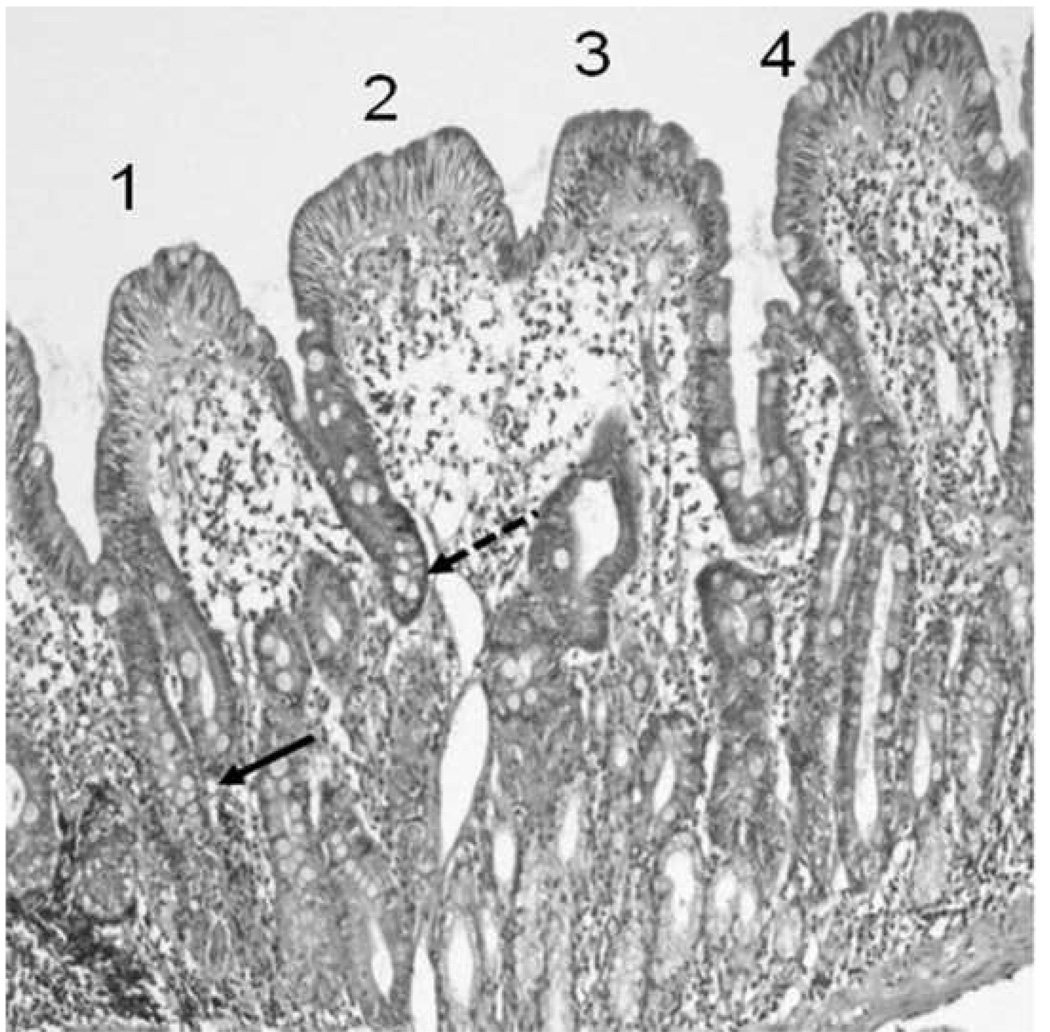
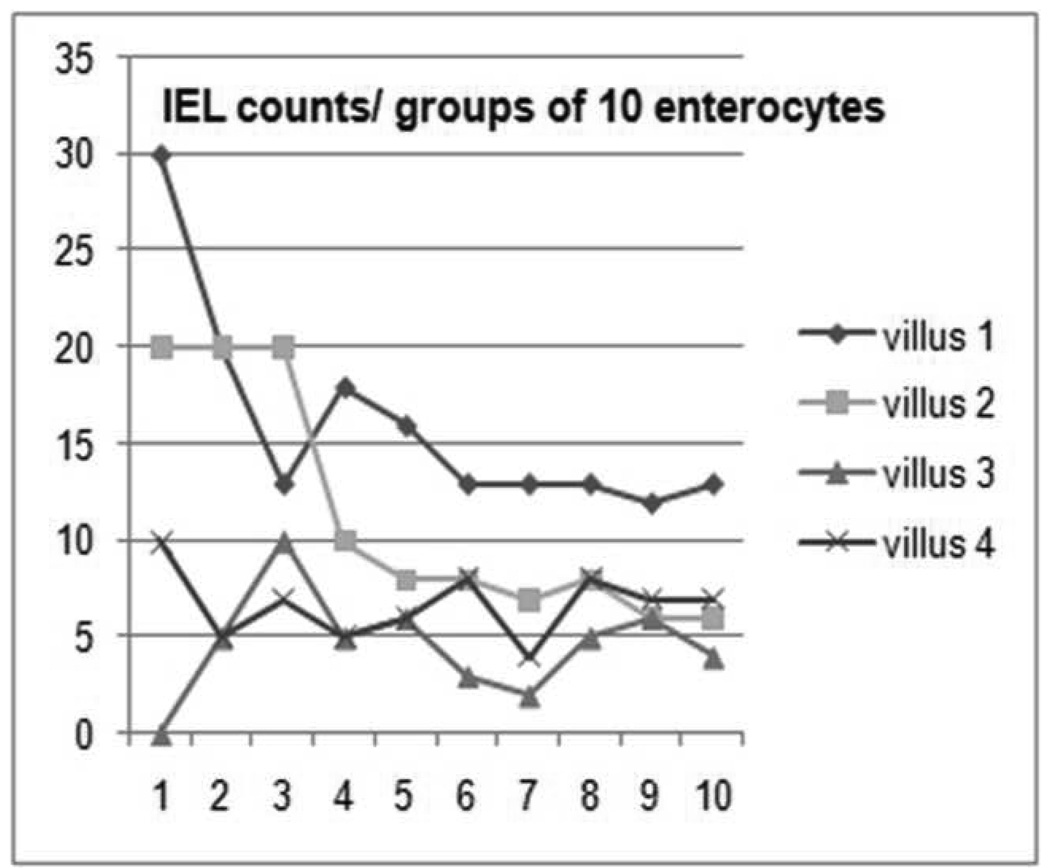
A validated method was developed to ensure time efficient and reproducible method of determining IEL counts in paired duodenal biopsies from both D1 and D2 Five samples were subjected to detailed counts. Observers (2) each performed IEL counts, in groups of 10 enterocytes and selected 4 villi with epithelial nuclei aligned to the basement membrane, marked 1 – 4. At villus 1, IELs/10 enterocytes were counted and recorded, starting at the base of the crypt (lowest point between two adjacent villi) and continuing till the next base, (Figure 1) IEL counts of villi marked 1– 4 were recorded, to establish intravillus differences in IEL counts. The IEL count/100 ECs was extrapolated for cumulative groups of 10 enterocytes and plotted on a graph to find at which point the counts became stable. (Figure 2) We also studied additional benefit in immunostaining lymphocytes with CD3, but found no additional sensitivity which gave largely similar findings (data not shown)
Figure 1.
Select 4 villi with epithelial nuclei aligned to basement membrane (marked 1 – 4). To count IELs, from villus 1: count and record IELs/10 enterocytes, starting at base of crypt (arrow, lowest point between two adjacent villi) continue till next base (dashed arrow), continue counts in of villi marked 2 – 4
Figure 2.
Extrapolation of IEL counts/ 10 enterocytes in 4 villi, the graph levels out at 50 enterocytes.
Quantitative analysis of IELs in 500 subjects
To determine the prevalence of LD in a larger sample of seronegative subjects in the cohort (500/1000) subjects were examined for D1 and D2 pathologies. IEL counts in D1 and D2 were compared. Architecture was assessed and IELs/ 100 ECs counted. A threshold of 25 IELs per 100 ECs was used as the threshold for intraepithelial lymphocytosis as quoted in European and other studies and hence defined LD in those without villous atrophy. 21, 26, 37
Symptom correlation
Symptoms as reported in the validated questionnaires were analyzed individually or in groups that represented dyspepsia, or irritable bowel syndrome by Rome II. 38 The ASQ has been applied in a previous study of celiac disease. 39
H. pylori infection
Samples from the antrum and corpus were cultured for H. pylori and analysed as previously reported. 40 Current H. pylori infection was defined as a positive culture or histological finding.
HLA typing
HLA-DQ risk types were predicted using the method described,41 and validated in several populations of European origin.42 Briefly, this method uses six HLA-tagging single nucleotide polymorphisms (SNPs), to identify DQ2.2, DQ2.5, DQ7 and DQ8 risk variants based on strong linkage disequilibrium (LD) at HLA-loci. SNPs rs2187668, rs2395182, rs4713586, rs7775228, rs4639334 and rs7454108 were genotyped with TaqMan assays (Applied Biosystems, Foster City, California, USA) on a 7500 Fast Real-Time PCR system (Applied Biosystems). All markers had a genotyping success rate > 99% and did not deviate (p > 0.05) from Hardy-Weinberg Equilibrium (HWE), with the exception of SNP rs4713586 which failed to produce clear allele clusters in the TaqMan assay, and was therefore excluded from further analyses. Genotype at the rs4713586 locus allows discrimination between DQ2.2 and the rare DQ4 type, and is therefore needed to predict DQ2 risk carriage in DQ7/DQ2.2 individuals. Only two individuals carried DQ2.2 (or DQ4) in combination with DQ7 in our sample, and these were excluded from further analyses. DQ2 and DQ8 risk variants were predicted for all other subjects as previously described. 41
Statistical analysis
Descriptive statistics were used to summarize the data, including counts and percentages for categorical data and medians and ranges for continuous data. The chi-square or Fisher’s exact test was used to test for associations between two categorical variables, while the Wilcoxon Rank Sum test was used to test for continuous data differences between two groups. For two continuous parameters, the strength of their linear relationship was measured using Spearman’s correlation coefficients (ρ). To assess the agreement between two measurements or the reliability between two raters, Lin’s concordance correlation coefficients were used for continuous data and Cohen’s kappa (κ) statistics for categorical data. The level of significance for statistical testing was defined as P<.05 (2-sided). All analyses were carried out using SAS version 8 software (SAS Institute Inc, Cary, NC).
Results
Serology
There were 33 subjects seropositive for tTg-IgA, widely dispersed across age (median=60, range 25–75) and evenly distributed by gender (16 males). Two known celiac subjects who had been on a gluten free diet were negative. EMA were tested on these 33 samples; 16 were positive for EMA, 3 of whom also had smooth muscle staining. All but one of the EMA positive samples were moderately or strongly positive on tTg-IGA testing. The EMA positives had higher tTg-IgA levels than the EMA negative subjects (median [range]: 89.3 [22.3 – 178.0] vs. 26.7 [20.8 – 87.1], respectively; P=0.0002)
Histopathology I
Initial review of histology using the presence of at least partial villous atrophy and 40 IELs/ 100 enterocytes as the cut off, found 7 /1000 subjects with celiac disease based on histology: 4 had total villous atrophy and 3, partial villous atrophy. All 7 were positive for tTg-IgA and 6/7 were EMA positive. However, by serology, a further 26 were positive for tTg-IgA and of these, 7/26 were also EMA positive.
Histopathology II, Nested case control study
The nested case control study comprised a blinded review of D1 and D2 biopsies of 99 subjects. One patient’s slides previously found to have total villous atrophy and IELS > 40 could not be retrieved nor could the D1 slides for 2 seronegative controls. A total of 16 subjects had an IEL count of >25/ 100 enterocytes and all of these were tTg-IgA positive (14 were EMA positive, and 2 negative). Of these, 6 had total villous atrophy on at least one sample, 7 had partial villous atrophy and 3 had normal architecture (LD). Two of 19 tTg-IgA positive but EMA negative had partial villous atrophy and IELs> 25. One subject with positive tTg and strong smooth muscle staining on EMA had normal D1 and D2 biopsies. There was high agreement between serology and indication of histological abnormality associated with celiac disease (κ=0.956) though the density of IELs correlated weakly with tTg-IgA levels (ρ = 0.36, P=0.0003)
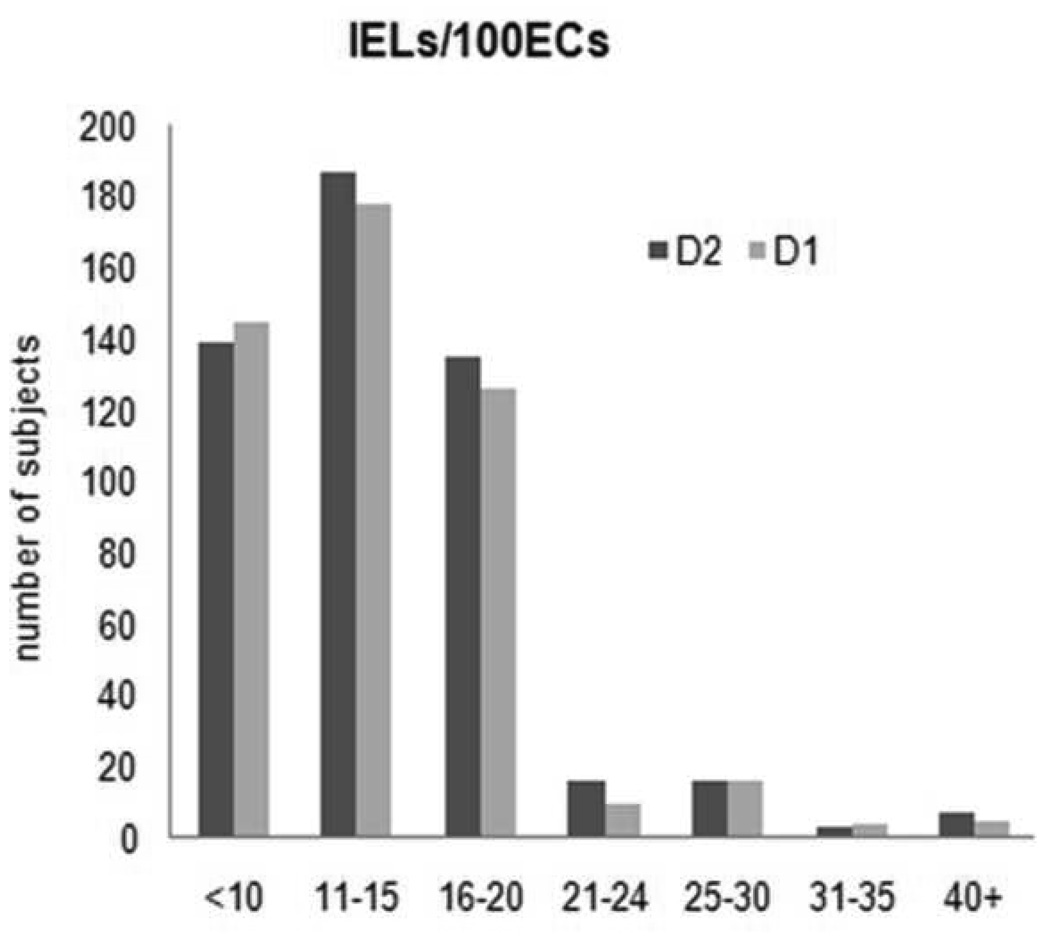
Two patients in the control group had partial villous atrophy but neither of these or the rest of seronegative controls (n=66) had > 25 IELs/100 enterocytes. The mean counts for IELs for all subjects were 14.0 (+/− 6.6) in D1 and 14.2 (+/− 6.4) in D2 (Figure 2). The mean IEL count/ 100 enterocytes in the tTg-IgA and EMA positive subjects was 33 (7–70) in D1 and 33 (7–70) in D2. The IEL counts in the biopsies from the first and second part of the duodenum were highly concordant (Lin’s concordance correlation coefficient=0.854, 95% CI, 0.788– 0.901). Results are shown in Table 1
Table 1.
Celiac Disease Serology and Histology
| Celiac Disease by histology/ serology n=16 | ||||
|---|---|---|---|---|
| Serology +ve |
IELs>25 | D1 or/ and 2 Atrophic Grade B1 = 6, Atrophic Grade B2 = 6 |
Crypt hyperplasia | Villus height: crypt depth ratio 1:1 =6 (B1) 2:1 =6 (B2) |
| 11 | 12 | 12 | 12 | 12 |
| IELs>25 | D1 or 2 Non-Atrophic Grade A |
Crypt hyperplasia | Villus height: crypt depth ratio 3:1 |
|
| 4 | 3 | 3 | 2 | 3 |
| IELs<25 | D1 or 2 Non- Atrophic Grade A |
Crypt hyperplasia | Villus height: crypt depth ratio 3:1 |
|
| 1 | 1 | 1 | 0 | 1 |
Celiac Disease, Composite Results
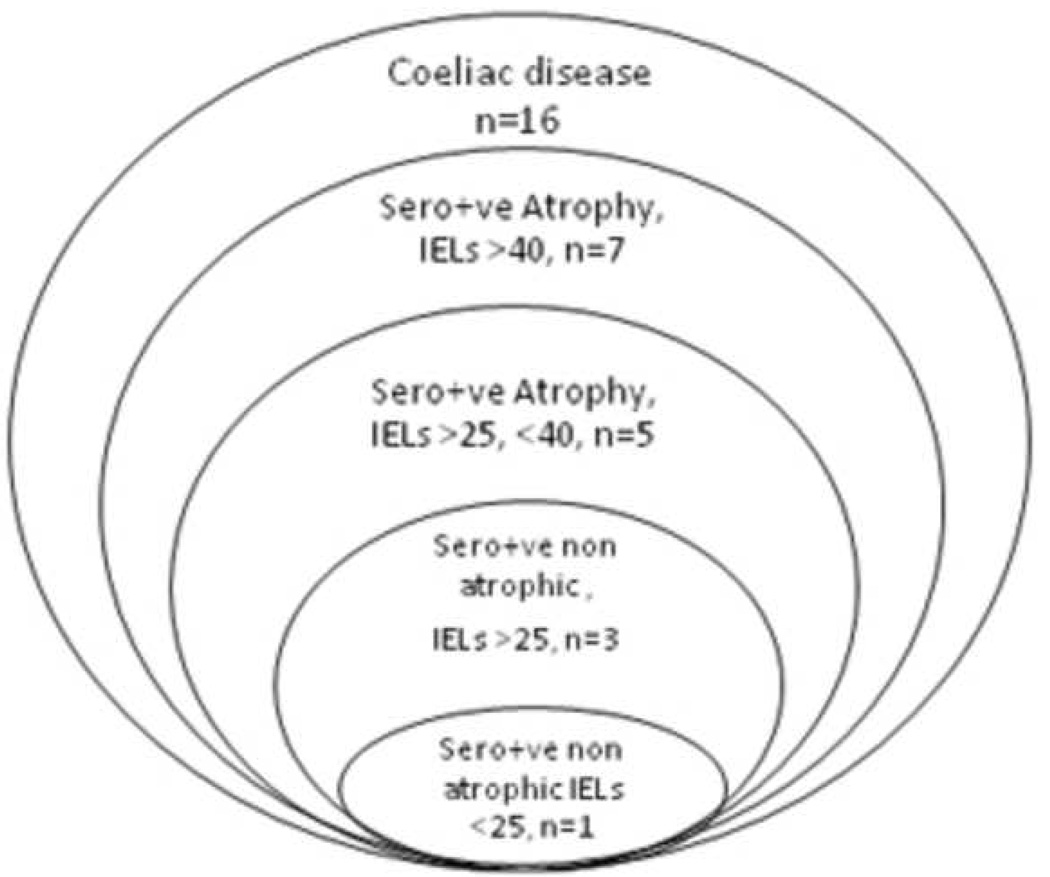
Thus, in total, 16/1000 subjects had serological and histological evidence of gluten sensitive enteropathy (> 25 IELs/100enterocytes with both tTg-IgA and EMA-IgA double positivity. (Figure 3) In this population the prevalence of undiagnosed celiac disease (based on tTg-IgA positive, EMA positive and IELs> 25 was 1.6% (95% CI, 0.92–2.58). If we include the 2 subjects that were previously diagnosed and treated the prevalence is 1.8%.
Figure 3.
Comparison of D1 and D2 IEL counts in all subjects
HLA genotyping
From the case control study, (n=97 available) with measured genotype data, 16 were seropositive. All 16 had the presence of DQ2 compared to only 17% of 81 seronegatives (p<.001, Fisher's Exact test). There was no association between seropositivity and DQ8 (12.5% vs. 21.0%, p=0.43 Chi-Square test). The median (IQR) IEL counts of those with DQ2 vs. without are 25.5 (11.0, 34.0) vs. 10.8 (8.5, 14.0), respectively with a strong correlation between the DQ2 and the IEL count and the serology status (Rank sum test p<0.0001)
Accurate assessment of IELs by histology
Extrapolation of IEL counts/group of 10 enterocytes showed that when 50 enterocytes have been counted there is a consistent count/100 ECs with minor variation per villus. (Figure 2) Analysis of interobserver concordance in IEL counts showed substantial agreement with an unweighted kappa value of 0.74 (95% CI 0.69–0.78).
Prevalence of Lymphocytic Duodenosis (raised IELs with normal villous architecture and negative celiac serology)
In seronegative subjects, 19/ 500 subjects had LD in either D1 or D2. In this group, 72% with LD had H. pylori infection compared to only 30% without LD (p value = 0.0001). The rate of H. pylori infection did not differ according to tTg result, with 12 of 33 (36.4%) tTg-positive subjects with infection compared to 33.9% subjects in the tTg-negative group (p =0.77). One subject had taken NSAIDs and one had associated lymphocytic gastritis. No concurrent autoimmune disease, Giardia or inflammatory bowel disease was reported in these subjects. The prevalence of LD in seronegative subjects is 3.8%. (95% CI: 2.3%–6.0%)
Symptom correlation with Serology and Histology
Symptoms did not influence participation in the endoscopy study, and there was no evidence of selection bias based on a careful review of socio-demographic and symptom data as previously documented. 32
In general, there was a lack of association between seropositivity and bowel disease symptoms, adjusted for age and gender. Among the symptoms that did show a trend, constipation with hard stool (OR=6.61, 95% CI, 1.06–41.24, p=0.043) and poor appetite (OR=4.60, 95% CI, 0.95–22.20, p=0.058) were each associated with EMA-confirmed seropositivity. Interestingly, a BMI over 25 was found to be protective from positive serology (OR=0.34, 95% CI, 0.12–0.96, p=0.042). Factors showing a trend toward association with a positive tTg result included poor appetite (OR=3.41, 95% CI, 0.96–12.11, p=0.058) and weight loss (OR=3.73, 95% CI, 0.81–17.12, p=0.090). Abdominal pain any time in the last 3 months (OR=0.49, 95% CI, 0.23–1.04, p=0.062) and symptoms of irritable bowel syndrome any time in the last 3 months (OR=0.44, 95% CI, 0.17–1.15, p=0.092) were negatively associated with tTg positivity, as was an elevated BMI (OR=0.46, 95% CI, 0.22–0.98, p=0.045). In addition to serology, most gastrointestinal symptoms including diarrhea were not positively associated with having an increased IEL count (>25). The one exception were any minor symptoms, not amounting to criteria for functional disorders, which corresponded to a greater than 3-fold higher odds of an increased IEL count (OR=3.63, 95% CI, 1.74–7.58, p=0.001). Abdominal pain any time in the last 3 months, (OR=0.27, 95% CI, 0.12–0.62, p=0.002), dyspepsia any time in the last 3 months (OR=0.43, 95% CI, 0.19–0.98, p=0.045) and having retrosternal pain (OR=0.30, 95% CI, 0.09–0.99, p=0.049) were negatively associated with having an increased IEL count. Correlation for height, weight, and BMI with IEL counts showed no results that were close to significance (height had nearly no correlation with IELs, r=0.01, p-value=0.87)
Discussion
This study is the first endoscopic population-based study to examine the prevalence and spectrum of celiac disease by parallel histology and serology with confirmation by HLA genotyping. Whilst prior studies have examined prevalence of celiac disease by counting clinically detected cases, serological testing of groups at especially high risk or serum surveys of the general population, biopsies have largely been reserved for confirmation of seropositive individuals. These previous studies have failed to detect potential gluten sensitive enteropathy in the absence of serological markers as may be seen in clinically detected disease. 15, 43.
This study demonstrates one of the highest prevalence rates of celiac disease found in any population except for Mexico and similar to that in Finland. 44–46 Including patients with serology and increased IELs without atrophy and 2 previously treated patients, the prevalence approaches 2%. The high prevalence of celiac disease in this population likely is the result of the carriage of the at risk genotype that is common in Finnish and Swedish populations. 47 If one also assumes that some of the seronegative subjects with increased IELs and normal architecture may also belong to the celiac spectrum then the prevalence of intestinal gluten sensitivity or latent celiac disease may be even higher. 48 Such a remarkably high prevalence may represent longstanding celiac disease though it has recently been suggested that the historic rate of celiac disease based on serology may be increasing especially in adults. 45, 49, 50
It is remarkable that so few subjects had symptoms of celiac disease, so called “silent” celiac disease. This makes it more difficult to identify those in a population who may have celiac disease beyond those already identified in high risk groups but also suggests that undiagnosed celiac disease may have little clinical impact, at least as detected by our questionnaire, which has been used in celiac disease, but not validated in this condition. 51
This observation is supported by work from the Cambridge heart health study, which found a lower body mass index, cholesterol and higher quality of life in seropositive individuals despite anemia and low bone density 52. Given these subtle manifestations of celiac disease and the increasing age at diagnosis, there may be a long presymptomatic phase prior to the development of symptoms. This hypothesis is supported by a high seroprevalence that developed by age 7 in a US birth cohort. 53
In this study there was a strong correlation between quantitative morphological analysis of the duodenal biopsy (with a threshold of 25 IELs/100 ECs) and human tTg-IgA serology. EMA provided important confirmatory secondary testing in our population. It may be reasonable to use this sequential testing strategy when screening a general population sample where the pretest prevalence of celiac disease is less than 2%.
The cut off for IEL counts in duodenal biopsies is controversial and normal ranges are cited as 10–30/100 ECs in the UK and up to 40/ 100 ECs in Europe and the United States. 6, 25, 26, 38, 54, 55 We found using counts of >25 IELs/ 100 ECs is the likely cut-off for abnormality. Higher thresholds of IELs would have missed 50% of cases in the population sample evaluated. .
A raised IEL count with normal villous architecture is not uncommon. One study found 2.2% (14/ 626) of duodenal biopsies revealed LD in routine practice. 22 If the IEL count is raised then celiac disease needs consideration. 56– 58
Lymphocytic enteropathy (celiac disease and LD) affected over 5% of this population. The estimated prevalence of LD in subjects seronegative for celiac disease in this setting is 3.8%. Here, there was no concurrent autoimmune disease, or inflammatory bowel disease reported, and one case with Giardia had normal duodenal pathology. There are many disease associations of a raised IEL count, including cow’s milk protein sensitivity, IgA deficiency, tropical sprue and post infective malabsorption 11, 12, 15, 16, 27, 30, 31, 59– 61 dermatitis herpetiformis, bacterial overgrowth, NSAIDs, systemic immune disease, microscopic colitis, lymphoma and neoplastic lymphoid disease are also possible associations. 25, 62, 63
Our observation of a strong association between lymphocytic duodenosis and H. pylori raises one such explanation. This suggests the small intestine can produce a distinct chronic inflammatory response to a variety of immune stimuli. The understanding of how this occurs would provide insight into basic immune homeostasis of the proximal small intestine that differs from those of IBD, which typically affects more distal parts of the intestine where the major luminal antigens are bacterial. Some experimental evidence also suggests that gluten may induce functional changes in the setting of mild lymphocytic duodenosis. 64
In summary, lymphocytic enteropathy affecting the proximal small intestine is common, and often but not exclusively associated with celiac disease. Celiac disease appears to be more common than previously suspected, generally clinically silent, and most cases can be detected by modern serologic techniques. Specificity can be maximized by the use of the endomysial antibody test as a second line. The impact of undiagnosed celiac disease and its natural history is crucial to determining the need for detection. We also need to understand which perturbation of the intestinal milieu lead to lymphocytic enteropathy and its consequences. Clinicians should be aware of the diversity of these associations when contemplating the management of patients in whom lymphocytic enteropathy is discovered.
Figure 4.
Comparison of serology, villous architecture and intraepithelial lymphocyte (IELs) counts/ 100 enterocytes of >40 versus <25/ 100 enterocytes in 16 subjects with diagnosed celiac disease. Sero+ve = positive serology for tTg and EMA, Atrophy = atrophy of villi, (total, B1 or partial, B2)
Acknowledgements
Dr R. Goldin for valuable advice and participation in IEL count validation, Patricia Krause with assistance with serological testing for celiac disease.
-
Funding: The Kalixanda study
-
–
Swedish Research Council
-
–
The Swedish Society of Medicine
-
–
Mag-tarm sjukas forbund
-
–
Norrbotten County Council, Sweden
-
–
AstraZeneca R&D Sweden
-
–
NIH grant DK 57872 to JAM
The Karolinska Institutet received only unrestricted grants from AstraZeneca. All work was done by an independent research team, including the study design.
Abbreviations
- IELs
intraepithelial lymphocytes
- ECs
enterocytes
- tTg
tissue transglutaminase
- EMA
endomysial antibody
- LD
Lymphocytic duodenosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, Macneil J, Mack D, Patel D, Moher D. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57–S67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clinical Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Valdovinos M, Petterson TM, Carpenter HA, Melton LJ., 3rd Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol. 1994;89:843–846. [PubMed] [Google Scholar]
- 4.Rossi TM, Albini CH, Kumar V. Incidence of celiac disease identified by the presence of serum endomysial antibodies in children with chronic diarrhea, short stature, or insulin-dependent diabetes mellitus. J Pediatr. 1993;123:262–264. doi: 10.1016/s0022-3476(05)81699-3. [DOI] [PubMed] [Google Scholar]
- 5.Stenhammar L, Ansved P, Jansson G, Jansson U. The incidence of childhood celiac disease in Sweden. J. Pediatr Gastroenterol Nutr. 1987;6:707–709. doi: 10.1097/00005176-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Weile B, Cavell B, Nivenius K, Krasilnikoff PA. Striking differences in the incidence of childhood celiac disease between Denmark and Sweden: a plausible explanation. J Pediatr Gastroenterol Nutr. 1995;21:64–68. doi: 10.1097/00005176-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hawkes ND, Swift GL, Smith PM, Jenkins HR, Caramaschi P. Incidence and presentation of coeliac disease in South Glamorgan. Eur J Gastroenterol Hepatol. 2000;12:345–349. doi: 10.1097/00042737-200012030-00013. [DOI] [PubMed] [Google Scholar]
- 8.Stevens FM, Egan-Mitchell B, Cryan E, McCarthy CF, McNicholl B. Decreasing incidence of coeliac disease. Arch Dis Child. 1987;62:465–468. doi: 10.1136/adc.62.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PHR, Guandalini S, Hill I, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Int Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 10.Catassi C, Kryszak D, Louis-Jacques O, Duerksen DR, Hill I, Crowe SE, Brown AR, Procaccini NJ, Wonderly BA, Hartley P, Moreci J, Bennett N, Horvath K, Burk M, Fasano A. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102:1454–1460. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 11.Catassi C, Fabiani E, Ratsch IM, Coppa GV, Giorgi PL, Pierdomenico R, Alessandrini S, Iwanejko G, Domenici R, Mei E, Miano A, Marani M, Bottaro G, Spina M, Dotti M, Montanelli A, Barbato M, Viola F, Lazzari R, Vallini M, Guariso G, Plebani M, Cataldo F, Traverso G, Ventura A, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 12.Rostami K, Kerckhaert J, von Blomberg BM, Meijer JW, Wahab P, Mulder CJ. SAT and serology in adult coeliacs, seronegative coeliac disease seems a reality. Neth J Med. 1998;53:15–19. doi: 10.1016/s0300-2977(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 13.Abrams JA, Diamond B, Rotterdam H, Green PH. Seronegative celiac disease: increased prevalence with lesser degrees of villous atrophy. Dig Dis Sci. 2004;49:546–550. doi: 10.1023/b:ddas.0000026296.02308.00. [DOI] [PubMed] [Google Scholar]
- 14.Murray JA, Herlein J, Mitros F, Goeken JA. Serologic testing for celiac disease in the United States: results of a multilaboratory comparison study. Clin Diagn Lab Immunol. 2000;7:584–587. doi: 10.1128/cdli.7.4.584-587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashtak S, Ettore MW, Homburger HA, Murray JA. Comparative usefulness of deamidated gliadin antibodies in the diagnosis of celiac disease. Clin Gastroenterol Hepatol. 2008;6:426–432. doi: 10.1016/j.cgh.2007.12.030. quiz 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araya M, Mondragon A, Rios G, Alarcon T, Roessler JL, Santos JL, Rostami K. The relationship between anti-endomysium antibodies and villous atrophy in coeliac disease using both monkey and human substrate. Hum Immunol. 1999;60:262–267. [Google Scholar]
- 17.Kaukinen K, Collin P, Holm K, Karvonen AL, Pikkarainen P, Maki M. Small-bowel mucosal inflammation in reticulin or gliadin antibody-positive patients without villous atrophy. Scand J Gastroenterol. 1998;33:944–949. doi: 10.1080/003655298750026967. [DOI] [PubMed] [Google Scholar]
- 18.Kaukinen K, Peraaho M, Collin P, Partanen J, Woolley N, Kaartinen T, Nuutinen T, Halttunen T, Maki M, Korponay-Szabo I. Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol. 2005;40:564–572. doi: 10.1080/00365520510023422. [DOI] [PubMed] [Google Scholar]
- 19.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 20.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Hayat M, Cairns A, Dixon MF, O'Mahony S. Quantitation of intraepithelial lymphocytes in human duodenum: what is normal? J Clin Pathol. 2002;55:393–394. doi: 10.1136/jcp.55.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahadeva S, Wyatt JI, Howdle PD. Is a raised intraepithelial lymphocyte count with normal duodenal villous architecture clinically relevant? J Clin Pathol. 2002;55:424–428. doi: 10.1136/jcp.55.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green PH, Cellier C. Medical progress: Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 24.Collin P, Wahab PJ, Murray JA. Intraepithelial lymphocytes and coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:341–350. doi: 10.1016/j.bpg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Kakar S, Nehra V, Murray JA, Dayharsh GA, Burgart LJ. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol. 2003;98:2027–2033. doi: 10.1111/j.1572-0241.2003.07631.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown I, Mino-Kenudson M, Deshpande V, Lauwers GY. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: an increasing diagnostic problem with a wide differential diagnosis. Arch Path Lab Med. 2006;130:1020–1025. doi: 10.5858/2006-130-1020-ILIAPP. [DOI] [PubMed] [Google Scholar]
- 27.Stern M, Dietrich R, Muller J. Small intestinal mucosa in coeliac disease and cow's milk protein intolerance: morphometric and immunofluorescent studies. Eur J Pediatr. 1982;139:101–105. doi: 10.1007/BF00441490. [DOI] [PubMed] [Google Scholar]
- 28.Klemola T. Immunohistochemical findings in the intestine of IgA-deficient persons: number of intraepithelial T lymphocytes is increased. Journal of Pediatr Gastroenterol Nutr. 1988;7:537–543. doi: 10.1097/00005176-198807000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Memeo L, Jhang J, Hibshoosh H, Green PH, Rotterdam H, Bhagat G. Duodenal intraepithelial lymphocytosis with normal villous architecture: common occurrence in H. pylori gastritis. Mod Pathol. 2005;18:1134–1144. doi: 10.1038/modpathol.3800404. [DOI] [PubMed] [Google Scholar]
- 30.Oberhuber G, Kastner N, Stolte M. Giardiasis: a histologic analysis of 567 cases. Scandinavian Journal of Gastroenterology. 1997;32:48–51. doi: 10.3109/00365529709025062. [DOI] [PubMed] [Google Scholar]
- 31.Ross IN, Mathan VI. Immunological changes in tropical sprue. Q J Med. 1981;50:435–449. [PubMed] [Google Scholar]
- 32.Aro P, Ronkainen J, Storskrubb T, Bolling-Sternevald E, Carlsson R, Johansson SE, Vieth M, Stolte M, Engstrand L, Talley NJ, Agreus L. Valid symptom reporting at upper endoscopy in a random sample of the Swedish adult general population: the Kalixanda study. Scand J Gastroenterol. 2004;39:1280–1288. doi: 10.1080/00365520410008141. [DOI] [PubMed] [Google Scholar]
- 33.Aro P, Ronkainen J, Storskrubb T, Bolling-Sternevald E, Svardsudd K, Talley NJ, Junghard O, Johansson SE, Wiklund I, Agreus L. Validation of the translation and cross-cultural adaptation into Finnish of the Abdominal Symptom Questionnaire, the Hospital Anxiety and Depression Scale and the Complaint Score Questionnaire. Scand J Gastroenterol. 2004;39:1201–1208. doi: 10.1080/00365520410008132. [DOI] [PubMed] [Google Scholar]
- 34.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Oberhuber G, Caspary WF, Kirchner T, Borchard F, Stolte M. [Diagnosis of celiac disease and sprue. Recommendations of the German Society for Pathology Task Force on Gastroenterologic Pathology] Pathologe. 2001;22:72–81. doi: 10.1007/s002920000428. [DOI] [PubMed] [Google Scholar]
- 36.Corazza GR, Villanacci V. Coeliac disease. J Clin Pathol. 2005;58:573–574. doi: 10.1136/jcp.2004.023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickson BC, Streutker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol. 2006;59:1008–1016. doi: 10.1136/jcp.2005.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson WGLG, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. In: Drossman DACE, Talley NJ, Thompson WG, Whitehead WE, editors. The Functional Gastrointestinal Disorders. 2nd edn ed. Washington: Degnon; 2000. pp. 351–432. [Google Scholar]
- 39.Agréus L, Svärdsudd K, Tibblin G, Lavö B. Endomysium antibodies are superior to gliadin antibodies in screening for coeliac disease in patients presenting supposed functional gastrointestinal symptoms. Scand J Prim Health Care. 2000;18:105–110. doi: 10.1080/028134300750018990. [DOI] [PubMed] [Google Scholar]
- 40.Storskrubb T, Aro P, Ronkainen J, Wreiber K, Nyhlin H, Bolling-Sternevald E, Talley NJ, Engstrand L, Agréus L. Antimicrobial susceptibility of Helicobacter pylori strains in a random adult Swedish population. Helicobacter. 2006;11:224–230. doi: 10.1111/j.1523-5378.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 41.Monsuur AJ, de Bakker PI, Zhernakova A, Pinto D, Verduijn W, Romanos J, Auricchio R, Lopez A, van Heel DA, Crusius JB, Wijmenga C. Effective detection of human leukocyte antigen risk alleles in celiac disease using tag single nucleotide polymorphisms. PLoS One. 2008;3:e2270. doi: 10.1371/journal.pone.0002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koskinen L, Romanos J, Kaukinen K, Mustalahti K, Korponay-Szabo I, Barisani D, Bardella MT, Ziberna F, Vatta S, Széles G, Pocsai Z, Karell K, Haimila K, Adány R, Not T, Ventura A, Mäki M, Partanen J, Wijmenga C, Saavalainen P. Immunogenetics.Cost-effective HLA typing with tagging SNPs predicts celiac disease risk haplotypes in the Finnish. Hungarian, and Italian populations. 2009;61:247–256. doi: 10.1007/s00251-009-0361-3. [DOI] [PubMed] [Google Scholar]
- 43.Rostami K, Kerckhaert J, Tiemessen R, von Blomberg BM, Meijer JW, Mulder CJ. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am J Gastroenterol. 1999;94:888–894. doi: 10.1111/j.1572-0241.1999.983_f.x. [DOI] [PubMed] [Google Scholar]
- 44.Remes-Troche JM, Rios-Vaca A, Ramirez-Iglesias MT, Rubio-Tapia A, Andrade-Zarate V, Rodriguez-Vallejo F, Lopez-Maldonado F, Gomez-Perez FJ, Uscanga LF. High prevalence of celiac disease in Mexican Mestizo adults with type 1 diabetes mellitus. J Clin Gastroenterol. 2008;42:460–465. doi: 10.1097/MCG.0b013e318046ea86. [DOI] [PubMed] [Google Scholar]
- 45.Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, Lohi O, Bravi E, Gasparin M, Reunanen A, Maki M. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 46.Kondrashova A, Mustalahti K, Kaukinen K, Viskari H, Volodicheva V, Haapala AM, Ilonen J, Knip M, Maki M, Hyoty H, Epivir Study G. Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med. 2008;40:223–231. doi: 10.1080/07853890701678689. [DOI] [PubMed] [Google Scholar]
- 47.Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T, Hopfl P, Knip M. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 48.Kurppa K, Collin P, Viljamaa M, Haimila K, Saavalainen P, Partanen J, Laurila K, Huhtala H, Paasikivi K, Maki M, Kaukinen K. Diagnosing Mild Enteropathy Celiac Disease: A Randomized, Controlled Clinical Study. Gastroenterology. 2009;136:816–823. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 49.Vilppula A, Collin P, Maki M, Valve R, Luostarinen M, Krekela I, Patrikainen H, Kaukinen K, Luostarinen L. Undetected coeliac disease in the elderly: a biopsy-proven population-based study. Dig Liver Dis. 2008;40:809–813. doi: 10.1016/j.dld.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, Zinsmeister AR, Melton LJ, III, Murray JA. Increased Prevalence and Mortality in Undiagnosed Celiac Disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agréus L, Svärdsudd K, Tibblin G, Lavö B. Endomysium antibodies are superior to gliadin antibodies in screening for coeliac disease in patients presenting supposed functional gastrointestinal symptoms. Scand J Prim Health Care. 2000;18:105–110. doi: 10.1080/028134300750018990. [DOI] [PubMed] [Google Scholar]
- 52.West J, Logan RF, Hill PG, Lloyd A, Lewis S, Hubbard R, Reader R, Holmes GK, Khaw KT. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut. 2003;52:1070–1071. doi: 10.1136/gut.52.7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, Emery LM, Sokol RJ, Erlich HA, Eisenbarth GS, Rewers M. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343–2351. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 54.Veress B, Franzen L, Bodin L, Borch K. Duodenal intraepithelial lymphocyte-count revisited. Scand Jf Gastroenterol. 2004;39:138–144. doi: 10.1080/00365520310007675. [DOI] [PubMed] [Google Scholar]
- 55.Vande Voort JL, Murray JA, Lahr BD, Van Dyke CT, Kroning CM, Moore SB, Wu TT. Lymphocytic duodenosis and the spectrum of celiac disease. Am J Gastroenterol. 2009;104:142–148. doi: 10.1038/ajg.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Farrelly C, Graeme-Cook F, Hourihane DO, Feighery C, Weir DG. Histological changes associated with wheat protein antibodies in the absence of villous atrophy. J Clin Pathol. 1987;40:1228–1230. doi: 10.1136/jcp.40.10.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maki M, Holm K, Collin P, Savilahti E. Increase in gamma/delta T cell receptor bearing lymphocytes in normal small bowel mucosa in latent coeliac disease. Gut. 1991;32:1412–1414. doi: 10.1136/gut.32.11.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldstein NS, Underhill J. Morphologic features suggestive of gluten sensitivity in architecturally normal duodenal biopsy specimens. Am J Clin Pathol. 2001;116:63–71. doi: 10.1309/5PRJ-CM0U-6KLD-6KCM. [DOI] [PubMed] [Google Scholar]
- 59.Phillips AD, Rice SJ, France NE, Walker-Smith JA. Small intestinal intraepithelial lymphocyte levels in cow's milk protein intolerance. Gut. 1979;20:509–512. doi: 10.1136/gut.20.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klemola T, Savilahti E, Arato A, Ormala T, Partanen J, Eland C, Koskimies S. Immunohistochemical findings in jejunal specimens from patients with IgA deficiency. Gut. 1995;37:519–523. doi: 10.1136/gut.37.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor CJ. Predictive value of intraepithelial lymphocyte counts in childhood coeliac disease. J Pediatr Gastroenterol Nutr. 1988;7:532–536. doi: 10.1097/00005176-198807000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Kosnai I, Karpati S, Savilahti E, Verkasalo M, Bucsky P, Torok E. Gluten challenge in children with dermatitis herpetiformis: a clinical, morphological and immunohistological study. Gut. 1986;27:1464–1470. doi: 10.1136/gut.27.12.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Overbeke L, Ectors N, Tack J. What is the role of celiac disease in enteropathy-type intestinal lymphoma? A retrospective study of nine cases. Acta Gastroenterol Belg. 2005;68:419–423. [PubMed] [Google Scholar]
- 64.Verdu EF, Huang X, Natividad J, Lu J, Blennerhassett PA, David CS, McKay DM, Murray JA. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G217–G225. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]