Abstract
Objective
Metabolic syndrome (MetS) is characterized by low-grade inflammation and confers an increased risk for cardiovascular disease. Endothelial progenitor cells (EPCs) are a measure of vascular health and are decreased in patients with various risk factors for cardiovascular disease (CVD). There is a paucity of data examining the EPC status especially in terms of their functionality in MetS subjects without diabetes or cardiovascular disease. We aimed to enumerate and functionally characterize EPCs in subjects with MetS in comparison to healthy controls.
Methods
The study was performed at the University of California Davis Medical Center. Healthy controls (n=31) and MetS (n=46) subjects were included in the study. EPCs were ennumerated in fasting blood by KDR/CD34 dual positivity. Functionality was assessed by the colony forming units (CFU) assay, migration and tubule formation.
Results
Subjects with MetS had significantly decreased number of EPCs compared to control subjects. Furthermore, EPCs from MetS subjects depicted significantly impaired clonogenic capacity i.e., decreased colony forming units, and impaired capacity to incorporate into tubular structures suggesting functional impairment of EPCs from MetS subjects.
Conclusions
We make the novel observation that MetS subjects without diabetes or CVD have decreased EPC number and impaired functionality as compared to control subjects. These findings could contribute to the increased CV risk in this population.
Keywords: CD34+KDR+ cells, EPC functions, CRP, Metabolic Syndrome
Introduction
Metabolic syndrome (MetS) is a cluster of dysglycemia, dyslipidemia, obesity and hypertension that has reached epidemic proportions in industrialized countries [1]. Patients with Metabolic Syndrome have an increased risk for cardiovascular disease (CVD)[2,3].
Endothelial progenitor cells (EPCs) are a subtype of bone marrow–derived progenitor cells expressing surface antigens of both hematopoietic stem cells and endothelial cells: they are involved in adult neovasculogenesis and maintenance of vascular integrity. An altered status of circulating EPCs represents a marker of endothelial dysfunction and vascular health [4–6]. It has been reported that persons at risk for coronary artery disease (CAD) have decreased number of circulating EPCs [7]. Also, lower number of EPCs has been reported in a wide range of conditions including chronic kidney disease, rheumatoid artritis (RA), diabetes, obesity and CAD [7–14]. Increasing age is also strongly associated with decreased EPCs number. Furthermore, functional properties of EPC may be of equal or probably greater importance than quantitative alterations [15]. Importantly, various features of MetS have been reported to individually correlate with decreased number and functionality of EPCs [16,17]. Although a few investigators have described EPC number or progenitor cell (PC) number in subjects with MetS having either diabetes [18,19], CAD [20] and obesity (males only) [21], however, no data is available on functionality of EPCs in drug-naïve MetS subjects especially in the absence of diabetes and CAD [21]. Furthermore, various pharmacological agents, such as statins, angiotensin receptor blockers (ARBs), PPAR gamma agonists increase EPC number [8,22,23] and thus were an exclusion criterion.
We hypothesized that EPC might be numerically and/or functionally impaired in drug naïve MetS subjects (both males and females), which may account for the increased CV risk in this subject population. We quantitated the number and functional capacity of EPCs obtained from the MetS subjects, not on statins, angiotensin receptor blockers (ARBs) or PPAR gamma agonists, and compared them with the matched control subjects.
Subjects and Methods
All subjects were recruited from Sacramento county through fliers and advertisements in the newspaper. The subjects (age 21–70 yrs) with the MetS (n=46) and healthy controls (n=31) were studied. Metabolic syndrome was defined using the criteria of the National Cholesterol Education Program Adult Treatment Panel III was used [24]. Briefly, the subject classified as MetS must have at least three risk factors to sustain the diagnosis, including either central obesity, hypertension, dyslipidemia (low HDL, high triglycerides) and or hypertension on anti-hypertensive medications. The control subject needed to have ≤2 features of MetS and not be on blood pressure (BP) medications. Other exclusion criteria for controls were: a fasting plasma glucose (>100 mg/dl) and, a triglycerides (TG >200 mg/dl).
For both groups, other exclusion criteria were: diabetes, clinical atherosclerosis (CAD, PAD, CVD etc), smoking, hypo- or hyperthyroidism, malabsorption, anticoagulant therapy, steroid therapy, anti-inflammatory drugs, statin & other hypolipidemic therapy, hypoglycemic agents, ARBs, TG>400mg/dl (for MetS subjects), oral contraceptives, use of antioxidant supplements in the past 6 months; pregnancy, abnormal complete blood count and alcohol consumption >1 oz/day; consumption of N-3 PUFA, postmenopausal women on estrogen replacement therapy, wounds,, retinopathy, recent surgery, inflammatory or malignant disease which may influence EPC kinetics, CRP >10mg/l, chronic high intensity exercisers (exercise>100 min per week).
Informed consent was obtained from participants in the study, which was approved by the Institutional Review Board at University of California Davis. After history and physical examination, fasting blood was obtained. A complete blood count, plasma lipid and lipoprotein profile, urea nitrogen, creatinine, aspartate aminotransferase, alanine aminotransferase, glucose and TSH were assayed by standard laboratory techniques in the Clinical Pathology Laboratory. Insulin levels were assayed by ELISA (Linco Biosystems) and HOMA-IR was calculated from glucose and insulin levels as described previously [19]. After subjects were screened, if they fit the selection criteria, they were requested to come to the UC Davis CCRC for a fasting blood draw.
Enumeration of Peripheral blood EPCs by Flow Cytometry
One ml of whole blood (heparinized) was used to quantitate the number of EPCs by the analysis for the expression of surface antigens by flow cytometry. In brief, 100 µL of peripheral blood was incubated initially with Fc-gamma receptor blocking agent followed by separate incubation with a phycoerythrin-conjugated antibody against human KDR and PE-Cy5-conjugated antibody against human CD34, and both antibodies together. Control stainings were performed with isotype-matched and species- specific antibodies. Incubation was followed by lysis with BD lysing solution, washing and fixation in 1% paraformaldehyde. Acquisition was then performed on a Becton Dickinson FACS Array flow cytometer (FACScan) and included 100,000 events per sample. Cells positive for both CD34 and KDR were characterized as EPCs. Also, the number of progenitor cells (CD34+ only) was quantitated. These analyses were performed for all the subjects (C, n=30 and Met S, n=46) in duplicate. Intra-assay coefficient of variation was performed in 5 different samples performed 10 times and was 11%.
Cell Culture Enrichment of EPCs
EPC isolation and culture were performed as described [12,19, 25]. Briefly, mononuclear cells (MNCs) were isolated from 20 ml of fasting heparinized blood by Ficoll Hypaque centrifugation. MNCs (4×106 cells in 3 ml medium) were plated on fibronectin-coated 6-well dishes (Becton Dickinson Biosciences) and cultivated in growth medium 199 (Gibco) containing 20% fetal calf serum plus 100 ng/ml vascular endothelial growth factor (VEGF) and penicillin (100 U/mL)/streptomycin (100 µg/mL, Gibco) for 48 hours. Nonadherent cells were then recollected, centrifuged, resuspended in fresh medium and 106 cells/well in 1 ml medium were replated onto fibronectin-coated, 24-wells plates and cultured in triplicate for 7 days in growth medium that was changed every 3 days.
EPC characterization
On the 7th day, a subset (n=7/group from matched pairs of C and MetS subjects whose EPCs were cultivated and processed together) of adherent cells underwent cytochemical analysis after incubation with DiI-LDL and FITC Ulex-lectin as described [10]. After staining, the cells were viewed under inverted fluorescent microscope.
To further confirm endothelial cell phenotype of EPC, cells from 24 well plates were removed from surface with PBS-EDTA and were surface stained for KDR and CD31.
Functional Assays
The cells growing in 24 well plates were used for the following assays.
Colony-forming Unit Assay
The ability to clonally expand and create colonies in an endothelial-specific medium is a key functional feature of EPCs. The CFU assay [7] was performed in all the subjects. CFUs, characterized by a central cluster surrounded by emerging cells were counted at day 7 in 24 well plates in a minimum of 3 wells per subject (C, n=25, MetS, n=43) and the average count was recorded.
EPC Migration Assay
EPC migratory function, which is essential for angiogenesis, was examined using a modified Boyden chamber technique in 16 Control subjects and 36 MetS subjects. A 24-well Transwell apparatus (Chemicon) with 8-µm pores was used. The chamber was immersed in a 24-well plate, which was filled with growth factor-free 199 media or medium with 100 ng/ml of human VEGF (R and D systems). EPCs (~4×104) were stained with CFDA (10 uM final conc) and placed in the inserts in duplicate along with one blank well. Also, one well of the 24 well plate was used for recording total number of cells added. The cells in this well were lysed by adding 1% Tris-Triton at the start of incubation itself. After incubation for 12 hours, the membrane was washed briefly with PBS and the upper side of membrane was wiped gently with a cotton ball. To the lower chamber was added Tris-Triton to lyse the cells which had migrated to the bottom of inserts. Migration was measured as MFI using spectrofluorometer. The results are expressed in MFI units as % migration of the cells with respect to total number of cells used.
Matrigel Tube Formation Assay
A Matrigel tube formation assay was performed to assess the ability of EPC to incorporate into endothelial cell vascular structures, which is believed to be important in new vessel formation. Growth factor reduced matrigel (Becton Dickinson) was layered into 48 well plates for ½ hr at 37°C. EPCs were stained with DiI (5uM conc; Molecular Probes) to distinguish them from HAEC. DiI-labeled EPC (1×104) and HAEC (4×104) were mixed and plated together followed by incubation at 37°C overnight with complete culture medium. Incorporated cells were counted from 4 random microscope fields per each subject (C, n=15, MetS, n=15).
Statistical Analysis
Data are expressed as mean ± SD or, for skewed variables, as median and interquartile range. Log transformations were applied to skewed data prior to parametric analyses. We were not able to get complete data sets for EPC functionality on all subjects due to the inadequacy of the EPCs obtained from each subject. Comparisons between the control and metabolic syndrome groups were made with two-sample t-tests. Spearman’s rank correlation coefficients were computed to assess the association between metabolic risk factors and EPC numbers and their functionality. Multiple regression models were constructed to evaluate predictors of EPC dependent variables. Independent variables assessed included age, BMI, waist circumference, blood pressure, plasma glucose, triglycerides, HOMA-IR, LDL-C, HDL-C, and CRP. Data were analyzed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
Table 1 shows the salient characteristics of the study subjects. There were no significant differences in age and gender between the 2 groups. MetS subjects had significantly higher body mass index (BMI), waist circumference, blood pressure (both systolic and diastolic) as well as fasting plasma glucose than those in the control group. Total cholesterol, triglycerides LDL and HDL, insulin and HOMA-IR were also significantly different in MetS subjects than controls. The MetS subjects were on hypertensive medications including beta blockers, ACE inhibitors, Ca channel blockers and diuretics (27%). Furthermore, MetS had significantly higher levels of CRP, a down stream marker of inflammation depicting higher systemic inflammation than control subjects.
Table 1.
Anthropometric, Clinical, and Biochemical Characteristics of the Subjects
| Control (n=31) |
MetS (n=46) |
P value | |
|---|---|---|---|
| Age (yrs) | 49 ± 11 | 54 ± 11 | 0.08 |
| Females/Males | 25/6 | 36/10 | 0.99 |
| Waist circumference (cm) | 94 ± 15 | 110±14 | 0.0001 |
| Weight (kg) | 85±22 | 102±19 | 0.0005 |
| BMI (kg/m2) | 30±6 | 36±6 | 0.0002 |
| Systolic BP(mmHg) | 121 ± 12 | 130± 13 | 0.001 |
| Diastolic BP (mmHg) | 74 ± 8 | 82 ± 10 | 0.0003 |
| Fasting Glucose (mg/dl) | 90 ± 7 | 101± 10 | 0.0001 |
| Total Cholesterol (mg/dl) | 185 ± 33 | 199 ± 27 | 0.048 |
| HDL-Cholesterol (mg/dl) | 65 ± 13 | 41 ± 12 | 0.0001 |
| LDL-Cholesterol (mg/dl) | 114 ± 27 | 129± 20 | 0.0001 |
| Triglycerides (mg/dl) | 75 (62,95) | 134 (106,172) | 0.0001 |
| Fasting Insulin (uIU/mL) | 8.6 ± 4.9 | 16.6 ± 9.9 | 0.0001 |
| HOMA-IR | 1.7 (0.9,2.8) | 1.1 (2.3,5.8) | 0.0001 |
| hsCRP (mg/L) | 1.3 (0.5,3) | 3.5(1.6,5.7) | 0.005 |
Data are summarized as mean ± SD except for hsCRP, HOMA and triglycerides which are presented as median (25th percentile, 75th percentile)
We quantitated the number of CD34+ only and CD34+KDR+ cells (EPCs) in the peripheral blood of all the subjects in duplicate using flow cytometry. In addition to decreased CD34 (progenitor cells) counts in MetS subjects compared to controls [Control 12.7 (11,21) mfi vs. MetS 8.2 (6.4,12), p<0.001], CD34+KDR+ EPCs were significantly (p<0.001) reduced by 34% in subjects with MetS compared with their age-matched healthy volunteers (Fig 1). Representative scatterplots are depicted in Supplementary Fig 1. Regression analysis revealed that CRP, triglycerides, age, and plasma glucose were the strongest predictors of reduced circulating CD34+KDR+ cells (adjusted R-squared=0.147, model p=0.01) (Table 2).
Fig 1.
Enumeration of EPCs by FACS. CD34/KDR dual positive cells in all the subjects were assessed as described in Methods. * p<0.001 compared to control subjects.
Table 2.
Summary of multiple regression models
| Dependent variable CD 34+KDR+† | |||
| Variable | Parameter Estimate, β |
Standard Error |
p-value |
| Intercept | 3.93 | 0.90 | 0.002 |
| Age | 0.01 | 0.007 | 0.07 |
| Glucose | −0.017 | 0.009 | 0.05 |
| Triglycerides† | −0.36 | 0.21 | 0.07 |
| CRP† | −1.46 | 0.74 | 0.05 |
| Interaction (triglycerides x CRP) |
0.3 | 0.16 | 0.05 |
| Dependent variable EPC CFU† | |||
| Variable | Parameter Estimate, β |
Standard Error |
p-value |
| Intercept | 1.96 | 1.69 | 0.25 |
| BMI | 0.04 | 0.02 | 0.03 |
| Glucose | −0.02 | 0.01 | 0.09 |
| HDL | 0.02 | 0.009 | 0.02 |
| DBP | −0.02 | 0.01 | 0.07 |
| Triglycerides | 0.41 | 0.28 | 0.14 |
| CRP† | −0.29 | 0.11 | 0.01 |
| Dependent variable Tubules | |||
| Variable | Parameter Estimate, β |
Standard Error |
p-value |
| Intercept | 7.71 | 9.31 | 0.42 |
| Age | 0.23 | 0.19 | 0.24 |
| HDL | 0.14 | 0.10 | 0.19 |
| CRP† | −3.16 | 1.50 | 0.04 |
loge transformed prior to regression analysis
Decreased number of EPCs in the MetS group compared with control group was also evident when analyzed for Ulex-lectin and DiI-LDL staining from cells growing on 24 well plates on 7th day after culture (Supplementary Fig 2). These cells also stained positive for the endothelial lineage markers, KDR and PECAM-1.
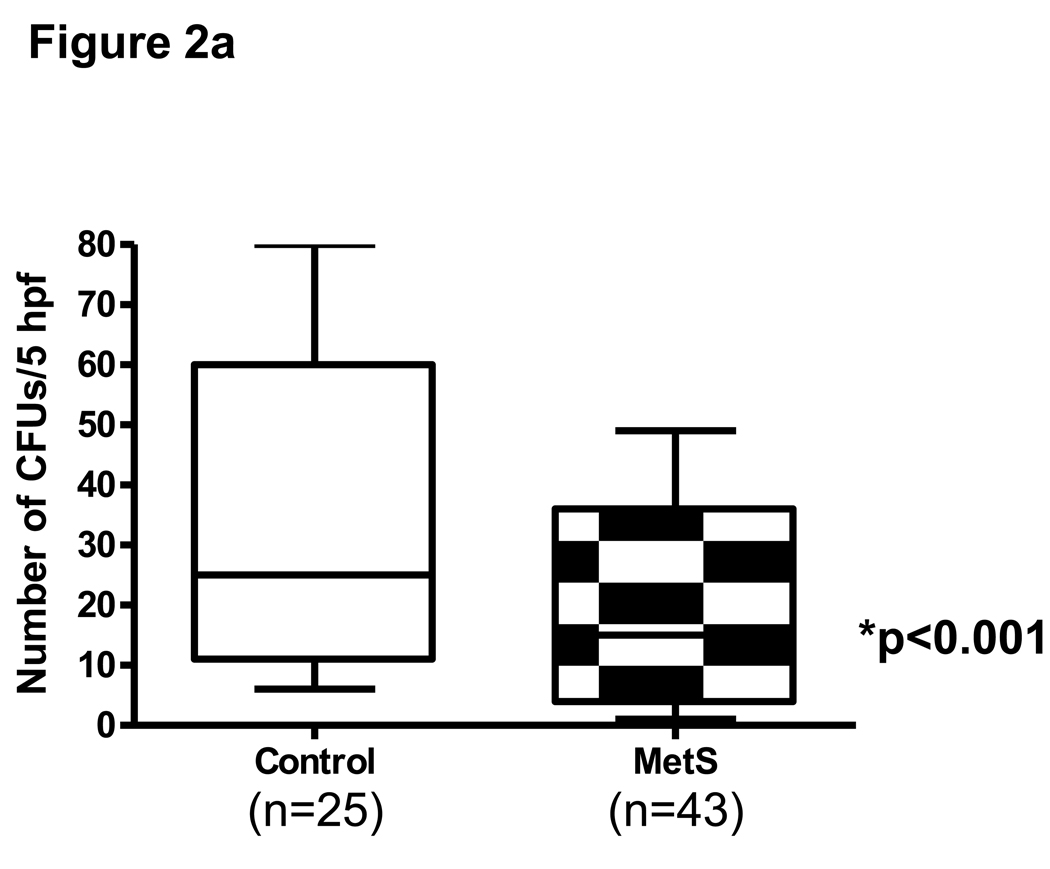
Significantly fewer EPC CFUs were found from MNCs culture of MetS subjects compared with normal controls (48% decrease) at day 7 p<0.001 (Fig 2a). These CFUs stained positive for Di-LDL and FITC- Ulex lectin consistent with endothelial lineage of cells. Moreover, regression analysis revealed that CRP was a strong predictor (p=0.01) of impaired clonogenic capacity, followed by adiposity, HDL, and BP variables, (adjusted R-squared=0.18, model p=0.005) (Table 2).
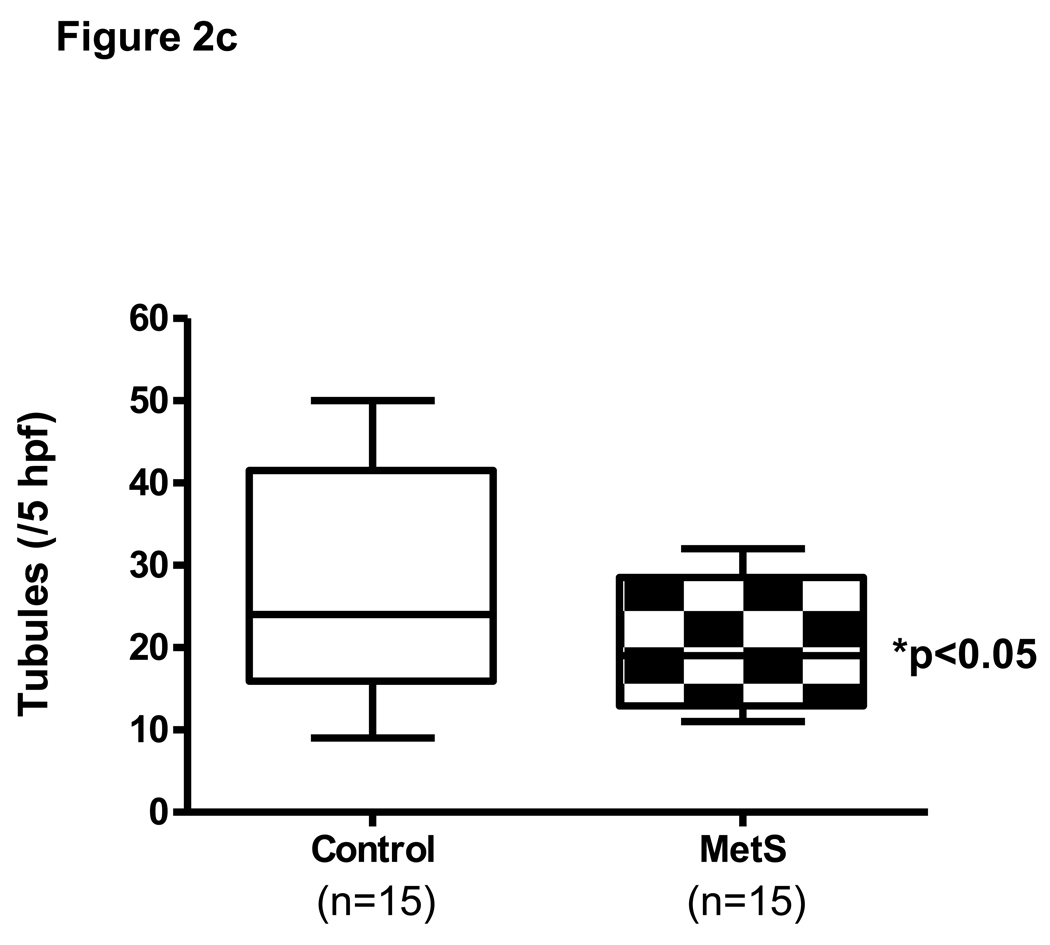
Fig 2.
Fig 2a: CFU in control and MetS subjects. Number of CFUs were ennumerated as described in Methods * p<0.001 compared to control EPCs
Fig 2b: Migration assay in control and MetS subjects. A Boyden chamber assay was used with growth factor-free medium or VEGF (100 ng/ml) supplemented medium.
Fig 2c: Vasculogenic capacity of EPCs (DiI-labeled) as measured by their incorporation into tubules formed by HAEC on matrigel. Fluorescence and phase contrast images are shown in the figure. Significantly fewer EPCs from MetS subjects were incorporated into the tubules formed by HAEC than those of the normal controls. EPCs incorporation in the MetS group was reduced by 33%. *P<0.05.
The migratory function of EPC in response to VEGF, which is believed to be important during neovascularization, was evaluated using a modified Boyden chamber. The migration capacity of EPCs from MetS, whilst lower, was not significantly different from control subjects (Fig 2b).
EPC incorporation into the tubular networks formed by HAEC was evaluated in culture using Matrigel, which is used to evaluate vasculogenic capacity. The data presented is representative of 15 paired subjects in C and MetS groups for whom the EPC isolation and matrigel assay was performed on the same day and time since these assays are time dependent and results vary largely on culture conditions [18,19,25]. Significantly fewer EPCs of MetS subjects were incorporated into tubules compared with the EPCs from normal controls (Fig 2c). Multiple regression analysis identified CRP as a significant predicator (p=0.04) when adjusted for age and HDL-C (adjusted R-squared=0.203, model p=0.03) (Table 2).
Discussion
Several recent reports indicate that the number of circulating EPCs is an independent indicator of overall cardiovascular health. Common conditions predisposing to atherosclerosis, such as hypercholesterolemia, hypertension, diabetes, and smoking are associated with endothelial dysfunction. There is a decrease in the number of EPCs in chronic inflammatory states and in individuals with cardiovascular risk factors [7,10,21]. Furthermore, subjects with MetS have been shown to have endothelial dysfunction. Also, individual features of MetS have been reported to contribute to decreased EPC number. A few investigators [18–21] have analyzed progenitor cell number and EPCs number in subjects with MetS and reported decreased cell numbers; however, no data exist in literature about the functionality of EPCs in MetS [21]. Also, it is important to emphasize that MetS subjects studied earlier were on variety of medications, including oral hypoglycemics, ARBs, statins etc, and these medications have been reported to affect EPC number and functionality [4,22,23]. Overall, there has been a paucity of data on EPCs in drug naïve MetS subjects, free of diabetes, hypercholestrolemia and/or CAD which prompted the current study. Our patients were not on statins, ARBs and PPAR agonists, since these drugs interfere with EPC number and functionality. In this study, we demonstrate that the number of circulating PCs and EPCs is decreased in MetS subjects. Circulating functional EPCs are thought to derive from immature undifferentiated progenitor cells. There are several controversies in the literature regarding the phenotypic characterization of the EPCs. Fadini et al have shown that CD34+KDR+ cell number is reduced in early and late atherosclerotic disease and correlate negatively with endothelial function [25]. A sound rationale to choose the CD34+KDR+ combination is that this is the only putative EPC phenotype that has demonstrated repeatedly and convincingly to be an independent predictor of cardiovascular outcomes [25–28]. In a 10-month follow-up study, Schmidt-Lucke et al [27] showed that the level of CD34+KDR+ cells independently predicted cardiovascular events and progression of atherosclerosis in a mixed population of healthy subjects and cardiovascular patients. In a larger study, Werner et al. [28] have reported that CD34+KDR+ cell count predicted cardiovascular events and cardiovascular death during a 12-month follow-up in 519 patients with CAD. Overall, it has been reported that these EPCs i.e., CD34+KDR+ cells, which are CD45- represent a subpopulation of circulating EPCs [29] and due to their functional properties, may represent the “true” EPCs. Whilst the major focus of this study was to examine EPC functionality in MetS, our findings with respect to decreased progenitor cell numbers accord with the larger study by Fadini et al [19] and contrasts with the smaller study in 18 obese males with Metabolic Syndrome [20]. Thus, it appears that both progenitor cells and EPCs are decreased in MetS. Our data of decreased number of EPCs in MetS is consistent with those reported by Fadini et al in diabetics [18,19] and Westerweel et al [20] in obese males. However, it needs to be emphasized that none of our subjects were diabetic and the majority with MetS were females (77%). On the other hand, Satoh et al [30] have reported increased EPCs in MetS subjects; however, the majority of their patients were studied after an acute coronary event. Despite this increase, the EPCs manifested decreased telomere length and increased oxidative DNA damage, suggesting a paradoxically dysfunctional population of EPCs. Importantly, our study population of MetS did not include patients with diabetes or CVD. One person had an LDL-C > 160 mg/dL, exclusion of the subject data from analyses did not change any of the results, thus these results hold true for MetS subjects without hypercholesterolemia also. Therefore, we extend further and underscore the decreased PC and EPC number in MetS previously reported in MetS patients with diabetes and obese males. Furthermore, using CD34+KDR+ as a dependent variable, age, plasma glucose, triglycerides, and CRP were found to be variables predictive of EPC (CD34+KDR+) measurements.
The EPC characterization for its endothelial lineage in the current study was done for multiple markers such as DiI uptake and concomitant Ulex-lectin. The endothelial lineage was further documented by the expression of KDR and CD31. Endothelial dysfunction (ED) is an early step in the pathogenesis of atherosclerosis. Importantly, CRP which is not only a cardiovascular risk marker but also a mediator in atherogenesis has been earlier reported by our group to cause ED mediated by uncoupling of eNOS in HAECs [31]. CRP treatment of EPCs in vitro resulted in altered eNOS activity [32]. There is growing evidence that bone marrow-derived EPC participates in endothelial repair. This process can be divided into 3 stages: mobilization from bone marrow, homing into the sites of vascular injury, and incorporation into the endothelium of the injured or newly formed blood vessels [28]. Importantly, not only the extent of the EPC pool is an indicator of vascular health, but also normal functions of EPC are required for adequate homeostasis [28]. The novelty of our study is the functional characterization (CFUs, migration and tubulogenesis) of EPCs in MetS subjects which has not been reported earlier in comparison to controls.
We report a highly significant (p<0.001) decrease in the number of CFUs in MetS compared to controls. Multivariate analysis revealed that BMI, HDL-C, and CRP were independent predictors for the reduction in the number of EPCs colonies. In support of our findings, earlier George et al [33] have reported a negative correlation between CRP levels and circulating EPC numbers (defined as CFU assay) in patients with unstable angina.
Current data suggest that EPC may be incorporated into damaged endothelium and may work in concert with existing endothelial cells to form blood vessels rather than forming entirely new vessels de novo [19,26]. Our results reveal that the incorporation of EPCs into EC was reduced in MetS subjects compared to controls which suggest that EPC incorporation into damaged endothelium or neovascularization foci may be impaired in MetS condition. These phenomena could contribute to the deterioration in the repair of damaged endothelium or angiogenesis in MetS.
Interestingly, individual risk factors seem to differentially affect the number and functional capacity of EPCs. These data suggest that different mechanisms contribute to the impairment in functional activity compared with the reduced levels of circulating EPCs. Overall, CRP emerged as a major predictor in most of the parameters analyzed in the current study. Our study is in line with and extends the role of CRP not only as an inflammatory biomarker but also biomediator in the disease process. Our data implicate CRP in dysregulation of EPCs and based on multivariate analysis, this observation is significant for calling attention to a possible effect of CRP in contributing to atherosclerosis in MetS. This effect may result from impaired ability for endothelial repair and regeneration due to reduced EPCs number. Verma’s group [32] has shown experimentally that CRP attenuates EPC survival, function and differentiation by induction of ROS. Future studies will examine mechanisms by which increased CRP levels may be linked to EPC dysfunction.
In conclusion, our study demonstrates for the first time decreased number of EPCs along with impaired functionality in both male and female subjects with MetS without diabetes, hypercholestrolemia or manifest cardiovascular disease when compared to matched controls and clearly fills a hiatus with respect to EPC functionality in MetS. Also, these subjects were non-smokers and drug naïve except for anti-hypertensive medications. These findings may explain in part, the increased CV risk in MetS population. Future studies will focus on mechanistic insights and intervention strategies to ameliorate altered EPC status in this high risk population.
Supplementary Material
Acknowledgments
The study was supported by ADA grant (IJ) and NIH K24 (AT00596, IJ) and UL1 RR024146 from the National Center for Research Resources(NCRR), a component of the National Institutes of Health (NIH), and to CTSC. Authors acknowledge assistance of Long Wang, PhD in assisting with subject recruitment and Manpreet Kaur for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors report any conflicts of interest.
References
- 1.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 4.Tousoulis D, Andreou I, Antoniades C, et al. Role of inflammation and oxidative stress in endothelial progenitor cell function and mobilization: therapeutic implications for cardiovascular diseases. Atherosclerosis. 2008;201:236–247. doi: 10.1016/j.atherosclerosis.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Agostini C, Avogaro A. Endothelial progenitor cells and vascular biology in diabetes mellitus: current knowledge and future perspectives. Curr Diabetes Rev. 2005;1:41–58. doi: 10.2174/1573399052952640. [DOI] [PubMed] [Google Scholar]
- 7.Hill JM, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 8.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 9.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 10.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 11.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67:1672–1676. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 12.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 13.Chen JZ, Zhang FR, Tao QM, et al. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 14.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 15.Bauersachs J, Thum T. Endothelial progenitor cell dysfunction: mechanisms and therapeutic approaches. Eur J Clin Invest. 2007;37:603–606. doi: 10.1111/j.1365-2362.2007.01833.x. [DOI] [PubMed] [Google Scholar]
- 16.MacEneaney OJ, Kushner EJ, Van Guilder GP, et al. Endothelial progenitor cell number and colony forming capacity in overweight obese adults. Int J Obesity (Lond) 2009;33:219–225. doi: 10.1038/ijo.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller – Ehmsen J, Braun D, Schneider T, et al. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J. 2008;29:1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- 18.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 19.Fadini GP, Kreutzenberg SV, Coracina A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Ht J. 2006;27:2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 20.Westerweel PE, Visseren FL, Hajer GR, et al. Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. Simvastatin / ezetimibe combination therapy. Eur Heart J. 2008;29:2808–2817. doi: 10.1093/eurheartj/ehn431. [DOI] [PubMed] [Google Scholar]
- 21.Fadini GP, Agostini C, Boscaro E, Avogaro A. Mechanisms and significance of progenitor cell reduction in the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:5–10. doi: 10.1089/met.2008.0067. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Fukuda N, Yao EH, et al. Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am J Hypertens. 2008;21(1):72–77. doi: 10.1038/ajh.2007.5. [DOI] [PubMed] [Google Scholar]
- 23.Werner C, Kamani CH, Gensch C, Böhm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56(10):2609–2615. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Fadini GP, de Kreutzenberg S, Agostini C, et al. Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis. 2009;207:213–219. doi: 10.1016/j.atherosclerosis.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 26.Fadini GP, Sartore S, Albiero M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Lucke C, Rössig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 28.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 29.Fadini GP, Baesso I, Albiero M, et al. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 30.Satoh M, Ishikawa Y, Takahashi Y, et al. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Hein TW, Singh U, Vasquez-Vivar J, et al. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. 2009;206:61–68. doi: 10.1016/j.atherosclerosis.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma S, Kuliszewski MA, Li SH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between CRP and cardiovascular disease. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 33.George J, Goldstein E, Abashidze S, et al. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25:1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.